Abstract
Background/Aim:
To investigate the geographic occurrence of mucosa-invading Fusobacteria in acute appendicitis.
Patients and Methods:
Carnoy- and formalin-fixated appendices from Germany, Russia, and China were comparatively investigated. Bacteria were detected using fluorescent in situ hybridization. Cecal biopsies from patients with inflammatory bowel disease and other conditions were used as disease controls.
Results:
Fusobacteria represented mainly by Fusobacterium nucleatum were the major invasive component in bacterial infiltrates in acute appendicitis but were completely absent in controls. The occurrence of invasive Fusobacteria in Germany, Russia, and China was the same. The detection rate in Carnoy-fixated material was 70–71% and in formalin-fixated material was 30–36%.
Conclusions:
Acute appendicitis is a polymicrobial infectious disease in which F. nucleatum and other Fusobacteria play a key role.
Keywords: Acute appendicitis, fluorescence in situ hybridization, Fusobacteria, Fusobacterium necrophorum, Fusobacterium nucleatum, inflammatory bowel disease, infection, intestinal microbiota, invasive bacteria, mucosal flora, polymicrobial infection
Acute appendicitis is a common potentially life-threatening purulent disease in which multiple bacterial species are involved; the etiology, however, is unknown.
We recently published a study on mucosa-invading bacteria in acute appendicitis.[1] The tissue samples from patients obtained during surgery for acute appendicitis were compared with biopsies from inflamed cecum of patients with inflammatory bowel disease (IBD), self-limiting colitis, diverticulosis, and biopsies from healthy controls. Bacteria were assessed using fluorescence in situ hybridization (FISH). Fusobacteria were found to be invasive in 62% of patients with proven appendicitis but in none of the controls.
Studying the literature for data indicating the infectious origin of the disease, we found an article by Guo et al. published in 2004 in The American Journal of Surgery “Cluster of acute hemorrhagic appendicitis among high school students in Wuhan, China.”[2] Those authors concluded that the observed “cluster of acute appendicitis represent a special kind of appendicitis, with features of an infectious disease in epidemiology.”
To compare our findings of fusobacterial invasion in acute appendicitis, we asked the authors to send us sections of the resected appendices. In addition, we asked a pathologist from Moskau, Russia, to provide us the samples from patients with acute appendicitis fixated both in Carnoy solution and formalin.
PATIENTS AND METHODS
Appendices
Materials from appendices removed during laparoscopic emergent appendectomy were investigated. None of the patients had received preoperative antibiotic treatment. All patients were operated within 24 h after onset of symptoms for suspected acute appendicitis. They had no history of previous episodes, ruling out chronic appendicitis.
The surgeries in China and Russia as our previous German study were performed without preoperative antibiotics.
Germany
The material of appendices and biopsies obtained in Germany were described in a previous publication.[1] Altogether 52 appendices with proven acute appendicitis were investigated.
Cecal biopsies from 100 patients with ulcerative colitis, 100 patients with Crohn's disease, 50 patients with self-limiting colitis, 50 patients with diverticulosis, 50 patients with irritable bowel syndrome, and 50 healthy controls were investigated.[1]
Russia
Twenty-three appendices with intraoperatively confirmed acute appendicitis were removed during laparoscopic surgery in Russia Tushinskaya Children's Hospital, Moscow. Patients were 6–14 years old (mean age, 11 years). The study was prospective and included a general surgeon and a pathologist. The resected appendices were divided into 2 parts. One part was fixed in Carnoy solution (6/6/1 vol. ethanol/glacial acetic acid/chloroform) and the other in formalin, and then both were embedded in paraffin using standard techniques. All patients were operated within 24 h after onset of symptoms for suspected acute appendicitis. Forty cecal biopsies were taken from children, 12–18 years of age (mean age, 14 years), with confirmed active IBD from macroscopically inflamed regions. All biopsies were fixated in Carnoy and embedded in paraffin.
China
Sections of 11 appendices were received from the Department of Surgery, No. 457, Hospital of People's Liberation Army, where the patients were operated during an outbreak of acute appendicitis among high school students in China.[2] The resected appendices were previously fixated in formalin and then embedded in paraffin using standard techniques.
The initial paraffin blocks from the resections were not available, since they were used up for the extended histopathologic evaluation of an appendicitis outbreak published previously.[2] Of the initial sections of material 1–3 sections of each patient had remained intact and were used for this study.
Fluorescent in situ hybridization
Sections of 4 μm thickness were placed on SuperFrost plus slides (R. Langenbrinck, Emmendingen, Germany). Multicolor FISH was performed according to previously described protocols for evaluation of tissue specimens and identification of bacteria[3,4] with a Nikon e600 fluorescence microscope (Nikon, Tokyo, Japan) and Nikon DXM1200 camera. Altogether 7 FISH probes were applied.
