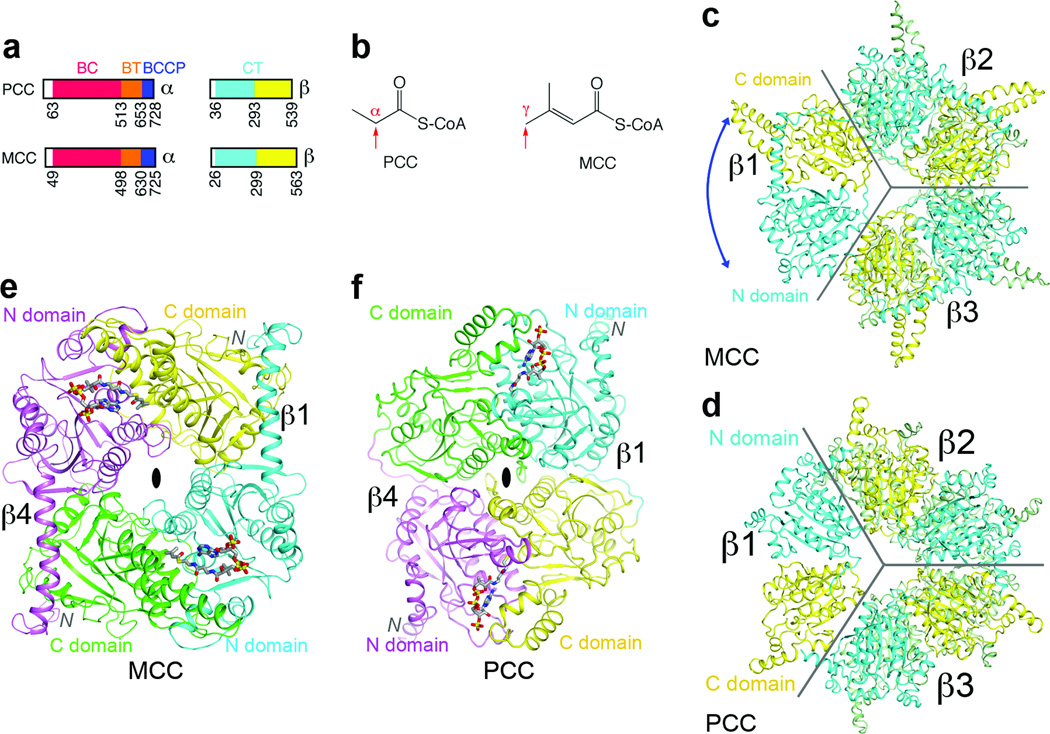
Figure 1. The domains of MCCβ are swapped compared to PCCβ.
(a). Domain organization of human MCC and PCC. (b). Distinct carboxylation targets of MCC and PCC, indicated by the red arrow. (c). Crystal structure of the β6 hexamer of PaMCC. The subunit beneath β1 is omitted for clarity, and the other two subunits in the bottom layer are colored in green. The blue arrow indicates the swapping of the positions of the N and C domains relative to PCCβ. Gray lines mark the boundaries of the subunits. (d). Structure of Roseobacter denitrificans PCCβ 9. (e). Structure of the β2 dimer of PaMCC. The N and C domains of the subunit in the bottom layer (β4) are colored in magenta and green, respectively. (f). Structure of the β2 dimer of PCC. All the structure figures were produced with PyMOL (www.pymol.org) unless stated otherwise.