Abstract
Regulation of organ growth is critical during embryogenesis. At the cellular level, mechanisms controlling the size of individual embryonic organs include cell proliferation, differentiation, migration, and attrition through cell death. All these mechanisms play a role in cardiac morphogenesis, but experimental studies have shown that the major determinant of cardiac size during prenatal development is myocyte proliferation. As this proliferative capacity becomes severely restricted after birth, the number of cell divisions that occur during embryogenesis limits the growth potential of the postnatal heart. We summarize here current knowledge concerning regional control of myocyte proliferation as related to cardiac morphogenesis and dysmorphogenesis. There are significant spatial and temporal differences in rates of cell division, peaking during the pre-septation period and then gradually decreasing towards birth. Analysis of regional rates of proliferation helps to explain the mechanics of ventricular septation, chamber morphogenesis, and the development of the cardiac conduction system. Proliferation rates are influenced by hemodynamic loading, and transduced by autocrine and paracrine signaling via growth factors. Understanding the biological response of the developing heart to such factors and physical forces will further our progress in engineering artificial myocardial tissues for heart repair and designing optimal treatment strategies for congenital heart disease.
Keywords: cell proliferation, cardiac development, embryo, heart
Introduction
At the cellular level, mechanisms controlling size of individual embryonic organs include cell proliferation, differentiation, migration, and cell death. All these mechanisms play a role in cardiac morphogenesis, but experimental studies have shown that the major determinant of cardiac size during prenatal development is myocyte proliferation (Clark et al., 1989; Saiki et al., 1997; Sedmera et al., 2002). The adult heart has traditionally been regarded as a postmitotic organ. Although it is clear today that this is not entirely correct, there is still agreement that most myocyte proliferation occurs during prenatal development. Proliferative activity in the heart not only increases its mass to match the increasing circulatory demands of the developing embryo, but together with programmed cell death and migration is a main factor shaping the developing heart. Under most notable pathological conditions, some changes in cell proliferation are usually detectable. Numerous studies utilizing varied methodological approaches have systematically mapped cell proliferation in different compartments during development. Since some of them are rather old, and in languages other than English, we here provide an overview of these precious bits of information, both to summarize findings to date and to serve as a methodological guideline for investigators willing to analyze proliferation in specific cardiac compartments. We also put into historical perspective some recent studies using computer-assisted methods to decipher proliferative structure of the entire organ.
Methods of assessing cell proliferation
DNA labeling
One of the most common methods of measuring cell proliferation is DNA pulse-labeling. Its principle lies in incorporation of labeled nucleotide into DNA of proliferating cells, specifically those going through DNA replication (S-phase). The length of such pulse depends on proliferative activity (cell cycle and S-phase length) of the tissue under study. To simplify the counting process and minimize numbers of counted cells to ensure robust statistics, length of pulse resulting in labeling index in the order of tens of percent is desirable. If the labeling index is below 5%, large numbers of cells must be counted to detect any differences, similar to apoptotic indices in the heart reported by the Anversa group (Kajstura et al., 1996; Anversa et al., 1998). Prior to the development of anti-bromodeoxyuridine immunohistochemistry, cell nuclei in the S-phase of cell cycle were detected by labeling with [3H]-thymidine followed by autoradiography (Figure 1). This technique retains merit today as being quantitative, yielding estimates of nuclear doubling under controlled conditions (Sedmera et al., 2003b). The earliest sampling time allowing incorporation of detectable amount of label into DNA is about 30 minutes, and in general, the labeling period is in the order of hours. The bioavailability of the label for in vivo incorporation does not commonly exceed two hours in mammals, but can be one to two days in the chick embryo (Yurkewicz et al., 1981) due to immature liver metabolism. This technique can be modified to “label-dilution” mode, in which the sampling is delayed by several days after label administration (Figure 1A), thus preferentially detecting cells that stopped dividing shortly after labeling. Since this time point is commonly narrowed, or sharpened, by administration of an excess amount of unlabeled thymidine, this technique is referred to as a “pulse-chase” experiment. Methodologically easier (and cheaper) is labeling with halogenated thymidine analogs such as 5-bromodeoxyuridine or 5-iododeoxyuridine that can be distinguished immunohistochemically (Burns and Kuan, 2005). This technique is widely applicable in systems ranging from cultured cells in vitro to whole animal labeling in vivo (Figure 1C) and allows identification of proliferating cell type by double immunohistochemistry (i.e. for DNA label and specific cell marker).
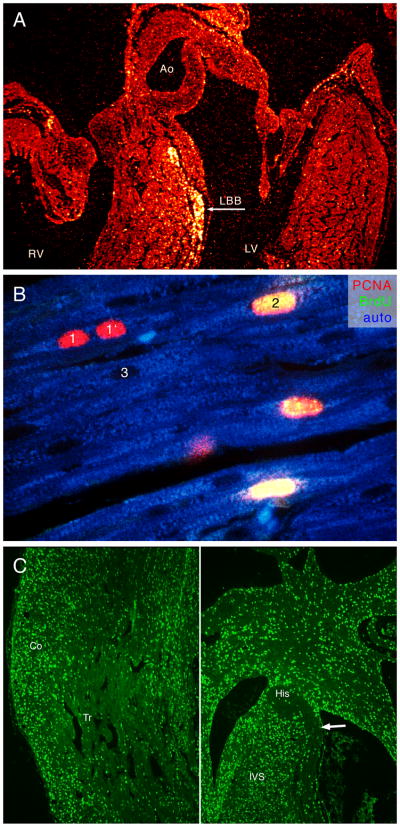
Figure 1.
A. Label dilution of [3H] thymidine in chick embryonic heart. Label was applied on ED2, and the heart sampled at ED10. Most of the radiolabel detected by autoradiography is retained in the ventricular conduction system, while more rapidly dividing working myocardium diluted label through many rounds of cell division. Ao, aorta, LBB, left bundle branch, LV, left ventricle, RV, right ventricle. From Thompson et al., 2000. B. Illustration of different immunohistochemical techniques for myocyte proliferation. Bromodeoxyuridine labeling (secondary antibody in the green channel) gives all-or-nothing signal, and labels only cells passing through S-phase during the labeling period (2). Proliferating cell nuclear antigen (red channel) lingers in the nuclei for longer time, and labels also cells that recently completed karyokinesis or cytokinesis (1, 1′). Unlabeled nuclei (without counterstaining) appear as oval shadows in the autofluorescent cytoplasm (3). Sample from neonatal pig myocardium labeled for 2 hours with bromodeoxyuridine. C. Saturation labeling in the fetal heart. Sections from rat embryonic heart labeled via Alzet osmotic mini-pump from ED17.5 to ED18.5. While almost all nuclei are labeled in the compact myocardium (Co), the trabeculae (Tr) show much lower labeling. Central conduction system in the interventricular septum (IVS) stands out by its paucity of label. Arrow points to left bundle branch (compare with Figure 1, where this structure, in a different species, is also distinguished by its low proliferation). Note also that the working myocardium of the interventricular septum shows similar labeling intensity as the compact myocardium (see also Figure2B for comparison with fetal chick data).
If the pulse duration is equivalent to or longer than cell cycle length, one can obtain “saturation labeling” that highlights (with suitable nuclear counterstaining) the non-proliferating cell population (Figure 1C). This has proven useful in the study of quiescent or differentiating cell populations, such as newly committed myocardium (Sedmera et al., 2003a) or cardiac conduction system (Thompson et al., 1990a; Thompson et al., 1995; Cheng et al., 1999). A note of caution should be added for early postnatal studies. This period is characterized by a shift from hyperplastic to hypertrophic myocyte growth, and often involves binucleation, i.e. karyokinesis without cytokinesis (Clubb and Bishop, 1984; Kellerman et al., 1992; Li et al., 1996). Additionally, DNA synthesis is often activated during development of cardiac hypertrophy in the adult heart, so interpretation of labeling studies in such cases needs to be complemented by another method (cell counts, cell volume measurements).
Intrinsic markers of cell proliferation
For archival tissue or human biopsies, several endogenous cell cycle markers can be detected by immunochemistry. One popular marker used in pathology is PCNA (proliferating cell nuclear antigen, Figure 1B), which works well but presents variable intensity of labeling in comparison with “all or nothing” nature of anti-bromodeoxyuridine. PCNA is unfortunately of limited value for quantification of proliferation in rapidly proliferating embryonic tissues where almost all cells are positive (except for detecting quiescent cell populations, see below). Other popular markers of proliferation include Ki-67 (Orlic et al., 2001b; Lynch et al., 2007), and phospho histone-H3 (Poolman and Brooks, 1998; Suzuki et al., 2005), that provides a sharper, less laborious alternative to earlier mitotic counts (Grohmann, 1961; Rychter et al., 1979). Various techniques and their principles are summarized in Table 1.
Table 1.
