Abstract
The annotated genome of Streptomyces coelicolor A3(2) revealed 18 cytosolic cytochromes P450 (CYPs) with six ferredoxin (fdx) proteins and two soluble ferredoxin reductases (fpr), their putative electron transport proteins. mRNA expression was observed for all 18 CYPs throughout growth and secondary metabolism, from 3 h after spore germination, and all CYP proteins examined also were present. Expression of members of the fdx complement was detected from the same time point, yet both fpr were detected only at 12 h. Six-hour exposure to dimethylbenzanthracene and benzo[a]pyrene xenobiotics resulted in the absence of some CYP mRNAs and expression of a specific fpr, FR2. This finding and the expression pattern during growth suggested that CYP activity may be regulated by availability of specific reductases. To test this proposal, we expressed in Escherichia coli and purified to homogeneity five CYPs: CYP105D5 (involved in xenobiotic metabolism) and CYP154A1, CYP154C1, CYP158A1, and CYP158A2 (putatively involved in secondary metabolism). Also the two soluble fpr (FR2 and FR3) proposed to shuttle electrons by means of fdx were purified, and specific interactions were observed so that FR2 preferentially reduced CYP105D5 (>90% reduction) compared with the other CYPs (>20% reduction), whereas FR3 preferentially reduced the other CYPs (>85% reduction) compared with CYP105D5 (>10%). Furthermore FR2 was shown to efficiently bind CYP105D5 and drive benzo[a]pyrene hydroxylation in contrast to FR3. These data show that control of CYP activity in S. coelicolor A3(2) involves specific interactions with fpr and their availability during the life cycle and, after xenobiotic exposure, represents a unique mechanism for regulating CYP function.
In contrast to most bacteria, Streptomyces species exhibit a multicellular life cycle. This complexity involves the formation of a mycelium, giving rise to aerial hyphae that differentiate into chains of reproductive spores. Preceding or simultaneously with the formation of the aerial hyphae and spores, a transition from primary to secondary metabolism occurs, resulting in production of an array of complex molecules. This process is highly regulated and involves specific regulatory and metabolic processes (for review see refs. 1 and 2). Some secondary metabolites provide defense against invading microorganisms such as bacteria and fungi, which compete for nutrients released from the degenerating substrate mycelium during sporulation (3). Medicine has taken advantage of this ability of streptomycetes to produce such secondary metabolites that account for over two-thirds of microbially derived therapeutics, including antibacterial, antifungal, antiviral, antiparasitic, antitumor, and immunosuppressant compounds (4). Structural diversity is observed among these compounds, in which common metabolic intermediates such as amino acids, carboxylic acids, and sugars are condensed into carbon skeletons, which then undergo final chemical tailoring. The latter is often by means of oxidative processes to produce the bioactive compound and involves cytochromes P450 (CYPs) (for review see ref. 5).
CYPs are a superfamily of heme-containing monooxygenases involved in a wide array of NADPH/NADH- and O2-dependent reactions (for review see refs. 6 and 7). In eukaryotes, CYPs have long been established as having a pivotal role in the biosynthesis of many physiologically important compounds such as steroid hormones and other sterols, vitamins, and eicosinoids (7). They also participate in the detoxification of diverse xenobiotic compounds (for review see ref. 8). Recently, whole-genome sequencing has revealed the extraordinary biodiversity of this superfamily of genes and enzymes throughout eukaryotes and prokaryotes (for review see refs. 9 and 10). In streptomycetes and other bacteria, CYPs are often located in macrolide antibiotic biosynthetic gene clusters where they catalyze stereo- and regio-specific oxidation of precursors leading to structural diversity among these molecules. This diversity has been shown for the antibacterial agents erythromycin (11), picromycin (12), and oleandomycin (13); the antifungal agent amphotericin (14); the antitumor agent epothilone (15); the antiparasitic agent avermectin (16); and the immunosuppressant rapamycin (17). The biological importance of the resulting hydroxyl and/or epoxide substituent introduced by the CYP often produces a significant increase in antibiotic potency. To date, specific functions of individual CYPs have been described in at least 21 different species of Streptomyces, and, in the majority of these, the individual CYP described is directly involved in biosynthesis of the antibiotic produced by that specific strain (http://drnelson.utmem.edu/CytochromeP450.html).
