Abstract
Background/Aim:
We aim to investigate the safety of outpatient blind percutaneous liver biopsy (BPLB) in infants and children with chronic liver disease (CLD).
Patients and Methods:
BPLB was performed as an outpatient procedure using the aspiration Menghini technique in 80 infants and children, aged 2 months to 14 yrs, for diagnosis of their CLD. Patients were divided into three groups: Group 1 (<1 year), group 2 (1–6 yrs), and group 3 (6–14 yrs). The vital signs were closely monitored 1 hr before biopsy, and then 1, 2, 6, and 24 hrs after biopsy. Twenty-four hours pre- and post-biopsy complete blood counts, liver enzymes, prothrombin time (PT), and abdominal ultrasonography, searching for a biopsy-induced hematoma, were done for all patients.
Results:
No mortality or major morbidities were encountered after BPLB. The rate of minor complications was 17.5% including irritability or “pain” requiring analgesia in 10%, mild fever in 5%, and drowsiness for >6 hrs due to oversedation in 2.5%. There was a statistically significant rise in the 1-hr post-biopsy mean heart and respiratory rates, but the rise was non-significant at 6 and 24 hrs except for group 2 where heart rate and respiratory rates significantly dropped at 24 hrs. No statistically significant difference was noted between the mean pre-biopsy and the 1, 6, and 24-hrs post-biopsy values of blood pressure in all groups. The 24-hrs post-biopsy mean hemoglobin and hematocrit showed a significant decrease, while the 24-hrs post-biopsy mean total leucocyte and platelet counts showed non-significant changes. The 24-hrs post-biopsy mean liver enzymes were non-significantly changed except the 24-hrs post-biopsy mean PT which was found to be significantly prolonged, for a yet unknown reason(s).
Conclusions:
Outpatient BPLB performed by the Menghini technique is safe and well tolerated even in infants and young children. Frequent, close monitoring of patients is strongly recommended to achieve optimal patient safety and avoid potential complications.
Keywords: Blind percutaneous liver biopsy, complications, infants, children
Liver biopsy is one of the most specific tests to assess the nature and severity of liver diseases. It can also be useful in monitoring the efficacy of various treatments. Blind percutaneous liver biopsy (BPLB) was first reported in pediatric practice in the late 1950s[1–5] and was regarded safe in this age group.[1,6] Paul Ehrlich is credited with performing the first percutaneous liver biopsy in 1883 in Germany,[7,8] therefore BPLB has been considered a helpful diagnostic procedure for more than a 100 yrs.[9,10] It is the most commonly used procedure to obtain tissue for histopathological assessment of liver disease.[11] BPLB is an important procedure in diagnosing liver disease in infants and children as it often provides diagnostic information not obtainable by other methods.[1,12,13] Recent reports have confirmed the low incidence of complications of liver biopsy as long as results of coagulation tests are normal.[1,14–16] After Menghini reported a technique for “one-second needle biopsy of the liver” in 1958, the procedure became more widely used. The average duration of the intrahepatic phase of previous liver-biopsy techniques had been 6–15 min.[8,17]
The size of the biopsy specimen varies between 1 and 4 cm in length and between 1.2 and 1.8 mm in diameter and represents 1/50,000 of the total mass of the liver.[18] British guidelines on liver biopsy consider that most hepatopathologists are satisfied with a biopsy specimen containing at least six to eight portal triads,[18,19] especially in cases of chronic liver disease (CLD) in which the extent of injury may vary among portal triads. An adequate specimen is provided when Menghini needles used measure up to 2 mm in diameter.[8,20,21]
To our knowledge, no pediatric series specifically examined the morbidity and mortality of outpatient BPLB in Egyptian infants and children. We meticulously monitored a cohort of Egyptian infants and children with CLD undergoing BPLB before and after the procedure to assess its safety.
Aim of work
In this study, we aimed to investigate the safety and complications of the Menghini aspiration (suction) technique of BPLB among infants and children, conducted as an outpatient procedure at the Hepatology Outpatient Clinic at Cairo University Children Hospital, Cairo, Egypt. The value of pre- and post-biopsy ultrasonographic scanning in diagnosing post-biopsy complications, particularly hepatic hematomas, was also thoroughly investigated.
PATIENTS AND METHODS
This study included 80 infants and children (58 males and 22 females) aged 2 months to 14 yrs undergoing liver biopsy as an outpatient procedure. They were divided into three age groups: Group 1 (<1 yr), group 2 (1–6 yrs), and group 3 (>6–14 yrs) [Figure 1].
Figure 1.

Age groups and percentage of the studied population
Inclusion criteria
Pediatric age group upto 14 years.
Patients under investigations for hepatomegaly.
Patients under investigations for hepatosplenomegaly.
Patients under investigations for cholestasis.
Patients under investigations for portal hypertension.
Exclusion criteria
History of unexplained bleeding.
Tendency to bleed
Prothrombin time (PT) >3 sec more than control, uncorrectable by vitamin K1.
