Abstract
Background/Aim:
Hepatitis D virus (HDV) superinfection in patients with chronic hepatitis B leads to accelerated liver injury, early cirrhosis, and decompensation. It may be speculated that hepatocellular carcinoma (HCC) may differ in these patients from hepatitis B virus (HBV) monoinfection. The aim of this study was to compare clinical aspects of hepatocellular carcinoma in patients of hepatitis D with HBV monoinfection.
Patients and Methods:
A total of 92 consecutive HCC cases seropositive for antibody against HDV antigen (HDV group) were compared with 92 HBsAg-positive and anti-HDV-negative cases (HBV group).
Results:
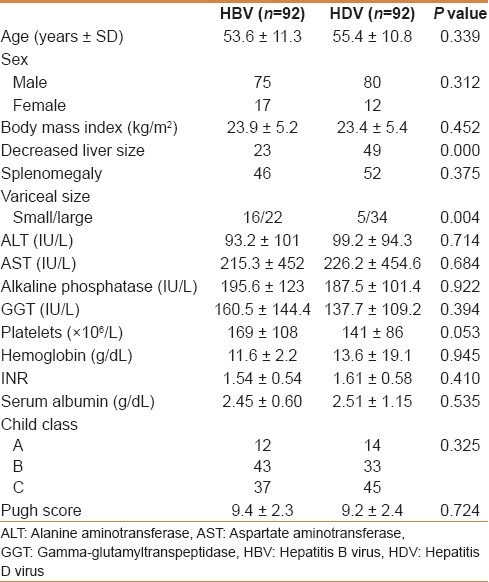
The features including sex, body mass index, presence of ascites, serum biochemistry, gross tumor appearance, child class, barcelona cancer liver clinic and okuda stages were not significantly different between the 2 groups. Decreased liver size was noticed more in cases of HDV compared with HBV group where the liver size was normal or increased (P=0.000). HDV patients had lower platelets (P=0.053) and larger varices on endoscopy (P=0.004). Multifocal tumors and elevated alpha-fetoprotein level >1000 IU/mL were more common in HBV group (P=0.040 and P= 0.061). TNM classification showed more stage III-IV disease in HBV group (P=0.000).
Conclusion:
Decreased liver size and indirect evidence of more severe portal hypertension and earlier TNM stage compared with HBV monoinfection indicate that HDV infection causes HCC in a different way, possibly indirectly by inducing inflammation and cirrhosis.
Keywords: Hepatocellular carcinoma, hepatitis D, hepatitis B
Chronic hepatitis B is a major cause of cirrhosis, hepatocellular carcinoma (HCC), and liver-related mortality worldwide. Coinfection with hepatitis D virus (HDV) is associated with more severe liver disease and poor prognosis.[1–3] HDV is a defective RNA virus that requires hepatitis B virus (HBV) as helper virus.[4] Two viruses share the same route of transmission, being transmitted by contaminated blood and body fluids. HDV can infect simultaneously with HBV (coinfection) or in a patient with already established HBV infection (superinfection).
Although the incidence of HDV infection has decreased in the endemic countries as a result of effective immunoprophylaxis against HBV and improvement in socioeconomic and hygienic conditions,[5–7] it remains a relevant cause of morbidity in the Asia Pacific region.[8] There is no satisfactory treatment of hepatitis D. HDV infection is a critical problem in our country. It is present in 16.6% of hepatitis B-infected patients in Pakistan, most commonly in younger males living in rural areas.[9]
HDV infection increases the risk for HCC threefold and mortality twofold in patients with HBsAg-positive cirrhosis.[10] In a study from Japan the overall relative risk for liver cirrhosis and HCC was 2.58 and 2.87, respectively.[11] However, these findings were contradicted by a retrospective analysis of 962 HBV patients that showed similar rates of HCC in the 82 HDV-infected patients and 880 non-infected patients.[12] According to another study, persistent HDV replication leads to cirrhosis and HCC at annual rates of 4% and 2.8%, respectively, and is the only predictor of liver-related mortality.[13] The aim of this study was to compare clinical features and tumor characteristics of HCC in hepatitis D antibody–positive patients with hepatitis B monoinfection in an Asian country with a high prevalence of HDV infection.