Overview of commonly used techniques for studying myocyte proliferation. Care should be taken to use an unambiguous (preferably nuclear) myocyte marker, as it is otherwise difficult to distinguish in vivo myocytes from non-myocytes (Dowell et al., 2003).
| Short name | Target labeled | Reference |
|---|---|---|
| Mitotic counts | Chromosomes in mitosis; laborious, requires patience | (Grohmann, 1961; Manasek, 1968; Rychter et al., 1979) |
| Phosphohistone H3 | Nuclear protein, phosphorylated during mitosis | (Poolman and Brooks, 1998; Suzuki et al., 2005) |
| [3H] thymidine autoradiography | DNA of nuclei in S-phase | (Thompson et al., 1990b; Sedmera et al., 2002) |
| BrdU immunolabeling | DNA of nuclei in S-phase | (Sedmera et al., 2002; van den Berg et al., 2009) |
| Ki-67 | Nuclear antigen, cell proliferation marker, positive in tumors | (Orlic et al., 2001b; Lynch et al., 2007) |
| PCNA | Nuclear antigen present in proliferating cells, good for quiescent cells in the embryo or cycling cells in tumors | (Thompson et al., 1990a; Thompson et al., 1995; Lin et al., 2000; Morritt et al., 2007) |
Viral and genetic techniques
In experimental systems, retroviral, adenoviral or genetic labeling reveals proliferative history and clonal origin of cells within myocardium. Infection with adenovirus, which has an incremental likelihood of being lost with subsequent cell division, has been used to highlight areas of slowly dividing myocardium such as the outflow tract in the avian heart ( Cheng et al 1999; Watanabe et al., 2001). Retroviral labeling has been used to prove the existence of common progenitors of both working and conducting myocardium, firmly establishing the myogenic origin of ventricular conduction system (Gourdie et al., 1995; Cheng et al., 1999). Conceptually similar studies were performed using nlaacZ transgene under the control of the alpha cardiac actin gene in the mouse (Meilhac et al., 2003), demonstrating that patterns of myocardial growth are similar in mammals and birds. Recently, this model was used to study the origin of ventricular conduction system, and it was shown that it is also myogenic (Miquerol et al., 2010). It is distinguished from the surrounding working myocardium by notably lower proliferation rates, as can be also appreciated from saturation labeling (Figure 1C).
Developmental changes in rates of myocyte proliferation
Embryonic period
Let us now review the dynamics of myocyte proliferation during development. During the earliest period of heart function, prior to peak proliferation at embryonic days 3–4 (in the chick embryo, Figure 2a), the tubular heart grows primarily by addition of newly committed mesenchymal cells (Moorman et al., 2010), whose high proliferative activity drops as they start to synthesize contractile proteins to become functional cardiomyocytes (Kelly et al., 2001; Sedmera et al., 2003a). In general, proliferative activity is inversely related to the level of cell differentiation. Thus, the rates of cell proliferation are the highest at the early, less differentiated developmental stages (ballooning period of chamber formation, Moorman et al., 2010) and then taper off until birth or hatching (Figure 2). There is a short spike in the early postnatal period, during adaptations to postnatal circulatory changes, followed by essentially permanent cell cycle arrest.
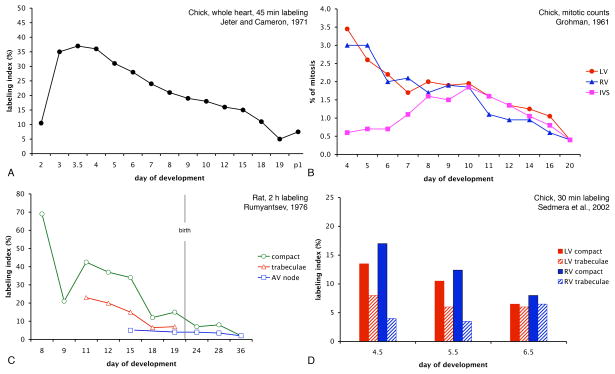
Figure 2.
Quantitative data of developmental trends in myocyte proliferation. A. Data from the entire chick embryonic heart (labeling with radioactive thymidine for 45 minutes show the peak in labeling index between embryonic day 3 and 4. B. Historical data of mitotic counts from chick embryonic heart showing similar declining trend from peak at embryonic day 4 towards hatching in both ventricles. This graph also shows clearly that the forming interventricular septum is distinguished by its low proliferative activity, while after completion of septation it behaves as the rest of the working myocardium. C. Regional differences in myocyte labeling in developing rat heart. Note the difference between the compact zone and the trabeculae as well as gradual decrease towards birth. D. Values from 2h bromodeoxyuridine labeling in the preseptation chick heart show decreasing gradient between the compact myocardium and trabeculae as well as decline in labeling with advancing development. The trends are identical in both left and right ventricle.
Regional differences in cardiac morphogenesis
The pattern of gradual decrease of proliferative activity during development has been noted by several investigators, including Ivan Cameron in the chick (Jeter and Cameron, 1971) and Pavel Rumyantsev in the rat (Rumyantsev, 1982). Other interesting temporal patterns emerge when one follows proliferative activity over time in different cardiac compartments. The path from the simple tubular heart nourished by diffusion from its lumen to mature ventricle supplied from the outside by coronary vessels is not straightforward. Ventricular trabeculae first appear as a sponge-like network of well-differentiated muscle, perhaps enabling to increase myocardial mass in the absence of coronary perfusion (Minot, 1901). Early ventricular trabeculae are fenestrated myocardial sheets (Icardo and Fernandez-Teran, 1987; Sedmera et al., 1997) that appear in histological section as finger-like (or villous) protrusions from the ventricular wall (Marchionni, 1995). Trabeculae not only decrease the diffusion distance for oxygen and nutrients, but also play a role in electrical conduction (de Jong et al., 1992). In the chicken, they also contribute to ventricular septation. Dieter Grohmann (Grohmann, 1961) noticed that the forming interventricular septum in the chick has a low mitotic activity, as it arises from coalescence of the slowly-growing ventricular trabeculae (Harh and Paul, 1975; Ben-Shachar et al., 1985). The difference between the ventricular compact myocardium and trabeculae (Figure 1C) also tends to decrease with advancing development and trabecular compaction (Figure 2D). Once septation and trabecular compaction are underway, the septal musculature adopts the kinetics of nearby working ventricular myocardium, cycling at higher rates (Figure 2B). The developing conduction system stands out by its low proliferative activity in chick (Cheng et al., 1999), mouse (Erokhina and Rumyantsev, 1988; Sedmera et al., 2003a) and rat (Thompson et al., 1995).
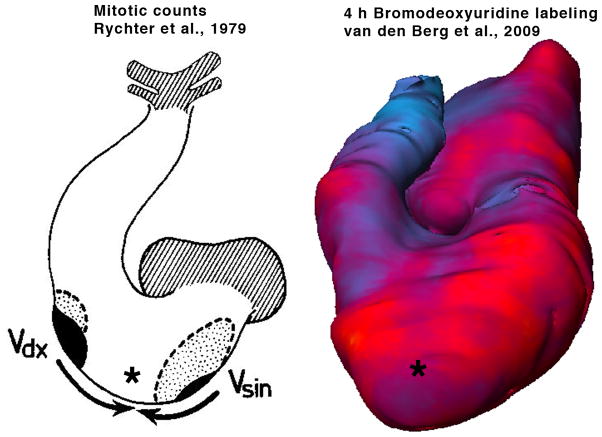
Differential growth of regions within the same compartment is important for molding of the entire organ. Proliferative regions within the ventricular free walls (Figure 3) have been highlighted by mitotic counts by Klaus Goerttler (Goerttler, 1956) and Rychter and associates (Rychterova, 1978; Rychter et al., 1979) and with BrdU labeling (Thompson et al 1990b), beautifully illustrated by the Moorman lab using three dimensional rendering (van den Berg et al., 2009). Regional differences exist even across the ventricular wall, with the highest proliferative activity localized in the parietal compact zone (Sedmera et al., 2002), lower proliferation in the trabeculae (Figure 2D), and virtually zero in the terminally differentiated parts of the central conduction system (Mikawa et al., 1992; Cheng et al., 1999). These local differences are confirmed by analysis of shape of clones derived from a single myocyte (Meilhac et al., 2003), which suggests that growth and shaping of the ventricular myocardium occur centripetally, i.e. by apposition of new cells (Jeter and Cameron, 1971) and by “ballooning” of the ventricular chambers in the period of cardiac chamber formation (Moorman and Christoffels, 2003).
Figure 3.
Differences in myocyte proliferation in the compact zone at the organ level. Two proliferative centers (black) in the apices of prospective left and right ventricle, together with lower activity in the interventricular septum (*), were noted by Rychter et al. (1979). Similar data (high proliferative activity in red, slowly dividing septum - *), together with regions of low myocyte proliferation in the atrioventricular canal and the outflow tract (blue) were recently reported by van den Berg et al (2009).
