Abstract
Background
Although a number of investigations have reported cognitive deficits in patients with bipolar disorder, relatively few have focused on the relationship between these impairments and clinical outcome.
Methods
In order to help clarify the pattern of and extent to which cognitive deficits are present at the onset of illness and their relationship to outcome, we examined 26 bipolar patients during their first hospitalization and 20 psychiatrically healthy control subjects. All subjects completed tests of frontal/executive control, psychomotor speed and memory function at baseline and self-reports of clinical recovery (time to recover in days) at 12 months post study enrollment.
Results
At baseline, first episode bipolar patients demonstrated greater deficits relative to control subjects on neurocognitive measures, and a significant association was detected between time to recover and performance on a measure of frontal/executive function (interference condition of the Stroop; p=.05; derived interference: p = .04). A trend towards significance was also demonstrated between time to clinical recovery and verbal fluency (p=.06).
Conclusions
These findings indicate that neuropsychological deficits are seen early in the course of bipolar disorder, prior to the effects of multiple or prolonged episodes, and may be associated with clinical outcome. Future studies are needed to determine whether changes in inhibitory processing or other cognitive function predict clinical outcome or are associated with treatment response.
Keywords: bipolar, neurocognition, recovery, inhibitory function, Stroop
INTRODUCTION
Bipolar disorder has been associated with significant and often persistent impairment in cognitive function (Altshuler et al., 1993) however, the relationship of these cognitive deficits to clinical outcome has not been well documented. Several investigations have reported cognitive deficits which are suggestive of impairments in executive function, attention, memory and psychomotor speed (Gurovitch et al., 1999; DuPont et al., 1995), yet relatively little research has focused on how these deficits relate to course of illness. It has been hypothesized that the cognitive deficits present in patients with bipolar disorder are due to residual effects caused by repeated or prolonged affective episodes, however, it is possible that these functional changes represent core features of the illness (Nordenson et al., 2004). Moreover, some studies have reported a progressive decline in cognitive performance over time while others have not, raising the question of whether these deficits are present at the onset of the illness (Altshuler, 1993). Several investigations have reported that bipolar patients with a more severe course of illness and higher numbers of affective episodes experience greater cognitive decrements (van Gorp et al., 1998; Kessing, 1998, Denicoff et al., 1999), and that specific variables including age of onset, duration of illness and number of hospitalilzations are associated with this neurocognitive profile. Given the importance of understanding the relationship between clinical symptoms and cognition to assess the potentially predictive value of cognitive variables with regard to functional recovery, recent studies have begun to examine neuropsychological performance of bipolar patients at variable points in their illness.
In an investigation by Rubinsztein et al (2000), investigators compared the performance of 14 bipolar patients in full or partial remission to that of 12 healthy control subjects on a battery of neurocognitive tests, which included a pattern and spatial recognition task, a delayed matching to sample task, and the One Touch Tower of London task, a spatial problem solving task (Elliot at al., 1996). Euthymic bipolar patients performed significantly worse on both the pattern and spatial recognition tasks as compared to controls and made more errors on the delayed matching to sample task. Performance was significantly correlated with total number of months of hospitalization, underscoring the importance of examining the associations between clinico-demographic factors and neurocognitive variables. In a study designed to assess the relationship between course of illness and neurocognitive function, Denicoff and colleagues (1999) examined 49 bipolar outpatients using a battery of tests, which included measures of inhibitory function (Stroop), verbal fluency (Controlled Oral Word Association Test [COWAT]), verbal learning and memory (California Verbal Learning Test [CVLT]), attention/vigilance (Continuous Performance Test [CPT]), psychomotor speed (Grooved Pegboard), executive function (Wisconsin Card Sorting Test [WCST]) and visual scanning/set maintenance (Letter Cancellation Test [LCT]). The authors reported that duration of symptoms was significantly predictive of the number of perseverative responses on the CVLT, total response time and number correct on the CPT, and performance on the LCT. Similarly, Zubieta and colleagues (2001) observed a negative correlation between executive function as measured by the WCST, and the number of past affective episodes and hospitalizations due to mania in bipolar I subjects. In a study by Lebowitz et al. (2001) bipolar patients with a history of multiple manic episodes generated significantly fewer words on a phonemic fluency task and made more errors on phonemic and semantic verbal fluency tasks than patients experiencing their first manic episode and healthy controls. Other investigations have reported inverse correlations between sustained attention and number of affective episodes and hospitalizations (Clark et al., 2001). Psychomotor speed has also been shown to be associated with number of past depressive episodes (MacQueen et al., 2001). In a study of euthymic bipolar patients, El-Badri and colleagues reported that the while total number of affective episodes was significantly related to cognitive impairment in multiple domains, no significant association was detected between cognitive performance and duration of illness or age of onset (El-Badri et al., 2001). In a recent study by Frangou and colleagues (2005), remitted bipolar patients showed impairment on a range of tasks requiring executive function relative to control subjects and found that duration of illness predicted loss of inhibitory control (Frangou et al., 2005). Finally, Nehra and colleagues examined cognitive performance in a group of euthymic bipolar patients following their first episode relative to euthymic multiple episode bipolar patients and control subjects (Nehra et al., 2006). While the study reported that overall, first episode patients performed more poorly than multiple episode patients or controls, mutiple episode patients demonstrated significantly worse performance on a subtest of executive functions, specifically perseverative errors on the WCST task, than either of the other two groups. Further, multiple episode patients also achieved lower overall scores on a memory scale than the other groups, raising the question of progressive cognitive impairment with repeated clinical episodes.
