Abstract
Induction of EBV lytic-phase gene expression, combined with exposure to an antiherpes viral drug, represents a promising targeted therapeutic approach to EBV-associated lymphomas. Short-chain fatty acids or certain chemotherapeutics have been used to induce EBV lytic-phase gene expression in cultured cells and mouse models, but these studies generally have not translated into clinical application. The recent success of a clinical trial with the pan-histone deacetylase (pan-HDAC) inhibitor arginine butyrate and the antiherpes viral drug ganciclovir in the treatment of EBV lymphomas prompted us to investigate the potential of several HDAC inhibitors, including some new, highly potent compounds, to sensitize EBV+ human lymphoma cells to antiviral agents in vitro. Our study included short-chain fatty acids (sodium butyrate and valproic acid); hydroxamic acids (oxamflatin, Scriptaid, suberoyl anilide hydroxamic acid, panobinostat [LBH589], and belinostat [PXD101]); the benzamide MS275; the cyclic tetrapeptide apicidin; and the recently discovered HDAC inhibitor largazole. With the exception of suberoyl anilide hydroxamic acid and PXD101, all of the other HDAC inhibitors effectively sensitized EBV+ lymphoma cells to ganciclovir. LBH589, MS275, and largazole were effective at nanomolar concentrations and were 104 to 105 times more potent than butyrate. The effectiveness and potency of these HDAC inhibitors make them potentially applicable as sensitizers to antivirals for the treatment of EBV-associated lymphomas.
Introduction
Latent infection with EBV, a γ-herpesvirus, is ubiquitous among human populations worldwide. Acute EBV infection results in the self-limiting illness infectious mononucleosis, although it can also lead to severe and sometimes fatal disease in immunocompromised patients.1 Latent EBV infection has also been associated with number of human malignancies such as Burkitt lymphoma (BL),2 nasopharyngeal carcinoma,3 posttransplantation lymphoproliferative disease (PTLD),4 Hodgkin lymphoma,5 non-Hodgkin lymphoma,6 and sporadic cancers of the gastrointestinal tract and breast.7,8 Commonly used antiherpes virus drugs, such as the nucleoside analogs ganciclovir (GCV) or acyclovir, are inefficient at eliminating EBV from chronically infected hosts because EBV maintains a latent state of infection in these tumors and lytic-phase proteins are required to convert these pro-drugs to active antiviral drugs.
In recent years, several studies have explored the concept that the induction of EBV lytic replication, with or without the addition of antiherpes virus drugs, could be therapeutically beneficial for EBV-associated tumors.9–11 This approach would have high tumor specificity because only EBV-containing cells would be targeted, whereas neighboring EBV− cells would remain unaffected. Several disparate agents have been used to induce lytic-phase EBV gene expression in tumor cells, including butyrate, valproic acid (VA), rituximab, bortezomib, cis-platinum, gemcitabine, 5-azacytidine, and γ-radiation.12–17 Although the specific mechanisms by which these agents induce EBV lytic-phase gene expression differ, they all modulate EBV gene transcription in infected cells. Butyrate and VA, in particular, are inhibitors of histone deacetylase (HDACs). Arginine butyrate in combination with GCV was used in a recent phase 1/2 multi-institutional clinical trial in patients with highly refractory EBV+ diverse lymphoid malignancies,18 and 10 of 15 patients showed significant tumor responses, including complete clinical and pathologic responses.
Chromatin structure and gene transcription are tightly regulated by the acetylation state of the histone molecules in the nucleosome. Histone acetyl transferases (HATs) and HDACs play a major role in this epigenetic control of cellular gene transcription.19 Whereas HATs acetylate conserved lysine residues in histone tails and associate with transcriptional coactivators and other HATs to facilitate gene transcription, HDACs typically associate with a different set of corepressor proteins such as SMRT, N-Cor, NURD, and others to remove the acetyl group from the acetylated lysines of histone tail, compact chromatin, and induce transcriptional repression.20,21 Certain small molecules with antiproliferative and proapoptotic activities in tumor cells were later identified as inhibitors of HDACs. Consequently, substantial effort has been made in the development of new HDAC inhibitors with potential therapeutic use.22 Many of the HDAC inhibitors developed to date have been found to have strong antitumor activity in laboratory models. Several HDAC inhibitors have already been clinically evaluated in multiple types of malignancies.23 Some of them have demonstrated efficacy in hematologic malignancies such as cutaneous T-cell leukemia, peripheral T-cell leukemia, acute myeloid leukemia, and Hodgkin lymphoma.24 Two HDAC inhibitors, suberoyl anilide hydroxamic acid (SAHA or Vorinostat)25 and FK-228 (Romidepsin)26 have been approved for the treatment of cutaneous T-cell leukemia.
Our previous studies demonstrated that butyrate, a general HDAC inhibitor, acts as an inducer of EBV lytic-phase gene expression and, together with GCV, efficiently kills EBV-infected cells. In the present study, we evaluated the efficacy of several newer and more potent inhibitors of multiple HDAC subclasses to induce EBV lytic-phase gene expression and GCV-dependent killing of infected cells. We report that the HDAC inhibitors MS275 (benzamide class), LBH589 (hydroxamic acid class), and largazole (cyclic depsipeptide class) efficiently killed EBV-infected BL cell P3HR1 in combination with GCV. We further demonstrate that some of these inhibitors also have potent activity in other EBV-infected BL cells and lymphoblastoid cell lines (LCLs).
Methods
Cells
Two BL cell lines, P3HR1 (an EBV-producing cell line originally obtained from the Jijoye cell line)27 and Daudi (an EBV+ but nonproducing line),28 were used in this study. The EBV-transformed lymphoblastoid cell line JY, also used in this study, was generated originally from a homozygous Indiana Amish population.29 Two EBV− B-cell lines, BJAB and Toledo, originally generated from a BL30 and a non-Hodgkin lymphoma,31 respectively, were also used in this study. Except for BJAB, all cells were maintained in RPMI 1640 with 10% FBS containing 100 U of penicillin and 100 μg of streptomycin per milliliter. BJAB cells were maintained in DMEM with 20% FBS and antibiotics.
HDAC inhibitors
A complete list of HDAC inhibitors used in this study is provided in Table 1 and their structures are shown in Figure 1. Sodium butyrate and SAHA were purchased from Sigma-Aldrich. Valproic acid, Scriptaid, apicidin, and oxamflatin were purchased from Calbiochem. MS275, LBH589, PXD101, and various largazole derivatives32 were synthesized at the Department of Chemistry, Colorado State University. Stock solutions of sodium butyrate were prepared fresh in sterile water. Valproic acid stock was prepared in water with the pH adjusted to 7.5 with 1M NaOH. Stock solutions of all other HDAC inhibitors were prepared in DMSO and stored in aliquots at −20°C.