Postnatal myocyte proliferation
As mentioned earlier, myocyte proliferation essentially stops early after birth or hatching, and further growth in volume of myocardial compartment occurs by increasing cell size (hypertrophy). In both chick and rat, this transition is rather abrupt (Clubb and Bishop, 1984; Kellerman et al., 1992; Kajstura et al., 1995a; Li et al., 1996). However, induced growth during this period (such as occurs after experimental changes in mechanical loading) is still based, at least in part, on cell proliferation (Sedmera et al., 2003b). It was shown recently that the early neonatal period in mouse is privileged in its myocyte regenerative capacity (Porrello et al., 2011) approaching that of lower vertebrates such as zebrafish (Lepilina et al., 2006; Kikuchi et al., 2010). This suggests that early corrective surgical interventions could potentially benefit through compensatory growth that is impossible to achieve with later interventions, thus enhancing the feasibility of such procedures as early biventricular repair for milder cases of hypoplastic left heart syndrome (Foker et al., 1999; Tchervenkov et al., 2000).
Cell cycle checkpoints
Postnatal proliferative block
What makes myocytes divide is interesting, but what makes them stop dividing is fascinating. The mitotic block in postnatal myocytes is generally attributed to inhibition of cyclins and cyclin dependent kinases (Horky et al., 1997; Brooks et al., 1998; Burton et al., 1999; Nagahama et al., 2001). There are other control mechanisms that might arrest cardiac myocytes in G0 phase of cell cycle, such as tumor suppressor protein p16ink associated with cell senescence (Kajstura et al., 2010), cyclin-dependent kinase inhibitor p21 (Poolman and Brooks, 1998), tumor suppressor protein p53 (Liu et al., 2010b) and its activator p14ARF, or retinoblastoma protein (Brooks et al., 1998), that must be phosphorylated by cyclin D-cdk4/6 in order to promote postnatal myocyte hypertrophy (Hinrichsen et al., 2008). Recent in vitro study of differentiated rat and mouse atrial and ventricular myocytes confirmed p21, p53 and 14-3-3 cell cycle regulators as key factors maintaining the myocytes in G0 phase (Zhang et al., 2010). Semiquantitative immunohistochemistry showed their down regulation during de-differentiation of myocytes in culture and ensuing cell cycle re-entry. This study also showed that atrial myocytes, expressing smaller amounts of those inhibitors, were more ready to renew proliferation. Notch signaling has also been shown to enable renewed cycling of quiescent embryonic stem cell-derived and neonatal ventricular cardiomyocytes through its action on cyclin D (Campa et al., 2008). Down-regulation of transcription factor C/EBPβ is associated with cell cycle reentry during exercise-induced myocardial growth (Bostrom et al., 2010). This proliferation was shown to be mediated in part by CITED4 up-regulation. A distinct control mechanism appears to be involved in maintenance of postnatal hypertrophy (Li et al., 1998; Poolman and Brooks, 1998). In contrast to this plethora of molecular players, the role of epigenetic factors, including oxygen tension, mechanical loading or metabolic changes that all show profound changes during postnatal myocyte maturation, remains underexplored.
Embryonic cell cycle arrest and development of conduction system
What forces a precisely defined population of embryonic cardiomyocytes destined to become conduction system to dramatically slow and even stop their proliferation at a specific time point amidst their rapidly cycling neighbors is indeed puzzling. While there is slowly emerging a molecular fingerprint of genes differentially expressed in the cardiac conduction system (Mommersteeg et al., 2007; Aanhaanen et al., 2009), little attention has been focused on the link between these and myocyte proliferation. The earliest precursors of ventricular conduction tissues are derived from the inner cell layer of the tubular single ventricle; some exit the cell cycle permanently during cardiac looping (Stage 13 in the chick), well before any known extracardiac cell population reaches the heart (Thompson et al., 1990b; Thompson et al., 2000; Thompson et al., 2003). This suggests a local mechanism of control, perhaps higher mechanical strain of the tissue (Damon et al., 2009) or signaling from the endocardium (Gourdie et al., 2003). Although it remains difficult to separate these two possibilities in vivo, recent experiments with unloaded chick embryonic hearts in culture have shown that passive myocyte strain, rather than shear stress-dependent signaling from the endocardium, is sufficient to stimulate differentiation of the primary ring myocardium (Sankova et al., 2010). In the chicken, recruitment of working myocytes to the conduction lineages occurs throughout development, with more distal Purkinje fibers added in the last third of incubation, as their proliferation slows or stops (Thompson et al., 2000). This hypothesis of distal recruitment based upon studies in the chick embryo (Cheng et al., 1999) has been challenged by more recent results of clonal analysis of ventricular myocytes in a transgenic mouse in which LacZ is randomly activated in cardiomyocytes and resulting clone size gives an estimate of proliferative history of cells in various myocardial compartments (Miquerol et al., 2010); small clones of cells were found limited to conduction tissues, suggesting that there is a non-zero proliferative activity going on even after commitment to the conduction lineage. However, comparison of their size with much larger clones found in the working myocardium suggests that slowed proliferation is definitely a common theme in conduction system formation in both birds and mammals. Results derived from clonal analysis and proliferative history as related to cardiac growth and conduction system formation have been recently reviewed and integrated by Moorman and associates (Moorman et al., 2010).
Long-term kinetic of ventricular conduction system
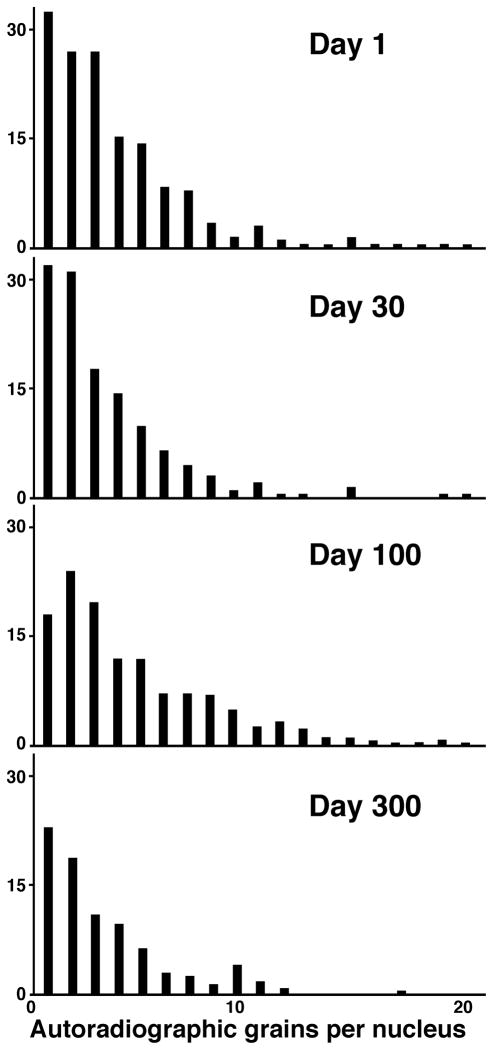
Long-term follow-up studies tracked the fate of the early-exiting ventricular conduction system myocytes throughout the live of the animal. In an extension of our earlier study (Thompson et al., 2000), a group of 81 chick embryos were pulse-labeled with one microcurie of [3H]-thymidine at embryonic day 3 (Hamburger-Hamilton Stage 15) and sampled at various stages during incubation and at days 1, 10, 30, 100 and 300 of postnatal development. Invariably, heavily labeled cells (with nuclear grain counts of 15–30 grains per nucleus) were detected in the His bundle, its bifurcation and proximal bundle branches. No evidence of division, detected either by 24-h bromodeoxyuridine labeling or decrease in grain counts was found in the most heavily labeled cells prenatally. Comparison of myocyte grain counts with those over nuclei of motor neurons in the neural tube suggested that one round of nuclear division may have occurred in labeled myocytes within ten days after hatching. This was confirmed by the rare occurrence of BrdU-positive nuclei with heavy thymidine label, usually two nuclei in one cell, suggesting binucleation without cytokinesis. In later life (days 30–300, Figure 4), only gradual decreases in counts of labeled cells were found in serial sections spanning the ventricular conduction system. This was likely due to apoptosis, which was found at low intensity (less then 1 in 1000 TUNEL-positive cells) in the myocardium including the conduction fascicles. No evidence of renewed DNA synthesis was found past day 30 in conduction system cardiomyocytes.
Figure 4.
Persistence of labeled cells in the ventricular conduction system in the chick. There is a gradual decrease in heavily labeled cells (over 10 grains per nucleus) with postnatal development, but both these and less intensively labeled cells can be found into adulthood, ten months after hatching. Values are means from three hearts, counting grains per nucleus in every tenth section. (See Thompson et al., 2000).