These investigations indicate a significant relationship between neurocognitive impairments and clinical variables, including frequency of affective episodes and number of hospitalizations. No study thus far has examined patients during their first hospitalization to document prospectively the relationship between neurocognitive performance and recovery. We examined bipolar patients during their first hospitalization, and hypothesized that compared to normal control subjects, even after becoming clinically stable, these patients would show deficits on neuropsychological tests sensitive to executive function. Further, it was hypothesized that that deficits in cognitive performance would be predictive of clinical recovery, therefore, we examined the extent to which performance on these measures was related to rehospitalization status and time to recover (in days) at 12 months post study enrollment.
METHODS
Forty six subjects were enrolled in this neuropsychological protocol which included 26 patients admitted to McLean Hospital for their first episode of bipolar disorder and 20 non-psychiatric control subjects, matched for age, sex, handedness and parental socioeconomic status (SES), recruited from the community. All subjects completed a structured clinical interview (SCID-P) to ensure accurate diagnoses as well as clinical rating scales to characterize the phase of their illness. These were administered by an experienced clinical interviewer with an established good inter-rater reliability (kappa >.90). Control subjects were free of Axis I pathologies and had no first degree relatives with any Axis I diagnosis. All subjects were native English speakers, and had no history of head trauma, alcohol or substance dependence. A neurocognitive test battery, which included tests of frontal/executive control was administered to each study subject, and for the bipolar patients, within one week of admission. All subjects signed an Informed Consent form approved by the McLean Hospital Institutional Review Board.
The neuropsychological tests were chosen because they are standard, well-known measures which can be administered and scored reliably, and have proven sensitive to frontal lobe dysfunction (Lezak 2004). The Stroop Color-Word Test (Stroop, 1935) measures the ability to inhibit inappropriate responses and resist interference and includes three sections (color naming, word reading and interference). The task is designed to establish competing response tendencies within the study subject and to assess the subjects’ ability to suppress the interfering stimuli (Comalli et al., 1962; Lezak, 2004). The Trail Making Test is a visual conceptual and visuomotor tracking task and involves first connecting consecutively numbered circles on one work sheet (Part A) and then connecting the same number of consecutively numbered and lettered circles on another work sheet by alternating between the two sequences (Part B). Trails B is the more sensitive of the two tests, particularly to frontal-lobe dysfunction, as scores on this section are indicative of the subject’s ability to shift sets and process concurrent stimuli (Reitan, 1958; Lezak, 2004). The Wisconsin Card Sorting Test assesses a person’s ability to form abstract concepts, utilize feedback, and to shift and maintain set. In the Controlled Oral Word Association (FAS) test, subjects are asked to generate as many different words as possible that begin with a particular letter during a 1 minute period. Three letters are used in succession (F, A, S); the total number of words generated for all three letters comprises the verbal fluency score. Finally, subjects completed the Vocabulary subtest of the Wechsler Adult Intelligence Scale- Revised (WAIS-R) as a measure of general intellectual ability (Wechsler, 1981). The Vocabulary subtest has been identified as the single best measure of both verbal and general mental ability (Lezak, 2004; Spreen and Strauss, 1991). All tests were administered by a trained psychometrician blind to subjects’ diagnostic group.