Streptomyces coelicolor A3(2) is the most studied streptomycete and is a model for genetic analysis of antibiotic production as well as for studying morphological changes during cell differentiation (for review see ref. 2). Recently, the genome sequence of S. coelicolor A3(2) was completed (4). Eighteen CYP sequences, six ferredoxin (fdx) sequences, and three ferredoxin reductase (fpr) sequences were tentatively revealed by sequence annotation (4) and characterized as the data were deposited (18). In the present work, we have utilized RT-PCR to monitor the expression of each CYP and reductase component throughout the life cycle of this organism to gain clues to CYP function. Further, through data described herein, we find that CYP transcription and translation are tightly coupled. Uniquely, we observed an expression profile whereby fpr emerges as a crucial component in the regulation of CYP activity. Furthermore, challenge of the organism with environmental xenobiotics resulted in the activation of a specific fpr gene, which in reconstituted CYP reduction experiments was shown to have a preference for CYP involved in xenobiotic metabolism over CYPs predicted to be involved in secondary metabolism. The importance of our findings is discussed in regard to the functional role of CYP in streptomycetes and for CYP biology in general.
Materials and Methods
Bacterial Strains and Media. Escherichia coli DH5α was used as a cloning host. Luria–Bertani medium was used in E. coli propagation. The S. coelicolor A3(2) culture was the M145 derivative of the wild-type A3(2) strain, the same as that used in the genome sequencing project (4). Fresh spores were collected and pregerminated as described (19). Cultures for RNA isolation were inoculated into yeast extract/malt extract (YEME) medium at ≈2 × 107 germinated spores per ml and grown at 30°C, 250 rpm on the Infors orbital shaker (19). Samples for RNA isolation were taken at specified time points (20).
Isolation of Total RNA from S. coelicolor A3(2) Spores, Germinated Spores, and Mycelium in the Absence and Presence of Xenobiotic. Spores, germinated spores, and mycelium were separated from broth by means of filtration and centrifugation and washed twice with water (19). Cell pellets were resuspended thoroughly in 10 mM Tris·HCl, pH 7.4, buffer containing 1 mM EDTA; lysozyme (3 mg/ml) was added and incubated for 10 min at room temperature. The lysate was added to Buffer RLT (Qiagen, Valencia, CA) containing 1% vol/vol 2-mercaptoethanol. The RNeasy Mini kit (Qiagen) was used for nucleic acid precipitation and isolation, according to the manufacturer's instructions. Nucleic acid preparations were treated with DNase I (Qiagen) to ensure complete removal of contaminating residual genomic DNA from purified RNA fractions. To monitor suppression and/or activation of CYP, fpr, and fdx gene expression in the presence of xenobiotics, benzo[a]pyrene (BAP) (dissolved in 100 mM dimethyl sulfoxide) and dimethylbenzanthracene (DMBA) (dissolved in 1.0 M dimethyl formamide) were used. Xenobiotics were added to germinated spore cultures at a final concentration of 0.5 mM, and RNA was extracted after 6 and 24 h of growth. Control cultures received solvent alone.
Gene Expression Analysis by RT-PCR. Transcript analysis was carried out by One Step RT-PCR and Q-solution (Qiagen) with 0.1 μg of total RNA as template. Conditions were as follows: first-strand cDNA synthesis, 50°C for 30 min followed by 94°C for 15 min; and amplification, 30 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 90 sec. Primers (www.aber.ac.uk/biology/research/wolfsonlab/web02.html) were designed to generate PCR products of the complete gene sequence for each CYP, fdx, and fpr. With each set of primers, negative controls were carried out with TaqDNA polymerase (Qiagen) in the absence of reverse transcriptase to confirm that amplified products were not derived from contaminating genomic DNA. Finally, the sequence of each amplified gene product was verified by DNA sequence analysis using one of the amplification primers. The CYP names were assigned by using the established nomenclature implemented by David Nelson, University of Tennessee, Memphis (18). For each fdx, assignments were applied for identification as follows: f1, SCO7110 (fdxA); f2, SCO5135 (fdxA1); f3, SCO7676; f4, SCO0773 (soyB2); f5, SCO3867 (soyB1); and f6, SCO1649. For each fpr, assignments were applied for identification as follows: FR1, SCO0681; FR2, SCO7117; and FR3, SCO2469.
