Abstract
Ca2+ and calmodulin (CaM) play a critical role in proliferation and viability of a wide variety of cells, including prostate cancer cells. We examined two prostate cancer cell lines, androgen-sensitive LNCaP and androgen-independent PC-3. Proliferation of LNCaP cells was six to eight times more sensitive to the inhibitory effect of the CaM antagonist N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) than were PC-3 cells. Because LNCaP cell proliferation is sensitive to stimulation by androgen, we assessed the physical and functional interaction between androgen receptor (AR) and CaM. We observed tight binding of AR to CaM when LNCaP cell extracts were subjected to CaM-affinity column chromatography. AR binding to CaM was Ca2+-dependent and was inhibited by pretreatment of the cell extracts with W-7. Using immunofluorescence staining and confocal microscopy, we demonstrated colocalization of AR and CaM in the nucleus of LNCaP cells. Furthermore, the functional relevance of AR-CaM interactions in intact cells was revealed by the observation that W-7 was as effective as Casodex, an antiandrogen, in blocking AR-regulated expression of prostate-specific antigen in LNCaP cells. AR seems to interact with CaM directly because purified human AR could bind to CaM-agarose, and CaM could be detected in AR-immunoprecipitate prepared from purified soluble proteins. These studies provide direct evidence for physical and functional interaction between AR and CaM and suggest the potential usefulness of CaM antagonists in blocking AR activity in prostate cancer.
Because of the important role that androgen plays in the viability and proliferation of prostate cancer cells, androgen ablation remains one of the most commonly used therapies for the treatment of metastatic prostate cancer (1–3). However, androgen ablation therapy is only palliative, and most patients receiving this treatment eventually succumb to hormone refractory disease that no longer responds to any of the currently available therapeutic agents (4). Identification of new targets or improved strategies for the treatment of metastatic prostate cancer may require further understanding of the molecular mechanism of androgen receptor (AR) action in prostate cancer cells.
Androgen is required for viability of prostate epithelial cells and the normal structural development and function of the prostate. Androgen also plays a role in the development of prostate carcinoma (5, 6). The effect of androgen is mediated by AR that translocates from cytoplasm into the nucleus and binds to specific promoter elements to modulate the expression of androgen-responsive genes (7–9). Identification and characterization of coactivators and corepressors involved in AR-regulated gene expression in prostate cancer cells have been the subjects of intense investigation in recent years (10–14).
Apoptosis of prostate epithelial cells induced by androgen deprivation is suppressed by drugs that block the influx of Ca2+ or its release from intracellular stores (15). Agents that cause a sustained increase in intracellular Ca2+ induce apoptosis in prostate cancer cells (16, 17). Changes in intracellular Ca2+ and the Ca2+-binding protein, calmodulin (CaM), affect the proliferative state and viability of prostate cancer cells (18). Enhanced expression of CaM is associated with proliferation-independent apoptosis of androgen-independent rat prostate cancer cells (18). Androgen affects CaM expression in rat prostate glandular cells and overexpression of a Ca2+-binding protein, calbindin D, protects prostate cancer cells against the cytotoxic effect of an intracellular Ca2+ surge (16). Intracellular Ca2+ also regulates AR expression in prostate cancer cells (19). Although Ca2+ and Ca2+-binding proteins are known to be intimately involved in prostate cancer cell survival and proliferation, their role in androgen and AR action remain elusive.
Ca2+/CaM levels are increased in human mammary tumor cells (20, 21), and their growth is highly sensitive to CaM antagonists (22). CaM stimulates estrogen receptor (ER) phosphorylation in mammary epithelial cells (23), and CaM levels are 2- to 3-fold higher in ER-positive than in ER-negative breast cancer cells (24). In addition, antiestrogens bind with high affinity to CaM (25), and both antiestrogens and CaM antagonists block breast cancer cell cycle progression at an identical point in late G1 phase (26). Furthermore, the interaction of antiestrogen with CaM is suggested to be responsible for antiestrogen inhibition of breast cancer cell growth (27). CaM exhibits structural and functional interaction with ER (28, 29) and stabilizes ER (30) in breast cancer cells. It is not known whether CaM interacts similarly with AR to affect either its activity and/or stability in prostate cancer cells.
In an attempt to understand the potential involvement of Ca2+/CaM in prostate cancer cell proliferation, we examined the effect of a specific cell-permeable CaM antagonist, N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide hydrochloride (W-7) (31), on androgen-sensitive (LNCaP) and androgen-independent (PC-3) prostate cancer cells. These studies revealed LNCaP cells to be six to eight times more sensitive than PC-3 cells to the antiproliferative effects of W-7, suggesting a possible role of AR in CaM action. This hypothesis is corroborated by observations that AR binds to CaM in a Ca2+-dependent manner, and that AR and CaM colocalize in the nuclei of LNCaP cells. Furthermore, W-7 blocks AR transcriptional activity in LNCaP cells as effectively as an antiandrogen, Casodex. Taken together, these observations suggest structural and functional interaction of AR with CaM in prostate cancer cells.