Platelet count <70,000/mm3.
Prolonged bleeding time (>10 min).
Use of any non-steroidal anti-inflammatory drug within previous 7–10 days.
Blood for transfusion unavailable.
Suspected hemangioma or other vascular tumor.
Inability to identify an appropriate site for biopsy by percussion or ultrasonography.
Suspected echinococcal cysts in the liver.
We considered morbid obesity, moderate to massive ascites, hemophilia, and infection in the right pleural cavity or below the right hemidiaphragm as relative contraindications.
All patients were subjected to the following:
Before biopsy
Thorough history taking including the main presenting symptoms and the time of onset of the disease.
Full general and local clinical examination for jaundice, abdominal distension, liver and spleen size, and the presence of ascites.
-
Laboratory investigations:
- Complete blood count (CBC): Including hemoglobin (Hb) level, haematocrit (Hct) value, total leucocytic count (TLC), and platelet count (Plat).
- Liver function tests (LFTs): Including total serum bilirubin (TSB) and direct serum bilirubin (DSB), alanine amino-transferase (ALT), aspartate amino-transferase (AST), and alkaline phosphatase (Alk Phos), PT, partial thromboplastin time, and bleeding time were done within 48 hrs before the biopsy for all patients. All patients were routinely given a single shot of 2–10 mg vitamin K1 intramuscularly (IM). Patients with PT more than 3 sec above control were given 10 mg vitamin K1 IM daily, and if PT was not corrected, they were excluded from the study.
Abdominal ultrasonography: For all patients using commercially available real-time machine (Toshiba Sonolayer V model SSL – 53 M, Toshiba Corporation, Tokyo, Japan). The studied data included measuring the hepatic, splenic, and renal spans and echo textures as well as searching for hepatic hematoma alongside the needle track. The gallbladder, bile duct, Portal vein, hepatic veins, and the peritoneal cavity were all meticulously examined.
Needle liver biopsy using the aspiration or suction Menghini technique[17] with a modified needle in which the nail of the original needle is absent. The bore of the needle ranged from 1.6 to 1.8 mm in internal diameter and a needle length of 7–9 cm depending on the age of the patient.
Biopsy procedure
Baseline vital signs including the heart rate (HR), respiratory rate (RR), arterial blood pressure (BP), and core body temperature were recorded 1 hr before the biopsy. Diazepam in a dose of 0.1 mg/kg body weight was used as a sedative in 68 patients 15–20 min before the procedure. Under local infiltration anesthesia by lidocaine hydrochloride, the intercostal right midaxillary approach was used in most patients (70 patients), whereas the subcostal midclavicular approach was used in 10 patients only. The needle was introduced in the right midaxillary line above the rib, just 1 intercostal space below the maximum liver dullness (in the intercostal approach) or below the costal margin in the midclavicular line (in the subcostal approach). After piercing the skin and subcutaneous tissues, reaching the surface of the liver, the trocar was retracted by the attached syringe, creating negative pressure and a length of 3–7 cm of the whole needle was introduced in the liver substance and withdrawn rapidly within 1 sec as originally described by Menghini.[17] The direction was downward, forward, and medially toward the umbilicus in the intercostal approach and directly posteriorly in the subcostal approach. The biopsies were fixed in 10% formol saline and stained using hematoxylin and eosin. After the biopsy, patients were placed in the right lateral decubitus. Fasting for at least 4 hrs after the biopsy was done for observation and early detection of the possible complications. Patients were allowed to go home 8 hrs after the procedure.
After biopsy
Vital signs (HR, RR, BP, and temperature) were closely monitored and recorded hourly for the first 4 hrs, two hourly for the following 4 hrs and then at 24 hrs after the procedure.
Oxygen saturation was measured using simple pulse oximetry before, during, and at 1, 2, 3, 4, 6, and 24 hrs post-biopsy. It should be ≥94%. If it drops below 94%, blood gases should be done.
All symptoms suggesting complications including irritability in infants or right hypochondrial pain in older children, pallor and fever were observed. CXR should be done if pulmonary symptoms develop or if a small pneumothorax is suspected.
CBC, LFTs, and PT were performed at 24 hrs after the biopsy.
Abdominal ultrsonography at 24 hrs after the procedure for early detection of complications with special concern on possible hematoma development whether intraparenchymal, subcapsular, or intraperitoneal, using both convex linear probe with a frequency of 5 MHz and sector probe for confirmation of the absence of haematomas.
RESULTS
This study included 80 infants and children undergoing outpatient BPLB who were subdivided into three age groups. All patients except four had hepatomegaly, 32 had splenomegaly, 16 had jaundice, and none had ascites. Patients included were for investigations and differential diagnosis of isolated hepatomegaly (28 patients), hepatosplenomegaly (22 patients), cholestasis (16 patients), glycogen storage disease (10 patients), and portal hypertension with esophageal varices (4 patients).
