Abstract
Purpose
To determine whether Toll-like receptor (TLR) ligands regulate the activation of retinal astrocytes (RACs) and the possible role of RACs in the polarization of interphotoreceptor retinoid-binding protein (IRBP)-specific T cells.
Methods
TLR expression on RACs isolated from C57BL/6 mice was examined using real-time PCR and flow cytometry. The ability of RACs before or after treatment with TLR ligands to interact with T cells was assessed by measuring major histocompatibility complex class II and costimulatory molecule expression and cytokine production. The stimulatory effect of RACs, with or without TLR stimulation, on responder IRBP-specific T cells was examined by T-cell proliferation, cytokine production, and disease-inducing ability.
Results
Cultured mouse RACs expressed TLR2, TLR3, and TLR4. Different TLR ligands had distinct stimulatory effects on RACs. PolyI:C (a TLR3 ligand) had the greatest effect in stimulating RACs to acquire antigen-presentation function, whereas BLP (a TLR2 ligand) had the lowest effect. TLR3 ligation increased the expression of MHC and costimulatory molecules and induced the production of IL-6, IL-12, and IL-23 by RACs. IRBP-specific T cells activated by polyI:C-treated RACs expanded vigorously, produced significant amounts of IFN-γ and IL-17, and induced experimental autoimmune uveitis when injected into naive mice.
Conclusions
The stimulatory effect of RACs on autoreactive T cells is regulated by TLR ligands. TLR3 had a marked effect on the ability of RACs to promote the activation of Th1 and Th17 IRBP-specific T cells. Thus, exposure to microbial antigen(s) may alter susceptibility to autoimmune uveitis by promoting the activation of autoreactive T cells.
Reactivation of autoreactive T cells within the target organ, such as the CNS or eye, is one of the critical pathogenic events in the development of autoimmunity.1,2 Tissue-invading autoreactive T cells require major histocompatibility complex (MHC) class II antigens for their reactivation in the organ.3–5 It is generally thought that such molecules are provided by macrophages and dendritic cells (DCs) that have infiltrated the inflamed organ. However, residential glial cells in the CNS and eye, such as microglia and astrocytes, have the ability to express MHC molecules6,7 and can initiate cross-talk between effector T cells and parenchymal cells of the organ.
We have previously reported that activated RACs have the potential to stimulate T cells in vitro.7 However, their T-cell stimulatory effect varies, depending on whether the RACs are preactivated and whether they are derived from disease-prone or disease-resistant strains of mice. For example, RACs from B10RIII mice, which are highly susceptible to the induction of experimental autoimmune uveitis (EAU), are effective in promoting interphotoreceptor retinoid-binding protein (IRBP)-specific T-cell expansion and cytokine production, whereas RACs from C57BL/6 (B6) mice, which are less susceptible to EAU induction, are not as effective.
Recent studies have shown that microbial proteins can act as ligands for Toll-like receptors (TLRs), a family of well-characterized, pattern-recognition receptors recognizing pathogen-associated molecular motifs.8,9 Different TLRs recognize distinct classes of ligands, and this results in changes in innate and adaptive immune responses. Thirteen TLRs have been described in humans. Some of these, such as TLRs 1, 2, 4, 5, and 6, preferentially bind bacterial products (e.g., lipopolysaccharide [LPS] or flagellin), whereas others, such as TLRs 3, 7, 8, and 9, mainly bind nucleic acids (e.g., single- and double-stranded RNA).10 TLR3 mediates responses to double-stranded (ds) RNA or its synthetic analogue polyinosinic:polycytidylic acid (polyI:C). dsRNA makes up the genome of one class of viruses (e.g., Reoviridae) and is also a replication intermediate of most other viruses, such as West Nile, respiratory syncytial, and influenza11,12; thus, TLR3 plays a key role in antiviral defense. TLR triggering of antigen-presenting cells (APCs) results in activation of these cells, as evidenced by the upregulation of maturation markers (e.g., CD83) and costimulatory molecules (e.g., CD86, CD80, and CD40).13 Functional differences in APCs and in the subsequent APC-T-cell responses may result from differences in the TLR triggered.
CD4+ T cells, both Th1 and Th17, are involved in the pathogenesis of animal models of uveitis. The polarization of infiltrating T cells into Th1 and Th17 subsets is contingent on the availability of proinflammatory cytokines, such as IL-12 for Th1 and TGF-β and IL-6 and IL-23 for Th17.14,15 Thus, the ability of RACs to sense ocular environmental cues and to translate them to T lymphocytes is important in inducing not only the expansion of cell populations but for CD4 T-cell polarization to phenotypes with either pathogenic or non-pathogenic potential.
In this study, we showed that RACs derived from B6 mice were able to recognize and respond to TLR ligands. Depending on the type of TLR ligand to which they were exposed, RACs expressed different levels of MHC II and costimulatory molecules, produced different amount of cytokines, and had different effects in promoting the polarization of CD4+ IRBP-specific T cells. Of the TLR ligands examined, a TLR3 ligand (namely, polyI:C) was most effective in activating RACs, leading to the production of cytokines of the Th1 and Th17 types that induce uveitis in naive mice.