Myocyte regeneration potential
The topic of heart regeneration is currently controversial, as reviewed in a recent book on the topic of cardiac development and regeneration in particular (Rosenthal and Harvey, 2010). During the past two decades, a paradigm shift has occurred from considering the adult myocardium as entirely post-mitotic (the only population of cells capable of proliferation being fibroblasts, as evidenced by scar formation during myocardial infarction), to admitting a potentially important role of continued proliferation and differentiation of myocytes from resident stem cells for maintenance of homeostasis. It was recently postulated (Zhang et al., 2010) that the cell material for regeneration might derive from (i) resident cardiac stem cells, (ii) bone marrow-derived stem cells, and/or (iii) stem cells resultant from de-differentiation of existing cardiomyocytes. Let us further explore these three options that are not mutually exclusive.
Cell cycle re-entry in adult myocytes
Early studies in a rat infarction model showed that there is small myocyte proliferative activity, which can increase in some compartments after experimental perturbation (Rumyantsev and Kassem, 1976). Next it was posited from rates of programmed cell death (apoptosis) in normal myocardium that cardiac muscle must somehow turnover within months or several years, depending on age and species under study, as well as the technique used for detecting dying cells (Kajstura et al., 1995b; Kajstura et al., 1996). Cell division in bona fide cardiomyocytes has been observed in the early chick (Manasek, 1968), in pathological conditions such as heart failure (Anversa et al., 1998; Kajstura et al., 1998), and also in normal hearts, with very high numbers of cells counted (in the order of tens of thousands, for detecting both cell death and cell division, as these are indeed not very common events).
Resident cardiac stem cells
Discovery of clusters of cells that are negative for traditional myocyte markers such as contractile proteins but positive for stem cell markers such as c-kit (Beltrami et al., 2003) made an important contribution to elucidation of this story. Indeed, well-differentiated binucleated working myocytes have not been found reentering the cell cycle, but those cells caught in the process of DNA synthesis appear to be new, small myocytes possibly derived from these resident stem cells. An alternative ingenious approach used radiocarbon dating of normal human tissues from before and after the era of atomic weapons testing to show that a substantial number of myocytes appear younger than the person (Bergmann et al., 2009). Calculated turnover rate was found to decrease from 1% in youth to less than half a percent in the senium, suggesting that even adult myocardium possesses a self-renewing capability. A unique study on patients treated with iododeoxyuridine for cancer showed large differences between individual patients, but this very long-term (weeks) “saturation labeling” showed rates of positive myocyte nuclei between 2.5 and 46%, suggesting rather active turnover of myocytes with half-life between 4 and 8 years (Kajstura et al., 2010). Meticulously designed control experiments exploring alternative explanations excluded increased myocyte ploidy or cell fusion as quantitatively important explanations of these observations. Despite rather atypical human population and small sample size (eight), these numbers (considerably higher than those demonstrated by radiocarbon study of Bergman and associates) clearly show that the adult mammalian heart possesses a significant self-renewing potential. An important question remaining is whether, and how, might this potential be harnessed for regeneration of myocardium lost during myocardial infarction. Genetic fate-mapping study in mice (Hsieh et al., 2007) have shown that while the stem cells contribute to replacement of myocytes after injury, they do not play a major role in normal homeostasis and renewal during aging. This accords with the above observations showing proliferative capacity of adult cardiomyocytes sufficient to guarantee physiological renewal. Overview of studies in the adult weighting the relative contribution of differentiated cardiomyocytes and multipotent cardiovascular progenitor cells was recently provided by Sturzu and Wu (2011). Possible molecular players capable of inducing adult myocytes to re-enter the cell cycle are discussed in the next section.
Myocytes from other sources?
Extracardiac cell sources such as bone marrow stem cells were reported to be beneficial post-infarct ten years ago (Orlic et al., 2001a; Orlic et al., 2001b; Orlic et al., 2001c; Dawn et al., 2005), but ensuing clinical trials in humans show that such effects, while real and sustained, are rather modest, and more likely due to paracrine factors produced by the transplanted cells rather than the addition of a significant mass of “new” myocardium from the transplant (summarized by (Jiang et al., 2010)). Experimental studies from other labs have shown that the cardiogenic potential of hematopoietic cells, especially in vivo, might be overstated, and suggest either cell fusion (Alvarez-Dolado et al., 2003) as a possible explanation of observations of the Anversa lab, or that the presence of circulation-derived cells in the myocardium after infarction does not have to mean that they differentiate into myocytes (Balsam et al., 2004; Murry et al., 2004). Clearly, many more basic issues need to be resolved before myocardial regeneration comparable to the zebrafish amputation model (Lepilina et al., 2006) can be seen in clinically relevant model of myocardial infarction in mammals. One step in this direction is the observation of the ability of one-day-old mice to regenerate amputated apex of the ventricle, an ability lost as early as one week after birth (Porrello et al., 2011).
One of the unresolved questions we would like to pose here is the actual developmental origin of those resident stem cells: at the early tubular stages, there is no evidence of non-myocytes being interspersed in the early myocardium; hence, it is possible that their source is extracardiac. Possible contributors could be 1) epicardium-derived cells, which were found to be capable of differentiating into myocytes in vitro (Kruithof et al., 2006), although controversy about applicability of this mechanism in vivo has been raised (Christoffels et al., 2009); 2) neural crest cells that are known to be multipotent (Yelbuz et al., 2003; Sieber-Blum et al., 2004; Kirby, 2007; Krejci and Grim, 2010) and contribute to multiple cell types in the heart; or 3) cells derived from the circulation (possibly originating in bone marrow or other hematopoietic locations), shown to preferentially invade a perivascular niche during the second half of incubation in the quail-chick parabiosis system (Zhang et al., 2006).
Alternatively, the cardiac resident stem cells can derive from myocytes by their dedifferentiation, as was shown in vitro (Zhang et al., 2010). This decision can be dictated by the local microenvironment, since physical factors such as low oxygen and nutrient availability are known to contribute to reversal of working myocyte fate. Another possibility is the contribution from second heart field cells expressing Isl1 (Cai et al., 2003; Zhou et al., 2008) that were shown to contribute to multiple cardiac lineages (myocytes, smooth muscle, endothelium, and epicardium).
Molecular control mechanisms of myocyte proliferation
Adult myocytes
The regulation of proliferation remains one of the major questions in developmental biology of cardiac muscle (Pasumarthi and Field, 2002), especially as related to heart repair (Oh et al., 2004). Interested readers should also consult other reviews focused on cell proliferation after infarction (Dowell et al., 2003), during regeneration in adult animals (Rubart and Field, 2006) or during development (Moorman et al., 2010). Postnatal cell cycle arrest appears to be species-specific (Wills et al., 2008) and potentially reversible, as indicated by significant endogenous regenerative capability of fish heart in response to injury (Lepilina et al., 2006). Recent experiments in zebrafish have shed some light on the cellular nature of this process. Amputation of part of the ventricle leads to myocyte dedifferentiation and proliferation involving polo-like kinase 1 (Jopling et al., 2010). These newly generated myocytes become electrically coupled with the remainder of the ventricle within a month from injury (Kikuchi et al., 2010). Recent studies in mammals have also shown that adult myocytes can be induced to proliferate by inhibition of p38 mitogen-activated protein kinase or stimulation with FGF1 (Engel et al., 2005), by stimulation by periostin (Kuhn et al., 2007), or through a neuregulin/ErbB signaling cascade (Bersell et al., 2009). Such studies also point out various signaling pathways regulating myocyte proliferation during normal development as well as during remodeling.
Regulation of growth in prenatal development
Multiple studies, both in vivo and in vitro, agree on a pivotal role of the FGF/FGFR cascade in immature myocytes (Speir et al., 1992; Sugi et al., 1993; Kardami et al., 1995; Mima et al., 1995; Sugi et al., 1995; Sheikh et al., 1999; Franciosi et al., 2000; Lavine et al., 2005). Among 23 members of the FGF family and 4 different FGF receptors, FGF2 and FGFR1 are the ones most often implicated. We have shown recently that exogenous addition of depleted FGF2 can rescue myocyte proliferation in the context of experimental left ventricular hypoplasia, indicating the therapeutic potential of soluble factors controlling myocyte proliferation (Dealmeida and Sedmera, 2009).