PATIENTS AND METHODS
All adult patients (age ≥18 years) with HCC associated with chronic HDV infection who were admitted under Gastroenterology Hepatology Services in a tertiary care hospital between January 1999 and June 2009 were identified by ICD coding. These consecutive cases were reviewed and compared with consecutive HBV monoinfection-related HCC patients who were positive for HBsAg, but seronegative for HDV and managed during the same time period.
Due to high prevalence of hepatitis D, it is an institutional policy to rule out hepatitis D in every case of hepatitis B. HBV and HDV infections were diagnosed by commercially available enzyme-linked immunosorbent assays for HBsAg and anti-HDV antibody. Antibody to hepatitis C virus was assayed by a third-generation test system. Patients were labeled as suffering from HBV monoinfection if antibodies for both hepatitis C and D were found negative in a patient with reactive HBsAg. The diagnosis of HCC was defined as either the presence of a hepatic lesion >2cm in diameter on triphasic CT with typical vascular pattern for HCC (hypervascular with washout in the portal/venous phase) with or without elevated alpha-fetoprotein >200 ng/mL or the presence of a lesion1–2 cm in diameter with typical vascular pattern for HCC on two dynamic imaging techniques.[14]
Demographic data (age, sex, body mass index were extracted from patients’ records. Patients with cirrhosis were identified based on clinical features of cirrhosis and/or radiologic evidence of cirrhosis in the context of portal hypertension (ascites, varices, thrombocytopenia, or hepatic encephalopathy). Patient data from the first clinic visit or admission with HCC were used to calculate Child–Turcotte–Pugh score and stage of HCC by Okuda,[15] Barcelona Cancer Liver Clinic (BCLC),[16] and TNM staging systems.[17] Alpha-fetoprotein levels during the same admission were also noted.
All of these patients underwent ultrasound examination followed by a triphasic CT scan.
Ultrasound examinations were carried out by experienced radiologists with the fasting subjects lying in the supine position. Measurement of the liver diameter was done in the right midclavicular line during deep inspiration. The size of the liver was measured from the hepatic dome to the inferior hepatic tip. The liver diameter of 12–15 cm was taken as normal.[18] Numbers of the tumor lesions were counted from the CT scan.
Endoscopic evaluation of varices was recorded where available. Endoscopy was performed by the same group of physicians. Grading system to document the size of varices being followed in our hospital evaluates varices as follows: Grade I, varices present but flatten completely with air insufflations; grade II, nonflatteningvarices that occupy 10%–30% of the esophageal luminal radius; grade III, varices occupy 31%–60% of the esophageal luminal radius; grade IV, varices occupy 61%–100% of the esophageal luminal radius.[19,20] For this study Grade I–II varices were grouped together as small and Grade III–IV as large varices.
Statistical analysis
Descriptive statistics were computed for all variables. Chi-square test was used for dichotomous variables and Mann–Whitney U test for continuous variables. P value of less than 0.05 was taken as significant. SPSS version 17 software (SPSS, Chicago, IL, USA) was used for all analyses.
RESULTS
Total number of patients of HCC seropositive for HDV identified were 92 (HDV group). They were compared with 92 consecutive HBsAg positive but anti-HDV negative HCC cases (HBV group). Combining two groups the study includes 184 patients, 84% of patients were males (male 155 and females 29). Their age was 54.6 ± 11.1 years (median 55, range 18-85). Most of these patients were in Child class B or C (A=26, B=76, C=82) with a median Child-Pugh score of 9 (5–14). Their Okuda stage was II in 106 (57.6%), III in 54 (29.4%), and I in 24 (13%).