Materials and Methods
Animals and Reagents
Pathogen-free female B6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Louisville. All animal studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Institutional approval was obtained, and institutional guidelines regarding animal experimentation were followed. Mouse TLR1–9 agonist kit was obtained from InvivoGen (catalog no. TLRL kit 1m; San Diego, CA). It included TLR1/2 agonist Pam3CSK4, TLR2 agonist HKLM (heat-killed Listeria monocytogenes), TLR3 agonist polyI:C, TLR4 agonist LPS-EK (Escherichia coli K12), TLR5 agonist ST-FLA (flagellin form Salmonella typhimurium), TLR6/2 agonist FSL1 (a synthetic lipoprotein representing the N-terminal part of the 44-kDa lipoprotein LP44 of Mycoplasma salivarium), TLR7 agonist ssRNA40, and TLR8 agonist ODN1826.
Isolation of Mouse Astrocytes
The isolation and culture of RACs have been reported previously.7 Briefly, eyes from B6 mice (age range, 4–6 weeks) were collected, and the connective tissue was removed. Then the eyes were immersed for 3 hours in Ca2+/Mg2+-free phosphate-buffered saline (PBS) containing 100 IU/mL penicillin and 100 μg/mL streptomycin on ice. Under a dissection microscope, the anterior segment and vitreous were removed and discarded, and the neural retina was incubated for 30 minutes at 37°C with 0.25% trypsin/1 mM EDTA (Invitrogen, Carlsbad, CA). Digestion was terminated by the addition of complete medium (CM) (RPMI 1640 medium containing 10% fetal bovine serum, 2 mM glutamax II, 100 IU/mL penicillin, and 100 μg/mL streptomycin; Sigma, St. Louis, MO). The cells were centrifuged at 400g for 5 minutes and then dissociated by gentle trituration through a fire-polished Pasteur pipette. After three washes, the cells were resuspended in CM and seeded on poly D-lysine-coated six-well plates at a density of 5 × 106 cells/well. After incubation for 30 days, the purity of the astrocytes was greater than 95%, as assessed by staining with antibody against glial fibrillary acidic protein (GFAP). Cells cultured for three to five passages were used in experiments.
Actively Induced and Adoptively Transferred Experimental Uveitis in B6 Mice
For active induction of disease, the animals were immunized subcutaneously with 100 μL emulsion containing human IRBP1–20 (300 μg) and 500 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in incomplete Freund’s adjuvant (Sigma), distributed over six spots on the tail base and flank. Concurrently, 0.2 μg pertussis toxin was injected intraperitoneally.
For adoptive transfer, recipient animals were injected intraperitoneally with 0.2 mL PBS containing 5 × 106 IRBP1–20 –specific T cells, prepared as described16,17 but using RACs as the APCs. The clinical course of the disease was assessed by indirect funduscopy twice a week and was graded as described previously. The pathology of the disease was confirmed by histology.17
Preparation of IRBP1–20–Specific T Cells
IRBP1–20–specific T cells were prepared from IRBP-immunized mice as described previously.16 Briefly, T cells were isolated from the lymph nodes or spleens of B6 mice at 13 days after immunization by passage through a nylon wool column. The cells (1 × 107) were stimulated with 20 μg/mL IRBP1–20 in 2 mL medium in a six-well plate (Costar, Cambridge, MA) in the presence of 2 × 107 irradiated syngeneic spleen cells as APCs. After 2 days, the activated lymphoblasts were isolated by gradient centrifugation (Lymphoprep; Robbins Scientific, Mountain View, CA) and were cultured in RPMI 1640 supplemented with 20 U/mL IL-2.
Generation of Bone Marrow–Derived Dendritic Cells
DCs were generated by the method of Lutz et al.18 with some modification. Femurs and tibiae of 6- to 8-week-old B6 mice were aseptically removed and cleared of surrounding muscle. Then the bones were cut and placed in cold medium, and the bone marrow was flushed out with a syringe. Bone marrow cells (1 × 106/well) in 1 mL CM containing 10 ng/mL granulocyte macrophage– colony-stimulating factor were plated in 24-well plates and fed every other day. On day 6, nonadherent cells were collected for phenotyping. The purity of the bone marrow DCs, determined by staining with anti-CD11c antibody and flow cytometry analysis, was greater than 95%.
Real-Time Quantitative RT-PCR Assay of TLR Expression
Total RNA from RACs was extracted using an RNA isolation kit (Invitrogen, Carlsbad, CA), treated with DNase I (GE Healthcare, Piscataway, NJ), and reverse transcribed into cDNA using an MMLV-RT kit (Invitrogen). Each cDNA sample was amplified for the gene of interest and β -actin (TaqMan assays; Mx3000P system; Stratagene, La Jolla, CA). The concentration of the gene of interest was determined using the comparative threshold cycle number and normalized to that of the internal β -actin control. Primers and probes used were: β -actin, forward primer, 5′-ATC-TACGAGGGCTATGCTCTCC-3′, reverse primer, 5′-ACGCTCGGTCAG-GATCTTCAT-3′, probe, 5′-CCTGCGTCTGGACCTG-GCTGGC-3′; TLR2, forward primer, 5′-TTCAACAAGATCACCTACATTGGC-3′, reverse primer, 5′-CAAGACTGCCCAGAGAAT-AAAAGG-3′, probe, 5′-TGACCTC-CGAGCGTGTGCGAACCT-3′; TLR3, forward primer, 5′-GGTGTTTCCA-GACAATTGGCAAG-3′, reverse primer, 5′-TGGAGGTTGTTGTAGGAA-AGATCG-3′, probe, 5′-CGCCCTCCTCTTGAACAACGCCCA-3′; TLR4, forward primer, 5′-CTGATGACATTCCTTCTTCAACC-3′, reverse primer, 5′-TTTCCTGTCAGTATCAAGT-TTGAG-3′, probe, 5′-AAGCCATGCCAT-GCCTTGTCTTCA-3′; and TLR9, forward primer, 5′-TATCCACCACCTG-CACAACTC-3′, reverse primer, 5′-GGCTCAGGCTCAGATTCACC-3′, probe, 5′-CGTCCACCTGTCCAACCTGCGGC-3′.