Materials and Methods
Cell Culture. LNCaP cells were grown in RPMI medium 1640 (GIBCO/BRL) containing 10 nM testosterone, whereas PC3 cells were grown in DMEM. Both cultures were supplemented with 10% FBS, 2.5 mM glutamine, 100 μg/ml streptomycin, and 100 units/ml penicillin. Cells were maintained in a humidified incubator with 5% CO2 and 95% air at 37°C.
Measurement of [3H]Thymidine Incorporation. Cells were pulse-labeled with 2 μCi (1 Ci = 37 GBq)/ml [3H]thymidine (ICN) for 30 min at 37°C in a humidified incubator. The radioactivity incorporated into acid-precipitable material was then determined as described (32). The effect of W-7 was assessed by treating cells with given concentrations of W-7 for 48 h before [3H]thymidine labeling.
Preparation of Nuclear and Cytoplasmic Fractions. Exponentially growing LNCaP and PC-3 cells were subjected to nuclear and cytoplasmic fractionation as described (33).
Preparation of Whole-Cell Extracts. Trypsinized cells were pelleted by centrifugation and resuspended in buffer A (50 mM Tris·HCl, pH 7.4/0.1% Triton X-100/5 mM EDTA/250 mM NaCl/50 mM NaF/0.1 mM Na3VO4) supplemented with protease inhibitor mixture (P-8340, Sigma) at a density of 2.5 × 107 cells per ml. Cells were then subjected twice to 30 pulses of sonication with a Branson Sonifier 250 set at an output control of 2 and a duty cycle of 20, with intermittent cooling on ice. The sonicated cell extract was cleared by centrifugation in an Eppendorf centrifuge at 12,500 rpm for 1 min. Protein concentration in cleared extracts was assessed using Bio-Rad Protein Assay reagent.
CaM-Agarose Affinity Column Chromatography. CaM-affinity chromatography was performed essentially as described (34). Briefly, whole-cell lysate of LNCaP cells prepared as described above was diluted 20-fold in buffer B (20 mM Tris, pH 7.4/4 mM MgCl2/2 mM CaCl2/10 mM KCl/1 mM PMSF) and applied to a CaM-agarose (Sigma) affinity column. After passing the diluted cell extract through the CaM-agarose column, the column was washed with buffer B until OD280 returned to baseline. Proteins retained on the column were then eluted with modified buffer B (20 mM Tris, pH 7.4/4 mM MgCl2/10 mM EGTA/10 mM KCl/1 mM PMSF) in which the Ca2+-chelator EGTA is substituted for Ca2+. All chromatography procedures including cell lysate application, washing, and elution were carried out at a flow rate of 1 ml/min. To assess the effect of W-7 and Casodex, diluted cell lysate was divided into three aliquots containing equal volumes and protein concentrations, and individual aliquots were treated in parallel with diluent (control), 20 μM W-7, or 100 μM Casodex, before application to the CaM-agarose column.
Direct interaction between AR and CaM was assessed by incubating purified recombinant human AR (kind gift from Zhong-Xun Zhou and Elizabeth M. Wilson, University of North Carolina, Chapel Hill) with CaM-agarose in buffer B containing 100 μg/ml BSA for 30 min at room temperature. In some cases, buffer B was either supplemented with 20 μM W-7 or 100 μM Casodex, or its Ca2+ was replaced by 10 mM EGTA. Agarose beads were sedimented, washed three times in buffer B, and suspended in electrophoresis sample buffer for Western blot analysis.
Immunoprecipitation. Purified recombinant human AR and CaM (35) incubated together in buffer B containing 100 μg/ml BSA for 30 min at room temperature were treated with anti-AR monoclonal antibodies (sc-7305x, Santa Cruz Biotechnology) followed by protein A-Sepharose. Protein A-Sepharose beads were then sedimented, washed three times in buffer B, and suspended in electrophoresis sample buffer for Western blot analysis.
Western Blot Analysis. Samples were subjected to denaturing 10% or 12% SDS/PAGE and then transferred to nitrocellulose membranes. Individual membranes were probed with rabbit polyclonal antibody against AR (Santa Cruz Biotechnology) or mouse monoclonal antibodies against prostate-specific antigen (PSA) (DAKO), cyclin A (Transduction Laboratories, Lexington, KY), β-actin (Santa Cruz Biotechnology), calcineurin (BD Pharmigen), or CaM (35). Immunoreactive bands were developed by using horseradish peroxidase-conjugated secondary antibodies and SuperSignal WestPico chemiluminescent substrate (Pierce) and visualized by using x-ray film.