Antibody Production and Protein Expression. Polyclonal antibodies were raised against S. coelicolor A3(2) CYP154A1, CYP154C1, CYP158A1, CYP158A2, and CYP105D5. CYPs were purified by two passes over Ni2+-nitrilotriacetic acid and one step of Q-Sepharose chromatography purification, and antibodies were commercially raised in ovine hosts (MicroPharm, Llandysul, United Kingdom). Antibody was purified from antiserum as described (21). Immunoprecipitation and Western analysis were carried out by standard procedures (22). S. coelicolor A3(2) cytosolic fraction was prepared from cell cultures grown for 6 h after germination. Cells were broken after two passages through the C5 homogenizer (Avestin, Ottawa) by using an operating pressure of 15,000 psi (1 psi = 6.89 kPa). The lysed cells were centrifuged at 10,000 × g to remove unbroken cells and cell debris. The cytosolic fraction was separated from the membrane fraction by ultracentrifugation at 100,000 × g for 1 h.
CYP, fdx, and fpr Purification. The ORFs of the CYPs, fdx, and fpr in the S. coelicolor A3(2) genome were cloned into the E. coli expression vector pET17b and heterologously expressed as described (18). Four histidine residues were engineered into the C terminus of the recombinant proteins for ease of purification. Three liters of culture expressing S. coelicolor CYPs CYP105D5, CYP154A1, CYP154C1, CYP158A1, and CYP158A2, S. coelicolor fdx, and fpr FR2 and FR3 were pelleted and resuspended in 200 ml of TES buffer (100 mM Tris·HCl, pH 7.5/10 mM sucrose/1 mM EDTA). FR1 is a putative secreted/membrane-associated protein and was excluded from consideration because bacterial CYP systems are soluble and cytosolic. After addition of lysozyme (0.5 mg/ml) and stirring at 4°C for 15 min, 1 vol of ice-cold water containing 0.1 mM EDTA was added slowly and continuously stirred for 30 min. Spheroplasts were pelleted at 3,000 × g, resuspended in 50 ml of 2-fold diluted TES buffer, and sonicated with a Branson sonifier. After centrifugation at 100,000 × g for 45 min, the recombinant protein was purified as described above.
Electron Donor System Investigation. Spectral analysis of fpr binding to CYP was measured as described (23). The split-cell technique (with purified CYP in one chamber of the sample and reference cuvettes and buffer A in the other chambers) was used. After recording the baseline, FR2 or FR3 was added incrementally to CYP in the sample cuvette and to the buffer in the reference cuvette. The subsequent spectrum was recorded between 350 and 500 nm. For catalytic function, CYPs require a source of reducing equivalents transferred to the CYP through ancillary reductase proteins. For prokaryotes, such as streptomycetes, such electrons are transferred from a FAD-containing fpr by means of a fdx to the CYP. Because the specific combination of individual fdx and fpr required to support individual CYPs of Streptomyces was unknown, the capacity of combinations of fdx/fpr to reduce Streptomyces CYP105D5, CYP154A1, CYP154C1, CYP158A1, and CYP158A2 was determined by the formation of the reduced CO spectrum by using methods previously described for other CYPs (18, 24, 25). Specific CYPs (200 pmol), fdx (2 nmol), and fpr (600 pmol) were incubated in 10 mM potassium phosphate, pH 7.4. After several cycles of degassing and bubbling with CO, NADPH (Sigma) was added (1 mM final concentration), and the reduced CO spectrum was recorded. Results were compared with Na2S2O4-induced CO difference spectra for each CYP, and the percentage efficiency of reduction was calculated.