Coculture of Astrocytes and T Cells and Determination of Cytokines Released by the T Cells
The RAC monolayer was incubated for 48 hours with IRBP1–20 –specific T cells (3 × 105) in the presence of IRBP1–20, after which the culture supernatants were collected for cytokine assay by ELISA (R&D Systems, Minneapolis, MN). To exclude the possibility that the cytokines were produced by the astrocytes, rather than the T cells, the astrocytes were treated with mitomycin C (MMC; 100 μg/mL) for 1 hour at 37°C before they were mixed with the responder T cells.
Proliferation Assay
T cells from IRBP1–20 –immunized B6 mice were prepared and seeded at 4 × 105 cells/well in 96-well plates and cultured at 37°C for 72 hours in a total volume of 200 μL medium with or without IRBP1–20 in the presence of irradiated syngeneic spleen APCs (1 × 105) or MMC-treated RACs, and [3H]thymidine incorporation during the last 8 hours was assessed using a microplate scintillation counter (Packard Instrument/Perkin Elmer, Boston, MA). The proliferative response was expressed as the cpm (mean ± SD) of triplicate determinations.
Flow Cytometry Analysis
Astrocytes or T cells were incubated for 30 minutes at 4°C in staining buffer (PBS containing 3% FCS and 0.1% sodium azide) containing isotype-matched control IgG or phycoerythrin-conjugated antibodies against mouse TLR2, TLR3 (see below), TLR4, MHC class II molecules, CD80, CD86, or CD40 (eBioscience, San Diego, CA). For staining of TLR3 (an intracellular molecule), before antibody incubation, the cells were fixed and permeabilized using a kit (Cytofix/Cytoperm Plus; BD PharMingen, San Diego, CA) according to the manufacturer’s protocol. The cells were then washed, resuspended in staining buffer, and analyzed by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ) using appropriate software (CellQuest; BD Biosciences).
Statistical Analysis
Data are expressed as the mean ± SEM for the indicated number of individual experiments. Student’s t-test was used to analyze the results.
Results
Expression of TLR2, TLR3, and TLR4 mRNA and Protein by Cultured B6 RACs
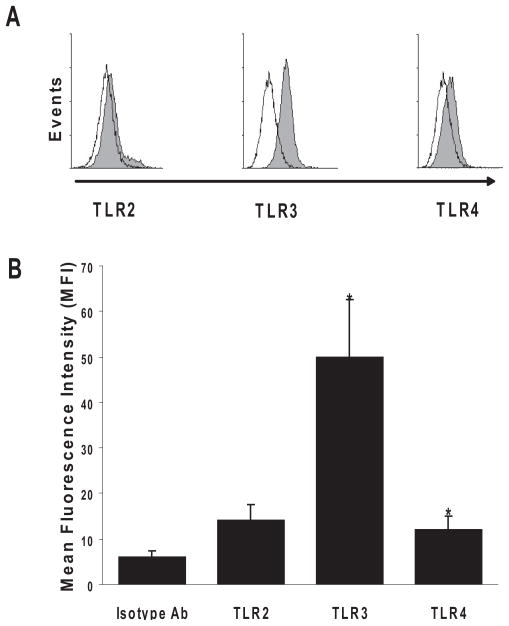
The parenchymal cells of the eye are known to express TLRs.19 To determine whether B6 RACs (GFAP-positive) expressed TLRs, we isolated RACs from the retina of naive B6 mice and cultured them in CM for 14 days, as described previously.7 With the use of RT-PCR assay to detect mRNA, we found the expression of TLR2, TLR3, and TLR4 (data not shown). However, analyzing their protein expression on RACs by flow cytometry revealed that TLR3 was highly expressed intracellularly, whereas TLR4 and TLR2 were weakly detected on the cell surface.