Immunofluorescent Staining and Confocal Microscopy. LNCaP cells were grown on glass slides and processed for immunocytochemistry as described (36). Slides were washed once with PBS, followed by fixation in 3.7% (vol/vol) formaldehyde for 20 min at 22°C. Cells were permeabilized in 0.5% (vol/vol) Triton X-100 for 15 min and blocked for1hin2%(wt/vol) BSA at 22°C. Slides were then incubated for 1 h at 22°C with anti-AR rabbit polyclonal (Santa Cruz Biotechnology) and anti-CaM mouse monoclonal (35) antibodies, followed by both goat-anti-rabbit-FITC- and goat-anti-mouse-tetramethylrhodamine B isothiocyanate-labeled secondary antibodies, for AR and CaM, respectively. After four washes with PBS, slides were mounted with Aqua Poly/Mount (Polysciences). Confocal laser scanning microscopy was performed with a Zeiss LSM 510W upright confocal microscope. Images were processed, and colocalization analysis (which is colored yellow) was performed with the Zeiss LSM 510 Meta Excitation system with collection at 488 and 543 nm (BP505–550 and LP560).
Results
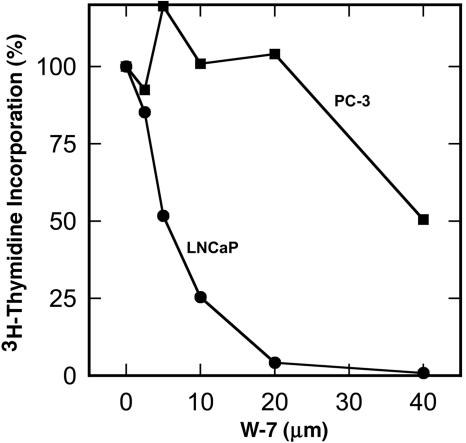
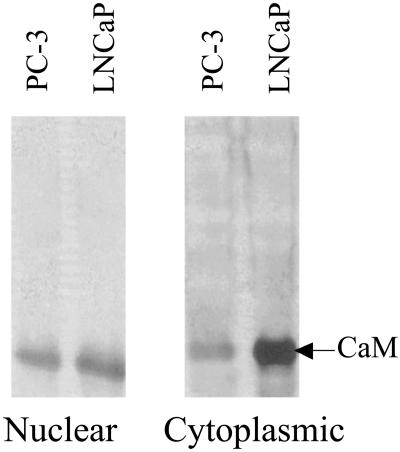
Differential Effect of W-7 on Proliferation of Androgen-Sensitive (LNCaP) and Androgen-Independent (PC-3) Prostate Cancer Cells. To investigate the potential role of Ca2+/CaM in androgen action, we examined the effect of a specific CaM-antagonist, W-7, on proliferation of androgen-sensitive (LNCaP) and androgen-independent (PC3) prostate cancer cells. Cells were treated with or without W-7 for 48 h before pulse-labeling for 30 min with [3H]thymidine. As shown in Fig. 1, the concentration of W-7 required for 50% inhibition (IC50) of thymidine incorporation into LNCaP cells was ≈5 μM, which is substantially lower than the 30–40 μM required for 50% inhibition of PC-3 cells. A similar difference in IC50 was seen between LNCaP and PC-3 cells when they were treated with W-7 for 24 h (data not shown). Thus, androgen-sensitive LNCaP cells are six to eight times more sensitive to the inhibitory effect of W-7 than the androgen-independent PC-3 cells. To explore the molecular mechanism for this inhibition, we initially examined the relative amounts of CaM in LNCaP and PC-3 cells. Western blot analysis revealed 2- and 3-fold greater amounts of CaM in the nuclear and cytoplasmic fractions, respectively, of LNCaP cells than in those of PC-3 cells (Fig. 2).
Fig. 1.
Differential sensitivity of the proliferation of LNCaP and PC-3 prostate cancer cells to anti-calmodulin W-7. Proliferation of androgen-sensitive LNCaP (•) and androgen-independent PC3 (▪) prostate cancer cells was assessed by [3H]thymidine incorporation. Cells were treated with or without the indicated concentrations of W-7 for 48 h before pulse labeling for 30 min with [3H]thymidine. Data are the average of duplicate determinations. There was <10% variation between duplicate determinations.
Fig. 2.
Western blot analysis of CaM in nuclear and cytoplasmic fractions of LNCaP and PC-3 cells. Equal amounts of protein from nuclear and cytoplasmic fractions of LNCaP and PC-3 cells were resolved by SDS/PAGE, and CaM was detected by using anti-CaM monoclonal antibodies on Western blots as described in Materials and Methods.