The mean pre- and post-biopsy vital signs were recorded [Table 1]. At 1 hr post-biopsy follow-up, there was a significant rise in the mean HR in the three groups with P value 0.002, 0.001, and 0.000, respectively. All patients in group 1, 42 of 48 patients in group 2 and 14 of 16 cases in group 3 had an increase in HR by <20 beats/minute. In contrast, the mean HR counted at 6 and 24 hrs post-biopsy showed no statistically significant difference from the pre-biopsy HR in all three groups. The 1-hr post-biopsy mean RR showed a significant rise in the three groups with P value 0.000, 0.022, and 0.000, respectively. The 6-hrs post-biopsy mean RR showed no statistically significant difference from the pre-biopsy mean RR in all three groups. However, the 24-hrs post-biopsy mean RR dropped significantly compared with the pre-biopsy mean RR among group 2 only (P 0.002).
Table 1.
Results of the pre- and post-biopsy vital signs of the studied population
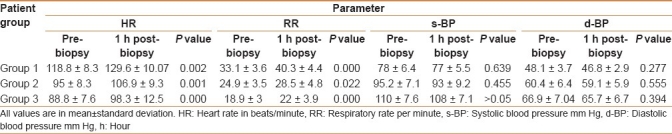
The mean systolic and diastolic BP in the three groups were nearly similar to the pre-biopsy measurements with the exception of an occasional slight drop; however, no statistically significant difference was noted between the mean pre-biopsy and the three post-biopsy values of BP in all three groups.
Oxygen saturation measured by pulse oximetry remained stable all over pre-, during, and post-biopsy with its value ≥94%. No patient required blood gas analysis.
The pre- and post-biopsy values of the different laboratory tests done to our patients and their significance are shown in Table 2. The pre-biopsy mean Hb level ranged between 6.9 and 13.9 gm/dl. Reassessment at 24-hrs post-biopsy showed a significant decrease (P=0.002). Similar results were obtained for the Hct value (P=0.001). The pre-biopsy TLC ranged from 3,100 to 18,800/mm3. At 24-hrs post-biopsy, the mean TLC was decreased, but this decrease did not reach a statistically significant difference. Fifty-two patients (65%) showed a decrease in the TLC and 28 patients (35%) showed an increase in the TLC. The pre-biopsy platelets count ranged from 140,000 to 584,000. The overall 24-hrs post-biopsy mean platelets count decreased but did not reach a statistically significant difference.
Table 2.
Pre- and post-biopsy laboratory tests values and significances
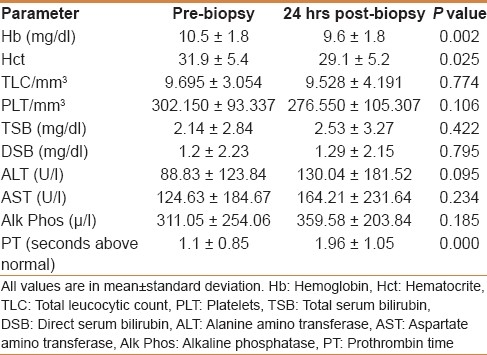
Regarding the LFTs, the pre-biopsy TSB ranged from 0.2 to 11.5 mg/dl and DSB 0.0 to 10.3 mg/dl. At 24-hrs post-biopsy, there was a non-statistically significant rise in the mean TSB and the mean DSB. ALT level before biopsy was between 9 and 508 U/l (normal value up to 20). Twenty-four hours after biopsy, the mean ALT showed a non-statistically significant increase. AST levels before biopsy ranged from 14 to 812 U/l (normal value up to 20), while 24 hrs after biopsy, the mean AST showed a non-statistically significant increase. Pre-biopsy serum Alk Phos level range was 118 to 1586 U/l (normal 120–400), and 24 hrs after biopsy, the mean serum Alk Phos was increased but did not reach a statistical significance.
In this study, liver biopsy was not performed if PT was prolonged by more than 3 sec above the control. In the 80 cases, the pre-biopsy prolongation of PT above the control ranged from 0.0 to 2.5 sec. The 24-hrs post-biopsy PT was significantly increased in 50 patients (65.5%, P 0.000).
As regards the intra- and post-procedure problems, two needle passes were needed in 10 pateints (12.5%). Among two of these cases, the liver was not palpable below the costal margin. Irritability or pain requiring medication (paracetamol) was observed in 8 cases (10%), drowsiness for more than 6 hrs probably due to oversedation in 2 patients (2.5%), and mild fever managed by cold compresses in 4 patients (5%). No mortality or major morbidity such as bile leak, pneumothorax, and bleeding requiring transfusion were detected (in spite of the significant drop in both Hb% and Ht and the significant prolongation of the PT 24 hrs after biopsy). In those with irritability or pain, CXR was done to rule out the possibility of small pneumothorax and it was normal in all of the eight patients.
Abdominal ultrasonograhy was done 24 hrs after biopsy in all patients (both by convex linear and sector probes) with no detectable complications particularly hematomas and intraperitoneal bleeding.