Increased Expression of MHC and Costimulatory Molecules on RACs after Exposure to TLR Ligands
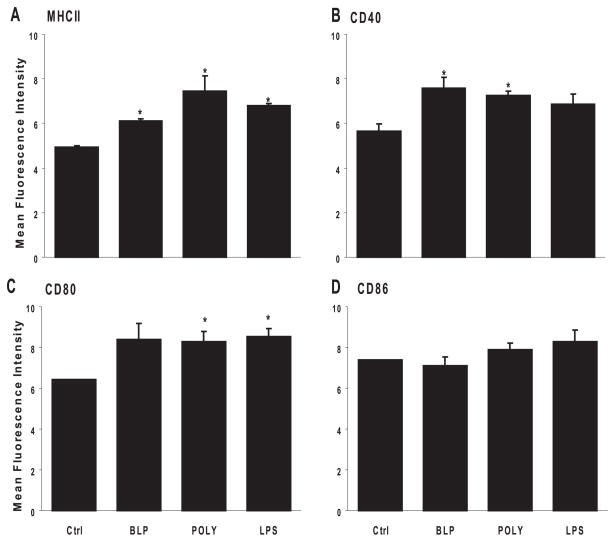
MHC and costimulatory molecules are required for T-cell activation. Therefore, we studied the expression of these molecules on RACs after exposure to TLR ligands. We treated RACs in vitro with BLP (a TLR2 ligand), dsRNA (poly:IC; a TLR3 ligand) or E. coli LPS (a TLR4 ligand) for 48 hours, then stained the cells for MHC and costimulatory molecules. RACs cultured in the absence of TLR ligands were included as controls. Figure 1 shows that untreated RACs expressed undetectable levels of MHC and costimulatory molecules and that exposure to the ligands mentioned significantly upregulated the expression of MHC class II molecules, CD40, and CD80 but not CD86. Each of these ligands had an approximately equal effect on expression.
Figure 1.
MHC class II and co-stimulatory molecule expression on TLR ligand-treated RACs. RACs (5 × 105) were incubated in medium alone or medium containing 0.1 μg/mL BLP, polyI:C, or LPS (respective ligands for TLR2, TLR3, or TLR4) for 48 hours, trypsinized, stained with monoclonal antibodies against MHC class II, CD80, CD86, or CD40, and analyzed by flow cytometry. Negative control samples were stained with an isotype-matched control mouse IgG antibody. Results are pooled fluorescence intensity data (mean ± SE) from three experiments. (A) MHC class II (control [Ctrl] vs. BLP, P = 0.001; Ctrl vs. PIC, P = 0.02; Ctrl vs. LPS, P < 0.001). (B) CD40 (Ctrl vs. BLP, P = 0.03; Ctrl vs. PIC, P = 0.02; Ctrl vs. LPS, P = 0.09). (C) CD80 (Ctrl vs. BLP, P = 0.007; Ctrl vs. PIC, P = 0.02; Ctrl vs. LPS, P = 0.006). (D) CD86 (Ctrl vs. BLP, P = 0.55; Ctrl vs. PIC, P = 0.18; Ctrl vs. LPS, P = 0.17). *P < 0.05.
Cytokine Production by RACs after Exposure to TLR Ligands
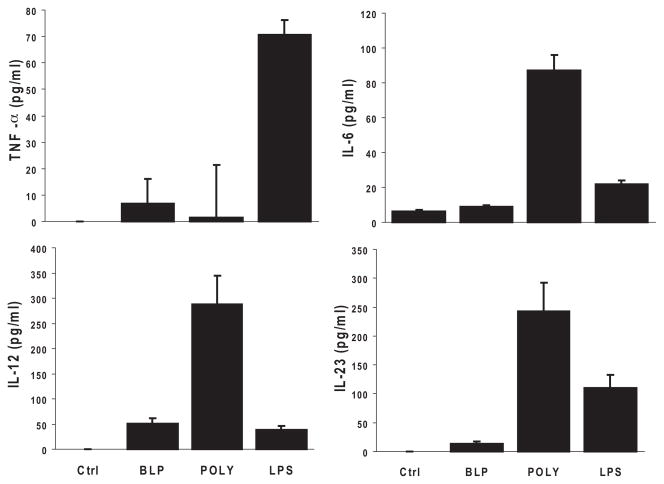
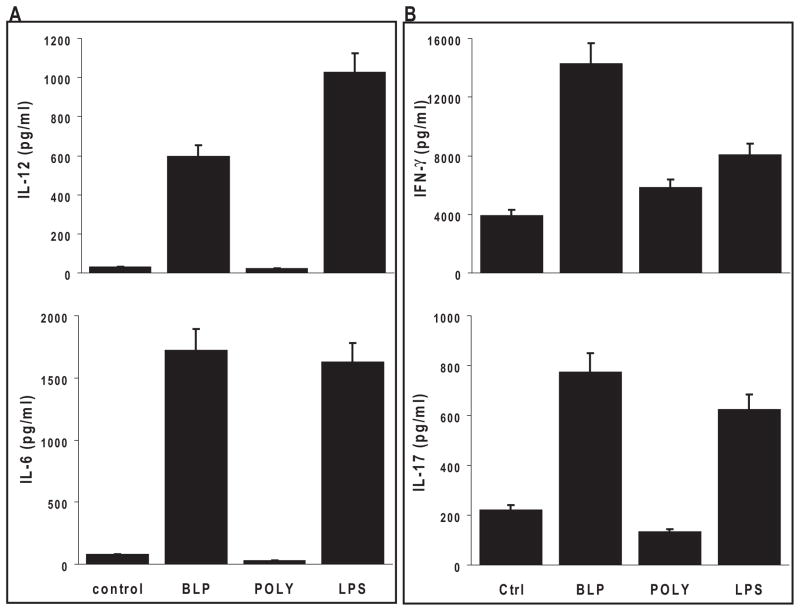
We next investigated the ability of RACs to produce cytokines known to affect the polarization of IRBP-specific T cells. The supernatant from the RACs was collected after 48-hour exposure to the different TLR ligands and was assayed for cytokine production. As shown in Figure 2, after the addition of LPS (TLR 4 ligand), RACs produced TNF-α and IL-23, whereas poly:IC (TLR 3 ligand) resulted in the production of IL-6, IL-12, and IL-23. In contrast, BLP (TLR 2 ligand) induced very low levels of cytokines compared with the other two.