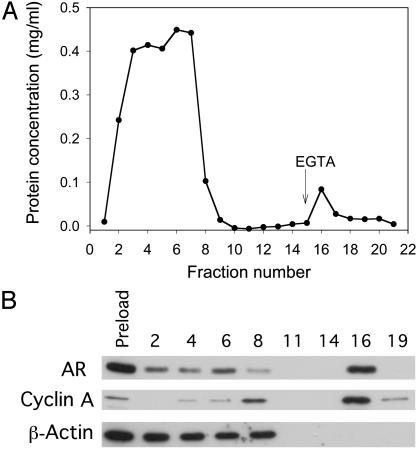
AR and Cyclin A in LNCaP Cell Extract Exhibit Ca2+-Dependent Binding to CaM. In addition to the differences in AR status, LNCaP and PC-3 cells exhibit a range of differences in their genetic makeup and are derived from two different sources, one from a lymph node metastasis (LNCaP) and the other from a brain metastasis (PC-3) (37). In an attempt to investigate the relationship between AR status and the exquisite sensitivity of prostate cancer cells to CaM-antagonist, we tested the ability of AR in LNCaP cell extracts to bind to CaM. We subjected whole-cell lysates of exponentially growing LNCaP cells containing AR to CaM-affinity chromatography with a CaM-agarose column. As shown in Fig. 3A, the bulk of cellular proteins passed freely through the column without binding to CaM in the presence of Ca2+ (fractions 1–8). However, a minor fraction of protein that was retained on the column in the presence of Ca2+ was eluted when Ca2+ was chelated with EGTA. The small peak of proteins observed after elution with EGTA-containing buffer represents proteins that exhibit Ca2+-dependent binding to CaM. Western blot analysis (Fig. 3B) of an equal amount of protein from each fraction eluted from the column (in the case of fractions 11 and 14, maximum volume was used) revealed a significant presence of AR in the peak fraction that eluted with EGTA. This fraction also contained cyclin A but not β-actin, an abundant protein in LNCaP cell extracts (Fig. 3B). β-Actin was recovered exclusively in the flow-through fractions. These latter data confirm the specificity of AR and cyclin A binding to CaM. Some AR and cyclin A were also detected in some of the flow-through fractions (Fig. 3B). The failure to bind to the CaM column was not due to saturation of the CaM affinity column with CaM-binding proteins in the cell extract, because reapplication of the pooled flow-through fractions to a fresh CaM-agarose column did not remove additional AR or cyclin A (data not shown). Whether AR in the flow-through fractions may be stably complexed to CaM and/or other proteins remains to be determined.
Fig. 3.
CaM-affinity column chromatography of LNCaP cell extracts. The whole-cell extract of exponentially growing LNCaP cells was subjected to CaM-agarose column chromatography in a Ca2+-containing buffer, and the proteins bound to CaM were then eluted by using EGTA-containing buffer as described in Materials and Methods. (A) Elution profile of proteins. (B) Western blot analysis of AR, cyclin A, and β-actin in equal amount of protein in individual fractions (for fractions 11 and 14, because there was no detectable protein, maximum volume was used). Data are representative of four different experiments. Preload, cell extract before applying to the column.
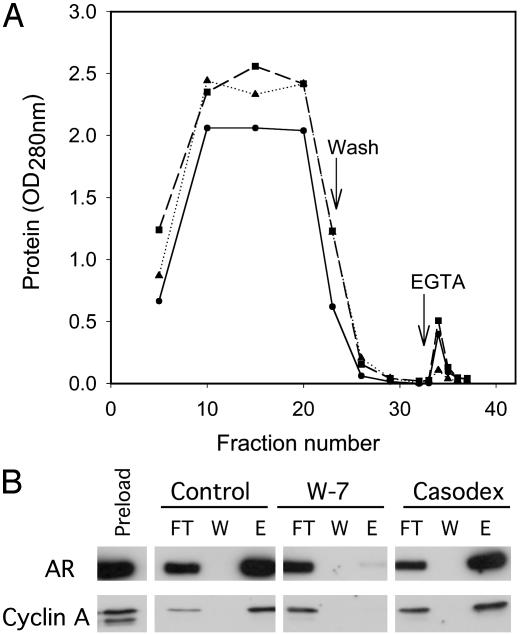
Selective Inhibition of AR Binding to CaM by W-7. To further test the specificity of interaction of AR and cyclin A with CaM, whole-cell lysates from exponentially growing LNCaP cells were treated with W-7 or Casodex before being applied to a CaM-agarose affinity column. The superimposed elution profile of proteins from control, W-7, and Casodex-treated cell extracts showed a substantially smaller EGTA elution peak for the lysate pretreated with W-7 (Fig. 4A), suggesting that CaM inactivation with W-7 inhibited binding of proteins to the CaM column. In contrast, the elution peak with Casodex pretreatment was indistinguishable from control (Fig. 4A). Western blot analysis of an equal volume of eluted fractions from the column confirmed that CaM-antagonist W-7 inhibited binding of AR and cyclin A to the CaM column (Fig. 4B). The antiandrogen Casodex did not block binding of AR or cyclin A to the CaM column.
Fig. 4.
Effect of W-7 and Casodex on AR binding to CaM on CaM-agarose affinity column. Whole-cell lysates from exponentially growing LNCaP cells were treated with 20 μM W-7 (▴), 100 μM Casodex (▪), or control (•) before applying to a CaM-agarose affinity column as described in Materials and Methods. (A) Elution profile of proteins. (B) Western blot analysis of AR and cyclin A in equal volumes of indicated fractions. Preload, cell extract; FT, flow-through; W, wash; E, eluate.