Sources of such growth factors may be multiple. They are produced by myocytes themselves and released in response to increased stretch (Clarke et al., 1995). The epicardium is often cited as a source of signaling molecules modulating myocyte proliferation, e.g retinoic acid (Chen et al., 2002) and PDGF (Kang et al., 2008). Perhaps not surprisingly, a variety of other molecules influence myocyte proliferation, as evidenced by often lethal phenotypes resulting from their deletion. In some cases, it is not clear whether these effects are primary or secondary, since myocyte proliferation is intimately linked with cardiac function and will of course drop prior to embryonic demise. Some molecules derived from the endocardium, such as endothelin or neuregulin with their signaling cascade, are required for both proliferation and differentiation into chamber myocardium (Asai et al., 2010) or trabecular formation (Liu et al., 2010a). Neuregulin1 signaling via its receptors ErbB2 and ErbB4, together with FGF1 and periostin, are also able to induce adult rat myocyte proliferation in vitro (Bersell et al., 2009). In Table 2 we compile evidence for growth factor signals affecting myocyte proliferation in both developing and mature cardiomyocytes.
Table 2.
Growth factors affecting myocyte proliferation. Cell cycle controlling factors were also summarized previously by Dowell et al. (2003).
| Prenatal heart | |||
|---|---|---|---|
| Factor | Species | Study design | Reference |
| ANF | Chick | Cell culture stimulation and inhibition | (Koide et al., 1996) |
| CT-1 | Mouse | Cell culture, expression studies | (Sheng et al., 1996) |
| EGF | Human Chick |
Cell culture Cell culture |
(Goldman et al., 1996; Hornberger et al., 2000) (Lau, 1993) |
| FGF2 | Chick | Soaked beads in vivo Adenoviral overexpression |
(Franciosi et al., 2000) (Dealmeida and Sedmera, 2009) |
| FGFR1 | Chick | Retroviral expression of DN receptor, antisense | (Mima et al., 1995) |
| IGF | Chick | Cell culture | (Lau, 1993) |
| NT-3 | Chick | Retroviral overexpression of truncated receptor | (Lin et al., 2000) |
| PDGF | Chick, mouse Mouse Rat Human |
Expression studies, induced by overload Alpha subunit receptor deletion Epicardium-conditioned media Decreased in left heart hypoplasia |
(Jedlicka et al., 1991; Lau, 1993; Pexieder et al., 1995) (Schatteman et al., 1995) (Kang et al., 2008) (Burton et al., 1991) |
| Retinoids (indirectly?) | Mouse | Targeted deletion of receptors | (Kastner et al., 1994; Gruber et al., 1996; Chen et al., 2002) |
| Postnatal heart | |||
|---|---|---|---|
| Factor | Species | Study design | Reference |
| EPO | Rat | Cell culture | (Wald et al., 1996) |
| FGF1 | Rat Newt |
Primary culture Primary culture |
(Engel et al., 2005) (Soonpaa et al., 1994) |
| FGFs | Zebrafish | Inhibition of signaling leads to scarring instead of regeneration | (Wills et al., 2008) |
| IGF-1 | Rat Mouse |
Correlation of expression of ligand and receptor with proliferation Recombinant protein administered in vivo post- infarction |
(Cheng et al., 1995) (Kofidis et al., 2004) |
| Neuregulin1 | Rat, mouse | Primary culture, in vivo injection, ErbB4 receptor null or overexpression; expression studies | (Zhao et al., 1998; Bersell et al., 2009) |
| Periostin | Rat | Cell culture, in vivo infarction | (Kuhn et al., 2007) |
Epigenetic control mechanisms
Mechanical loading is one important epigenetic factor affecting myocardial growth. Like skeletal muscle, myocardium adapts to increased functional demands, either by hyperplasia during the prenatal period (Clark et al., 1989; Saiki et al., 1997) or by hypertrophy in adulthood. This is true even for myocytes grown in culture, which show much higher rates of proliferation and differentiation if appropriately stretched (Miller et al., 2000). Even in the absence of detailed knowledge about the molecular nature of such strain sensitive signals, discussed by Damon et al (2009), mechanical loading or use of various conditioned media could be exploited for generation of artificial myocardial constructs (Evans et al., 2003). Once the critical thickness of approximately 50–100 microns is reached, vascularization becomes necessary for further growth. Indeed, in myocardial constructs in unstirred bioreactors, hypoxia appears to be a limiting factor beyond thickness of about 150 microns (Radisic et al., 2005; Tobita et al., 2006).
Normally, the process of growth of the myocardium and coronary bed is tightly coupled (Tomanek et al., 1999). Interestingly, the factors controlling the growth of these two compartments are similar, with FGF2 emerging as a major player (Tomanek et al., 1996; Tomanek et al., 2008; Lavine and Ornitz, 2009). This is perhaps not surprising, since both myocardium and vascular tissues are mesodermally derived and therefore likely to respond to similar stimuli in similar fashion.
In conclusion, myocyte proliferation in the developing heart certainly plays a critical role in growth, morphogenesis and remodeling of the organ. It is also an important part of the equation controlling homeostasis in the adult. Because of the complexity of the patterns and pronounced differences between developmental periods, care must be taken in designing studies to measure its rate. Identification of control mechanisms, as well as those directing the exit from the cell cycle, will help in designing strategies for repair of diseased adult myocardium, including formation of artificial myocardial tissue.
Acknowledgments
We would like to express our sincere thanks to Drs. Rob Gourdie, Christi Kern and Vojtech Melenovsky for critical reading of the manuscript.
Grant support:
Ministry of Education VZ 0021620806; Academy of Sciences AV0Z50450515 and AV0Z50110509; Grant Agency of the Czech Republic 304/08/0615, P302/11/1308. D. Sedmera is also a recipient of the Purkinje Fellowship from the Academy of Sciences of the Czech Republic. National Institutes of Health, HL50582, HL91452, RR16434.
References
- Aanhaanen WT, Brons JF, Dominguez JN, Rana MS, Norden J, Airik R, Wakker V, de Gier-de Vries C, Brown NA, Kispert A, Moorman AF, Christoffels VM. The Tbx2+ primary myocardium of the atrioventricular canal forms the atrioventricular node and the base of the left ventricle. Circ Res. 2009;104:1267–1274. doi: 10.1161/CIRCRESAHA.108.192450. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Anversa P, Leri A, Beltrami CA, Guerra S, Kajstura J. Myocyte death and growth in the failing heart. Lab Invest. 1998;78:767–786. [PubMed] [Google Scholar]
- Asai R, Kurihara Y, Fujisawa K, Sato T, Kawamura Y, Kokubo H, Tonami K, Nishiyama K, Uchijima Y, Miyagawa-Tomita S, Kurihara H. Endothelin receptor type A expression defines a distinct cardiac subdomain within the heart field and is later implicated in chamber myocardium formation. Development. 2010;137:3823–3833. doi: 10.1242/dev.054015. [DOI] [PubMed] [Google Scholar]
- Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar G, Arcilla RA, Lucas RV, Manasek FJ. Ventricular trabeculations in the chick embryo heart and their contribution to ventricular and muscular septal development. Circ Res. 1985;57:759–766. doi: 10.1161/01.res.57.5.759. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, Panakova D, Gupta RK, Xiao C, MacRae CA, Rosenzweig A, Spiegelman BM. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G, Poolman RA, Li JM. Arresting developments in the cardiac myocyte cell cycle: role of cyclin-dependent kinase inhibitors. Cardiovasc Res. 1998;39:301–311. doi: 10.1016/s0008-6363(98)00125-4. [DOI] [PubMed] [Google Scholar]
- Burns KA, Kuan CY. Low doses of bromo- and iododeoxyuridine produce near-saturation labeling of adult proliferative populations in the dentate gyrus. Eur J Neurosci. 2005;21:803–807. doi: 10.1111/j.1460-9568.2005.03907.x. [DOI] [PubMed] [Google Scholar]
- Burton PB, Hauck A, Nehlsen-Cannarella SL, Gusewitch GA, Sorensen CM, Gundry SR, Bailey LL. Hypoplastic left heart syndrome: some clues to its aetiology. Lancet. 1991;338:1148. doi: 10.1016/0140-6736(91)92006-n. [DOI] [PubMed] [Google Scholar]
- Burton PB, Raff MC, Kerr P, Yacoub MH, Barton PJ. An intrinsic timer that controls cell-cycle withdrawal in cultured cardiac myocytes. Dev Biol. 1999;216:659–670. doi: 10.1006/dbio.1999.9524. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W, Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. J Cell Biol. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Chang TC, Kang JO, Choudhary B, Makita T, Tran CM, Burch JB, Eid H, Sucov HM. Epicardial induction of fetal cardiomyocyte proliferation via a retinoic acid-inducible trophic factor. Dev Biol. 2002;250:198–207. doi: 10.1006/dbio.2002.0796. [DOI] [PubMed] [Google Scholar]