Figure 2.
TLR ligands induced proinflammatory cytokine secretion by RACs. RAC cultures were treated for 24 hours with the TLR2, TLR3, or TLR4 ligand described in Figure 1, and then the cell supernatants were tested by ELISA for the cytokines TNF-α, IL-6, IL-12, and IL-23. Results are the mean ± SEM for three independent experiments, each in duplicate.
Effect of TLR Ligands on RACs Shapes IRBP-Specific T-Cell Polarization
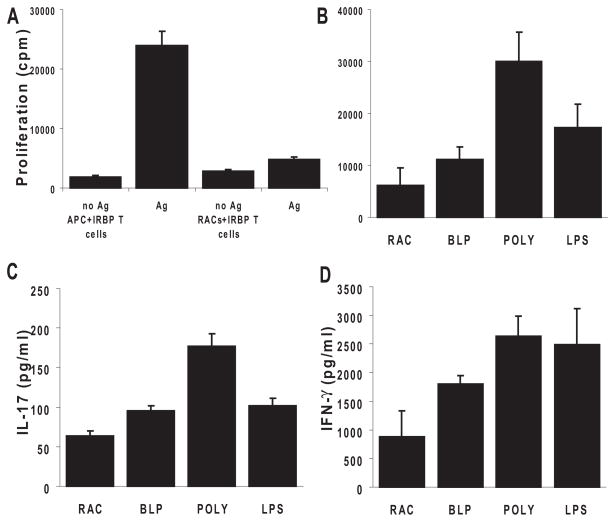
To investigate whether TLR ligand-exposed RACs gained the ability to increase the proliferation of IRBP-specific T cells, we cocultured B6 RACs with IRBP1–20 –specific T cells and assessed the proliferation of the T cells. Responder T cells isolated from the spleens of IRBP1–20 –immunized B6 mice were added to 96-well plates seeded with RACs pretreated with medium, BLP, polyI:C, or LPS and were incubated for 72 hours in the presence of IRBP1–20. For controls, the same number of T cells was incubated with IRBP1–20 in the presence of B6 splenic APCs. Untreated RACs had no T-cell stimulatory effect (Fig. 3A), but, after exposure to polyI:C (TLR3 ligand) or LPS (TLR4 ligand), the RACs greatly increased the proliferation of IRBP-specific T cells (Fig. 3B). PolyI:C-treated RACs were the most effective at stimulating T-cell proliferation; BLP had a much lower effect.
Figure 3.
RACs activated by TLRs shaped antigen-specific uveitogenic T-cell proliferation and differentiation into Th1 and Th17 cells. (A) Nonactivated RACs lack the ability to present antigen to IRBP-specific T cells compared with peripheral APCs. T cells (3 × 105) from in vivo IRBP1–20 –primed B6 mice were cultured with 1 × 105 syngeneic irradiated spleen cells or MMC-treated RACs in the presence of 10 μg/mL IRBP1–20; proliferation was measured by the incorporation of [3H]-thymidine (0.5–1 μCi/well) during the last 8 hours of the 72-hour incubation period. (B) PolyI:C-treated RACs were effective in presenting uveitogenic peptide, leading to the proliferation of IRBP1–20 –specific T cells. Cultured RACs were treated for 24 hours with TLR ligands (the same concentrations as in Fig. 1), washed, and MMC-treated before coculture with T cells. (C) TLR ligand-pre-treated RACs induced IRBP-specific T cells to produce proinflammatory cytokines. The experimental paradigm was as in (D). After 48 hours, the supernatants from triplicate cultures were pooled, and cytokines were measured by ELISA. Values are the mean ± SEM for three individual experiments.
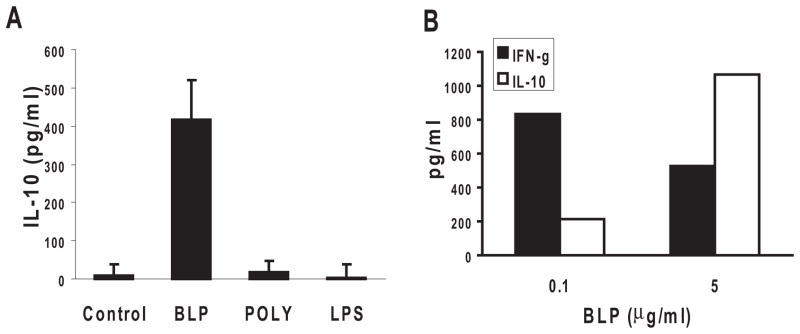
To determine whether TLR ligand-exposed RACs favored Th1, Th17, or Treg polarization of IRBP-specific T cells, supernatants from the cell cultures were collected after 48 hours, and the IFN-γ, IL-17, and IL-10 produced by the responder IRBP1–20 –specific T cells were measured by ELISA. As shown in Figures 3C and D, polyI:C-treated RACs strongly stimulated T-cell production of IL-17 and IFN-γ, whereas LPS-treated RACs had a similar effect on IFN-γ but not on IL-17 production. In contrast, BLP-stimulated RACs produced only limited amounts of IFN-γ and IL-17 (Figs. 3C, D) but significant amounts of IL-10 (Fig. 4A). The level of IL-10 production was dose dependent; a dose of 5 μg/mL of BLP resulted in a higher IL-10/IFN-γ ratio in the culture supernatant than a dose of 0.1 μg/mL (Fig. 4B).