The Cy3-labeled probe (orange fluorescence) that characterized a bacterial group of interest: F. nucleatum (Fnuc),[5] F. necrophorum (Fnec),[5] or Fusobacteria (Fuso)[5] was simultaneously hybridized with the universal for virtually all bacteria Eubacteria-specific (Eub388)[6] Alexa488-labeled probe (green fluorescence) and a mix of Cy5-labeled probes (red fluorescence) that included the main faecal bacterial groups such as Faecalibacterium prausnitzii (Fprau),[7] Bacteroides (Bac303),[8] Clostridium group and XIVa/Roseburia (Erec482).[9] The fluorescence signals of specific probes, universal probe, and probes for Fprau+Bac303+Erec482 groups were overlaid within the same microscopic field.
RESULTS
Histopathology
Germany
Twenty-seven of 52 patients with the clinical and intraoperative diagnosis of acute appendicitis had suppurative appendicitis and 25 had catarrhal appendicitis by histology.
Russia
Eleven of 23 patients with clinical and intraoperative diagnosis of acute appendicitis had suppurative appendicitis and 12 had catarrhal appendicitis by histology.
China
The initial paraffin blocks from the resections were not available, since they were used up for extended histopathologic examination of an appendicitis outbreak published previously. One to three sections of the initial resected appendices from each patient remained intact and were used for this study. All 11 patients had a suppurative appendicitis.
Infiltration of the mucosa by Fusobacteria in acute appendicitis
The occurrence of invasive Fusobacteria in Carnoy-fixated material from appendices in Germany and Russia were nearly the same [Table 1]. In the German material, the invasive Fusobacteria were mainly represented by F. nucleatum (79%), F. necrophorum (12%), and Fusobacteria (9%) that could not be determined at species level as they were positive with Fuso but negative with the Fnuc and Fnec FISH probes. F. nucleatum composed 85% and F. necrophorum 15% of the invasive Fusobacteria in the appendical material from Russia.
Table 1.
Occurrence of Fusobacteria in the mucosa
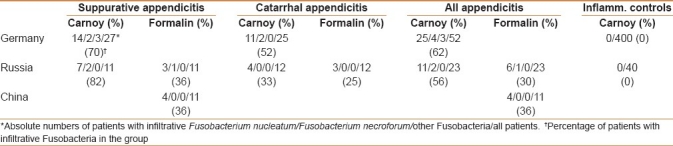
The invasion of Fusobacteria had an appearance of a needle-like infiltration of single epithelial cells within an intact appearing epithelial layer in patients with catarrhal appendicitis. We called them “pinned cells” because of the marked appearance of these infiltrates [Figure 1]. A deep infiltration of the submucosa with Fusobacteria was seen in suppurative appendicitis [Figure 2].
Figure 1.
Infiltration of single epithelial cells by Fusobacterium nucleatum (pinned cells) in the appendix of a patient with catarrhal appendicitis (hybridization with the Fnuc Cy3 probe, orange fluorescence, ×400)
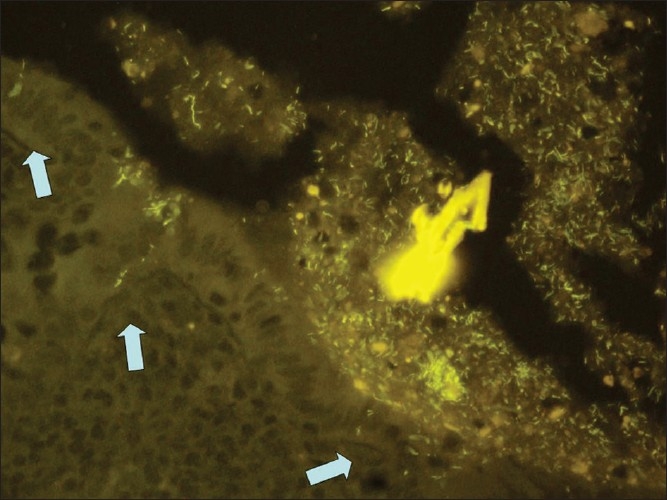
Figure 2.