Table 1.
HDAC inhibitors used in the study
| Name | Class | HDAC specificity class (isoform)45,46 | Clinical trials23,47 | EBV clinical trial |
|---|---|---|---|---|
| Butyrate | Short-chain fatty acid | pan-HDAC | β-thalassemia, sickle cell anemia | Refractory EBV lymphoma18 |
| Valproate | Short-chain fatty acid | HDACI (weak) HDACII (weak) | AML/MDS and solid tumors | N/A |
| MS275 (Entinostat) | Benzamide | HDACI (1,2,3) HDACII (9) | Refractory solid tumors, lymphoma and AM | N/A |
| Apicidin | Cyclic tetrapeptide | HDACI (1,2,3,8) | Preclinical PK | N/A |
| Largazole | Macrocyclic depsipeptide | HDACI (1,2,3) | None | N/A |
| Oxamflatin | Hydroxamic acid | HDAC (1,2,3,8) | None | N/A |
| Scriptaid | Hydroxamic acid | HDACI (1,2,3) | None | N/A |
| HDACII (6) | ||||
| SAHA (Vorinostat) | Hydroxamic acid | HDACI (1,2,3,8) | CTCL, MDS, MM | N/A |
| HDACII (4,6,7,9) | ||||
| LBH589 (Panobinostat) | Hydroxamic acid | HDACI (1,2,3,8) HDACII (5,6) | CTCL and solid tumors | N/A |
| PXD101 (Belinostat) | Hydroxamic acid | HDACI (1,2,3,8) | Hematologic and solid tumors | N/A |
| HDACII (6,7) |
N/A indicates not available.
Figure 1.
Chemical structures and chemical classes of the HDAC inhibitors used.
Analysis of lytic gene expression
To determine EBV lytic-phase gene expression, levels of thymidine kinase (TK) and BGLF4 gene transcripts were analyzed by reverse transcription and real-time PCR. Total RNA from P3HR1, Daudi, or JY cells, either untreated or treated with various HDAC inhibitors, was isolated by TRIzol reagent (Invitrogen) extraction following the manufacturer's protocol. Genomic or episomal DNA contamination from the RNA preparations were removed by RNase-free DNase treatment at a concentration of 0.1 U/μL. Five micrograms of total RNA was reverse transcribed by Superscript III (Invitrogen) using random hexamer primers. The TK, BGLF4, or β-actin cDNA from these preparations was then amplified using SYBR Green PCR amplification technology in an ABI PRISM 7500 sequence detection system (Applied Biosystems). The primers used in the real-time PCR amplification are listed in Table 2. Relative quantification of gene expression was determined by the comparative threshold method (ΔCT), as described previously.33 Expression of the β-actin mRNA in each individual sample was used to normalize the dataset.
Table 2.
Primers used in the study
| Transcripts | Sequence (5′ to 3′) | PCR product | EBV genomic region (Genbank or Virus) | Reference |
|---|---|---|---|---|
| Actin-F | TCCCTGGAGAAGAGCTACGA | 194 bp | None | Ghosh et al33 |
| Actin-R | AGCACTGTGTTGGCGTACAG | |||
| Thymidine kinase-F | TCCGGAGCCAGCTTCTCTCC | 275 bp | 131353-131334 (NC_007605) | Ghosh et al33 |
| Thymidine kinase-R | CGTGATTGTTGTTAGACCGG | 131079-131098 | ||
| EBER1-F | GGACCTACGCTGCCCTAGAGG | 166 bp | 6649-6631 (NC_007605) | Present study |
| EBER1-R | AAACATGCGGACCACCAGCTG | 6794-6774 | ||
| BGLF4-F | TGCGGAGTTGAGCCCGACGA | 263 bp | 111309-111290 (NC_007605) | Present study |
| BGLF4-R | CATACACGGCCCCGTAGCTC | 111047-111066 | ||
| Qp-F | AGGCGCGGGATAGCGTGCGCTACCGGA | 339 bp | 62426-62452 (B95-8) | Komano et al48 |
| Qp-R | TCCTCGTCCATGGTTATCAC | 108075-108056 | ||
| Cp-F | CACTACAAGACCTACGCCTCTCCATCCATC | 297 bp | 11425-11454 (B95-8) | Komano et al48 |
| Cp-R | TCTCCCCTAGGCCCTGAAGGTGAACCGCTT | 14832-14813, 17636-17626 | ||
| Wp-F | TCAGAGCGCCAGGAGTCCACACAAAT | 235 bp | 14384-14410 (B95-8) | Komano et al48 |
| Wp-R | TCTCCCCTAGGCCCTGAAGGTGAACCGCTT | 14832-14813, 17636-17626 |
PCR assay for the presence of EBV
The presence of EBV genome in the cell lines used in the study was determined by PCR analysis of EBV EBER1 sequences in the genomic and extrachromosomal DNA extract from these cells. One microgram of DNA was subjected to PCR amplification with β-actin– or EBER1-specific primer sets (Table 2) for 35 cycles, and products were analyzed on 2% agarose gel.
Cell viability assay
The viability of EBV+ lymphoma cells after treatment with various HDAC inhibitors in presence or absence of GCV was enumerated by the Trypan blue dye-exclusion method using a Countess automated cell counter (Invitrogen).
