Abstract
Background/Aim:
To identify the prevalence of gluten sensitivity in a healthy adult Saudi population within a low endemic area of celiac disease using IgA tissue transglutaminase antibody. The study was conducted as a prospective pilot study for Saudi attendees of a blood donation centre at King Faisal Specialist Hospital & Research Centre in Jeddah, Saudi Arabia.
Patients and Methods:
Individuals were invited to participate in the study and screened for gluten sensitivity using immunoglobulin A tissue transglutaminase antibody (IgA TTG) along with serum IgA level. Descriptive data was presented and expressed as mean value; correlation between variables was estimated using Pearson correlation, and nonparametric data using Pearson rho correlation (level of P value <0.05 is considered to be statistically significant).
Results:
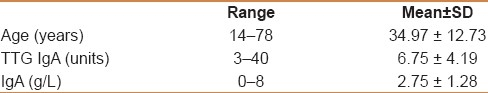
Two hundred and four individuals (122 males and 82 females, mean age 35 years) attending the blood donation centre were screened. Three individuals tested positive for IgA TTG showing normal IgA level (1 female and 2 males) with a 1.5% prevalence in the cohort.
Conclusions:
Positive celiac screening is present at a low prevalence rate in our adult population, in which the individuals’ age and their serum IgA levels are not associated with the positivity level. A study on a larger scale with the application of histologic confirmation of positive cases is needed.
Keywords: Celiac, duodenal, gluten, tissue transglutaminase antibody
Celiac disease (CD) prevalence, often considered uncommon outside the western hemisphere, tends to be misdiagnosed or underdiagnosed. Interestingly, even in high-prevalence areas, a large survey revealed CD as underdiagnosed by primary care physicians compared with gastroenterologists (11% vs 65%). Furthermore, the manifestations of the disease were deemed uncommon (32%) in adulthood.[1] Most often, CD presents with nongastroenterological features; therefore, sound knowledge of the diversity of the disease and its prevalence in the region is essential. This pilot study would explore the prevalence of this disease in a silent group or asymptomatic individuals.
PATIENTS AND METHODS
Between April and July 2010, Saudi attendees of the Blood Donation Centre of King Faisal Specialist Hospital and Research Centre, Jeddah, were assessed by the principal investigator for their current medical issues and possible coexisting manifestations of CD using a short self-administered questionnaire. Subsequently, the purpose of this study was explained and informed consent obtained from all the participants. Blood samples were drawn to screen for CD using tissue transglutaminase antibody immunoglobulin A (TTG IgA) and immunoglobulin A (IgA) to rule out IgA deficiency. TTG is measured using the commercial enzyme-linked immunonosorbent assay (ELISA, QUANTA lite, Inova Diagnostics, San Diego, CA, USA) Positive TTG IgA is defined at a level above 20.00 units. Serum IgA is measured using immunoturbidimetric assay (COPAS INTEGRA® 400 plus, Roche, Mannheim, Germany). The reference range of serum IgA is 0.70–4.00 g/L. Sample size calculation for determination of the desired sample in consideration to the reported prevalence in the middle eastern area showed that 300 individuals are needed to be included to determine the prevalence. This study is approved and monitored by the Institutional Review Board of King Faisal Specialist Hospital and Research Centre, Jeddah.
RESULTS
Two hundred and four individuals (122 males and 82 females) attending the blood donation center were screened [Table 1]. Based on the screening questionnaire, no individual was found to have features to suggest CD. No short stature was identified in the cohort (defined as a standing height more than 2 standard deviations (SDs) below the mean (or below the 2.5 percentile) for gender). Three individuals tested positive for TTG IgA revealing normal IgA level (1 female and 2 males); their values are as follows: A 22-year-old female, 22 units; a 31-year-old male, 32 units; and a 33-year-old male, 40 units, with the prevalence rate in the cohort being 1.5%. No IgA-deficient individuals were reported. A significant association was found to exist between serum IgA and anti-TTG levels (P=0.0001) and serum IgA levels and the age of the individuals (P=0.010). A non significant association was noted between age and the TTG IgA level (P=0.554) as well as between serum IgA level and TTG status (P=0.153).