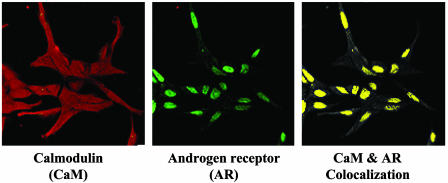
CaM Localized with AR in the Nucleus of LNCaP Cells. The binding of AR to CaM suggested that the two proteins may interact in cells. This hypothesis was examined by immunocytochemistry. Confocal analysis of exponentially growing LNCaP cells showed a diffuse distribution of CaM throughout the cell (Fig. 5). CaM was present in the nucleus, cytoplasm, and membranes. In contrast, AR was found predominantly in the nucleus. Analysis with a colocalization software program revealed that CaM in the nucleus is colocalized with AR (Fig. 5).
Fig. 5.
Confocal immunofluorescent detection of AR and CaM in exponentially growing LNCaP cells. Exponentially growing LNCaP cells were plated on glass slides and processed for immunocytochemistry as described in Materials and Methods. Cells were stained with an anti-CaM antibody, followed by a tetramethylrhodamine B isothiocyanate-labeled secondary antibody (CaM). In addition, slides were incubated with anti-AR antibody, followed by FITC-labeled secondary antibody (AR). Colocalization of CaM and AR was analyzed with the Zeiss LSM 510 Meta Excitation system and is represented in yellow. Data are representative of three independent experimental determinations.
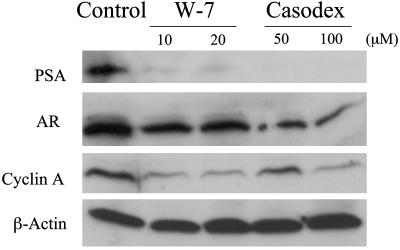
Anti-CaM Drug W-7 Is as Effective as Antiandrogen Casodex in Inhibiting Androgen-Responsive Gene (PSA) Expression in LNCaP Cells. To assess the functional relevance of the physical interaction between AR and CaM, exponentially growing LNCaP cells were treated for 24 h with either W-7 or Casodex, and cell extracts were then tested for PSA levels on Western blots. Treatment of LNCaP cells with W-7 resulted in a dramatic decrease in PSA (Fig. 6). This decrease was similar to that seen in cells treated with Casodex. Both W-7 and Casodex also caused a partial decrease in AR levels, but this reduction was more noticeable in LNCaP cells treated with Casodex than with W-7. Interestingly, W-7 and Casodex also caused a significant reduction in cyclin A levels (Fig. 6). The inhibitory effects of W-7 and Casodex on PSA, AR, and cyclin A levels are specific, because neither of these drugs had any effect on β-actin levels (Fig. 6).
Fig. 6.
Effect of W-7 and Casodex on expression of PSA, AR, cyclin A, and β-actin in LNCaP cells. Western blot analysis of whole-cell lysates from exponentially growing LNCaP cells treated with the indicated concentrations of W-7 or Casodex for 24 h was performed as described in Materials and Methods.
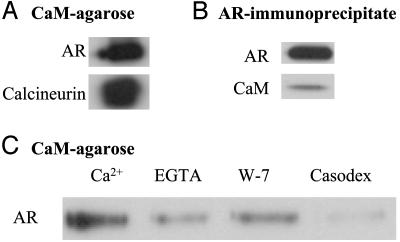
AR Interacts Directly with CaM. Binding of AR in LNCaP cell extracts to CaM-agarose could be direct or indirect involving other proteins in the cell extract. To distinguish between these possibilities, purified recombinant human AR was tested for its ability to bind to CaM-agarose. As shown in Fig. 7A, purified AR bound to CaM-agarose, as did calcineurin, a known CaM-binding protein. Purified AR also bound to purified CaM in soluble form, because CaM coimmunoprecipitated with AR (Fig. 7B). The binding of purified AR to CaM-agarose was Ca2+-dependent and was reduced by the Ca2+-chelator EGTA and the CaM antagonist W-7 (Fig. 7C). Interestingly, antiandrogen Casodex also inhibited purified AR from binding to CaM (Fig. 7C); by contrast, Casodex did not inhibit the binding of AR to CaM in LNCaP cell extract (Fig. 4B) (see Discussion).
Fig. 7.
Purified AR interacts with CaM. (A) Purified AR binds to CaM-agarose as effectively as calcineurin. Purified AR or calcineurin was incubated with CaM-agarose, and proteins bound to CaM-agarose were prepared and subjected to Western blot analysis as described in Materials and Methods to detect AR and calcineurin. (B) CaM is associated with AR immunoprecipitate. Purified AR and CaM were incubated together in the presence of Ca2+, AR immunoprecipitate was prepared, and AR and CaM in the immunoprecipitate were detected by Western blot analysis as described in Materials and Methods. (C) Effect of EGTA, W-7, and Casodex on binding of purified AR to CaM-agarose. Purified AR was incubated with CaM-agarose in the presence of 2 mM Ca2+ (control), 10 mM EGTA, 20 μM W-7, or 100 μM Casodex, and AR bound to CaM-agarose was determined as described in Materials and Methods.