BAP Oxidation Assays. Purified CYP105D5 (1 μM final concentration) and the fdx associated downstream in their operon (f4) were incubated with either purified FR2 or FR3 (P450/fdx/fpr ratio 1:10:3) at room temperature for 5 min. BAP and NADPH (1 mM final concentration) was added to initiate the reaction. Reactions were incubated for various times at 30°C and terminated by rapid freezing of the samples in dry ice/acetylaldehyde, and samples were extracted twice with ethyl acetate. Organic layers were transferred to clean tubes and dried under a N2 stream. BAP and metabolites were separated by HPLC (Waters) on a 25-cm Zorbax C18 column (5 mm particle size, 4.6 mm i.d.; DuPont) previously equilibrated with a methanol/5 mM potassium phosphate, pH 6.8, mixture (55:45 vol/vol). The methanol concentration was increased to 100% over 50 min with a linear gradient (flow rate, 1.0 ml·min–1) (26). For whole-cell metabolism, fresh spores were collected and pregerminated as described (19) and inoculated into yeast extract/malt extract liquid medium. BAP was added at a final concentration of 0.5 mM. After incubation at 30°C, 250 rpm on the Infors orbital shaker, for 6 or 24 h, reactions were terminated, extracted, and analyzed as described above.
General Methods. Na2S2O4-reduced CO difference spectra for quantification of CYP contents were measured and calculated according to the method of Omura and Sato (27). Protein quantification was performed with the bicinchoninic acid assay (Sigma). DNA sequencing was performed by using the Applied Biosystems PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit and Applied Biosystems PRISM 377 DNA Sequencer.
Results
Gene Expression Analysis. Total RNA was prepared from S. coelicolor A3(2) spores, germinated spores after 3 h of growth, and mycelium (72 h of growth after germination), i.e., both before and after the onset of secondary metabolism, and used for gene expression analysis by RT-PCR. Identification of PCR products was performed by DNA sequencing. Expression of HrdB, the major sigma factor of S. coelicolor A3(2), was used as a positive control. With each set of primers, negative control experiments in the absence of reverse transcriptase confirmed that amplified products were not generated by contaminating genomic DNA.
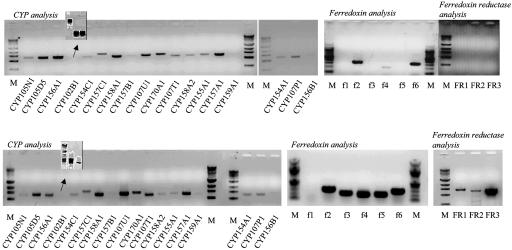
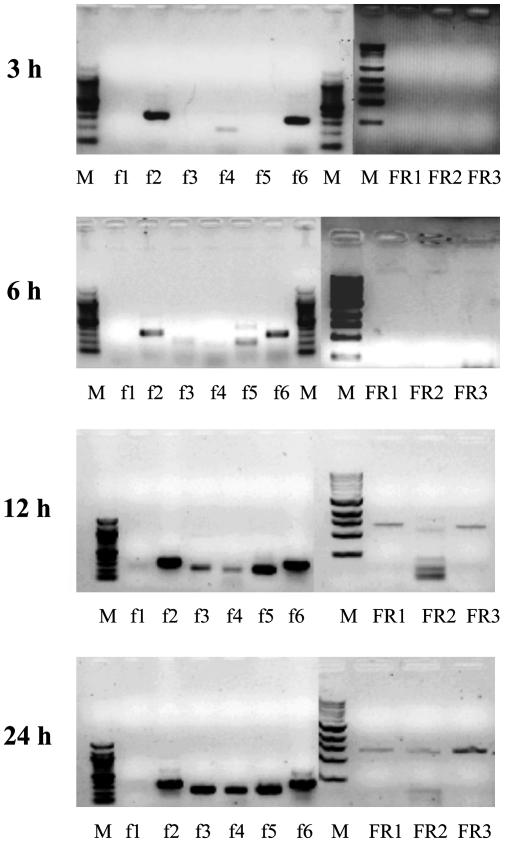
Transcripts for each CYP, fdx, and fpr at 3 h of growth after germination are shown in Fig. 1. Transcripts for each CYP were detected, except mRNA for CYP159A1. Subsequently, mRNA for CYP159A1 could not be detected at 3 h but was detectable at 6 h in two of four samples and always was seen at later times. Expression of CYP102B1 was detected subsequently by using three pairs of primers to produce short partial cDNAs (Fig. 1 Inset). In contrast, although fdx transcripts were detectable, no fpr could be detected until after 6 h (Fig. 2). At 12 h, fpr transcripts were detectable (Fig. 2), subsequent to the organism beginning the transition from primary to secondary metabolism (1, 2). This finding indicated that the CYP transcripts were expressed before their complementary reductase partners, which are essential for CYP activity. Expression of all of the 18 CYP genes early in the life cycle of S. coelicolor A3(2) was surprising and the absence of reductase mRNA was even more so. After 72 h of growth, well beyond the onset of secondary metabolism, transcripts for the 18 CYPs could still be detected. Transcripts for fdx 2–6 were clearly detected at this time, as were transcripts for each fpr (FR1, FR2, and FR3). These data showing the absence of reductase expression before the transition to secondary metabolism reflect a previously uncharacterized mechanism for regulation of CYP activity based on availability of the appropriate reductase.