Table 1.
Summary of celiac disease screening
DISCUSSION
CD is an autoimmune disorder prevalent in patients with hypersensitivity to a gluten component and its derivatives in agricultural crops, most typically observed in European descendants; however, the current evolutionary data showed that in areas of the fertile crescent in Iraq, Syria, and Iran, this component is present and has been consumed by the locals over several centuries,[2] and therefore, the possibility of CD development is at present due to the continuous exposure to this antigen. Various studies have shown, in fact, that CD prevalence is reaching those in developing countries with a reported prevalence of 3%–20% in at- risk populations and 3%–5% in those with type I diabetes mellitus.[3] Great interest in estimating the prevalence of the disease is growing in what were formerly considered low-prevalence areas, including the Middle East. The diversity of CD manifestations triggered physicians’ awareness of the disease, as well as Richard Logan's iceberg principle developed in 1991 in Europe, in which a large group of patients went underdiagnosed, as they were asymptomatic. He found that the ratio of diagnosed to undiagnosed CD was 5:1–13:1, the total size of the iceberg being more or less the same worldwide, despite differences in the waterline from continent to continent.[4] Very few studies exist addressing the prevalence of CD in Saudi Arabia, particularly in an adult population. Al-Attas identified the prevalence of CD in different group categories, including high-risk and average populations. Among the high-risk group based on clinical suspicion, autoimmune thyroid diseases and inflammatory bowel disease, the prevalence rate using the IgA Endomysial Ab is 7.6%;11%, nondetected in inflammatory bowel disease. Also, CD could not be detected in healthy donors using the IgA Endomysial Ab.[5] Insulin-dependent diabetes mellitus type 1 is a high-risk group in which the prevalence of CD using antigliadin and antireticulin antibodies in 10 out of 123 diabetic pediatric patients is 8.1%.[6] When CD prevalence in the Middle East region was studied, its prevalence in low-risk individuals using serology of different markers indicated that CD was present in 0.14%—1.3%, using the duodenal biopsy ranges between 0.033% and 1.17%.[7] In Iran, CD reported in apparently healthy blood donors was 1/166 using IgA antigliadin and IgA antiendomysial antibodies.[8] This study reports a similar prevalence in the adult population with tissue transglutaminase antibody IgA of 1.5%, over a wide representative population age range. TTG IgA was chosen for this study because of its high specificity, above 95% and sensitivity of 90%–96%. The common presence of IgA deficiency estimated at 1:500–700, and particularly in those with CD, the reported incidence being 2%–3%,[9] the IgA value in this study was mandated for each patient. No IgA-deficient individual was detected. Considering the limitations imposed by reporting the prevalence of CD in this study, including those not biopsied or who had declined biopsy, the prevalence in the western populations appears to be approximately 1:100 (1%) with a range of 1:80–1:140 (1.25%–0.71%).[10] The question that arises is whether the diagnosis of CD was satisfactorily arrived at, based on serology alone or in combination with duodenal biopsy? Also, could serology alone be done to discuss the prevalence of CD? The answer has been addressed in several prior studies. Sanders and colleagues reported through a decision tool applied to a large group that had undergone upper endoscopy and duodenal biopsy, that in a given positive TTG of a high-risk group, based on the referral symptoms, have a sensitivity, specificity, positive predictive value, and negative predictive value for a positive antibody result to diagnose CD are 90.9%, 90.9%, 28.6%, and 99.6%, respectively.[11] When two tests are employed together, such as TTG and DPG (deaminated gliadin peptide), it will be possible to reach or rule out a diagnosis of CD without biopsy in 92% of cases in pretest populations of low- and high-risk individuals for CD, with better results in the high-risk individuals.[12] Higher levels of TTG IgA antibodies in conjunction with serum total IgA levels are associated with the increased likelihood of a diagnosis of CD.[13] The observations in this study also showed a statistically significant association between serum IgA and TTG IgA levels that would support the importance of serum IgA measurement.