- Cheng G, Litchenberg WH, Cole GJ, Mikawa T, Thompson RP, Gourdie RG. Development of the cardiac conduction system involves recruitment within a multipotent cardiomyogenic lineage. Development. 1999;126:5041–5049. doi: 10.1242/dev.126.22.5041. [DOI] [PubMed] [Google Scholar]
- Cheng W, Reiss K, Kajstura J, Kowal K, Quaini F, Anversa P. Down-regulation of the IGF-1 system parallels the attenuation in the proliferative capacity of rat ventricular myocytes during postnatal development. Lab Invest. 1995;72:646–655. [PubMed] [Google Scholar]
- Christoffels VM, Grieskamp T, Norden J, Mommersteeg MT, Rudat C, Kispert A. Tbx18 and the fate of epicardial progenitors. Nature. 2009;458:E8–9. doi: 10.1038/nature07916. discussion E9–10. [DOI] [PubMed] [Google Scholar]
- Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ. Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am J Physiol. 1989;257:H55–61. doi: 10.1152/ajpheart.1989.257.1.H55. [DOI] [PubMed] [Google Scholar]
- Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circ Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- Clubb FJ, Jr, Bishop SP. Formation of binucleated myocardial cells in the neonatal rat. An index for growth hypertrophy. Lab Invest. 1984;50:571–577. [PubMed] [Google Scholar]
- Damon BJ, Remond MC, Bigelow MR, Trusk TC, Xie W, Perucchio R, Sedmera D, Denslow S, Thompson RP. Patterns of muscular strain in the embryonic heart wall. Dev Dyn. 2009;238:1535–1546. doi: 10.1002/dvdy.21958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong F, Opthof T, Wilde AA, Janse MJ, Charles R, Lamers WH, Moorman AF. Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res. 1992;71:240–250. doi: 10.1161/01.res.71.2.240. [DOI] [PubMed] [Google Scholar]
- Dealmeida A, Sedmera D. Fibroblast Growth Factor-2 regulates proliferation of cardiac myocytes in normal and hypoplastic left ventricles in the developing chick. Cardiol Young. 2009:1–11. doi: 10.1017/S1047951109003552. [DOI] [PubMed] [Google Scholar]
- Dowell JD, Field LJ, Pasumarthi KB. Cell cycle regulation to repair the infarcted myocardium. Heart Fail Rev. 2003;8:293–303. doi: 10.1023/a:1024738104722. [DOI] [PubMed] [Google Scholar]
- Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erokhina IL, Rumyantsev PP. Proliferation and biosynthetic activities of myocytes from conductive system and working myocardium of the developing mouse heart. Light microscopic autoradiographic study. Acta Histochem. 1988;84:51–66. doi: 10.1016/S0065-1281(88)80010-2. [DOI] [PubMed] [Google Scholar]
- Evans HJ, Sweet JK, Price RL, Yost M, Goodwin RL. Novel 3D culture system for study of cardiac myocyte development. Am J Physiol Heart Circ Physiol. 2003;285:H570–578. doi: 10.1152/ajpheart.01027.2002. [DOI] [PubMed] [Google Scholar]
- Foker JE, Berry J, Steinberger J. Ventricular growth stimulation to achieve two-ventricular repair in unbalanced common atrioventricular canal. Progress in Pediatric Cardiology. 1999;10:173–186. [Google Scholar]
- Franciosi JP, Bolender DL, Lough J, Kolesari GL. FGF-2-induced imbalance in early embryonic heart cell proliferation: a potential cause of late cardiovascular anomalies. Teratology. 2000;62:189–194. doi: 10.1002/1096-9926(200010)62:4<189::AID-TERA4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Goerttler K. Die Stoffwechseltopograpie des embryonalen Huhhnerherzens und ihre Bedeutumg fur die Entstehung angeborener Herzfehler. Deutsche Gesellschaft fur Pathologie Verhandlungen. 1956;40:181–186. [Google Scholar]
- Goldman B, Mach A, Wurzel J. Epidermal growth factor promotes a cardiomyoblastic phenotype in human fetal cardiac myocytes. Exp Cell Res. 1996;228:237–245. doi: 10.1006/excr.1996.0322. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Harris BS, Bond J, Justus C, Hewett KW, O’Brien TX, Thompson RP, Sedmera D. Development of the cardiac pacemaking and conduction system. Birth Defects Research. 2003;69C:46–57. doi: 10.1002/bdrc.10008. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Mima T, Thompson RP, Mikawa T. Terminal diversification of the myocyte lineage generates Purkinje fibers of the cardiac conduction system. Development. 1995;121:1423–1431. doi: 10.1242/dev.121.5.1423. [DOI] [PubMed] [Google Scholar]
- Grohmann D. Mitotische Wachstumsintensitat des embryonalen und fetalen Hunchenherzens und ihre Bedeutung fur die entstehung von Herzmissbildungen. Z f Zellforschung. 1961;55:104–122. [Google Scholar]
- Gruber PJ, Kubalak SW, Pexieder T, Sucov HM, Evans RM, Chien KR. RXR alpha deficiency confers genetic susceptibility for aortic sac, conotruncal, atrioventricular cushion, and ventricular muscle defects in mice. J Clin Invest. 1996;98:1332–1343. doi: 10.1172/JCI118920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harh JY, Paul MH. Experimental cardiac morphogenesis. I. Development of the ventricular septum in the chick. J Embryol Exp Morphol. 1975;33:13–28. [PubMed] [Google Scholar]
- Hinrichsen R, Hansen AH, Haunso S, Busk PK. Phosphorylation of pRb by cyclin D kinase is necessary for development of cardiac hypertrophy. Cell Prolif. 2008;41:813–829. doi: 10.1111/j.1365-2184.2008.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horky M, Kuchtickova S, Vojtesek B, Kolar F. Induction of cell-cycle inhibitor p21 in rat ventricular myocytes during early postnatal transition from hyperplasia to hypertrophy. Physiol Res. 1997;46:233–235. [PubMed] [Google Scholar]
- Hornberger LK, Singhroy S, Cavalle-Garrido T, Tsang W, Keeley F, Rabinovitch M. Synthesis of extracellular matrix and adhesion through beta(1) integrins are critical for fetal ventricular myocyte proliferation. Circ Res. 2000;87:508–515. doi: 10.1161/01.res.87.6.508. [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icardo JM, Fernandez-Teran A. Morphologic study of ventricular trabeculation in the embryonic chick heart. Acta Anat. 1987;130:264–274. doi: 10.1159/000146455. [DOI] [PubMed] [Google Scholar]
- Jedlicka S, Finkelstein JN, Paulhamus LA, Clark EB. Increased PDGF-like protein in banded embryonic ventricle. Pediatr Res. 1991;29:19A. [Google Scholar]
- Jeter JR, Jr, Cameron IL. Cell proliferation patterns during cytodifferentiation in embryonic chick tissues: liver, heart and erythrocytes. J Embryol Exp Morphol. 1971;25:405–422. [PubMed] [Google Scholar]
- Jiang M, He B, Zhang Q, Ge H, Zang MH, Han ZH, Liu JP, Li JH, Zhang Q, Li HB, Jin Y, He Q, Gong XR, Yin XY. Randomized controlled trials on the therapeutic effects of adult progenitor cells for myocardial infarction: meta-analysis. Expert Opin Biol Ther. 2010;10:667–680. doi: 10.1517/14712591003716437. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464:606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Mansukhani M, Cheng W, Reiss K, Krajewski S, Reed JC, Quaini F, Sonnenblick EH, Anversa P. Programmed cell death and expression of the protooncogene bcl-2 in myocytes during postnatal maturation of the heart. Exp Cell Res. 1995a;219:110–121. doi: 10.1006/excr.1995.1211. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D’Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, Anversa P. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107:305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kajstura J, Zhang X, Liu Y, Szoke E, Cheng W, Olivetti G, Hintze TH, Anversa P. The cellular basis of pacing-induced dilated cardiomyopathy. Myocyte cell loss and myocyte cellular reactive hypertrophy. Circulation. 1995b;92:2306–2317. doi: 10.1161/01.cir.92.8.2306. [DOI] [PubMed] [Google Scholar]
- Kang J, Gu Y, Li P, Johnson BL, Sucov HM, Thomas PS. PDGF-A as an epicardial mitogen during heart development. Dev Dyn. 2008;237:692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- Kardami E, Liu L, Kishore S, Pasumarthi B, Doble BW, Cattini PA. Regulation of basic fibroblast growth factor (bFGF) and FGF receptors in the heart. Ann N Y Acad Sci. 1995;752:353–369. doi: 10.1111/j.1749-6632.1995.tb17444.x. [DOI] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kellerman S, Moore JA, Zierhut W, Zimmer HG, Campbell J, Gerdes AM. Nuclear DNA content and nucleation patterns in rat cardiac myocytes from different models of cardiac hypertrophy. J Mol Cell Cardiol. 1992;24:497–505. doi: 10.1016/0022-2828(92)91839-w. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464:601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Cardiac Development. New York: Oxford University Press; 2007. p. 273. [Google Scholar]
- Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22:1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- Koide M, Akins RE, Harayama H, Yasui K, Yokota M, Tuan RS. Atrial natriuretic peptide accelerates proliferation of chick embryonic cardiomyocytes in vitro. Differentiation. 1996;61:1–11. doi: 10.1046/j.1432-0436.1996.6110001.x. [DOI] [PubMed] [Google Scholar]
- Krejci E, Grim M. Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol (Praha) 2010;56:149–157. [PubMed] [Google Scholar]
- Kruithof BP, van Wijk B, Somi S, Kruithof-de Julio M, Perez Pomares JM, Weesie F, Wessels A, Moorman AF, van den Hoff MJ. BMP and FGF regulate the differentiation of multipotential pericardial mesoderm into the myocardial or epicardial lineage. Dev Biol. 2006;295:507–522. doi: 10.1016/j.ydbio.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- Lau CL. Behavior of embryonic chick heart cells in culture. 2. Cellular responses to epidermal growth factor and other growth signals. Tissue Cell. 1993;25:681–693. doi: 10.1016/0040-8166(93)90050-u. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Ornitz DM. Shared circuitry: developmental signaling cascades regulate both embryonic and adult coronary vasculature. Circ Res. 2009;104:159–169. doi: 10.1161/CIRCRESAHA.108.191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell. 2006;127:607–619. doi: 10.1016/j.cell.2006.08.052. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- Li JM, Poolman RA, Brooks G. Role of G1 phase cyclins and cyclin-dependent kinases during cardiomyocyte hypertrophic growth in rats. Am J Physiol. 1998;275:H814–822. doi: 10.1152/ajpheart.1998.275.3.H814. [DOI] [PubMed] [Google Scholar]
- Lin MI, Das I, Schwartz GM, Tsoulfas P, Mikawa T, Hempstead BL. Trk C receptor signaling regulates cardiac myocyte proliferation during early heart development in vivo. Dev Biol. 2000;226:180–191. doi: 10.1006/dbio.2000.9850. [DOI] [PubMed] [Google Scholar]
- Liu J, Bressan M, Hassel D, Huisken J, Staudt D, Kikuchi K, Poss KD, Mikawa T, Stainier DY. A dual role for ErbB2 signaling in cardiac trabeculation. Development. 2010a;137:3867–3875. doi: 10.1242/dev.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yue S, Chen X, Kubin T, Braun T. Regulation of cardiomyocyte polyploidy and multinucleation by CyclinG1. Circ Res. 2010b;106:1498–1506. doi: 10.1161/CIRCRESAHA.109.211888. [DOI] [PubMed] [Google Scholar]
- Lynch P, Lee TC, Fallavollita JA, Canty JM, Jr, Suzuki G. Intracoronary administration of AdvFGF-5 (fibroblast growth factor-5) ameliorates left ventricular dysfunction and prevents myocyte loss in swine with developing collaterals and ischemic cardiomyopathy. Circulation. 2007;116:I71–76. doi: 10.1161/CIRCULATIONAHA.106.681866. [DOI] [PubMed] [Google Scholar]
- Manasek FJ. Mitosis in developing cardiac muscle. J Cell Biol. 1968;37:191–196. doi: 10.1083/jcb.37.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchionni MA. Cell-cell signalling. neu tack on neuregulin. Nature. 1995;378:334–335. doi: 10.1038/378334a0. [DOI] [PubMed] [Google Scholar]
- Meilhac SM, Kelly RG, Rocancourt D, Eloy-Trinquet S, Nicolas JF, Buckingham ME. A retrospective clonal analysis of the myocardium reveals two phases of clonal growth in the developing mouse heart. Development. 2003;130:3877–3889. doi: 10.1242/dev.00580. [DOI] [PubMed] [Google Scholar]
- Mikawa T, Cohen-Gould L, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. III: Polyclonal origin of adjacent ventricular myocytes. Dev Dyn. 1992;195:133–141. doi: 10.1002/aja.1001950208. [DOI] [PubMed] [Google Scholar]
- Miller CE, Donlon KJ, Toia L, Wong CL, Chess PR. Cyclic strain induces proliferation of cultured embryonic heart cells. In Vitro Cell Dev Biol Anim. 2000;36:633–639. doi: 10.1290/1071-2690(2000)036<0633:CSIPOC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mima T, Ueno H, Fischman DA, Williams LT, Mikawa T. Fibroblast growth factor receptor is required for in vivo cardiac myocyte proliferation at early embryonic stages of heart development. Proc Natl Acad Sci U S A. 1995;92:467–471. doi: 10.1073/pnas.92.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minot CS. On a hitherto unrecognised circulation without capillaries in the organs of Vertebrata. Proc Boston Soc Nat Hist. 1901;29:185–215. [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Moreno-Rascon N, Beyer S, Dupays L, Meilhac SM, Buckingham ME, Franco D, Kelly RG. Biphasic development of the Mammalian ventricular conduction system. Circ Res. 2010;107:153–161. doi: 10.1161/CIRCRESAHA.110.218156. [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Hoogaars WM, Prall OW, de Gier-de Vries C, Wiese C, Clout DE, Papaioannou VE, Brown NA, Harvey RP, Moorman AF, Christoffels VM. Molecular Pathway for the Localized Formation of the Sinoatrial Node. Circ Res. 2007;100:354–362. doi: 10.1161/01.RES.0000258019.74591.b3. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. Cardiac chamber formation: development, genes, and evolution. Physiol Rev. 2003;83:1223–1267. doi: 10.1152/physrev.00006.2003. [DOI] [PubMed] [Google Scholar]
- Moorman AF, van den Berg G, Anderson RH, Christoffels VM. Early Cardiac Growth and the Ballooning Model of Cardiac Chamber Formation. In: Rosenthal N, Harvey RP, editors. Heart Development and Regeneration. London: Elsevier; 2010. pp. 219–236. [Google Scholar]
- Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Cardiac tissue engineering in an in vivo vascularized chamber. Circulation. 2007;115:353–360. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- Nagahama H, Hatakeyama S, Nakayama K, Nagata M, Tomita K. Spatial and temporal expression patterns of the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p57Kip2 during mouse development. Anat Embryol (Berl) 2001;203:77–87. doi: 10.1007/s004290000146. [DOI] [PubMed] [Google Scholar]
- Oh H, Chi X, Bradfute SB, Mishina Y, Pocius J, Michael LH, Behringer RR, Schwartz RJ, Entman ML, Schneider MD. Cardiac muscle plasticity in adult and embryo by heart-derived progenitor cells. Ann N Y Acad Sci. 2004;1015:182–189. doi: 10.1196/annals.1302.015. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001a;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 229–230. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001b;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001c;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- Pexieder T, Jedlicka S, Sugimara K, Tatimatsu A, Sato H. Immunohistochemical localization of platelet derived growth factor (PDGF) during cardiac morphogenesis in chick and mouse embryos and fetuses. In: Clark EB, Markwald RR, Takao A, editors. Developmental Mechanisms of Heart Disease. Armonk, NY: Futura Publishing; 1995. pp. 207–212. [Google Scholar]
- Poolman RA, Brooks G. Expressions and activities of cell cycle regulatory molecules during the transition from myocyte hyperplasia to hypertrophy. J Mol Cell Cardiol. 1998;30:2121–2135. doi: 10.1006/jmcc.1998.0808. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, Vunjak-Novakovic G. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. Am J Physiol Heart Circ Physiol. 2005;288:H1278–1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Harvey RP. Heart Development and Regeneration. Vol. 2. London: Elsevier; 2010. p. 496. [Google Scholar]
- Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- Rumyantsev PP. Cardiomyocytes in the processes of reproduction, differentiation, and regeneration. Leningrad: Nauka; 1982. p. 285. [Google Scholar]
- Rumyantsev PP, Kassem AM. Cumulative indices of DNA synthesizing myocytes in different compartments of the working myocardium and conductive system of the rat’s heart muscle following extensive left ventricle infarction. Virchows Arch B Cell Pathol. 1976;20:329–342. doi: 10.1007/BF02890352. [DOI] [PubMed] [Google Scholar]
- Rychter Z, Rychterova V, Lemez L. Formation of the heart loop and proliferation structure of its wall as a base for ventricular septation. Herz. 1979;4:86–90. [PubMed] [Google Scholar]
- Rychterova V. Development of proliferation structure of the ventricular heart wall in the chick embryo between the 6th and 14th day of embryogenesis. Folia Morphol (Praha) 1978;26:131–143. [PubMed] [Google Scholar]
- Saiki Y, Konig A, Waddell J, Rebeyka IM. Hemodynamic alteration by fetal surgery accelerates myocyte proliferation in fetal guinea pig hearts. Surgery. 1997;122:412–419. doi: 10.1016/s0039-6060(97)90034-9. [DOI] [PubMed] [Google Scholar]