Discussion
Our study demonstrates Ca2+-dependent binding of AR to CaM and shows that AR colocalizes with CaM in the nucleus of androgen-sensitive prostate cancer cells. These physical interactions between AR and CaM appear to have functional significance, because CaM-antagonist could suppress PSA levels. Our study also reveals the involvement of CaM in the expression, and possibly function, of cyclin A in prostate cancer cells. These interactions of CaM with AR may contribute to greater sensitivity to the inhibitory effect of anti-CaM W-7 on proliferation of androgen-sensitive LNCaP cells than of androgen-independent PC-3 prostate cancer cells.
It is intriguing to note in our studies that CaM levels, as determined by Western blot analysis, were 2- to 3-fold higher in LNCaP cells than in PC-3 cells (Fig. 2). Androgen and intracellular Ca2+ are known to regulate the expression of several Ca2+-binding proteins, including calreticulin, calbindin D, and CaM (15, 16, 18, 38). Androgen-induced calreticulin expression was shown to protect LNCaP, but not PC-3 cells, against Ca2+-ionophore-induced apoptosis (39). Furthermore, if CaM is involved in AR action, as demonstrated in the present study, then it is conceivable that CaM levels are higher in LNCaP cells because of AR than in PC-3 cells that lack AR. Consistent with this possibility is the observation that CaM levels are two to three times higher in ER-positive human breast tumors than in ER-negative tumors (24).
The interaction of CaM with ER in human mammary tumor cells has been characterized. CaM stimulates tyrosine phosphorylation and activation of ER (23). Gel mobility-shift assays revealed a CaM requirement in ER–estrogen response element complex formation and in transcriptional activation of an estrogen-responsive promoter (28). Inactivation of CaM function in the nucleus of MCF-7 cells eliminates estrogen-stimulated ER transcriptional activation (29). A direct interaction of ERα, but not ERβ, with CaM makes ERα activity susceptible to the inhibitory effect of CaM antagonists (40). Ca2+-dependent, but estradiol-independent, binding of CaM to ER is shown to enhance the stability of ER in MCF-7 human breast epithelial cells (30). We now report that, like ER, AR interacts both physically and functionally with CaM in prostate cancer cells.
AR binding to CaM seems to be a direct binding because, like AR in LNCaP cells, purified AR also binds to CaM-agarose in a Ca2+-dependent manner (Fig. 7). It is, however, intriguing to note that Casodex was effective in preventing the binding of recombinant wild-type human AR (Fig. 7), but not AR in LNCaP cells (Fig. 4), to CaM-agarose. AR in LNCaP cells has a mutation in the ligand-binding domain, which is known to affect the activity, affinity, and responsiveness of AR to various ligands (41–45). These observations, taken together, suggest that the ligand-binding domain of AR may play an important role in AR interaction with CaM.
CaM is also known to interact with and regulate the activity and/or nuclear localization of several cell-cycle regulatory proteins, including p21Cip1, cyclin D1-Cdk4, and CaM kinase II (46–48). Furthermore, CaM is involved in the expression of cell-cycle regulatory proteins Cdk2, Cdk4, cdc2, p21Cip1, cyclin B, and cyclin A and the enzymes of DNA replication, DNA polymerase α and proliferating cell nuclear antigen (49, 50). In this study, we found that cyclin A in LNCaP cell extracts binds to CaM either directly or indirectly, and that the expression of cyclin A is sensitive to the inhibitory effect of the anti-CaM drug W-7. This observation reveals an important role of CaM not only in AR transcriptional activity but also in regulating the expression and activity of cell-cycle regulatory proteins required for the progression of prostate cancer cells through S phase.
AR-positive prostate cancer cells, even those that become androgen-independent, require AR activity for their proliferation (51). Based on the observations presented in this article, we speculate that AR activity in such androgen-refractory cells could be stimulated by accessory proteins, such as CaM, that interact with AR in an androgen-independent manner. Inhibiting such interactions between accessory proteins and AR (for example, with CaM antagonists as shown in this study) may provide an effective strategy for blocking AR-regulated events necessary for proliferation and viability of androgen-sensitive as well as androgen-independent prostate cancer cells.
Acknowledgments
We thank Richard Croxen for excellent technical assistance. This work was supported by National Institutes of Health Grants DK57864 (to G.P.-V.R.) and CA93645 (to D.B.S.).
Abbreviations: AR, androgen receptor; CaM, calmodulin; ER, estrogen receptor; PSA, prostate-specific antigen; W-7, N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide hydrochloride.