Limitations of this study stem from it being a small sample coupled with the inability to confirm the positive serologic cases with a corresponding duodenal histology. This will provide a more appropriate representation of the prevalence and support the statistical conclusions regarding the presence of CD in the silent population.
CONCLUSION
In conclusion, the prevalence of CD compares favourably with the low-prevalent regions within the neighbouring countries, and in the areas having a higher disease incidence, duodenal biopsy is needed for confirmation of the disease, especially in individuals with low serology titres, particularly in the low-prevalence areas. With increased serology titres the precision and accuracy to diagnose CD improve, with probably a lesser need to perform duodenal biopsy.
Footnotes
Source of Support: Umm AlQura University Research Grant 43009008
Conflict of Interest: None declared.
REFERENCES
- 1.Zipser RD, Farid M, Baisch D, Patel B, Patel D. Physican awareness of celiac disease: A need for further education. J Gen Intern Med. 2005;20:644–6. doi: 10.1111/j.1525-1497.2005.0107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malekzadeh R, Sachdev A, Fahid Ali A. Coeliac disease in developing countries: Middle East, India and North Africa. Best Pract Res Clin Gastroenterol. 2005;19:351–8. doi: 10.1016/j.bpg.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Catassi C, Rätsch IM, Gandolfi L, Pratesi R, Fabiani E, El Asmar R, et al. Why is coeliac disease endemic in the people of the Sahara? Lancet. 1999;354:647–8. doi: 10.1016/s0140-6736(99)02609-4. [DOI] [PubMed] [Google Scholar]
- 4.Issue 2. Vol. 10. Munich, Germany: World Gastroenterology Organiziation; 2005. WGO-OMGE Practice Guideline, Celiac Disease, World Gastroenterology News; pp. 1–8. [Google Scholar]
- 5.Al Attas RA. How common is celiac disease in Eastern Saudi Arabia? Ann Saudi Med. 2002;22:315–9. doi: 10.5144/0256-4947.2002.315. [DOI] [PubMed] [Google Scholar]
- 6.Al-Ashwal AA, Shabib SM, Sakati NA, Attia NA. Prevalence and characteristics of celiac disease in type I diabetes mellitus in Saudi Arabia. Saudi Med J. 2003;24:1113–5. [PubMed] [Google Scholar]
- 7.Barada K, Bitar A, Mokadem MA, Hashash JG, Green P. Celiac disease in Middle Eastern and North African countries: A new burden? World J Gastroenterol. 2010;16:1449–57. doi: 10.3748/wjg.v16.i12.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Moghadam KF, Farhadi M, Ansari R, et al. High prevalence of coeliac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–8. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Jarzabek-Chorzelska M, Sulej J, Karnewska K, Farrell T, Jablonska S. Celiac disease and immunoglobulin a deficiency: How effective are the serological methods of diagnosis? Clin Diagn Lab Immunol. 2002;9:1295–300. doi: 10.1128/CDLI.9.6.1295-1300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Hopper AD, Cross SS, Hurlstone DP, McAlindon ME, Lobo AJ, Hadjivassiliou M, et al. Pre-endoscopy serological testing for coeliac disease: Evaluation of a clinical decision tool. BMJ. 2007;334:729. doi: 10.1136/bmj.39133.668681.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugai E, Moreno ML, Hwang HJ, Cabanne A, Crivelli A, Nachman F, et al. Celiac disease serology in patients with different pretest probabilities: Is biopsy avoidable? World J Gastroenterol. 2010;16:3144–52. doi: 10.3748/wjg.v16.i25.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeersch P, Coenen D, Geboes K, Mariën G, Hiele M, Bossuyt X. Use of likelihood ratios improves clinical interpretation of IgA anti-TTG antibody testing for celiac disease. Clin Chim Acta. 2010;411:13–7. doi: 10.1016/j.cca.2009.09.030. [DOI] [PubMed] [Google Scholar]