At the time of neuropsychological evaluation, first hospitalized bipolar patients had received no more than two weeks of pharmacologic treatment, and most were seen within one week of entering the hospital. All subjects agreed to be enrolled in a naturalistic, longitudinal study which required patients to be evaluated at monthly intervals. At 12 months post study enrollment, first episode patients completed a second clinical interview (to confirm diagnosis) and provide information regarding clinical state and recovery.
Statistical Analyses
We examined differences in neuropsychological functioning between bipolar patients and healthy controls using one-tailed Student’s t-tests (as the direction of the group differences were hypothesized a priori); to account for multiple comparisons we used a Bonferroni adjusted significance level, one-tailed, of .005. Neuropsychological test scores found to differ significantly between the groups were entered as dependent variables in analyses of covariance (ANCOVAs) that included age, sex and education as covariates. Finally, to examine the association of neuropsychological functioning and clinical outcome (time to recovery), we used Spearman’s Rho correlations. Variables that departed significantly from a Gaussian distribution were log-transformed.
RESULTS
Demographic characteristics for the study subjects are listed in Table 1. The study groups were matched for parental socioeconomic status, age and sex, however, bipolar patients completed slightly fewer years of education compared to control subjects (14.0 vs. 15.8, p=.01). With regard to estimated intellectual function, bipolar patients performed in the high average range (VIQ=108.4) while normal controls achieved scores in the superior range (VIQ =120.9), yielding a significant between-group difference (p=.02). Given that patients were evaluated within the first two weeks of their hospitalization, and that scores may reflect elements of the current clinical state, a correction for VIQ was not included in the current data. Additionally, the primary outcome variable in this study is the relationship between neurocognitive performance and recovery within the patient cohort, which would not be affected by a between-group correction for IQ.
Table 1.
Demographic and Clinical Features in First Episode Bipolar Patients and Healthy Comparison Subjects (Mean ± SD or N(%))
| Variable | Healthy Controls (N = 20) | Bipolar Patients (N = 26) |
|---|---|---|
| Age (years) | 25.3 ± 4.7 | 24.4 ± 5.5 |
| Sex (Male) | 15 (75) | 19 (73) |
| Education | 15.8 ± 2.1 | 14.0 ± 2.3* |
| Handedness (Right) | 19 (95) | 24 (92) |
| Estimated VIQ | 120.9 ± 16.3 | 108.7 ± 18.2* |
| Age of Onset (years) | -- | 23.8 ± 5.0 |
| Duration of Illness (days) | -- | 26.2 ±2.9 |
p<.05
Table 2 shows the results of the t-tests of group differences. First hospitalized bipolar patients demonstrated significantly poorer performance on Trail Making Test, Part A, a test of psychomotor speed, than control subjects (p=.002). Patients also performed more poorly than controls on the Trail Making Test Part B, which has an additional requirement of maintaining and shifting of mental set (p = .002). Both these group differences survived Bonferroni correction. Subtraction of the time to complete Trails A from the time to complete Trails B was calculated to examine the latency effect of shifting set. This score was not significantly different between groups.
Table 2.
Neuropsychological Measures of Executive Functioning in First Episode Bipolar Patients and Healthy Controls
| Frontal-Executive Task | Bipolar Patients N = 26 Mean ± SD | Healthy Subjects N = 20 Mean ± SD | p-value* (t-test) |
|---|---|---|---|
| Trail Making (seconds) | |||
| Trails, PartA | 44.0 ± 14.2 | 32.5 ± 9.8 | .002 |
| Trails, Part B | 64.8 ± 28.1 | 44.7 ± 14.1 | .002 |
| Trails B-A | 21.1 ± 19.1 | 12.3 ± 8.9 | .24 |
|
| |||
| Stroop Color Word Test (seconds) | |||
| Color Naming | 69.4 ± 15.0 | 56.7 ± 9.6 | <.001 |
| Word Reading | 48.1 ± 9.7 | 43.6 ± 6.6 | .05 |
| Interference | 120.4 ± 32.4 | 100.8 ± 22.7 | .01 |
| Derived Interference | 51.0 ± 21.5 | 44.1 ± 17.6 | .15 |
|
| |||
| Wisconsin Card Sorting Test | |||
| Total Categories | 7.05 ± 5.1 | 8.7 ± 1.3 | <.001 |
| Total Perseverations | 17.2 ± 11.4 | 7.1 ± 5.1 | <.001 |
|
| |||
| Verbal Fluency | |||
| Total Words | 40.1 ± 10.0 | 47.3 ± 13.6 | .03 |
p-values are one-tailed; those that remained significant after Bonferroni correction are printed in bold.