- Sankova B, Machalek J, Sedmera D. Effects of mechanical loading on early conduction system differentiation in the chick. Am J Physiol Heart Circ Physiol. 2010;298:H1571–1576. doi: 10.1152/ajpheart.00721.2009. [DOI] [PubMed] [Google Scholar]
- Schatteman GC, Motley ST, Effmann EL, Bowen-Pope DF. Platelet-derived growth factor receptor alpha subunit deleted Patch mouse exhibits severe cardiovascular dysmorphogenesis. Teratology. 1995;51:351–366. doi: 10.1002/tera.1420510602. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Hu N, Weiss KM, Keller BB, Denslow S, Thompson RP. Cellular changes in experimental left heart hypoplasia. Anat Rec. 2002;267:137–145. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Hu N, Clark EB. Developmental changes in the myocardial architecture of the chick. Anat Rec. 1997;248:421–432. doi: 10.1002/(SICI)1097-0185(199707)248:3<421::AID-AR15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, Thompson RP. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. Anat Rec. 2003a;274A:773–777. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- Sedmera D, Thompson RP, Kolar F. Effect of increased pressure loading on heart growth in neonatal rats. J Mol Cell Cardiol. 2003b;35:301–309. doi: 10.1016/s0022-2828(03)00011-7. [DOI] [PubMed] [Google Scholar]
- Sheikh F, Fandrich RR, Kardami E, Cattini PA. Overexpression of long or short FGFR-1 results in FGF-2-mediated proliferation in neonatal cardiac myocyte cultures. Cardiovasc Res. 1999;42:696–705. doi: 10.1016/s0008-6363(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development. 1996;122:419–428. doi: 10.1242/dev.122.2.419. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M, Grim M, Hu YF, Szeder V. Pluripotent neural crest stem cells in the adult hair follicle. Dev Dyn. 2004;231:258–269. doi: 10.1002/dvdy.20129. [DOI] [PubMed] [Google Scholar]
- Soonpaa MH, Oberpriller JO, Oberpriller JC. Factors altering DNA synthesis in the cardiac myocyte of the adult newt, Notophthalmus viridescens. Cell Tissue Res. 1994;275:377–382. doi: 10.1007/BF00319437. [DOI] [PubMed] [Google Scholar]
- Speir E, Tanner V, Gonzalez AM, Farris J, Baird A, Casscells W. Acidic and basic fibroblast growth factors in adult rat heart myocytes. Localization, regulation in culture, and effects on DNA synthesis. Circ Res. 1992;71:251–259. doi: 10.1161/01.res.71.2.251. [DOI] [PubMed] [Google Scholar]
- Sturzu AC, Wu SM. Developmental and regenerative biology of multipotent cardiovascular progenitor cells. Circ Res. 2011;108:353–364. doi: 10.1161/CIRCRESAHA.110.227066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Sasse J, Barron M, Lough J. Developmental expression of fibroblast growth factor receptor-1 (cek-1; flg) during heart development. Dev Dyn. 1995;202:115–125. doi: 10.1002/aja.1002020203. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Sasse J, Lough J. Inhibition of precardiac mesoderm cell proliferation by antisense oligodeoxynucleotide complementary to fibroblast growth factor-2 (FGF- 2) Dev Biol. 1993;157:28–37. doi: 10.1006/dbio.1993.1109. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Lee TC, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function and stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res. 2005;96:767–775. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- Tchervenkov CI, Jacobs ML, Tahta SA. Congenital Heart Surgery Nomenclature and Database Project: hypoplastic left heart syndrome. Ann Thorac Surg. 2000;69:S170–179. doi: 10.1016/s0003-4975(99)01283-7. [DOI] [PubMed] [Google Scholar]
- Thompson RP, Kanai T, Germroth PG, Gourdie RG, Thomas PC, Barton PJR, Mikawa T, Anderson RH. Organization and Function of Early Specialized Myocardium. In: Clark EB, Markwald RR, Takao A, editors. Developmental Mechanisms of Heart Disease. Armonk, NY: Futura Publishing; 1995. pp. 269–279. [Google Scholar]
- Thompson RP, Lindroth JR, Allen AJ, Fazel AR. Cell differentiation birthdates in the embryonic rat heart. Ann NY Acad Sci. 1990a;588:446–448. [Google Scholar]
- Thompson RP, Lindroth JR, Wong YM. Regional differences in DNA-synthetic activity in the presepatation myocardium of the chick. In: Clark EB, Takao A, editors. Developmental Cardiology: Morphogenesis and Function. Mount Kisco, NY: Futura Publishing; 1990b. pp. 219–234. [Google Scholar]
- Thompson RP, Reckova M, DeAlmeida A, Bigelow M, Stanley CP, Spruill JB, Trusk T, Sedmera D. The oldest, toughest cells in the heart. In: Chadwick DJ, Goode J, editors. Development of the cardiac conduction system. Chichester: Wiley; 2003. pp. 157–176. [PubMed] [Google Scholar]
- Thompson RP, Soles-Rosenthal PG, Cheng G. Origin and fate of cardiac conduction tissue. In: Clark EB, Takao A, Nakazawa M, editors. Etiology and Morphogenesis of Congenital Heart Disease: Twenty Years of Progress in Genetics and Developmental Biology. Armonk, NY: Futura Publishing; 2000. pp. 251–255. [Google Scholar]
- Tobita K, Liu LJ, Janczewski AM, Tinney JP, Nonemaker JM, Augustine S, Stolz DB, Shroff SG, Keller BB. Engineered early embryonic cardiac tissue retains proliferative and contractile properties of developing embryonic myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H1829–1837. doi: 10.1152/ajpheart.00205.2006. [DOI] [PubMed] [Google Scholar]
- Tomanek RJ, Hansen HK, Christensen LP. Temporally expressed PDGF and FGF-2 regulate embryonic coronary artery formation and growth. Arterioscler Thromb Vasc Biol. 2008;28:1237–1243. doi: 10.1161/ATVBAHA.108.166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomanek RJ, Haung L, Suvarna PR, O’Brien LC, Ratajska A, Sandra A. Coronary vascularization during development in the rat and its relationship to basic fibroblast growth factor. Cardiovasc Res. 1996;31(Spec No):E116–126. [PubMed] [Google Scholar]
- Tomanek RJ, Hu N, Phan B, Clark EB. Rate of coronary vascularization during embryonic chicken development is influenced by the rate of myocardial growth. Cardiovasc Res. 1999;41:663–671. doi: 10.1016/s0008-6363(98)00330-7. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Abu-Issa R, de Boer BA, Hutson MR, de Boer PA, Soufan AT, Ruijter JM, Kirby ML, van den Hoff MJ, Moorman AF. A caudal proliferating growth center contributes to both poles of the forming heart tube. Circ Res. 2009;104:179–188. doi: 10.1161/CIRCRESAHA.108.185843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald MR, Borda ES, Sterin-Borda L. Mitogenic effect of erythropoietin on neonatal rat cardiomyocytes: signal transduction pathways. J Cell Physiol. 1996;167:461–468. doi: 10.1002/(SICI)1097-4652(199606)167:3<461::AID-JCP10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Jafri A, Fisher SA. Apoptosis is required for the proper formation of the ventriculo-arterial connections. Dev Biol. 2001;240:274–288. doi: 10.1006/dbio.2001.0466. [DOI] [PubMed] [Google Scholar]
- Wills AA, Holdway JE, Major RJ, Poss KD. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development. 2008;135:183–192. doi: 10.1242/dev.010363. [DOI] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Zhang X, Zdanowicz M, Parker J, Creazzo TL, Johnson GA, Kirby ML. Myocardial volume and organization are changed by failure of addition of secondary heart field myocardium to the cardiac outflow tract. Dev Dyn. 2003;228:152–160. doi: 10.1002/dvdy.10364. [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, Lauder JM, Marchi M, Giacobini E. 3H-thymidine long survival autoradiography as a method for dating the time of neuronal origin in the chick embryo: the locus coeruleus and cerebellar Purkinje cells. J Comp Neurol. 1981;203:257–267. doi: 10.1002/cne.902030207. [DOI] [PubMed] [Google Scholar]
- Zhang N, Mustin D, Reardon W, Almeida AD, Mozdziak P, Mrug M, Eisenberg LM, Sedmera D. Blood-borne stem cells differentiate into vascular and cardiac lineages during normal development. Stem Cells Dev. 2006;15:17–28. doi: 10.1089/scd.2006.15.17. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li TS, Lee ST, Wawrowsky KA, Cheng K, Galang G, Malliaras K, Abraham MR, Wang C, Marban E. Dedifferentiation and proliferation of mammalian cardiomyocytes. PLoS One. 2010;5:e12559. doi: 10.1371/journal.pone.0012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- Zhou B, von Gise A, Ma Q, Rivera-Feliciano J, Pu WT. Nkx2–5- and Isl1-expressing cardiac progenitors contribute to proepicardium. Biochem Biophys Res Commun. 2008;375:450–453. doi: 10.1016/j.bbrc.2008.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]