Immunoblotting
Expression of EBV early antigen-diffuse or BMRF1 proteins after treatment of P3HR1 cells with individual HDAC inhibitors was determined by immunoblotting, essentially as described previously.33
Results
Induction of EBV lytic-phase gene expression with HDAC inhibitors
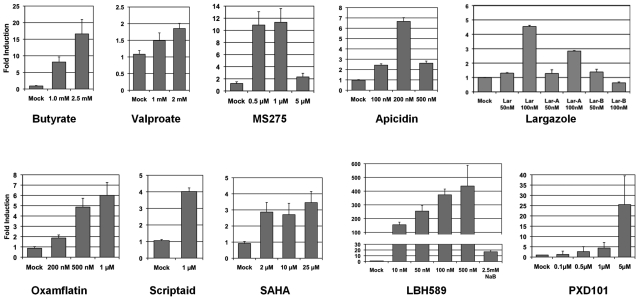
To investigate the efficiency of the several newer HDAC inhibitors for the induction of EBV lytic-phase gene expression, we measured their effect on the expression of the gene encoding the viral TK enzyme. The EBV-TK enzyme is normally expressed only during the lytic-replication stage of the virus life cycle. We initially studied the EBV+ BL cell line P3HR1, which typically maintains a type I latency in which only the EBV latency genes EBNA1, EBER1, and EBER2 are expressed. The cells were exposed to individual HDAC inhibitors (Figure 1) for 48 hours, followed by total RNA extraction. TK transcript expression in these preparations was then analyzed by reverse transcription and real-time PCR analysis. Exposure to most of the HDAC inhibitors produced increases in TK expression in a dose-dependent manner compared with the level of expression in vehicle-treated control cells (Figure 2). The highest level of increase in TK expression was produced by the hydroxamic acids LBH589 (panobinostat) and PXD101 (belinostat) (more than 250- and 26-fold, respectively). Butyrate, MS275, oxamflatin, apicidin, and largazole induced TK expression to a moderately high level (17-, 11-, 6-, 6.5-, and 4.5-fold, respectively). Scriptaid, SAHA, and VA induced TK expression only marginally above untreated cells. LBH589, PXD101, and apicidin were extremely toxic to the cells at the highest concentrations tested, which limited our ability to assess their potency in this assay. Therefore, these results demonstrate that most of the structurally diverse HDAC inhibitors used in this study are inducers of EBV TK gene expression and are therefore inducers of EBV lytic-phase gene expression.
Figure 2.
HDAC inhibitor–mediated induction of TK transcript in EBV+ lymphoma cells. Three million P3HR1 cells in 3 mL of RPMI 1640 medium were exposed to individual HDAC inhibitors for 48 hours. HDAC inhibitors used include short-chain fatty acids (butyrate and valproate), cyclic tetrapeptide (apicidin), cyclic depsipeptide (parent largazole and analogs A and B), benzamide (MS275), and hydroxamic acids (oxamflatin, LBH589, SAHA, PXD101, and Scriptaid). Inhibitor concentrations were determined empirically so that cytotoxicity remained minimal. Total RNA extraction, reverse transcription, and real-time PCR analysis were performed as described in “Analysis of lytic gene expression.” Real-time PCR was performed in triplicate on each HDAC inhibitor–treated sample for both TK mRNA and β-actin mRNA, and these values were used to determine respective ΔCt and the fold induction. RNA from P3HR1 cells treated with 1.0 and 2.5mM sodium butyrate were used as internal controls in each experiment (not shown for each inhibitor). Each assay was repeated 3 times and error bars in each individual figure represent SDs.
Cell growth analysis with HDAC inhibitors in combination with GCV
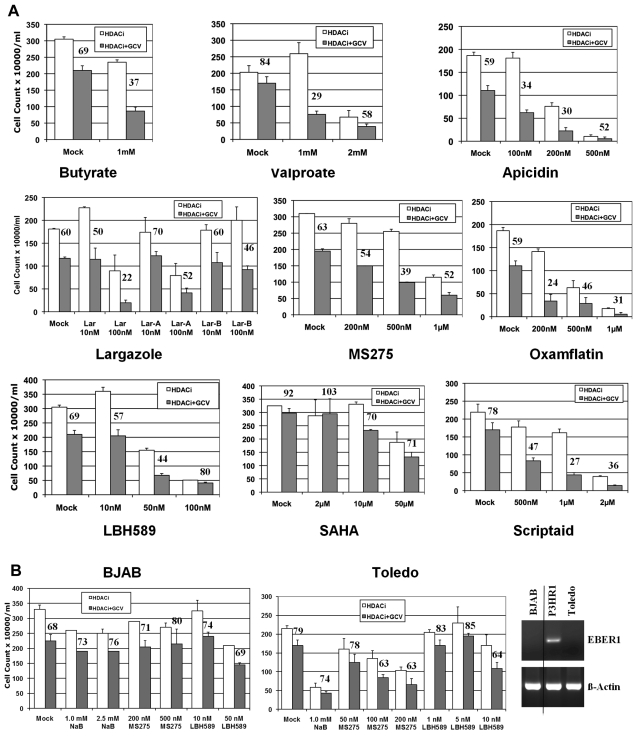
Because most of the HDAC inhibitors efficiently induced TK expression in P3HR1 cells, we next analyzed whether the presence of the antiherpes virus drug GCV during this induction of lytic-phase gene expression would facilitate killing of the EBV-infected cells. We exposed the cells to the individual HDAC inhibitor, GCV, or the combination for 72 hours. The HDAC inhibitors were then removed and the cells were maintained in fresh medium containing GCV for a further 72 hours. The short-chain fatty acid class HDAC inhibitor VA showed significant growth inhibition in combination with GCV, because only 29% of cells survived when treated with VA and GCV together, compared with 84% when treated with GCV alone (Figure 3A). However, a relatively high concentration of VA (up to 1.0mM), was necessary to achieve this effect. VA was therefore similar to butyrate, another short-chain fatty acid, in its potency. Higher concentrations of VA were cytostatic for the P3HR1 cells. The cyclic tetrapeptide apicidin also was efficient in cell killing, but the effective dose range was very limited. At 100nM, 34% of the cells survived with the combination treatment, whereas 59% survived with GCV treatment alone. Higher concentrations of apicidin were toxic to the cells. In contrast, the benzamide HDAC inhibitor MS275 was relatively nontoxic to the cells at the concentration ranges effective to induce sensitivity to GCV. In our assays, MS275 at 500nM was found to have optimal cell killing activity when used together with GCV (39% survival in combination treatment vs 63% with GCV alone). We also tested 3 cyclic depsipeptide HDAC inhibitors of the largazole class. Largazole was originally isolated from a marine cyanobacterium and showed strong cytotoxic activity, particularly against tumor cells.34 The 3 compounds largazole, largazole-A, and largazole-B were prepared by total synthesis, as described previously.32 Chemical structures of the largazole and its synthetic analogs, as well as all of the other HDAC inhibitors used in the study, are shown in Figure 1. Of these 3 largazole derivatives, the parent natural product (largazole itself) showed the most significant cell killing effect at a low concentration (at 100nM, 22% survival in combination treatment vs 60% with GCV alone). Largazole analog B also showed cytotoxic activity in combination, albeit at a lower level. At least 10 other largazole derivatives were tested for their ability to sensitize EBV-infected tumor cells to GCV, but only one other largazole analog had activity comparable to parent largazole (data not shown). Four additional HDAC inhibitors of the hydroxamic acid class (oxamflatin, SAHA, LBH589, and Scriptaid) were tested in the same assays. Of these 4 inhibitors, SAHA had little effect on cell killing in the presence of GCV. Scriptaid produced strong cytotoxic activity, requiring concentrations in the range of 1μM or higher. Oxamflatin showed efficient cytotoxic activity at a 200nM concentration (24% survival in combination treatment vs 59% with GCV alone). LBH589 was highly effective in the range of a 10 to 50nM concentration and was the most potent HDAC inhibitor found to sensitize the tumor cells in this study (44% survival in combination treatment vs 69% with GCV alone at a 50nM concentration). Our study clearly demonstrates that many of the HDAC inhibitors have strong cytotoxic activity in presence of the antiherpes virus drug GCV.