DISCUSSION
Liver biopsy is a well-established procedure in the diagnosis and follow-up of liver diseases. It is indicated in various clinical settings, to establish a diagnosis, to assess prognosis, and to monitor therapy.[23] It can change the clinical diagnosis in 8–14%, the management in 12–18%, and the frequency of LFT monitoring in 36% of cases.[24,25] Although the liver has a rich vascular supply, complications associated with percutaneous liver biopsy are rare. Sixty percent of complications occur within 2 hrs and 96% within 24 hrs after the procedure.[7,16] In a large study in adults, only 4% of complications occurred late (1–6 days after the procedure).[16] However, still BPLB is an invasive technique that carries a risk of complications. Although the rate of major complications is low, minor complications may occur, though not frequently.[23]
In this study, we kept our patients fasting for the first 4 hrs after biopsy for proper monitoring and early detection of complications. In the study carried out by Lichtman and his colleagues[1] on 174 infants, nearly all their babies were active and eating within 1–2 hrs after the procedure.
Follow-up of our patients after biopsy revealed a significant increase in the mean HR in the three study groups 1 hr after biopsy, while no significant change 6 hrs post-biopsy. There was a significant drop in the mean HR of the second group 24 hrs after biopsy. In the adult study conducted by Minuk and his colleagues,[26] there was a highly significant increase in the mean HR of their cases 24 hrs after biopsy.
The arterial BP showed a non-significant drop in the mean systolic and diastolic readings in the three age groups at 1, 6, or 24 hrs after biopsy. None of our cases suffered from drop of BP by more than 20 mm Hg. Similarly in another study, the investigators reported that only 1 out of 184 biopsies (0.5%) in children less than 1 yr old showed a drop of more than 20 mm Hg in the systolic BP.[1] Others reported a highly significant drop in both mean systolic and mean diastolic pressures in the 6-hrs period after biopsy. Their study included 40 adult patients, 31 patients diagnosed as cirrhosis and 5 neoplasia. In addition, 11 patients (27.5%) had a post-biopsy drop in systolic and diastolic BP >20 mm Hg.[26] The difference between our results and theirs may be attributed to the difference in age of the two studied groups as well as the difference in their histopathological findings. The higher incidence of cirrhosis and neoplasia among their groups might predispose to more post-biopsy blood loss which may account for the more significant drop in BP.
Regarding the mean Hb% and Hct, we detected a significant drop 24 hrs after biopsy with 5% showing a decrease by more than 2 g/dl. Other authors reported a decrease in Hb% of more than 2 g/dl among 3 children out of 184 (1.6%).[1]
The TLC in our study did not change significantly all through the 24 hrs after biopsy except in one patient; 65% showed a drop in the TLC, whereas 35% showed an increase in the TLC with one case showing an increase of more than 10,000/mm3. In this case, infection was suggested as the patient had fever of 38.5°C, but cultures were negative. Reddy and Schiff[27] stated that transient bacteremia occurs in 5.8–13.5% of patients after liver biopsy, and although such bacteremia is generally inconsequential, septicemia and shock can develop on rare occasions in patients with biliary obstruction and cholangitis. Currently, there are no recommendations for the routine use of prophylactic antibiotics in patients undergoing liver biopsy including those with prosthetic valves or joints.[28]
As regard the platelet count, there was a nonsignificant change in the platelets count 24 hrs after biopsy in this study with 70% of patients showing a drop of platelets and 30% showing a rise. This rise may be a reactive thrombocytosis. As regards the LFTs, this study showed a non-significant rise of liver enzymes (AST, ALT Alk Phos) at 24 hrs after the biopsy, whereas PT showed a highly significant prolongation which has a yet unknown reason(s) and needs to be validated in a bigger study.