On the Stroop Color Word Test, bipolar patients performed significantly slower on all three conditions (Color Naming, Word Reading, and Interference) relative to control subjects, although only the group difference in Color Naming remained significant after Bonferroni correction (p = .001). To better isolate inhibitory capacity from psychomotor speed, we also calculated a derived interference score (Interference score minus Color Naming score), but this score did not differ significantly between groups. On the Wisconsin Card Sorting Test (WCST), a test of abstraction and the ability to shift mental set, first hospitalized patients achieved fewer total categories than control subjects, a difference that was still significant with Bonferroni correction (p <.001). Interestingly, after a clarification of the rules, no significant differences were detected between the groups, indicating that first hospitalized patients were able to utilize feedback to improve their performance. Total number of perseverations on the WCST also differed between the groups, with first hospitalized bipolar subjects making significantly more errors than control subjects at the adjusted Bonferroni level (p <. 001).
To examine potential confounds of demographic variables on the above group differences, we conducted ANCOVAs predicting each of the five neuropsychological test scores that survived Bonferroni correction, with sex, age and education as covariates. None of these demographic variables were significantly associated with Stroop Color Naming, total categories on the WCST, total perseverations on the WCST, or time to complete Trails A or B.
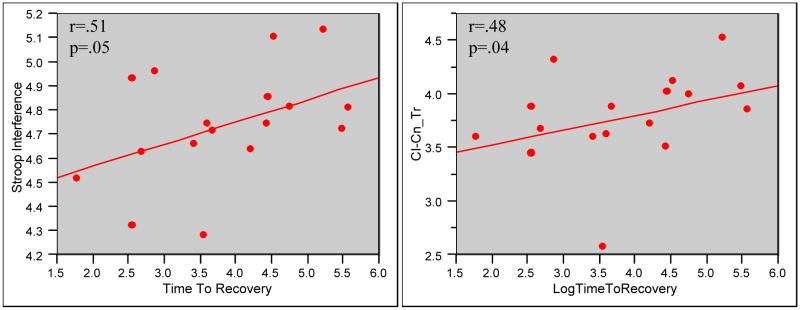
For the analyses examining neuropsychological functioning in relation to clinical outcome, “time to recovery” was defined as the number of days required for the patient to return to a baseline level of function, which was assessed using self-report data collected at 12 months post study enrollment. At the one year post discharge time point, bipolar patients completed comprehensive clinical follow-up interviews in the laboratory or by telephone. As shown in Figure 1, a significant association was detected between time to recovery and the score on the Interference condition of the Stroop test (rs = 0.51; p=.05), as well as for the derived Interference score (rs = 0.48; p=.04). A strong but not statistically significant negative correlation was found between recovery and the verbal fluency score (rs = −0.47; p=.06). Finally, age at onset and length of illness were not significantly correlated with time to recovery, nor were they significantly associated with the Stroop scores. Thus, the correlations between time to recovery and Stroop variables were not accounted for by these indicators of illness severity.
Figure 1.
Time to recovery was defined as the number of days required for the patient to return to a baseline of function, which was assessed via self-report collected at 12 months post study enrollment. A significant association was detected between time to recovery and the score on the Interference condition of the Stroop test (p=.05), as well as for the derived Interference measure (CI-CN:p=.04).
DISCUSSION
As hypothesized, bipolar patients experiencing their first hospitalization demonstrated a significant relationship between neuropsychological performance and time to recovery. Consistent with previous reports, bipolar patients performed more poorly on frontal/executive tasks when compared to healthy control subjects. Performance on tasks which require abstraction, executive control, inhibition, or shifting of mental set appeared to be altered early in the course of the illness in these patients, consistent with theories of anomalous prefrontal function (Yurgelun-Todd et al., 2000; Nordenson, Gruber, Yurgelun-Todd, 2004; Yurgelun-Todd et al., 2006). Results from this investigation demonstrate that patients who performed better on the interference condition of the Stroop test, a task requiring active inhibition of an overlearned tendency, required fewer days to return to a baseline level of function as defined by patients’ self-report. In fact, a significant association was also detected for the derived interference condition and time to recovery. The derived score is designed to control for the potential effects of psychomotor slowing, thus providing a precise measure of inhibitory capacity, therefore, these findings indicate that more efficient processing of tasks requiring executive function is related to a decrease in time to recovery.