Diffuse infiltration of the submucosa by Fusobacterium nucleatum in suppurative appendicitis (Fnuc Cy3 probe, orange fluorescence, ×400)
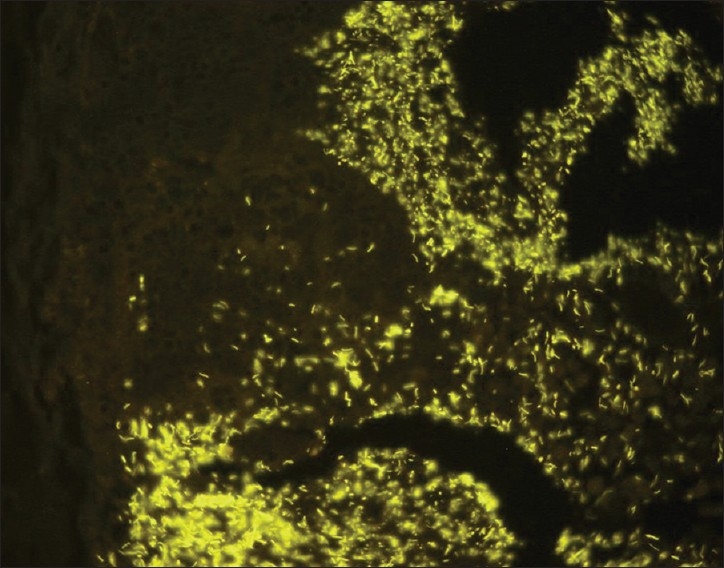
The detection of bacteria in formalin-fixated appendices was difficult. The hybridization signals even with the universal bacterial probe were often very weak. However, using the F. nucleatum-specific probe, we could definitively detect invasive Fusobacteria in 30% of appendices from Russia and in 36% appendices from China [Table 1 and Figure 3].
Figure 3.
Diffuse infiltration of the submucosa by Fusobacterium nucleatum (Fnuc Cy3 probe, orange fluorescence). The section is counterstained with DAPI stain, the nuclei of eukaryotic cells are seen as large blue spots
Invasive Fusobacteria were found in none of the Carnoy-fixated cecal biopsies from Germany (N=400) or Russia (N=40) [Table 1].
DISCUSSION
We had previously demonstrated that the occurrence and the mean percent of Fusobacteria in mucosal lesions correlated positively with the severity of acute appendicitis when investigating material of appendices resected for acute appendicitis in Germany and suggested an infectious origin of the disease.[1]
The present study raises nearly identical data in a group of patients undergoing surgery in a hospital in Moskau. Similar to our study in Germany, none of the biopsies taken from the inflamed regions of patients with IBD contained invasive Fusobacteria. We have now comparatively investigated the appendices fixated simultaneously immediately after surgery in Carnoy and formalin solution. The formalin fixation proved to be detrimental for FISH. The FISH signals in formalin-fixated tissues were very weak. Nevertheless, despite the overall low accessibility of bacteria to FISH probes in formalin-fixated material, we could definitively identify F. nucleatum as mucosa infiltrating bacterium in 30% of formalin-fixated samples from Russia and 36% of samples from China. Our data indicates, that the invasive microbial process underlying acute appendicitis is the same in such different geographic regions as Germany, Russia, and China and is in further support of the infectious origin of acute appendicitis both in sporadic cases and in clustered outbreaks.
The study was performed according to the ethical rules included in the Declaration of Helsinki. The investigations were approved by the Institutional Review Board of the Charité Universitätsmedizin Berlin. The investigations were performed concomitantly to acute surgery using material from appendices that would have been otherwise discarded.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Swidsinski A, Dörffel Y, Loening-Baucke V, Theissig F, Rückert JC, Ismail M, et al. Acute appendicitis is characterized by local invasion with Fusobacterium nucleatum/necrophorum. Gut. 2011;60:34–40. doi: 10.1136/gut.2009.191320. [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Xiao SY, Yan H, Sun ND, Jiang MS, Liu DY. Cluster of acute hemorrhagic appendicitis among high school students in Wuhan, China. Am J Surg. 2004;188:115–21. doi: 10.1016/j.amjsurg.2003.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol. 2005;43:3380–9. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swidsinski A, Loening-Baucke V, Vaneechoutte M, Doerffel Y. Active Crohn's disease and ulcerative colitis can be specifically diagnosed and monitored based on the biostructure of the fecal flora. Inflamm Bowel Dis. 2008;14:147–61. doi: 10.1002/ibd.20330. [DOI] [PubMed] [Google Scholar]
- 5.Sunde PT, Olsen I, Göbel UB, Theegarten D, Winter S, Debelian GJ, et al. Fluorescence in situ hybridization (FISH) for direct visualization of bacteria in periapical lesions of asymptomatic root-filled teeth. Microbiology. 2003;149:1095–102. doi: 10.1099/mic.0.26077-0. [DOI] [PubMed] [Google Scholar]
- 6.Amann R, Krumholz L, Stahl DA. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990;172:762–70. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suau A, Rochet V, Sghir A, Gramet G, Brewaeys S, Sutren M, et al. Fusobacterium prausnitzii and related species represent a dominant group within the human fecal flora. Syst Appl Microbiol. 2001;24:139–45. doi: 10.1078/0723-2020-00015. [DOI] [PubMed] [Google Scholar]
- 8.Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology. 1996;142:1097–106. doi: 10.1099/13500872-142-5-1097. [DOI] [PubMed] [Google Scholar]
- 9.Franks AH, Harmsen HJ, Raangs GC, Jansen GJ, Schut F, Welling GW. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998;64:3336–45. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