Regarding the ultrasonographic findings, no intrahepatic or subcapsular hematomas were detected in the 80 cases at 24 hrs after the biopsy. In the study of Lichtman and his colleagues, hematoma at the biopsy site was reported in only 1 out of 148 cases.[1]
Actually, BPLB has been adopted as a procedure in children without the same extensive examination of its safety as in adults.[1] In this study, no major complications were reported. Only minor complications such as irritability or pain requiring analgesia, oversedation, or drowsiness for more than 6 hrs, and mild fever occurred. Reports from the late 1940s of large numbers of children being investigated for malnutrition suggested that BPLB by a subcostal approach was safe.[29] Early reports of small series in children using the Menghini technique showed no major complications.[2–5] Walker et al.[6] reported no life-threatening complications in a series of 210 biopsies performed in 166 patients, of whom 67 were of age 1 yr or less and 24 were of age 1 week to 2 months. In a study of the risks of complications of the different pediatric gastroenterology procedures in 1981,[30] a rate of 4% in 584 liver biopsies performed at 25 centers was found. These complications included bleeding, pneumothorax, and pain. However, the clinical details including ages of the patients, number of infants with and without complications, condition of patients, and biopsy technique were not given in that study.[30]
Ultrasound was done to all our patients prior to biopsy to exclude the presence of liver cysts or huge dilatation of the bile ducts likely to be punctured by the biopsy needle. Any highly vascular mass such as a hemangioma, arterio-venous malformation, or vascular liver tumour were considered not suitable for BPLB; however, liver congestion per se was not a contraindication. Three patients were excluded based on the ultrasonographic findings; one with hepatic cyst and two with liver hemangiomas. Presence of infection of the adjacent lung, pleura, or skin, or peritonitis was also considered a contraindication for biopsy. Serious complications of liver biopsy can be avoided if patients at high risk of bleeding are excluded. Despite claims that hepatic bleeding after biopsy is a random event[31] patients with abnormal clotting studies seem to be at risk. In our series, no patients underwent biopsy if the PT was >3 sec above the control or platelet count less than 70,000/mm3. In the study of Litchman and his coworkers,[1] no patients underwent biopsy if the PT was >15 sec or the partial thromboplastin time was >49 sec. The platelet count was >60,000/mm3 in all but one in their series.[1] Although these criteria are considered “absolute” contraindications by most hepatologists, they can be corrected by transfusions of platelets or fresh-frozen plasma and are therefore not truly absolute.[22]
Investigators in adults encountered other minor complications as transient, localized discomfort at the biopsy site; pain requiring analgesia; and mild, transient hypotension (due to a vasovagal reaction). Approximately one fourth of patients had pain in the right upper quadrant or right shoulder after liver biopsy. The pain was usually dull, mild, and brief.[32] Ongoing, severe pain in the abdomen should alert the physician to the possibility of a more serious complication, such as bleeding or peritonitis. Although very rare, clinically significant intraperitoneal hemorrhage is the most serious bleeding complication of BPLB; it usually becomes apparent within the first 2–3 hrs after the procedure.[33] Free intraperitoneal blood may result from laceration caused by deep inspiration during the biopsy or may be related to a penetrating injury of a branch of the hepatic artery or portal vein. Risk factors for hemorrhage after liver biopsy are older age, more than three passes with the needle during biopsy, and the presence of cirrhosis or liver cancer.[34] Findings of free intraperitoneal fluid on ultrasonography or computed tomography should be correlated with the clinical assessment of the patient.[35] If hemorrhage is suspected, immediate arrangements for blood, platelets, and plasma should be made, and a surgeon and an angiographer should be alerted. Measures to improve the patient's hemodynamic status by the administration of intravenous fluids, blood products, or both may be sufficient. If hemodynamic instability persists for a few hours despite the use of aggressive resuscitative measures, angiography and embolization or surgical exploration are indicated. Small intrahepatic or subcapsular hematomas can be noted after liver biopsy even in asymptomatic patients.[36] Large hematomas may cause pain associated with tachycardia, hypotension, and a delayed decrease in the hematocrit. Conservative treatment of hematomas is generally sufficient.[33] A study by Lebensztejn et al.[37] conducted on 250 patients aged 1–17 yrs with the use of Menghini needles reported complications in 3 patients (1.2%).
We did all our BPLBs as an outpatient or as day cases. Currently, 4–70% of liver biopsies are performed as outpatient procedure, with a reported frequency of 30% in Romania, 29% in France, and 70% in the United States.[23,38–41] The complication rate is similar for inpatients and outpatients.[42,43] The safety of BPLB in adults has been discussed in numerous reports;[44–46] the most recent large study reported a mortality of 0.005% with the Menghini technique.[9] Other investigators found the mortality rate among patients after BPLB approximately 1 in 10,000 to 1 in 12,000.[33,47] Mortality is highest among patients who undergo biopsies of malignant lesions. Cirrhosis is another risk factor as it might lead to fatal bleeding after liver biopsy.[8] The least common of the hemorrhagic complications is hemobilia,[48] which usually presents with the classic triad of gastrointestinal bleeding, biliary pain, and jaundice[19,24] approximately 5 days after the biopsy.[49] Other rare complications of BPLB include biliary ascites, bile pleuritis, bile peritonitis, pneumothorax, hemothorax, subcutaneous emphysema, pneumoperitoneum, pneumoscrotum, subphrenic abscess, carcinoid crisis, anaphylaxis after biopsy of an echinococcal cyst, pancreatitis due to hemobilia, and breakage of the biopsy needle.[16,33] None of these complications were encountered in our series.