The mean age was not different in both groups of patients (55.4 years in HDV group and 53.6 in HBV group). Other features, including sex, presence of ascites, serum biochemistry, and Child class, were not significantly different between the two groups [Table 1]. Decreased liver size was noticed more in cases of HDV compared with HBV group where the liver size was normal or increased (P=0.000). HDV patients had lower platelets (P=0.053). Endoscopic evaluation of varices was available in 77 patients, 39 in the HDV group and 38 in the HBV group. HDV group had more “large” (grade III–IV) varices on endoscopy compared with HBV group (P=0.004).
Table 1.
Characteristics of patients with HBV and HDV hepatocellular carcinoma
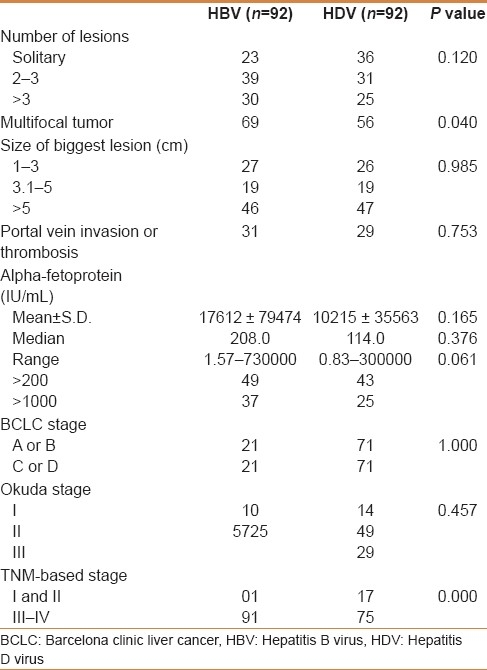
The mean alpha-fetoprotein levels were higher in HBV patients than in HDV patients (17612 vs 10215). However, there was no statistically significant difference. More patients in HBV group had elevated alpha-fetoprotein >1000; P value of 0.061 though not significant, did show a trend [Table 2]. Multifocal tumors were more common in HBV group (P=0.040). TNM classification showed more stage III-IV disease in HBV group (P=0.000),although there was no difference in Okuda or BCLC stage (P=0.764 and P=1.000).
Table 2.
Tumor characteristics of HBV and HDV hepatocellular carcinomas
DISCUSSION
HCC is a substantial complication of liver cirrhosis. It is recognized that HBV is one of the few human oncogenous viruses.[21] HCC incidence is higher in countries where hepatitis B is endemic. Addition of HDV infection seems to increase the risk of HCC development. HDV-related cirrhosis is usually an aggressive disease with a median time to decompensation less than 2 years and a median survival less than 5 years.[22] However, as with any immune-mediated disease, different patterns of progression, ranging from mild to severe progressive disease, are observed.[23] In these patients, it is the clinical decompensation and not HCC, which is the first dominant complication to appear. Severity of HDV infection may be an important predictor of HCC. Patients infected with genotype one HDV have a lower remission rate, more aggressive disease, and more adverse outcomes (cirrhosis, hepatocellular carcinoma, or mortality) than those with genotype II HDV.[24,25]
Unfortunately HDV genotype I is prevalent in our country and in one of our previous studies all the HDV patients belonged to this genotype.[26] HBV genotype D is prevalent in about 95% of our HBV patients.[27] Although the information about genotypes is not available in the present study, it may be presumed that most of our patients would be harboring HDV genotype I and HBV genotype D.
Hepatitis viruses may cause HCC through an indirect mechanism inducing inflammation and cirrhosis, whereas HBV has a direct oncogenic potential as well.[28] HBV DNA integrates into cellular DNA but is not an acutely transforming virus, because HCC usually develops decades after infection. Other factors, namely cirrhosis, inflammation, alcohol intake, and viral superinfections, could promote the oncogenetic process induced by HBV. HDV infection, superimposed on the oncogenetic background provided by chronic HBV infection appears to provide an additional promotion risk for HCC. Our HDV-positive patients had decreased liver size and indirect evidence of more severe portal hypertension and earlier TNM stage compared with HBV monoinfection, which may indicate that HDV infection causes HCC possibly indirectly by inducing inflammation and cirrhosis.