Performance on the Stroop interference condition, measured both by CI and CI-CN, is a process previously shown to be mediated, at least in part, by the anterior cingulate cortex (ACC) (Carter et al., 1995; Gruber et al., 2004), and was significantly associated with time to recovery. These results complement recent neuroimaging studies of bipolar patients which report alterations in frontal regions (Drevets et al., 1997; Blumberg et al., 1999). Structural MR studies have reported alterations of subregion specific prefrontal volume in bipolar patients (Lopez-Larson, 2002) and a number of functional imaging investigations have reported reductions of frontal activity during cognitive challenge tasks (Blumberg et al., 2000; Yurgelun-Todd et al., 2000; Adler et al., 2004; Strakowski et al., 2005). One recent structural MR study examined gray and white matter volumes of subregions of the ACC and performance on tests of executive function, which included the Trail Making Test and the Wisconsin Card Sorting Test, in bipolar patients and healthy subjects. Consistent with findings from the current investigation, the authors of that study reported poorer performance in bipolar patients relative to controls on tasks requiring executive function. Further, a significant interaction was detected between groups for the relationship between ACC gray and white matter volume and prediction of task performance, suggesting a different contribution for these regions in patients and control subjects.
While the precise role of the ACC and the dorsolateral prefrontal cortex (DLPFC) in bipolar disorder are not clear, both regions have been shown to be components of a neural network which plays a critical role in the completion of tasks requiring self monitoring and inhibition, functions often noted to be altered in bipolar patients (Gruber et al., 2004; Christodoulou et al., 2006). In one recent functional magnetic resonance imaging (fMRI) investigation, bipolar patients demonstrated significantly reduced signal intensity within a subregion of the anterior cingulate cortex which accompanied an increase in the dorsolateral prefrontal cortex relative to control subjects during the performance of the interference condition of the Stroop test (Gruber et al., 2004). The alterations in cingulate activation noted in patients with bipolar disorder may be reflective of an abnormality of the anterior cingulate itself, or inappropriate modulation of the cingulate by other interactive brain regions including the DLPFC. One possibility is that patients with bipolar disorder have an inherent disruption of the frontal network, which supports inhibitory functions (Mayberg et al., 1997). This functional change would be consistent with clinical observations in bipolar disorder (Clark et al., 2001; Sax et al., 1999), as bipolar patients have difficulty staying on task and maintaining mental focus. The current findings would suggest that bipolar patients who demonstrate the strongest inhibitory control are also the most likely to demonstrate shorter times to return to baseline levels of functioning.
Studies have begun to characterize the relationship between clinical symptoms and cognitive function in order to assess the predictive value of cognitive variables in terms of functional recovery. A number of investigations have identified a relationship between cognitive performance and number of past affective episodes and hospitalizations, suggesting that deficits in recall and recognition are associated with a longer duration of bipolar illness and a history of affective, particularly manic, episodes (Deckersbach et al., 2004; Cavanaugh et al., 2002; Donaldson et al., 2003). Recently, Martinez-Aran et al. (2004) identified a positive correlation between bipolar patients’ performance on all measures of the CVLT and their Global Assessment of Functioning (GAF) scores. Poor GAF scores were also associated with more perseverative errors on the WCST, reduced psychomotor speed, impaired verbal fluency, and deficits in delayed logical and visual recall. All measures of the CVLT except for recognition were inversely correlated with duration of illness and total number of hospitalizations, manic episodes, and suicide attempts suggesting a significant relationship between verbal memory performance and the course of bipolar illness. In the current investigation, the patient population was evaluated early in the course of illness, and had no prior history of hospitalizations, underscoring the likelihood that differences noted between the groups are independent of treatment effects or the result of course of illness.