We used the modified aspiration (suction) Menghini needles in all patients. Some investigators found that the Tru Cut needle produce has more complications than Menghini needle (3.5/1,000 versus 1/1,000).[16,49] This may be related to the shorter stay of the needle in the hepatic parenchyma in the Menghini technique, which allows short time for the patient to move minimizing the risk of tearing the capsule.[19] However, some authors believe that the risks for both needles are about the same.[50]
An important question regarding the percutaneous liver biopsy that should be addressed is blind or ultrasound-guided techniques? The answer depends on the skills of the gastroenterologist (hepatologist) and on the technical possibilities (accessibility to the ultrasound machine). However, it is still debatable whether ultrasound-guided LB has an advantage over the blind one or not.[51,52] In a prospective study in France, Cadranel et al.[38] showed that from 2084 liver biopsies, only 56% were echo-guided. Also, many studies showed that the complications of LB seem to be related to the type of the technique, blind, or echo-guided, respectively: (1) Younossi et al.[53] showed that the complications appeared in 4% of the cases with “blind” biopsies and in 2% of the cases with “ultrasound-guided” biopsies (the study revealed the cost-effectiveness of echo-guided biopsy); (2) Farrell et al.[54] showed complications in 1.8% of the cases with “ultrasound-guided” biopsies and in 7.7% of the cases with “blind” biopsies (P<0.05); and (3) Pasha et al.[52] showed that severe complications occurred in 0.5% of the cases with “ultrasound-guided” biopsies and in 2.2% of the cases with “blind” biopsies (P<0.05). The same author revealed that the pain appeared more often (50% of the cases) in the “blind” biopsy group as compared with the “ultrasound-guided” biopsy group (37% of the cases, P=0.003).
We believe that BPLB is a safe procedure with some acceptable minor complications. It is not time consuming. No special skills of the gastroenterologist (hepatologist) regarding performing and interpreting ultrasound or technical difficulties (accessibility to the ultrasound machine) are needed. This is of special value in centers where ultrasonography is not readily available all the time or where its use in invasive procedure is limited by the availability of experienced radiologist. The outpatient BPLB is also of great value in saving hospital resources and limiting hospitalization.
We obtained important histopathological information in 85% of our patients and no significant information in 15%. This finding is nearly similar to that reported by other studies who obtained diagnostically important data in 83% of cases.[1]
Actually the theoretical risk of liver biopsy in adults has been determined by pooling individual series of liver biopsies. Data on infants from other studies in which the Menghini method was used are difficult to interpret because of insufficient information, and if these are added to our series they raise the total number of patients, which is still too small to eliminate the β error entirely. Our data from one center with only one biopsy technique suggest that BPLB is safe in infants and children. The decision about whether to perform a liver biopsy in an infant should be based on the usefulness of the histological information to be gained as well as on technical considerations. Delay in diagnosis of CLD in infants and children can have serious consequences, and we strongly recommend biopsy without undue delay or hesitation from the pediatrician or the family side.
CONCLUSION
We conclude that BPLB performed by the aspiration Menghini technique is safe even in infants and small children. The patients should have normal coagulation, and there should be no other major contraindications to the procedure. Ultrasound examination of the liver should be performed before liver biopsy. Although the ease and safety of outpatient liver biopsy has been established, the procedure is not trivial. The 24-hrs-post-biopsy significant prolongation of PT needs to be looked into. Close, frequent monitoring of the patient undergoing BPLB is necessary to achieve optimal safety.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lichtman S, Guzman C, Moore D, Weber JL, Roberts EA. Morbidity after percutaneous liver biopsy. Arch Dis Child. 1987;62:901–4. doi: 10.1136/adc.62.9.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruton OC, Metzger JF Sprinz H. Experience with needle biopsy of the liver in infants and children. Pediatrics. 1955;16:836–41. [PubMed] [Google Scholar]
- 3.Kaye R, Koop CE, Wagner BM, Picou D, Yakovac WC. Needle biopsy of the liver, an aid in the differential diagnosis of prolonged jaundice in infancy. AMA J Dis Child. 1959;98:699–709. [PubMed] [Google Scholar]