Patients with florid infections from both HBV and HDV and active liver inflammation should develop HCC at a younger age than those infected by HBV alone.[29,30]
However, there was no significant age difference in our study. This may be because in endemic areas HDV may superinfect at any age and start the aggressive disease leading to cirrhosis and eventually HCC. It is also known that active replication of HBV promotes carcinogenesis[31] and HDV can inhibit HBV genome and replication[32,33] and could interfere with cancer development in HDV patients with less aggressive disease. This ability of HDV to suppress HBV replication could represent a protective mechanism to lower the risk of HCC development.
There was much difference in TNM-based staging among two groups. Our patients of hepatitis B were mostly in stage III-IV. This may reflect a more aggressive tumor behavior. This trend is also reflected by a higher number of HBV group patients having their alpha-fetoprotein level >1000 IU/mL. On the other hand, early stage in HDV HCC could be attributable to lead time bias; earlier diagnosis while investigating early decompensation or more severe portal hypertension. The latter was reflected in our cases by decreased liver size, lower platelet count, and larger varices. However, there was no difference in Child–Pugh score because this scoring system does not take into account the above factors. This may also explain why there was no difference in Okuda staging, which depends on the tumor size and three measures of the severity of cirrhosis (the amount of ascites and the serum albumin and bilirubin levels) included in the Child–Pugh scoring. There was also no difference in the BCLC staging, which is based on the extent of the primary lesion, performance status, vascular invasion, as well as Child class. The patient-centered approach in this system is more appropriate to segregate good surgical candidates for resection or radical therapy from those requiring palliative care while our patients in both groups presented late.
Our patients did not originate from a surveillance program, and diagnosis was made incidentally or for the presence of tumor-related symptoms. The patients were identified by the ICD coding system leading to the identification of hospitalized patients, with an advanced underlying liver disease as well as stage of the tumor. Most of our patients (except two in each group) had clinical cirrhosis. The diagnosis of liver cirrhosis was made by radiologic and clinical evaluation and biopsies were not done. This may influence the characterization of the tumor. However, in both groups cirrhosis stage was equally distributed. The possibility of early cirrhosis in four cases (two in each group) who did not have clinical cirrhosis cannot be ruled out.
The main strength of this paper is the large number of patients with HDV/HBV hepatocellular carcinoma evaluated. The diagnosis of HDV coinfection was made by measuring serum HDV antibodies and HDV RNA was not done. Considering this data it is not possible to determine if our patients were having an active form of hepatitis D at the time of presentation. Data concerning the serum levels of HBV-DNA and the status of HBe antigen and antibody was also not available in all cases. The patients were identified by the ICD coding system leading to the identification of hospitalized patients, with a severe underlying liver disease (high prevalence of Child–Pugh C patients), which may influence the characterization of the tumor. These are patients with really advanced tumors (small number of Okuda I patients in both groups). However, it is not possible to perform a case control prospective study as it would take years to get sufficient number of cases HDV-positive HCC cases. However, our findings may be confirmed in a large prospective multicenter study.