Fig. 1.
Gene expression analyzed by RT-PCR for the whole CYP, fdx, and fpr complement of S. coelicolor A3(2). Total RNA that was extracted 3 h postgermination (Upper) and 72 h postgermination (after onset of secondary metabolism) (Lower) and used as PCR template is shown. Products were amplified and authenticated by sequencing. In negative controls, containing DNA polymerase but lacking reverse transcriptase, amplified products were not detected. M represents DNA molecular weight marker: 1 kb for CYP/fpr and 100 bp for fdx.
Fig. 2.
Time course of gene expression by RT-PCR for the fdx and fpr complement of S. coelicolor A3(2). Total RNA was extracted and used as a template with each pair of specific primers 3, 6, 12, and 24 h postgermination. Products were authenticated by sequencing. In negative controls, containing DNA polymerase but lacking reverse transcriptase, amplified products were not detected.
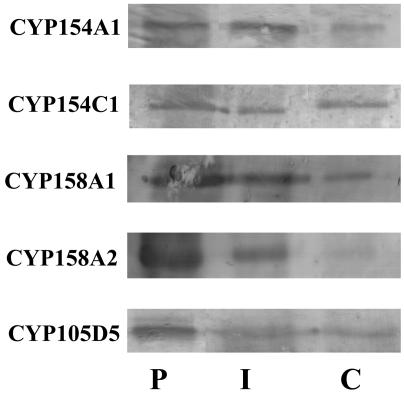
Detection of Protein Expression. As will be described below, BAP hydroxylation is observed when FR2 mRNA is detected, indicating close association of transcription and translation. Further, Fig. 3 demonstrates the presence of five CYP proteins within 6 h postgermination. CYP154A1 present in Fig. 3 also has been reported as present by 2D gel analysis (http://qbab.aber.ac.uk/s_coeli/referencegel) as has CYP156B1 and CYP157C1. Although not all 18 CYPs have been studied yet, this random analysis of 7 CYPs strongly argues for close temporal association of translation with transcription through the complete CYP/fdx/fpr complement in S. coelicolor A3(2).
Fig. 3.
Western blot analysis of CYP from S. coelicolor A3(2). Analysis of samples was carried out to detect the presence of individual CYPs by using purified recombinant CYP (lane P), immunoprecipitated CYP isolated from S. coelicolor A3(2) cytosolic extracts (lane I), and direct analysis of S. coelicolor A3(2) cytosolic extracts concentrated by Centricon-50 (Millipore) filtration (lane C).