- 4.Hong R, Schubert WK. Menghini needle biopsy of the liver. Am J Dis Child. 1960;100:42–6. doi: 10.1001/archpedi.1960.04020040044009. [DOI] [PubMed] [Google Scholar]
- 5.Porter M, Riley HD, Jr, Graham H. Needle biopsy of the liver in infants and children. J Pediatr. 1964;65:176–88. doi: 10.1016/s0022-3476(64)80518-7. [DOI] [PubMed] [Google Scholar]
- 6.Walker WA, Krivit W, Sharp HL. Needle biopsy of the liver in infancy and childhood.A safe diagnostic aid in liver disease. Pediatrics. 1967;40:946–50. [PubMed] [Google Scholar]
- 7.van Leeuwen DJ, Wilson L, Crowe DR. Liver biopsy in the mid-1990s: Questions and answers. Semin Liver Dis. 1995;15:340–59. doi: 10.1055/s-2007-1007286. [DOI] [PubMed] [Google Scholar]
- 8.Bravo A, Sheth SG, Chopra S. Liver biopsy. [Last accessed on 2011 Apr 9];N Engl J Med. 2001 344:495–500. doi: 10.1056/NEJM200102153440706. Available from: http://www.nejm.org . [DOI] [PubMed] [Google Scholar]
- 9.Sherlock S. 8th ed. Boston, Melbourne: Blackwell Scientific Publication; 1989. Diseases of the liver and biliary system: Needle biopsy of the liver; pp. 36–48. [Google Scholar]
- 10.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: An audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–41. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nobili V, Comparcola D, Sartorelli MR, Natali G, Monti L, Falappa P, et al. Blind and ultrasound-guided percutaneous liver biopsy in children. Pediatr Radiol. 2003;33:772–5. doi: 10.1007/s00247-003-1044-0. [DOI] [PubMed] [Google Scholar]
- 12.Balistreri WF. Neonatal cholestasis. J Pediatr. 1985;106:171–84. doi: 10.1016/s0022-3476(85)80282-1. [DOI] [PubMed] [Google Scholar]
- 13.Brough AJ, Bernstein J. Conjugated hyperbilirubinemia in early infancy.A reassessment of liver biopsy. Hum Pathol. 1974;5:507–16. doi: 10.1016/s0046-8177(74)80003-1. [DOI] [PubMed] [Google Scholar]
- 14.Mahal AS, Knauer CM, Gregory PB. Bleeding after liver biopsy: How often and why? Gastroenterology. 1979;76:1192. [Google Scholar]
- 15.Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: Complications in 1000 inpatients and outpatients. Gastroenterology. 1978;4:103–6. [PubMed] [Google Scholar]
- 16.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy.A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–73. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 17.Menghini G. One-second needle biopsy of the liver. Gastroenterology. 1958;35:190–9. [PubMed] [Google Scholar]
- 18.Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice.British Society of Gastroenterology. Gut. 1999;45(Suppl 4):41–411. doi: 10.1136/gut.45.2008.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klatskin G, Conn HO, editors. Histopathology of the liver. Vol. 1. New York: Oxford University Press; 1993. Techniques; pp. 3–8. [Google Scholar]
- 20.Sporea I, Popescu A, Sirli R. Why, who and how should perform liver biopsy in chronic liver diseases. World J Gastroenterol. 2008;14:3396–402. doi: 10.3748/wjg.14.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: How safe is it? Ann Intern Med. 1993;118:150–3. doi: 10.7326/0003-4819-118-2-199301150-00013. [DOI] [PubMed] [Google Scholar]
- 22.Brown KE, Janney CG, Brunt EM. Liver biopsy: Indications, technique, complications, and interpretation. In: Bacon BR, Di Bisceglie AM, editors. Liver disease: Diagnosis and management. New York: Churchill Livingstone; 2000. p. 47. [Google Scholar]
- 23.Sparchez Z. Complications after percutaneous liver biopsy in diffuse hepatopathies. Rom J Gastroenterol. 2005;14:379–84. [PubMed] [Google Scholar]
- 24.Friedman LS. Controversies in liver biopsy: Who, where, when, how, why? Curr Gastroenterol Rep. 2004;6:30–6. doi: 10.1007/s11894-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 25.McGill DB. Liver biopsy: When, how, by whom, and where? Curr Gastroenterol Rep. 2001;3:19–23. doi: 10.1007/s11894-001-0036-1. [DOI] [PubMed] [Google Scholar]
- 26.Minuk GY, Sutherland LR, Wiseman DA, MacDonald FR, Ding DL. Prospective study of the incidence of ultrasound-detected intrahepatic and subcapsular hematomas in patients randomized to 6 or 24 hours of bed rest after percutaneous liver biopsy. Gastroenterology. 1987;92:290–3. doi: 10.1016/0016-5085(87)90119-3. [DOI] [PubMed] [Google Scholar]
- 27.Reddy KR, Schiff ER. Complications of liver biopsy. In: Taylor MB, editor. Gastrointestinal emergencies. 2nd ed. Baltimore: Williams and Wilkins; 1997. pp. 959–68. [Google Scholar]
- 28.Reddy KR, Jeffers LJ. Evaluation of the liver: Liver biopsy and laparoscopy. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff's diseases of the liver. 8th ed. Vol. 1. Philadelphia: Lippincott-Raven; 1999. pp. 245–66. [Google Scholar]
- 29.Sherlock S. Needle biopsy of the liver: A review. J Clin Pathol. 1962;15:291–304. doi: 10.1136/jcp.15.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ament M. Prospective study of risks of complication in 6,424 procedures in pediatric gastroenterology. Pediatr Res. 1981;15:524. [Google Scholar]