References
- 1.Huggins, C. & Hodges, C. V. (1941) Cancer Res. 1, 293–297. [Google Scholar]
- 2.Huggins, C. (1967) Science 156, 1050–1054. [DOI] [PubMed] [Google Scholar]
- 3.Denis, L. J. & Griffiths, K. (2000) Semin. Surg. Oncol. 18, 52–74. [DOI] [PubMed] [Google Scholar]
- 4.Gittes, R. F. (1991) N. Engl. J. Med. 324, 236–245. [DOI] [PubMed] [Google Scholar]
- 5.Wilding, G. (1992) Cancer Surv. 14, 113–130. [PubMed] [Google Scholar]
- 6.Catalona, W. J. & Scott, W. W. (1986) in Campbell's Urology, eds. Walsh, P. C., Gittes, R. F., Perlmutter, A. D. & Stamey, T. A. (Saunders, Philadelphia), 5th Ed., pp. 1463–1534.
- 7.Lindzey, J., Kumar, M. V., Grossman, M., Young, C. & Tindall, D. J. (1994) Vitam. Horm. 49, 383–432. [DOI] [PubMed] [Google Scholar]
- 8.Heinlein, C. A. & Chang, C. (2002) Endocr. Rev. 23, 175–200. [DOI] [PubMed] [Google Scholar]
- 9.Perry, J. E., Grossmann, M. E. & Tindall, D. J. (1996) Prostate Suppl. 6, 79–81. [PubMed] [Google Scholar]
- 10.Yamamoto, A., Hashimoto, Y., Kohri, K., Ogata, E., Kato, S., Ikeda, K. & Nakanishi, M. (2000) J. Cell Biol. 150, 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reutens, A. T., Fu, M., Wang, C., Albanese, C., McPhaul, M. J., Sun, Z., Balk, S. P., Janne, O. A., Palvimo, J. J. & Pestell, R. G. (2001) Mol. Endocrinol. 15, 797–811. [DOI] [PubMed] [Google Scholar]
- 12.Reid, J., Murray, I., Watt, K., Betney, R. & McEwan, I. J. (2002) J. Biol. Chem. 277, 41247–41253. [DOI] [PubMed] [Google Scholar]
- 13.Loy, C. J., Sim, K. S. & Yong, E. L. (2003) Proc. Natl. Acad. Sci. USA 100, 4562–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman, M. M., Miyamoto, H., Lardy, H. & Chang, C. (2003) Proc. Natl. Acad. Sci. USA 100, 5124–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor, J., Sawczuk, I. S., Benson, M. C., Tomashefsky, P., O'Toole, K. M., Olsson, C. A. & Buttyan, R. (1988) Prostate 13, 119–130. [DOI] [PubMed] [Google Scholar]
- 16.Furuya, Y., Lundmo, P., Short, A. D., Gill, D. L. & Isaacs, J. T. (1994) Cancer Res. 54, 6167–6175. [PubMed] [Google Scholar]
- 17.Martikainen, P., Kyprianou, N., Tucker, R. W. & Isaacs, J. T. (1991) Cancer Res. 51, 4693–4700. [PubMed] [Google Scholar]
- 18.Furuya, Y. & Isaacs, J. T. (1994) Prostate 25, 301–309. [DOI] [PubMed] [Google Scholar]
- 19.Gong, Y., Blok, L. J., Perry, J. E., Lindzey, J. K. & Tindall, D. J. (1995) Endocrinology 136, 2172–2178. [DOI] [PubMed] [Google Scholar]
- 20.Chun, K. & Sacks, D. (2000) in Calcium: The Molecular Basis of Calcium Action in Biology and Medicine, eds. Pochet, R., Donato, R., Haiech, J., Heizmann, C. & Gerke, V. (Kluwer, Dordrecht, The Netherlands), pp. 541–563.