Figure 3.
Cytotoxic activity of HDAC inhibitors in the presence of an antiherpes virus drug. (A) Three hundred thousand P3HR1 cells were exposed to either 40μM GCV or vehicle, and the indicated concentrations of individual HDAC inhibitors in a 1-mL volume in 24-well plates in triplicate. Three days later, 800 μL of the medium was removed without disturbing the settled cells, 1 mL of fresh growth medium containing GCV (40μM) was added, and the cells were allowed to grow for another 3 days. HDAC inhibitors used included butyrate, valproate, apicidin, largazole and its analogs, MS275, oxamflatin, LBH589, SAHA, and Scriptaid. The number above the HDAC + GCV bar represents the percentage of cells surviving relative to the cultures exposed to that particular HDAC inhibitor alone (assigned a value of 100%). Error bars represent SDs in individual experiments. (B) Cytotoxic activity of selected HDAC inhibitors (butyrate, MS275, and LBH589) in the presence of GCV in the EBV− B-lymphoma lines BJAB and Toledo. Experiments were carried out essentially as in panel A. The right panel shows detection of EBER1- and β-actin–specific PCR products generated from cellular DNA of BJAB, P3HR1, and Toledo cells analyzed in a 2% agarose gel. A vertical line has been inserted to indicate a repositioned gel lane.
To verify that the combinatory effect of GCV and HDAC inhibitors we observed on P3HR1 cells was indeed because of the presence of EBV, we also tested the combination treatment on 2 EBV− B-lymphoma lines, BJAB and Toledo. As shown in Figure 3B, exposure to the HDAC inhibitors butyrate, MS275, or LBH589 did not produce additional cytotoxic activity in the presence of GCV in either BJAB or Toledo cells. When used as a single agent, most of the HDAC inhibitors studied generated higher cytotoxicity in Toledo cells compared with the other cell lines used in this study. This prompted us to use lower concentrations of HDAC inhibitors at which Toledo cells remained healthy. We confirmed by PCR analysis that BJAB and Toledo cells were indeed EBV−, whereas P3HR1 was EBV+ (Figure 3B right panel).
Induction of viral BGLF4 and BMRF-1 expression by HDAC inhibitors
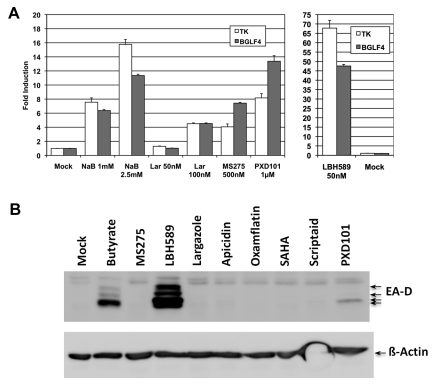
For GCV to act as an antiviral or cytotoxic drug, it requires initial monophosphorylation by an EBV-encoded kinase, with subsequently conversion to a triphosphate by a cellular kinase. The resulting GCV-TP then gets incorporated into an actively replicating DNA strand and causes premature termination of DNA synthesis,35 evoking apoptosis. A few studies have reported that GCV is a better substrate for the EBV serine-threonine protein kinase BGLF4 than for the viral TK, both of which are exclusively expressed during lytic-phase replication of EBV.36 We analyzed BGLF4 expression in P3HR1 cells after exposure to certain HDAC inhibitors in comparison with the TK expression profiles. Butyrate, largazole, MS275, PXD101, and LBH589 were tested because these HDAC inhibitors were found to be the most efficient at generating cytotoxicity in combination with GCV or at inducing TK expression. As shown in Figure 4A, for each of the HDAC inhibitors tested, the pattern of change of BGLF4 expression paralleled TK expression. Exposure to progressively higher concentrations of HDAC inhibitors (butyrate or largazole), which led to increases in TK induction, also increased BGLF4 expression. We have reported previously that exposure of P3HR1 cells to butyrate induces EBV early antigen-diffuse protein (EA-D or BMRF1), a major protein of the EBV lytic replication cycle.33 We evaluated EA-D protein expression in P3HR1 cells that were exposed to some of the individual HDAC inhibitors used in this study. As shown in Figure 4B, LBH589 strongly induced the EA-D protein, whereas PXD101 did so very weakly and the other HDAC inhibitors did not. Our results therefore demonstrate that although HDAC inhibitors induce TK and BGLF4 expression in a similar manner, induction of the EA-D protein may require coordinate expression of other factors that different HDAC inhibitors regulate differentially.
Figure 4.
HDAC inhibitor–mediated induction of EBV lytic-phase gene expression. (A) Comparison of TK and BGLF4 transcript expression in P3HR1 cells after exposure to different HDAC inhibitors. Cells were treated with individual HDAC inhibitors as indicated and total RNA was analyzed by reverse transcription and real-time PCR analysis as described in the legend to Figure 2. Expression of β-actin mRNA under similar treatment conditions was used to normalize the dataset. (B) Immunoblot analysis for EA-D protein. Thirty micrograms of whole-cell lysates from individual HDAC inhibitor–treated cells (for 48 hours) or untreated cells were separated in 10% SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with a 1:2000 dilution of mouse anti-EBV EA-D Ab (Millipore). Equal loading of proteins was verified by immunoblotting with 1:15 000 dilution of mouse anti–β-actin Ab (Sigma-Aldrich). Each assay was repeated 3 times and error bars in each individual figure represent SDs.