- 31.Ewe K. Bleeding after liver biopsy does not correlate with indices of peripheral coagulation. Dig Dis Sci. 1981;26:388–93. doi: 10.1007/BF01313579. [DOI] [PubMed] [Google Scholar]
- 32.Castera L, Negre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30:1529–30. doi: 10.1002/hep.510300624. [DOI] [PubMed] [Google Scholar]
- 33.van Thiel DH, Gavaler JS, Wright H, Tzakis A. Liver biopsy: Its safety and complications as seen at a liver transplant center. Transplantation. 1993;55:1087–90. doi: 10.1097/00007890-199305000-00029. [DOI] [PubMed] [Google Scholar]
- 34.Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118:96–8. doi: 10.7326/0003-4819-118-2-199301150-00003. [DOI] [PubMed] [Google Scholar]
- 35.Hederstrom E, Forsberg L, Floren CH, Prytz H. Liver biopsy complications monitored by ultrasound. J Hepatol. 1989;8:94–8. doi: 10.1016/0168-8278(89)90167-0. [DOI] [PubMed] [Google Scholar]
- 36.Raines DR, Van Heertum RL, Johnson LF. Intrahepatic hematoma: A complication of percutaneous liver biopsy. Gastroenterology. 1974;67:284–9. [PubMed] [Google Scholar]
- 37.Lebensztejn DM, Kaczmarski M, Sobaniec-Łotowska M, Barwijuk-Machała M. Blind liver biopsy in children–diagnostic significance and complications in authors’ own material. Med Sci Monit. 2000;6:1155–8. [PubMed] [Google Scholar]
- 38.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: Results of a prospective nationwide survey.For the Group of Epidemiology of the French Association for the study of the liver (AFEF) Hepatology. 2000;32:477–81. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 39.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: An audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–41. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith BC, Desmond PV. Outpatient liver biopsy using ultrasound guidance and the biopty gun is safe and cost effective. Aust N Z J Med. 1995;25:209–11. doi: 10.1111/j.1445-5994.1995.tb01524.x. [DOI] [PubMed] [Google Scholar]
- 41.Gheorghe L, Iacob S, Gheorghe C. Liver biopsy under ultrasound control for diffuse liver disease - toward a faster, safer, cost-effective and easy acceptable procedure. Rom J Gastroenterol. 2005;14:97–8. [PubMed] [Google Scholar]
- 42.Douds AC, Joseph AE, Finlayson C, Maxwell JD. Is day case liver biopsy underutilised? Gut. 1995;37:574–5. doi: 10.1136/gut.37.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiezia S, Salvio A, Di Somma C, Scelzi C, Assanti AP, Giannattasio F, et al. The efficacy of liver biopsy under ultrasonographic guidance on an outpatient basis. Eur J Ultrasound. 2002;15:127–31. doi: 10.1016/s0929-8266(02)00033-2. [DOI] [PubMed] [Google Scholar]
- 44.Zamcheck N, Klausenstock O. Liver biopsy (concluded). II. The risk of needle biopsy. N Engl J Med. 1953;249:1062–9. doi: 10.1056/NEJM195312242492605. [DOI] [PubMed] [Google Scholar]
- 45.Thaler H. [On advantages and dangers of the menghini method of liver biopsy] Wien Klin Wochenschr. 1964;76:533–8. [PubMed] [Google Scholar]
- 46.Lindner H. [Limitations and hazards of percutaneous liver biopsy with the Menghini needle.Experiences with 80,000 liver biopsies] Dtsch Med Wochenschr. 1967;92:1751–7. doi: 10.1055/s-0028-1106036. [DOI] [PubMed] [Google Scholar]
- 47.McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–400. doi: 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 48.Lee SP, Tasman-Jones C, Wattie WJ. Traumatic hemobilia: A complication of percutaneous liver biopsy. Gastroenterology. 1977;72:941–4. [PubMed] [Google Scholar]
- 49.Lin CL, Chang JJ, Lee TS, Lui KW, Yen CL. Gallbladder polyp as a manifestation of hemobilia caused by arterial-portal fistula after percutaneous liver biopsy: A case report. World J Gastroenterol. 2005;14:305–7. doi: 10.3748/wjg.v11.i2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malnick S, Melzer E. Routine ultrasound-guided liver biopsy: A time whose idea has come? J Clin Gastroenterol. 2005;39:900–3. doi: 10.1097/01.mcg.0000180803.49328.a7. [DOI] [PubMed] [Google Scholar]
- 51.Vautier G, Scott B, Jenkins D. Liver biopsy: Blind or guided? BMJ. 1994;309:1455–6. doi: 10.1136/bmj.309.6967.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pasha T, Gabriel S, Therneau T, Dickson ER, Lindor KD. Cost-effectiveness of ultrasound-guided liver biopsy. Hepatology. 1998;27:1220–6. doi: 10.1002/hep.510270506. [DOI] [PubMed] [Google Scholar]
- 53.Younossi ZM, Teran JC, Ganiats TG, Carey WD. Ultrasound-guided liver biopsy for parenchymal liver disease: An economic analysis. Dig Dis Sci. 1998;43:46–50. doi: 10.1023/a:1018815802500. [DOI] [PubMed] [Google Scholar]
- 54.Farrell RJ, Smiddy PF, Pilkington RM, Tobin AA, Mooney EE, Temperley IJ, et al. Guided versus blind liver biopsy for chronic hepatitis C: Clinical benefits and costs. J Hepatol. 1999;30:580–7. doi: 10.1016/s0168-8278(99)80187-1. [DOI] [PubMed] [Google Scholar]