- 21.Singer, A. L., Sherwin, R. P., Dunn, A. S. & Appleman, M. M. (1976) Cancer Res. 36, 60–66. [PubMed] [Google Scholar]
- 22.Wei, J. W., Hickie, R. A. & Klaassen, D. J. (1983) Cancer Chemother. Pharmacol. 11, 86–90. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio, A., Rotondi, A. & Auricchio, F. (1984) Proc. Natl. Acad. Sci. USA 81, 5921–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krishnaraju, K., Murugesan, K., Vij, U., Kapur, B. M. & Farooq, A. (1991) Br. J. Cancer 63, 346–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lam, H. Y. (1984) Biochem. Biophys. Res. Commun. 118, 27–32. [DOI] [PubMed] [Google Scholar]
- 26.Musgrove, E. A., Wakeling, A. E. & Sutherland, R. L. (1989) Cancer Res. 49, 2398–2404. [PubMed] [Google Scholar]
- 27.Gulino, A., Barrera, G., Vacca, A., Farina, A., Ferretti, C., Screpanti, I., Dianzani, M. U. & Frati, L. (1986) Cancer Res. 46, 6274–6278. [PubMed] [Google Scholar]
- 28.Biswas, D. K., Reddy, G. P.-V., Pickard, M., Makkad, B., Pettit, N. & Pardee, A. B. (1998) J. Biol. Chem. 273, 33817–33824. [DOI] [PubMed] [Google Scholar]
- 29.Li, L., Li, Z. & Sacks, D. B. (2003) J. Biol. Chem. 278, 1195–1200. [DOI] [PubMed] [Google Scholar]
- 30.Li, Z., Joyal, J. L. & Sacks, D. B. (2001) J. Biol. Chem. 276, 17354–17360. [DOI] [PubMed] [Google Scholar]
- 31.Hidaka, H., Sasaki, Y., Tanaka, T., Endo, T., Ohno, S., Fujii, Y. & Nagata, T. (1981) Proc. Natl. Acad. Sci. USA 78, 4354–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy, G. P.-V. (1982) Biochem. Biophys. Res. Commun. 109, 908–915. [DOI] [PubMed] [Google Scholar]
- 33.Subramanyam, C., Honn, S. C., Reed, W. C. & Reddy, G. P.-V. (1990) J. Cell. Physiol. 144, 423–428. [DOI] [PubMed] [Google Scholar]
- 34.Reddy, G. P.-V., Reed, W. C., Deacon, D. H. & Quesenberry, P. J. (1994) Biochemistry 33, 6605–6610. [DOI] [PubMed] [Google Scholar]
- 35.Sacks, D. B., Porter, S. E., Ladenson, J. H. & McDonald, J. M. (1991) Anal. Biochem. 194, 369–377. [DOI] [PubMed] [Google Scholar]
- 36.Swart-Mataraza, J. M., Li, Z. & Sacks, D. B. (2002) J. Biol. Chem. 277, 24753–24763. [DOI] [PubMed] [Google Scholar]
- 37.Webber, M. M., Bello, D. & Quader, S. (1997) The Prostate 30, 58–64. [DOI] [PubMed] [Google Scholar]
- 38.Wang, Z., Tufts, R., Haleem, R. & Cai, X. (1997) Proc. Natl. Acad. Sci. USA 94, 12999–13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu, N. & Wang, Z. (1999) Cancer Res. 59, 1896–1902. [PubMed] [Google Scholar]
- 40.Garcia Pedrero, J. M., Del Rio, B., Martinez-Campa, C., Muramatsu, M., Lazo, P. S. & Ramos, S. (2002) Mol. Endocrinol. 16, 947–960. [DOI] [PubMed] [Google Scholar]
- 41.Doesburg, P., Kuil, C. W., Berrevoets, C. A., Steketee, K., Faber, P. W., Mulder, E., Brinkmann, A. O. & Trapman, J. (1997) Biochemistry 36, 1052–1064. [DOI] [PubMed] [Google Scholar]
- 42.Veldscholte, J., Voorhorst-Ogink, M. M., Bolt-de Vries, J., van Rooij, H. C., Trapman, J. & Mulder, E. (1990) Biochim. Biophys. Acta 1052, 187–194. [DOI] [PubMed] [Google Scholar]
- 43.Veldscholte, J., Ris-Stalpers, C., Kuiper, G. G., Jenster, G., Berrevoets, C., Claassen, E., van Rooij, H. C., Trapman, J., Brinkmann, A. O. & Mulder, E. (1990) Biochem. Biophys. Res. Commun. 173, 534–540. [DOI] [PubMed] [Google Scholar]
- 44.Wang, C., Young, W. J. & Chang, C. (2000) Endocrine 12, 69–76. [DOI] [PubMed] [Google Scholar]
- 45.Ris-Stalpers, C., Verleun-Mooijman, M. C., Trapman, J. & Brinkmann, A. O. (1993) Biochem. Biophys. Res. Commun. 196, 173–180. [DOI] [PubMed] [Google Scholar]
- 46.Taules, M., Rius, E., Talaya, D., Lopez-Girona, A., Bachs, O. & Agell, N. (1998) J. Biol. Chem. 273, 33279–33286. [DOI] [PubMed] [Google Scholar]
- 47.Taules, M., Rodriguez-Vilarrupla, A., Rius, E., Estanyol, J. M., Casanovas, O., Sacks, D. B., Perez-Paya, E., Bachs, O. & Agell, N. (1999) J. Biol. Chem. 274, 24445–24448. [DOI] [PubMed] [Google Scholar]
- 48.Patel, R., Holt, M., Philipova, R., Moss, S., Schulman, H., Hidaka, H. & Whitaker, M. (1999) J. Biol. Chem. 274, 7958–7968. [DOI] [PubMed] [Google Scholar]
- 49.Bosch, M., Gil, J., Bachs, O. & Agell, N. (1998) J. Biol. Chem. 273, 22145–22150. [DOI] [PubMed] [Google Scholar]
- 50.Colomer, J., Lopez-Girona, A., Agell, N. & Bachs, O. (1994) Biochem. Biophys. Res. Commun. 200, 306–312. [DOI] [PubMed] [Google Scholar]
- 51.Zegarra-Moro, O. L., Schmidt, L. J., Huang, H. & Tindall, D. J. (2002) Cancer Res. 62, 1008–1013. [PubMed] [Google Scholar]