Figure 4.
BLP pretreated RACs induced IRBP-specific T cells to produce IL-10. (A) The experimental paradigm was as in Figure 3B, and the supernatants from each culture were analyzed for IL-10 by ELISA. (B) Supernatants from cocultures of IRBP-specific T cells, IRBP1–20, and RACs pretreated with a low (0.1 μg/mL) or high (5 μg/mL) dose of BLP were tested for IFN-γ (filled columns) and IL-10 (hatched columns) by ELISA.
IRBP1–20–Specific T Cells Restimulated by PolyI:C or LPS-Pretreated RACs Induced Uveitis on Adoptive Transfer into Naive Mice
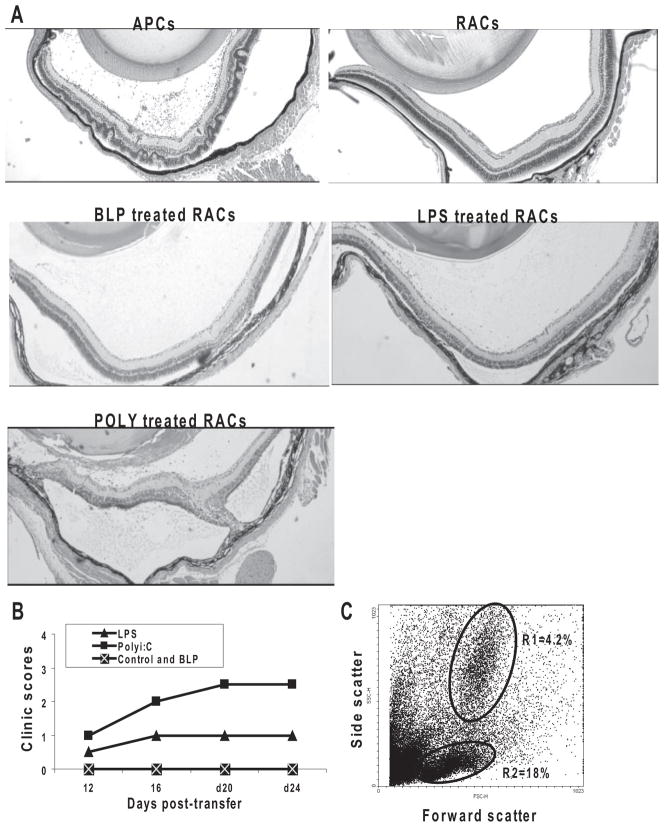
To determine whether IRBP-specific T cells gained disease-inducing ability after exposure to RACs pretreated with TLR ligands, IRBP-specific T cells were cocultured for 48 hours with RACs pretreated with BLP, polyI:C, or LPS, after which the T cells were separated from the RACs using gradient centrifugation and adoptively transferred into naive B6 mice. As shown in Figure 5, T cells stimulated by polyI:C-treated RACs induced severe and chronic uveitis, those stimulated with LPS-treated RACs were less effective, and those stimulated with BLP-treated RACs did not induce disease. Inflammatory cells in the eye of polyI:C-treated RACs on day 24 consisted of Gr-1–positive monocytes, polymorphonuclear leukocytes (gate R1), and T cells (gate R2). This result was similar to that in mice that received IRBP T cells stimulated with irradiated splenic APCs (data not shown).
Figure 5.
TLR pretreated RACs enhanced the disease-inducing ability of IRBP-specific T cells. IRBP1–20 –specific T cells from the draining lymph nodes and spleens of donor B6 mice immunized with IRBP1–20 were stimulated with IRBP1–20 and irradiated splenic APCs or RACs pre-treated with TLR ligands, as described in Figure 3B. T-cell blasts (3 × 106 cells/mouse) were then transferred to naive mice (n = 6/group). Disease severity was documented on day 24 after transfer by light microscopy (A). The course of ocular inflammation was recorded by funduscopy (B), and the number and type of eye-infiltrating cells on day 24 from mice adoptively were transferred with IRBP1–20 T cells (stimulated with RACs pretreated with polyI:C) by flow cytometry (C). The cytogram and gates for lymphocytes (gate R2) and monocytes/polymorphonuclear leukocytes (gate R1) are shown in the density plot. Numbers indicate the percentage of events in each gate.
Response of RACs to TLR Ligands Differs from Those of Bone Marrow DCs
To determine whether the response of RACs to TLR ligands was cell-type specific, we assessed the effect of TLR ligands on DCs prepared from the bone marrow of B6 mice. After 24-hour exposure to BLP, polyI:C, or LPS at the same concentration of 0.1 μg/mL used for RACs, the supernatants were collected for cytokine production and the DCs were harvested for coculture with responder IRBP-specific T cells. As shown in Figure 6A, in contrast to RACs (Fig. 2), DCs did not respond to polyI:C but gave a stronger response than RACs to BLP and LPS in the production of IL-12 and IL-6. As a result, polyI:C–pretreated DCs did not increase IL-17 and IFN-γ production by IRBP-specific T cells, whereas BLP- and LPS-pretreated DCs produced dramatic increases (Fig. 6B).