Several factors should be considered in interpreting the study findings. This investigation focused on the relationship between neurocognitive performance and time to recovery or outcome, as defined by the number of days required for the patient to return to a baseline level of function following their initial hospitalization. Given that many subjects were not rehospitalized within the first 12 months, this measure was based on patients’ self report of clinical state and their determination of “return to baseline” levels of function. Self -report measures rely on patients’ ability to be good and truthful historians, which may be a confounding variable in this study (Sainfort et al., 1996). It is of note, however, that subjects were enrolled in a longitudinal study and were contacted at several points during the investigation. Subjects were therefore “used to” thinking about their own clinical state and were able to determine the number of days to feeling better. Further, since subjects were contacted at multiple time points, it is likely that their reporting remained consistent, and for subjects who were rehospitalized, number of days were calculated based on hospital records. An additional consideration of the current investigation is the fact that some subjects were taking pharmacotherapeutic agents at the time of initial testing, which differed per individual. All testing was completed within two weeks of medication treatment, and in most cases, within a single week of being in the hospital. Although we cannot eliminate the possibility that some medication effects were present, it is probable that pharmacotherapy would have only hindered their cognitive performance as they were in the earliest stages of treatment; the relationship to outcome would likely have remained the same. In fact, the majority of subjects remained on the same therapeutic regime at their “recovery” point, although some of the dosages may have been altered. Thus, it is expected that any potential effect of medication would have remained throughout the investigation and not affected the relationship between cognitive performance and outcome. The significant between-group difference for VIQ is likely due to the fact that scores are an estimate, based on a single measure of the WAIS-R battery, and that control subjects, matched for patient age and socioeconomic status, were largely comprised of healthy individuals from the community, many of whom were from local colleges and universities. While the VIQ is commonly used to estimate IQ, patients’ scores were likely slightly reduced relative to premorbid levels, given their recent inpatient admission. Nevertheless, the relationship between Stroop performance and time to recovery is a within-patient analysis, which remains unaffected by a between-group difference in VIQ.
In summary, findings from the current investigation suggest an association between baseline inhibitory capacity, as measured by the Stroop test, and time to recovery in first episode bipolar patients. Neither age at onset or length of illness were significantly correlated with time to recovery, or performance on the Stroop;, the relationship between time to recovery and Stroop performance was not accounted for by these indicators of illness severity. These neuropsychological measures may be associated with dysfunction in frontal regions, particularly the anterior cingulate cortex. Furthermore, the neuropsychological deficits were seen in patients early in the course of the illness, prior to the potential neurotoxic effects of repeated affective episodes, or alterations secondary to chronic treatment with pharmacologic agents. Future studies are needed to determine whether changes in inhibitory processing or other cognitive function predict clinical outcome or are associated with treatment response.
Acknowledgments
The authors kindly thank Ms. Alexandra McCaffrey who assisted with the preparation of this manuscript.
Footnotes
Contributors: All authors have contributed to and approved the final manuscript. Specifically, Drs. Gruber and Yurgelun-Todd designed and implemented the study and managed all literature searches, and Dr. Gruber wrote the manuscript. Dr. Rosso completed statistical analyses and ensured integrity of the data included in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6(6):540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- Altshuler LL. Bipolar disorder: are repeated episodes associated with neuroanatomic and cognitive changes? Biol Psychiatry. 1993;33:8–9. 563–565. doi: 10.1016/0006-3223(93)90093-s. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Martinez D, Ricketts S, de Asis J, White T, Epstein J, McBride PA, Eidelberg D, Kocsis JH, Silbersweig DA. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48(11):1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156(12):1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. Neuroimage. 1995;2(4):264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Cavanagh JT, Van Beck M, Muir W, Blackwood DH. Case-control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. Br J Psychiatry. 2002;180:320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- Christodoulou T, Lewis M, Ploubidis GB, Frangou S. The relationship of impulsivity to response inhibition and decision-making in remitted patients with bipolar disorder. Eur Psychiatry. 2006;21(4):270–273. doi: 10.1016/j.eurpsy.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Clark L, Iversen SD, Goodwin GM. A neuropsychological investigation of prefrontal cortex involvement in acute mania. Am J Psychiatry. 2001;158(10):1605–1611. doi: 10.1176/appi.ajp.158.10.1605. [DOI] [PubMed] [Google Scholar]