In summary, we have tried to identify some differences in the presentation of HCC in hepatitis D versus hepatitis B. More HDV patients have decreased liver size and indirect evidence of more severe portal hypertension and earlier TNM stage compared with HBV monoinfection. Multifocal tumors are more common in hepatitis B. These facts may indicate that HDV infection possibly causes HCC indirectly by inducing inflammation and cirrhosis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wursthorn K, Manns MP, Wedemeyer H. Natural history: The importance of viral load, liver damage and HCC. Best Pract Res Clin Gastroenterol. 2008;22:1063–79. doi: 10.1016/j.bpg.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen YC, Sheen IS, Chu CM, Liaw YF. Prognosis following spontaneous HBsAgseroclearance in chronic hepatitis B patients with or without concurrent infection. Gastroenterology. 2002;123:1084–9. doi: 10.1053/gast.2002.36026. [DOI] [PubMed] [Google Scholar]
- 3.Seetlani NK, Abbas Z, Raza S, Yakoob J, Jafri W. Prevalence of hepatitis D in HBsAg positive patients visiting liver clinics. J Pak Med Assoc. 2009;59:434–7. [PubMed] [Google Scholar]
- 4.Rizzetto M, Canese M, Aricò S. Immunofluorescence detection of a new antigen antibody system (delta/anti-delta) associated to hepatitis B virus in liver and serum of HBsAg carriers. Gut. 1977;18:997–1003. doi: 10.1136/gut.18.12.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaeta B, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S, et al. Chronic hepatitis D: A vanishing Disease? An Italian multicenter study. Hepatology. 2000;32:824–7. doi: 10.1053/jhep.2000.17711. [DOI] [PubMed] [Google Scholar]
- 6.Değertekin H, Yalçin K, Yakut M, Yurdaydin C. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: A meta-analysis. Liver Int. 2008;28:494–8. doi: 10.1111/j.1478-3231.2008.01673.x. [DOI] [PubMed] [Google Scholar]
- 7.Huo TI, Wu JC, Lin RY, Sheng WY, Chang FY, Lee SD. Decreasing hepatitis D virus infection in Taiwan: An analysis of contributory factors. J Gastroenterol Hepatol. 1997;12:747–51. doi: 10.1111/j.1440-1746.1997.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 8.Abbas Z, Jafri W, Raza S. Delta hepatitis in Asia Pacific Region-Review. World J Gastroenterol. 2010;16:554–62. doi: 10.3748/wjg.v16.i5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mumtaz K, Hamid SS, Adil S, Afaq A, Islam M, Abid S, et al. Epidemiology and clinical pattern of hepatitis delta virus infection in Pakistan. J Gastroenterol Hepatol. 2005;20:1503–7. doi: 10.1111/j.1440-1746.2005.03857.x. [DOI] [PubMed] [Google Scholar]
- 10.Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B.The European Concerted Action on Viral Hepatitis (Eurohep) Gut. 2000;46:420–6. doi: 10.1136/gut.46.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamura I, Kurimura O, Koda T, Ichimura H, Katayama S, Kurimura T, et al. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: A study from Kure, Japan. J Gastroenterol Hepatol. 1993;8:433–6. doi: 10.1111/j.1440-1746.1993.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 12.Cross TJ, Rizzi P, Horner M, Jolly A, Hussain MJ, Smith HM, et al. The increasing prevalence of hepatitis D virus (HDV) infection in South London. J Med Virol. 2008;80:277–82. doi: 10.1002/jmv.21078. [DOI] [PubMed] [Google Scholar]
- 13.Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, de Franchis R, et al. A 28-year study of the course of hepatitis delta infection: A risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 2009;136:1629–38. doi: 10.1053/j.gastro.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, et al. Natural history of hepatocellular carcinoma and prognosis in relation to treatment.Study of 850 patients. Cancer. 1985;56:918–28. doi: 10.1002/1097-0142(19850815)56:4<918::aid-cncr2820560437>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin Liver Dis. 1999;19:329–38. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 17.Greene FL, Page DL, Fleming ID, editors. 6th ed. New York: Springer Verlag Inc; 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 18.Kratzer W, Fritz V, Mason RA, Haenle MM, Kaechele V Roemerstein Study Group. Factors affecting liver size: A sonographic survey of 2080 subjects. J Ultrasound Med. 2003;22:1155–61. doi: 10.7863/jum.2003.22.11.1155. [DOI] [PubMed] [Google Scholar]