Figure 6.
PolyI:C had less effect on peripheral DCs than RACs. (A) Bone marrow– derived DCs were incubated for 24 hours in 12-well plates with 0.1 μg/mL BLP, polyI:C, or LPS. Then culture supernatants were collected and assayed for IL-6 and IL-12 by ELISA. (B) DCs, treated as in (A), were irradiated and cocultured with IRBP1–20 and responder T cells derived from IRBP1–20 –immunized mice. Supernatants were collected after 48 hours, and IL-17 and IFN-γ release by responder T cells into the supernatants was measured. Data are presented as the mean ± SEM of three experiments.
Discussion
The activation of invading autoreactive T cells inside the target organ is important in determining the clinical outcome of autoimmune inflammation. Recent findings have highlighted the role of TLRs in the activation of autoreactive T cells and their ability to trigger autoreactivity or the switch to autoimmunity.20,21 In this study, we investigated the role of ligands for TLR2, TLR3, and TLR4 in the activation of RACs and the effect on T cell–mediated immune responses. In vitro studies showed that TLR ligands could activate RACs to produce IL-12, IL-23, TNF-α, and IL-6 (Fig. 2), the cytokines known to direct the polarization of autoreactive T cells. TLR3 and TLR4 ligands induced the production of significant levels of IL-23 by RACs, though the effect of the TLR3 ligand (polyI:C) was stronger. The TLR3 ligand also induced higher levels of IL-12 production than either of the other two ligands. These results showed that RACs can be a rich source of IL-12 and IL-23.
Furthermore, incubation of IRBP-specific T cells with RACs preexposed to polyI:C led to the production of higher levels of IFN-γ and IL-17 by IRBP1–20 –specific T cells, and adoptive transfer of these cells into naive mice induced severe uveitis. IL-10 production by IRBP-specific T cells was also detected, but only in T cells cocultured with TLR2-exposed RACs (Figs. 3, 4). These results suggest that the polarization of autoreactive T cells toward Th1, Th17, and Treg cells may be regulated by RACs after exposure to different TLR ligands.
We have previously shown that RACs from B10RIII mice, which are highly susceptible to EAU induction, readily express MHC class II and costimulatory molecules and promote IRBP-specific T-cell expansion and differentiation, suggesting that such cells may play an important role in regulating T-cell activation in the eye. However, RACs from B6 mice, which are less susceptible to EAU induction, are less effective. In the present study, the addition of TLR ligands, especially TLR3, had a significant effect in upregulating the expression of MHC class II molecules and the costimulatory molecules CD80 and CD40 and the production of cytokines. This suggested that TLR ligands are more potent inducers of the APC function of RACs in B6 mice than proinflammatory cytokines. The lower susceptibility of B6 mice to autoimmune uveitis may be attributed to the relative resistance of B6 RACs to proinflammatory cytokines released by effector T cells. However, a variety of infectious pathogens may have the ability to stimulate B6 RACs and to contribute to the development of autoimmune uveitis.
The eye is an immune privileged site because of the existence of several mechanisms regulating the intraocular immune response. Self-reactive effector T or B cells may not be sufficient to induce autoimmune disease in the absence of additional “inflammatory signals.” Inflammation (e.g., systemic infection with bacteria or viruses) may upregulate levels of costimulatory molecules in the eye and may break this tolerance. It has been reported that IFN-β, sE-selectin, and sICAM-1 levels are elevated in patients with retinal vasculitis, idiopathic uveitis, and Behçet’s disease. These molecules can be induced in retinal vascular endothelial cells in vitro by TLR-3.22 We found that a ligand for TLR3, the receptor for dsRNA (Fig. 7), had the strongest stimulatory effect on RACs, suggesting that during viral infections RACs may become activated. Accumulating evidence also indicates that endogenous ligands, such as heat-shock proteins, extracellular matrix fragments, fibrinogen, and β-defensin, as well as mRNA released from damaged tissues or apoptotic cells, can recognize self-TLRs and, thus, play an important role in autoimmunity.20,23,24 Our finding that TLR3 signaling in RACs favors a polarized proinflammatory adaptive immune response raises the possibility that endogenous TLR3 ligands can exacerbate intraocular inflammation.
Figure 7.
RACs from B6 mice expressed TLRs. GFAP-positive RACs were tested for the expression of TLR2, TLR3, and TLR4 by flow cytometry. (A) TLR expression by RACs is shown by the shift in fluorescence intensity of the anti–TLR antibody–treated cells (filled curve) compared with isotype (control)–treated cells (empty curve). Data are representative of three experiments. (B) Pooled data from three experiments demonstrating TLR expression (mean fluorescence intensity [MFI] ± SE) above baseline levels (isotype control; [Baseline vs. TLR2, P = 0.56; Baseline vs. TLR3, P = 0.03; Baseline vs. TLR4, P = 0.02]). *P < 0.05.