Prolonged exposure to HDAC inhibitors is not essential for efficient sensitization to antiviral agents
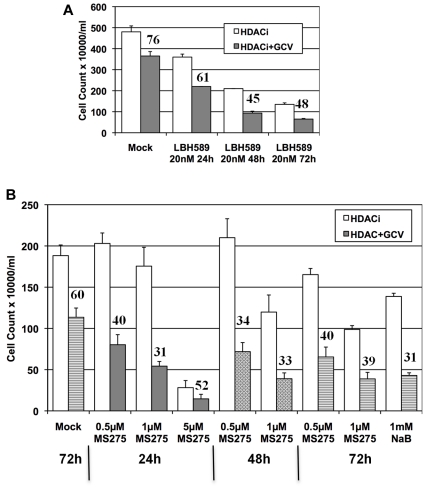
We rationalized that the potential clinical efficacy of the combination of an HDAC inhibitor and an antiviral agent would be improved if we could determine the minimal time of exposure to HDAC inhibitor required to sensitize the tumor cells to antiviral agents. To determine this, P3HR1 cells were exposed to HDAC inhibitors for 24 or 48 hours in the combination treatment protocols and the results were compared with those using 72 hours of exposure to HDAC inhibitors (Figure 2). As shown in Figure 5A, using a 20nM concentration of LBH589 in P3HR1 cells together with GCV, the relative tumor cell cytotoxicity induced by 48 hours of exposure to the HDAC inhibitor was similar to the cytotoxicity observed in cells that had been exposed for 72 hours (45% survival with 48 hours of exposure; 48% with 72 hours of exposure). The cytotoxicity induced by the HDAC inhibitor alone, however, also increased with each longer interval of exposure. When MS275 was used as the HDAC inhibitor at 1μM, total cytotoxicity with 48 hours of exposure was equivalent to that seen with 72 hours of exposure and the relative cytotoxicity conferred by the addition of GCV (measured as the fraction of surviving cells) was equivalent for all 3 intervals of HDAC inhibitor exposure. In this experiment, a higher concentration of the inhibitor (as identified in Figure 2) was also studied to determine whether a shorter exposure to a higher concentration of the HDAC inhibitor would effectively sensitize cells to GCV. We found that a 24-hour exposure to 5μM MS275 plus GCV was more cytotoxic than 48 or 72 hours of exposure to either 0.5 or 1μM MS275 plus GCV (Figure 5B). However, exposure to 5μM MS275 as a single agent produced substantial cytotoxicity within the 24-hour treatment period.
Figure 5.
Sensitization of EBV lymphoma cells to GCV-mediated killing by brief exposure to HDAC inhibitors. (A) Three hundred thousand P3HR1 cells in a 1-mL volume were treated with 20nM LBH589 for the indicated period of time in the presence or absence of 40μM GCV. The culture medium was completely removed after centrifugation at the end of incubation with LBH589 and replenished with fresh growth medium with or without GCV, as indicated. Media for all cells were replaced again at 72 hours. Cells were counted at 144 hours (day 6). (B) Similar protocol as in panel A, but MS275 was evaluated at 3 different concentrations. The overwhelming toxicity after exposure to MS275 at 5μM for 48 or 72 hours precluded any meaningful cell count. Cells exposed to sodium butyrate at 1mM were used as an internal control. The number above the HDAC + GCV bar represents the percentage of cells surviving relative to the cell count after exposure to the HDAC inhibitor alone (assigned a value of 100%). Experiments were repeated 3 times and error bars represent SDs in individual experiments.
HDAC inhibitors in combination with an antiviral induce efficient cell killing of other EBV+ lymphoma cells
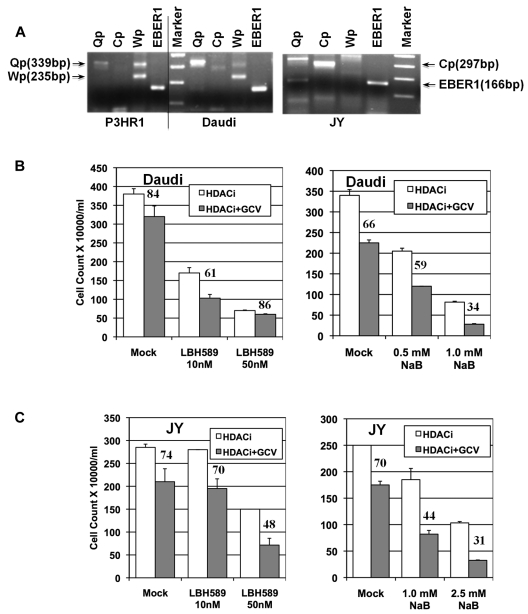
The experiments described in Figures 1 through 5 were carried out in the BL cell line P3HR1, which maintains an EBV type 1 latency. To determine whether the combination of HDAC inhibitor and GCV would be effective against other EBV+ lymphoma cells, we tested butyrate and one of the other most effective HDAC inhibitors, LBH589, in combination with GCV in cytotoxicity assays. We used another BL line, Daudi, and an LCL line, JY, for this purpose. EBV replication in LCLs is of type 3 latency, and all 11 latency gene products are expressed. EBV replication in LCLs resembles that found in the clonal or multiclonal B-cell populations in patients with PTLD. The EBV expression and latency status of these lines was confirmed by RT-PCR analysis of the expression of EBER1, Qp, Cp, and Wp-specific transcripts. As shown in Figure 6A, all 3 lines expressed EBER1 RNA abundantly. As expected, transcripts from the Qp promoter were observed in both of the BL cell lines, P3HR1 and Daudi. The Qp transcript was also detected in JY cells. Although it is not common, some type 3 latency LCLs do express the Qp transcript.37 Although wide variations of Wp expression among different latency types have been noted previously, expression of the Cp transcript is specific to cells with type 3 EBV latency.37 The JY cells, but neither the P3HR1 or Daudi cells, expressed Cp-specific transcripts. We analyzed the ability of butyrate and LBH589 to sensitize both Daudi and JY cells to an antiviral agent. As single agents, both HDAC inhibitors reduced the number of the Daudi cells significantly at both concentrations tested, but had less of a cytotoxic effect on the JY cells (Figure 6B). LBH589 in combination with GCV had a modest cytotoxic effect on Daudi cells (61% relative survival with combination treatment vs 84% with GCV alone), but a more significant effect on JY cells (48% relative survival in combination treatment vs 74% with GCV alone). Butyrate in combination with GCV produced a strong cytotoxic effect on both Daudi cells (34% relative survival in combination treatment vs 66% with GCV alone) and JY cells (44% relative survival in combination treatment vs 70% with GCV alone). These results demonstrate clearly that the combination of an HDAC inhibitor and GCV is effective at killing EBV+ lymphoma cells of diverse origins.
Figure 6.
Effect of HDAC inhibitor and GCV combination treatment on other EBV+ lymphoma cells. (A) Analysis of promoter use by the 3 different EBV lymphoma cell lines used in the study. Reverse transcription and PCR analysis of total RNA was carried out using primers that specifically detected the Qp, Wp, or Cp transcripts (Table 2). Products were analyzed on a 2% agarose gel with a 100-bp DNA ladder as a marker. A vertical line has been inserted to indicate a repositioned gel lane. (B) Effect of combination treatment on the BL line Daudi. Four hundred thousand cells/mL/well were used along with 60μM GCV in the appropriate wells. Assay parameters were as described in the legend for Figure 2. (C) Effect of combination treatment on the EBV-transformed lymphoblastoid cell line JY. In this case, 200 000 cells/mL/well were used, along with 60μM GCV as appropriate. Experiments were repeated 3 times and error bars represent SDs in individual experiments.