- 19.Miller LS, Schiano TD, Adrain A, Cassidy M, Liu JB, Ter H, et al. Comparison of high resolution endoluminalsonography to video endoscopy in the detection of esophageal varices. Hepatology. 1996;24:552–5. doi: 10.1002/hep.510240315. [DOI] [PubMed] [Google Scholar]
- 20.Kane L, Kahaleh M, Shami VM, Caldwell SH, Berg CL, Abdrabbo KM, et al. Comparison of the grading of esophageal varices by transnasalendoluminal ultrasound and esophagogastroduodenoscopy. Clin Gastroenterol Hepatol. 2005;3:806–10. doi: 10.1016/s1542-3565(05)00482-9. [DOI] [PubMed] [Google Scholar]
- 21.Ryu WS. Molecular aspects of hepatitis B viral infection and the viral carcinogenesis. J Biochem Mol Biol. 2003;36:138–43. doi: 10.5483/bmbrep.2003.36.1.138. [DOI] [PubMed] [Google Scholar]
- 22.Gheorghe L, Iacob S, Simionov I, Vadan R, Gheorghe C, Iacob R, et al. Natural history of compensated viral B and D cirrhosis. Rom J Gastroenterol. 2005;14:329–35. [PubMed] [Google Scholar]
- 23.Yurdaydın C, Idilman R, Bozkaya H, Bozdayi AM. Natural history and treatment of chronic delta hepatitis. J Viral Hepat. 2010;17:749–56. doi: 10.1111/j.1365-2893.2010.01353.x. [DOI] [PubMed] [Google Scholar]
- 24.Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, Chen SW, et al. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology. 2006;130:1625–35. doi: 10.1053/j.gastro.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 25.Niro GA, Smedile S, Andriulli A, Rizzetto M, Gerin JL, Casey JL. The predominance of hepatitis delta virus genotype I among chronically infected Italian patients. Hepatology. 1997;25:728–34. doi: 10.1002/hep.510250339. [DOI] [PubMed] [Google Scholar]
- 26.Moatter T, Abbas Z, Shabir S, Jafri W. Clinical presentation and genotype of hepatitis delta in Karachi. World J Gastroenterol. 2007;13:2604–7. doi: 10.3748/wjg.v13.i18.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas Z, Muzaffar R, Siddiqui A, Naqvi SA, Rizvi SA. Genetic variability in the precore and core promoter regions of hepatitis B virus strains in Karachi. BMC Gastroenterol. 2006;6:20. doi: 10.1186/1471-230X-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveri F, Brunetto MR, Actis GC, Bonino F. Pathobiology of chronic hepatitis virus infection and hepatocellular carcinoma (HCC) Ital J Gastroenterol. 1991;23:498–502. [PubMed] [Google Scholar]
- 29.Verme G, Brunetto MR, Oliveri F, Baldi M, Forzani B, Piantino P, et al. Role of hepatitis delta virus infection in hepatocellular carcinoma. Dig Dis Sci. 1991;36:1134–6. doi: 10.1007/BF01297460. [DOI] [PubMed] [Google Scholar]
- 30.Brunetto MR, Oliveri F, Colombatto P, Bonino F. Hepatocellular carcinoma and infections with multiple hepatitis viruses. Princess Takamatsu Symp. 1995;25:61–6. [PubMed] [Google Scholar]
- 31.Ikeda K, Arase Y, Kobayashi M, Someya T, Saitoh S, Suzuki Y, et al. Consistently low hepatitis B virus DNA saves patients from hepatocellular carcinogenesis in HBV related cirrhosis.A nested case-control study using 96 untreated patients. Intervirology. 2003;46:96–104. doi: 10.1159/000069744. [DOI] [PubMed] [Google Scholar]
- 32.Sagnelli E, Felaco FM, Rapicetta M, Stroffolini T, Petruzziello A, Annella T, et al. Interaction between HDV and HBV infection in HbsAg-chronic carriers. Infection. 1991;19:155–8. doi: 10.1007/BF01643238. [DOI] [PubMed] [Google Scholar]
- 33.Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Galimany R, et al. Role of hepatitis B, C, and D viruses in dual and triple infection: Influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404–10. doi: 10.1053/jhep.2001.26511. [DOI] [PubMed] [Google Scholar]