- Comalli PE, Jr, Wapner S, Werner H. Interfernce effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]
- Deckersbach T, Savage CR, Reilly-Harrington N, Clark L, Sachs G, Rauch SL. Episodic memory impairment in bipolar disorder and obsessive-compulsive disorder: the role of memory strategies. Bipolar Disord. 2004;6(3):233–244. doi: 10.1111/j.1399-5618.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- Denicoff KD, Ali SO, Mirsky AF, Smith-Jackson EE, Leverich GS, Duncan CC, Connell EG, Post RM. Relationship between prior course of illness and neuropsychological functioning in patients with bipolar disorder. J Affect Disord. 1999;56(1):67–73. doi: 10.1016/s0165-0327(99)00028-2. [DOI] [PubMed] [Google Scholar]
- Donaldson S, Goldstein LH, Landau S, Raymont V, Frangou S. The Maudsley Bipolar Disorder Project: the effect of medication, family history, and duration of illness on IQ and memory in bipolar I disorder. J Clin Psychiatry. 2003;64(1):86–93. [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Arch Gen Psychiatry . 1995;52(9):747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- El-Badri SM, Ashton CH, Moore PB, Marsh VR, Ferrier IN. Electrophysiological and cognitive function in young euthymic patients with bipolar affective disorder. Bipolar Disord. 2001;3(2):79–87. doi: 10.1034/j.1399-5618.2001.030206.x. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ, Robbins TW, Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26(5):975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- Frangou S, Donaldson S, Hadjulis M, Landau S, Goldstein LH. The Maudsley Bipolar Disorder Project: executive dysfunction in bipolar disorder I and its clinical correlates. Biol Psychiatry. 2005;58(11):859–864. doi: 10.1016/j.biopsych.2005.04.056. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol Psychiatry. 1999;45(5):639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82(2):191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. 1998;28(5):1027–1038. doi: 10.1017/s0033291798006862. [DOI] [PubMed] [Google Scholar]
- Lebowitz BK, Shear PK, Steed MA, Strakowski SM. Verbal fluency in mania: relationship to number of manic episodes. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14(3):177–182. [PubMed] [Google Scholar]
- Lezak M. Neuropsychological Assessment. 4. Oxford University Press; New York: 2004. [Google Scholar]
- Lopez-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52(2):93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Young LT, Galway TM, Joffe RT. Backward masking task performance in stable, euthymic out-patients with bipolar disorder. Psychol Med. 2001;31(7):1269–1277. doi: 10.1017/s0033291701004597. [DOI] [PubMed] [Google Scholar]
- Martinez-Aran A, Vieta E, Colom F, Torrent C, Sanchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugue E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Silva JA, McGinnis S, Glass TG, Martin CC, Fox PT. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- Nehra R, Chakrabarti S, Pradhan BK, Khehra N. Comparison of cognitive functions between first and multi-episode bipolar affective disorders. J Affect Disord. 2006;93:185–92. doi: 10.1016/j.jad.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nordenson B, Gruber SA, Yurgelun-todd D. Neurocognition in Bipolar Disorder: A review of the current research. Curr Psychos Therap Rpts. 2004;2:147–152. [Google Scholar]
- Reitan RM. The Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- Rubinsztein JS, Michael A, Paykel ES, Sahakian BJ. Cognitive impairment in remission in bipolar affective disorder. Psychol Med. 2000;30(5):1025–1036. doi: 10.1017/s0033291799002664. [DOI] [PubMed] [Google Scholar]
- Sainfort F, Becker M, Diamond R. Judgments of quality of life of individuals with severe mental disorders: Patient self-report versus provider perspectives. Am J Psychiatry. 1996;153(4):497–502. doi: 10.1176/ajp.153.4.497. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Jr, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156(1):139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. Oxford University Press; New York: 1991. [Google Scholar]
- Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162(9):1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- van Gorp WG, Altshuler L, Theberge DC, Wilkins J, Dixon W. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. A preliminary study. Arch Gen Psychiatry. 1998;55(1):41–46. doi: 10.1001/archpsyc.55.1.41. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale - Revised. The Psychological Corporation; New York: 1981. [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2(3 Pt 2):237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Ross AJ. Functional magnetic resonance imaging studies in bipolar disorder. CNS Spectr. 2006;11(4):287–297. doi: 10.1017/s1092852900020782. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Huguelet P, O’Neil RL, Giordani BJ. Cognitive function in euthymic bipolar I disorder. Psychiatry Res. 2001;102(1):9–20. doi: 10.1016/s0165-1781(01)00242-6. [DOI] [PubMed] [Google Scholar]