Discussion
In the present study, we have demonstrated that HDAC inhibitors of disparate classes and structures induce EBV lytic-phase gene expression and sensitize EBV+ tumor cells to cytotoxicity in presence of the antiherpes virus drug GCV. Effective sensitizing concentrations for some of the HDAC inhibitors, such as LBH589, apicidin, MS275, and largazole, were in the range of 20-500nM. Our previous in vitro and preclinical studies demonstrated that the HDAC inhibitor sodium or arginine butyrate strongly induced viral TK expression in a patient-derived EBV lymphoma cell line, as well as in the patient himself, and suggested the therapeutic potential of the approach.10,12 A phase 1/2 clinical trial later showed that this treatment strategy indeed significantly reduced the tumor burden in two-thirds of patients with very refractory EBV-associated lymphomas.18 The lymphomas that responded included posttransplantation lymphoproliferative diseases, T/NK lymphomas, EBV+ large B-cell lymphomas, and EBV+ cutaneous T-cell lymphomas. However, because of its poor oral bioavailability and short half-life in vivo, this regimen required that butyrate be infused continuously for many days and at a high dose for therapeutic activity. In contrast, some of the HDAC inhibitors investigated in the present study were 104- to 105-fold more potent than butyrate and have superior pharmacokinetics. Interestingly, a few of these novel HDAC inhibitors are already in clinical trials.23
Most of the HDAC inhibitors used in our study induced EBV TK expression, and their relative activity in TK induction was correlated with their ability to generate tumor cytotoxicity in presence of GCV. However, this correlation was not universal for all of the HDAC inhibitors studied. For example, VA did demonstrate cytotoxicity in combination with GCV, although its TK-inducing ability was modest compared with the newer HDAC inhibitors studied herein. Conversely, SAHA produced up to a 3-fold induction of TK, but did not augment cytotoxicity in the presence of GCV. This occasional lack of correlation with TK induction suggested that kinases other than TK might also be involved in the conversion of the pro-drug GCV. Some studies have reported that the EBV protein kinase coded by the BGLF4 gene is a more efficient kinase for the phosphorylation of GCV than TK.36,38 In our studies, the induction of BGLF4 always paralleled the induction TK by all of the HDAC inhibitors tested. It is therefore possible that HDAC inhibitor–mediated induction of BGLF4 and subsequent expression of EBV-PK may also have contributed to the phosphorylation of GCV. However, the differential induction of these 2 potential GCV kinases does not explain the lack of correlation between TK induction and sensitization to GCV by SAHA or VA. Irrespective of the mechanism of HDAC inhibitor–mediated sensitization, however, the control experiments with the EBV− lymphoma cell lines demonstrated that the presence of EBV is critical for the combination of agents to generate efficient cytotoxicity.
Although butyrate in combination with GCV was quite effective in killing EBV+ tumor cells in vitro and in clinical studies, the pharmacokinetic limitations of butyrate in a clinical application mandated infusion at high doses to patients continuously over several days. We demonstrated that a 2-day exposure to selected HDAC inhibitors was as effective at sensitizing tumors to GCV as a 3-day exposure. With MS275, even a 24-hour exposure produced cytotoxic activity in combination with GCV comparable to 2- or 3-day exposure. These findings suggest that prolonged exposure to HDAC inhibitors might not be necessary in the clinical setting, potentially limiting secondary toxicities. Indeed, we have reported previously that shorter durations of exposure to butyrate also efficiently killed EBV+ lymphoma cells in the presence of GCV.33 Based on these data, 1 patient with refractory EBV+ lymphoma was treated in a protocol using butyrate for 5 days and GCV or valganciclovir for 21 days, and the lymphoma burden was dramatically reduced within 1 cycle. Furthermore, previously high EBV viral loads, as well as the viral loads of 2 other herpesviruses (CMV and HHV6), became undetectable.39
The reported pharmacokinetics and pharmacodynamics for MS275 and LBH589 as single agents appear superior to butyrate. A phase 1 clinical trial with MS275 administered orally demonstrated that the area under the plasma concentration versus time curve easily reached 59-268 ng/h/mL for doses of 2-8 mg/m2 and was sustained for a minimum of 34 ± 26 hours across all dose levels.40 The administration of MS275 induced acetylation of histone H3 and H4 in circulating PBMCs in these studies and in a variety of tumor cell lines, including prostate, pancreas, and breast cancer lines, at these concentrations in vitro.41 LBH589 also displayed rapid absorption when administered orally in a phase 1 clinical trial, with a serum half-life of approximately 14.6 hours and an area under the curve of 134 ng/h/mL for a single 20-mg dose.42
The results of the present study demonstrate that the EBV latency type in the lymphoma is not crucial for the success of combination therapy approach with HDAC inhibitors and GCV. P3HR1 cells, a line originally derived from the BL cell line Jijoye, produce virus particles that are transformation defective.43 Daudi cells were also isolated from a BL patient and are a transformation-defective but EBV-nonproducing line.44 Our data demonstrate that the inherent viral defects of the P3HR1 or Daudi cells do not interfere with HDAC inhibitor–mediated induction of lytic-phase gene expression and cytotoxicity in the presence of an antiherpes viral drug. The JY cell line, an EBV-transformed LCL with a different latency pattern, responded equally well to the combination treatment approach with either butyrate or LBH589 as the viral-inducing agent. These results in the JY cell line mirror the observed responsiveness of PTLD patients to the combination of butyrate and GCV in a previous clinical trial.18 PTLD, commonly arising in immunosuppressed individuals, is caused by unchecked proliferation of EBV+ B cells in the absence of immune surveillance. In both LCL and PTLD, EBV maintains a type 3 latency in which all of the EBV latent gene products are expressed.
In summary, the results of the present study demonstrate that several structurally distinct HDAC inhibitors are efficient agents for sensitizing EBV+ lymphoma cells to antiherpes virus drugs. Only nanomolar concentrations of the most potent of these agents are necessary for optimal effect. Our previous work demonstrated that the HDAC inhibitor butyrate has impressive early-phase clinical activity in the treatment of patients with EBV+ lymphomas, and data from the present study suggest that there is also potential for the application of these new HDAC inhibitors in combination therapy with an antiherpes virus drugs for treating EBV+ lymphomas. In particular, some of the new HDAC inhibitors with substantial clinical and safety data might provide more convenient treatment regimens and even, in combination with oral antivirals, a completely outpatient-based protocol.