We observed that TLR ligands had a different effect on RACs and DCs to influence cytokine production or the polarization of IRBP-specific T cells. TLR ligands have also been reported to differentially affect the uptake and cross-presentation of cellular antigens by monocyte-derived DCs.25 Compared with LPS or R-848, polyI:C is less efficient in inducing IL-12 production by myeloid-derived DCs.26 In the present study, polyI:C was less efficient in activating bone marrow-derived DCs, whereas a similar concentration of polyI:C strongly stimulated RACs. Thus, the same TLR ligand may have different effects on different immune cells, raising the question why differences exist between cell types stimulated with the same ligand, a finding also reported by Lundberg.27 One obvious reason for the differences between cell types is that they have very different functions in the immune system; DCs are professional APCs involved in T-cell activation, whereas RACs are nonprofessional immune cells. Given their diverse roles in the immune response, it makes sense that the same ligand would induce cell type-specific responses. The characterization of the responses of different cell types to different TLR ligands should increase our understanding of the contribution of each cell to the innate host response to infection or tissue damage.
Acknowledgments
The authors thank Tom Barkas for editorial assistance.
Supported in part by National Institutes of Health Grants NEI EY12974, EY14599, and Vision Research Infrastructure Development (R24 EY015636); Research to Prevent Blindness career development award (HS); the Commonwealth of Kentucky Research Challenge Trust Fund (HK); and an unrestricted grant from Research to Prevent Blindness.
Footnotes
Disclosure: G. Jiang, None; Y. Ke, None; D. Sun, None; Y. Wang, None; H.J. Kaplan, None; H. Shao, None
References
- 1.Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thurau SR, Mempel TR, Flugel A, et al. The fate of autoreactive, GFP+ T cells in rat models of uveitis analyzed by intravital fluorescence microscopy and FACS. Int Immunol. 2004;16:1573–1582. doi: 10.1093/intimm/dxh158. [DOI] [PubMed] [Google Scholar]
- 3.Ehlers S. Commentary: adaptive immunity in the absence of innate immune responses? The un-Tolled truth of the silent invaders. Eur J Immunol. 2004;34:1783–1788. doi: 10.1002/eji.200425250. [DOI] [PubMed] [Google Scholar]
- 4.Prinz M, Garbe F, Schmidt H, et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclerosis. J Clin Invest. 2006;116:456– 464. doi: 10.1172/JCI26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khayyamian S, Hutloff A, Buchner K, et al. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci U S A. 2002;99:6198– 6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butovsky O, Landa G, Kunis G, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J Clin Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang G, Ke Y, Sun D, Han G, Kaplan HJ, Shao H. Reactivation of uveitogenic T cells by retinal astrocytes derived from experimental autoimmune uveitis-prone B10RIII mice. Invest Ophthalmol Vis Sci. 2008;49:282–289. doi: 10.1167/iovs.07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barton GM, Medzhitov R. Toll-like receptors and their ligands. Curr Top Microbiol Immunol. 2002;270:81–92. doi: 10.1007/978-3-642-59430-4_5. [DOI] [PubMed] [Google Scholar]
- 9.Cristofaro P, Opal SM. Role of Toll-like receptors in infection and immunity: clinical implications. Drugs. 2006;66:15–29. doi: 10.2165/00003495-200666010-00002. [DOI] [PubMed] [Google Scholar]
- 10.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 12.Schroder M, Bowie AG. TLR3 in antiviral immunity: key player or bystander? Trends Immunol. 2005;26:462– 468. doi: 10.1016/j.it.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Groschel S, Piggott KD, Vaglio A, et al. TLR-mediated induction of negative regulatory ligands on dendritic cells. J Mol Med. 2008;86:443– 455. doi: 10.1007/s00109-008-0310-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakae S, Iwakura Y, Suto H, Galli SJ. Phenotypic differences between Th1 and Th17 cells and negative regulation of Th1 cell differentiation by IL-17. J Leukoc Biol. 2007;81:1258–1268. doi: 10.1189/jlb.1006610. [DOI] [PubMed] [Google Scholar]
- 15.Reiner SL. Development in motion: helper T cells at work. Cell. 2007;129:33–36. doi: 10.1016/j.cell.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Shao H, Fu Y, Song L, Sun S, Kaplan HJ, Sun D. Lymphotoxin beta receptor-Ig fusion protein treatment blocks actively induced, but not adoptively transferred, uveitis in Lewis rats. Eur J Immunol. 2003;33:1736–1743. doi: 10.1002/eji.200323745. [DOI] [PubMed] [Google Scholar]
- 17.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20 –specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Lutz MB, Kukutsch N, Ogilvie ALJ, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 19.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 21.Carpentier PA, Duncan DS, Miller SD. Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun. 2008;22:140–147. doi: 10.1016/j.bbi.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MT, Hooper LC, Kump L, et al. Interferon-beta and adhesion molecules (E-selectin and s-intracellular adhesion molecule-1) are detected in sera from patients with retinal vasculitis and are induced in retinal vascular endothelial cells by Toll-like receptor 3 signalling. Clin Exp Immunol. 2007;147:71–80. doi: 10.1111/j.1365-2249.2006.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rifkin IR, Leadbetter EA, Busconi L, Viglianti G, Marshak-Rothstein A. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 24.Lau CM, Broughton C, Tabor AS, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 26.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundberg AM, Drexler SK, Monaco C, et al. Key differences in TLR3/polyI:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007;110:3245–3252. doi: 10.1182/blood-2007-02-072934. [DOI] [PubMed] [Google Scholar]