Acknowledgments
The authors thank Drs Jack Strominger, Thomas Gilbert, and Gerald Denis for their generous donation of the B-cell lines JY, BJAB, and Toledo, respectively, and Virginia Newman for technical assistance.
This study was supported by the V Foundation (to D.V.F.), the National Cancer Institute (grants CA153474 to D.V.F. and S.P.P. and CA152314 to R.M.W.), the Karin Grunebaum Foundation for Cancer Research (to D.V.F.), and HemaQuest Pharmaceuticals.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.K.G., S.P.P., and D.V.F. conceived and planned the study and analyzed the data; R.M.W. synthesized some of the compounds; S.K.G. performed the experiments; and all authors contributed to writing the manuscript.
Conflict-of-interest disclosure: S.P.P., D.V.F., and R.M.W. have had research or consulting agreements with and own equity in HemaQuest Pharmaceuticals. S.K.G. declares no competing financial interests.
Correspondence: Douglas V. Faller, PhD, MD, Cancer Center, Boston University School of Medicine, 72 East Concord St, K 701, Boston, MA 02118; e-mail: dfaller@bu.edu.
References
- 1.Straus SE, Cohen JI, Tosato G, Meier J. NIH conference. Epstein-Barr virus infections: biology, pathogenesis, and management. Ann Intern Med. 1993;118(1):45–58. doi: 10.7326/0003-4819-118-1-199301010-00009. [DOI] [PubMed] [Google Scholar]
- 2.Epstein M, Achong B, Barr Y. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 3.Liebowitz D. Nasopharyngeal carcinoma: the Epstein-Barr virus association. Semin Oncol. 1994;21(3):376–381. [PubMed] [Google Scholar]
- 4.Hopwood P, Crawford DH. The role of EBV in post-transplant malignancies: a review. J Clin Pathol. 2000;53(4):248–254. doi: 10.1136/jcp.53.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deacon EM, Pallesen G, Niedobitek G, et al. Epstein-Barr virus and Hodgkin's disease: transcriptional analysis of virus latency in the malignant cells. J Exp Med. 1993;177(2):339–349. doi: 10.1084/jem.177.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibata D, Weiss LM, Hernandez AM, Nathwani BN, Bernstein L, Levine AM. Epstein-Barr virus-associated non-Hodgkin's lymphoma in patients infected with the human immunodeficiency virus. Blood. 1993;81(8):2102–2109. [PubMed] [Google Scholar]
- 7.Bonnet M, Guinebretiere JM, Kremmer E, et al. Detection of Epstein-Barr virus in invasive breast cancers. J Natl Cancer Inst. 1999;91(16):1376–1381. doi: 10.1093/jnci/91.16.1376. [DOI] [PubMed] [Google Scholar]
- 8.Yuen ST, Chung LP, Leung SY, Luk IS, Chan SY, Ho J. In situ detection of Epstein-Barr virus in gastric and colorectal adenocarcinomas. Am J Surg Pathol. 1994;18(11):1158–1163. doi: 10.1097/00000478-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Gutiérrez MI, Judde JG, Magrath IT, Bhatia KG. Switching viral latency to viral lysis: a novel therapeutic approach for Epstein-Barr virus-associated neoplasia. Cancer Res. 1996;56(5):969–972. [PubMed] [Google Scholar]
- 10.Mentzer SJ, Perrine SP, Faller DV. Epstein-Barr virus post-transplant lymphoproliferative disease and virus-specific therapy: pharmacological re-activation of viral target genes with arginine butyrate. Transpl Infect Dis. 2001;3(3):177–185. doi: 10.1034/j.1399-3062.2001.003003177.x. [DOI] [PubMed] [Google Scholar]
- 11.Israel BF, Kenney SC. Virally targeted therapies for EBV-associated malignancies. Oncogene. 2003;22(33):5122–5130. doi: 10.1038/sj.onc.1206548. [DOI] [PubMed] [Google Scholar]
- 12.Mentzer SJ, Fingeroth J, Reilly JJ, Perrine SP, Faller DV. Arginine butyrate-induced susceptibility to ganciclovir in an Epstein-Barr-virus-associated lymphoma. Blood Cells Mol Dis. 1998;24(2):114–123. doi: 10.1006/bcmd.1998.0178. [DOI] [PubMed] [Google Scholar]
- 13.Westphal EM, Blackstock W, Feng W, Israel B, Kenney SC. Activation of lytic Epstein-Barr virus (EBV) infection by radiation and sodium butyrate in vitro and in vivo: a potential method for treating EBV-positive malignancies. Cancer Res. 2000;60(20):5781–5788. [PubMed] [Google Scholar]
- 14.Moore SM, Cannon JS, Tanhehco YC, Hamzeh FM, Ambinder RF. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother. 2001;45(7):2082–2091. doi: 10.1128/AAC.45.7.2082-2091.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daibata M, Bandobashi K, Kuroda M, Imai S, Miyoshi I, Taguchi H. Induction of lytic Epstein-Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV-positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J Virol. 2005;79(9):5875–5879. doi: 10.1128/JVI.79.9.5875-5879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng WH, Hong G, Delecluse HJ, Kenney SC. Lytic induction therapy for Epstein-Barr virus-positive B-cell lymphomas. J Virol. 2004;78(4):1893–1902. doi: 10.1128/JVI.78.4.1893-1902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu DX, Tanhehco Y, Chen J, et al. Bortezomib-induced enzyme-targeted radiation therapy in herpesvirus-associated tumors. Nat Med. 2008;14(10):1118–1122. doi: 10.1038/nm.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perrine SP, Hermine O, Small T, et al. A phase 1/2 trial of arginine butyrate and ganciclovir in patients with Epstein-Barr virus-associated lymphoid malignancies. Blood. 2007;109(6):2571–2578. doi: 10.1182/blood-2006-01-024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wade PA. Transcriptional control at regulatory checkpoints by histone deacetylases: molecular connections between cancer and chromatin. Hum Mol Genet. 2001;10(7):693–698. doi: 10.1093/hmg/10.7.693. [DOI] [PubMed] [Google Scholar]
- 20.Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21(18):6091–6101. doi: 10.1128/MCB.21.18.6091-6101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiagalingam S, Cheng KH, Lee HJ, Mineva N, Thiagalingam A, Ponte JF. Histone deacetylases: unique players in shaping the epigenetic histone code. Ann N Y Acad Sci. 2003;983:84–100. doi: 10.1111/j.1749-6632.2003.tb05964.x. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin F, La Thangue NB. Histone deacetylase inhibitors open new doors in cancer therapy. Biochem Pharmacol. 2004;68(6):1139–1144. doi: 10.1016/j.bcp.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Tan J, Cang S, Ma Y, Petrillo RL, Liu D. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol. 2010;3:5. doi: 10.1186/1756-8722-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15(12):3958–3969. doi: 10.1158/1078-0432.CCR-08-2785. [DOI] [PubMed] [Google Scholar]
- 25.Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R. FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist. 2007;12(10):1247–1252. doi: 10.1634/theoncologist.12-10-1247. [DOI] [PubMed] [Google Scholar]
- 26.Grant C, Rahman F, Piekarz R, et al. Romidepsin: a new therapy for cutaneous T-cell lymphoma and a potential therapy for solid tumors. Expert Rev Anticancer Ther. 2010;10(7):997–1008. doi: 10.1586/era.10.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinuma Y, Konn M, Yamaguchi J, Wudarski DJ, Blakeslee JR, Jr, Grace JT., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein E, Klein G, Nadkarni JS, Nadkarni JJ, Wigzell H, Clifford P. Surface IgM-kappa specificity on a Burkitt lymphoma cell in vivo and in derived culture lines. Cancer Res. 1968;28(7):1300–1310. [PubMed] [Google Scholar]
- 29.Terhorst C, Parham P, Mann DL, Strominger JL. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menezes J, Leibold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975;22(4):276–284. [PubMed] [Google Scholar]
- 31.Gabay C, Ben-Bassat H, Schlesinger M, Laskov R. Somatic mutations and intraclonal variations in the rearranged Vkappa genes of B-non-Hodgkin's lymphoma cell lines. Eur J Haematol. 1999;63(3):180–191. doi: 10.1111/j.1600-0609.1999.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 32.Bowers AA, West N, Newkirk TL, et al. Synthesis and histone deacetylase inhibitory activity of largazole analogs: alteration of the zinc-binding domain and macrocyclic scaffold. Org Lett. 2009;11(6):1301–1304. doi: 10.1021/ol900078k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh SK, Forman LW, Akinsheye I, Perrine SP, Faller DV. Short, discontinuous exposure to butyrate effectively sensitizes latently EBV-infected lymphoma cells to nucleoside analogue antiviral agents. Blood Cells Mol Dis. 2007;38(1):57–65. doi: 10.1016/j.bcmd.2006.10.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying Y, Taori K, Kim H, Hong J, Luesch H. Total synthesis and molecular target of largazole, a histone deacetylase inhibitor. J Am Chem Soc. 2008;130(26):8455–8459. doi: 10.1021/ja8013727. [DOI] [PubMed] [Google Scholar]
- 35.Ooka T, Calender A, de Turenne M, Daillie J. Effect of arabinofuranosylthymine on the replication of Epstein-Barr virus and relationship with a new induced thymidine kinase activity. J Virol. 1983;46(1):187–195. doi: 10.1128/jvi.46.1.187-195.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng Q, Hagemeier SR, Fingeroth JD, Gershburg E, Pagano JS, Kenney SC. The Epstein-Barr virus (EBV)-encoded protein kinase, EBV-PK, but not the thymidine kinase (EBV-TK), is required for ganciclovir and acyclovir inhibition of lytic viral production. J Virol. 2010;84(9):4534–4542. doi: 10.1128/JVI.02487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao Q, Young LS, Woodman CB, Murray PG. Epstein-Barr virus (EBV) and its associated human cancers–genetics, epigenetics, pathobiology and novel therapeutics. Front Biosci. 2006;11:2672–2713. doi: 10.2741/2000. [DOI] [PubMed] [Google Scholar]
- 38.Gustafson EA, Chillemi AC, Sage DR, Fingeroth JD. The Epstein-Barr virus thymidine kinase does not phosphorylate ganciclovir or acyclovir and demonstrates a narrow substrate specificity compared to the herpes simplex virus type 1 thymidine kinase. Antimicrob Agents Chemother. 1998;42(11):2923–2931. doi: 10.1128/aac.42.11.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faller DV, Ghosh S, Feldman T, et al. Short-term exposure to arginine butyrate, in combination with ganciclovir, is as effective as continuous exposure for virus-targeted therapy of EBV-positive lymphomas [abstract]. Blood. 2009;114(1):4754. [Google Scholar]
- 40.Kummar S, Gutierrez M, Gardner ER, et al. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res. 2007;13(18 pt 1):5411–5417. doi: 10.1158/1078-0432.CCR-07-0791. [DOI] [PubMed] [Google Scholar]
- 41.Hess-Stumpp H, Bracker TU, Henderson D, Politz O. MS-275, a potent orally available inhibitor of histone deacetylases–the development of an anticancer agent. Int J Biochem Cell Biol. 2007;39(7-8):1388–1405. doi: 10.1016/j.biocel.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Rathkopf D, Wong BY, Ross RW, et al. A phase I study of oral panobinostat alone and in combination with docetaxel in patients with castration-resistant prostate cancer. Cancer Chemother Pharmacol. 2010;66(1):181–189. doi: 10.1007/s00280-010-1289-x. [DOI] [PubMed] [Google Scholar]
- 43.Biggin M, Bodescot M, Perricaudet M, Farrell P. Epstein-Barr virus gene expression in P3HR1-superinfected Raji cells. J Virol. 1987;61(10):3120–3132. doi: 10.1128/jvi.61.10.3120-3132.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones MD, Foster L, Sheedy T, Griffin BE. The EB virus genome in Daudi Burkitt's lymphoma cells has a deletion similar to that observed in a non-transforming strain (P3HR-1) of the virus. EMBO J. 1984;3(4):813–821. doi: 10.1002/j.1460-2075.1984.tb01890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackwell L, Norris J, Suto CM, Janzen WP. The use of diversity profiling to characterize chemical modulators of the histone deacetylases. Life Sci. 2008;82(21-22):1050–1058. doi: 10.1016/j.lfs.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Bradner JE, West N, Grachan ML, et al. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6(3):238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrine SP, Faller DV. Butyrate-induced reactivation of the fetal globin genes: a molecular treatment for the beta-hemoglobinopathies. Experientia. 1993;49(2):133–137. doi: 10.1007/BF01989417. [DOI] [PubMed] [Google Scholar]
- 48.Komano J, Sugiura M, Takada K. Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata. J Virol. 1998;72(11):9150–9156. doi: 10.1128/jvi.72.11.9150-9156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]