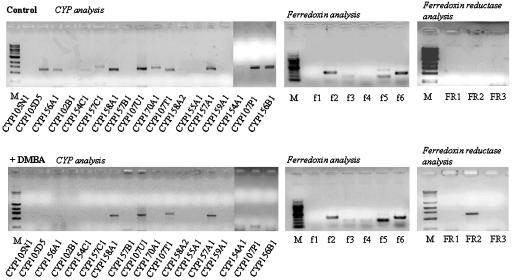
Specific mRNA Expression Analysis in the Presence of Xenobiotics. Germinated spores were exposed to the polycyclic aromatic hydrocarbons DMBA and BAP, and their effects on CYP, fdx, and fpr mRNA production/absence were compared with control samples in the absence of xenobiotic. After 6 h of DMBA treatment it was evident that the transcripts for seven CYPs (CYP105N1, CYP157C1, CYP154C1, CYP157B1, CYP158A2, CYP159A1, and CYP156B1) were absent (Fig. 4). A specific fpr transcript, FR2, was clearly expressed by 6 h, compared with control samples, by exposure of S. coelicolor A3(2) to DMBA (Fig. 4). In parallel experiments, treatment with BAP also altered the pattern of CYP and reductase transcript expression; absence of three CYP transcripts (CYP154C1, CYP157B1, and CYP159A1) compared with control was seen (www.aber.ac.uk/biology/research/wolfsonlab/index.html). Again, no differences in fdx transcript expression were observed. However, in an identical pattern to DMBA treatment, BAP exposure of cells led to the activation of FR2 by 6 h. These are clearly intriguing results, demonstrating that xenobiotic exposure had an effect on both CYP and reductase gene expression in S. coelicolor A3(2). The data also implicated FR2 as potentially a specific reductase partner for the functional activity of CYP(s) involved in the xenobiotic response.
Fig. 4.
Gene expression analysis by RT-PCR for the whole CYP, fdx, and fpr complement of S. coelicolor A3(2) after treatment of cells with DMBA. Total RNA, extracted from control cultures 6 h postgermination and from identical cultures exposed to 0.5 mM DMBA, was used as a template with each pair of specific primers. Products were authenticated by sequencing. In negative controls, containing DNA polymerase but lacking reverse transcriptase, amplified products were not detected.
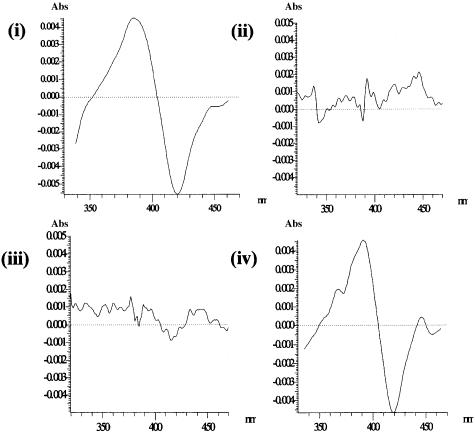
Spectral Analysis and Reduction of Purified Recombinant CYPs by Combinations of fdx and fpr. Initially, we investigated the binding of FR2 and FR3 to CYP105D5, CYP154A1, CYP154C1, CYP158A1, and CYP158A2 in solution by measuring their ability to induce a low- to high-spin change as has been observed with titrations of CYP17 with flavodoxin (23) and CYP11 with adrenodoxin (28). FR2 was able to induce a type I spin shift on CYP105D5 heme iron as observed at 386 and 420 nm. This result was in direct contrast to FR3, which had little effect in inducing a CYP105D5 spin change (Fig. 5); FR3 was shown to interact more favorably with a known secondary metabolizing CYP, being able to induce a type I spin shift on CYP158A2 heme iron as observed at 390 and 420 nm. In contrast, FR2 had little effect in inducing a CYP158A2 spin change (Fig. 5). Furthermore, we investigated reduction of the heme-iron of an S. coelicolor A3(2) xenobiotic CYP (CYP105D5) and putative secondary metabolizing CYPs (CYP154A1, CYP154C1, CYP158A1, and CYP158A2) by combinations of fdx and fpr to determine whether a specific fpr was used by specific CYPs and to relate to the transcript patterns observed. Each CYP had a typical reduced CO difference spectrum, as observed previously (18). In CO reduction experiments, CYP105D5 was efficiently reduced with FR2, ≈90% of the full reduction by Na2S2O4, with different isoforms of fdx interchangeable (Table 1) by using combinations of P450/fdx/FR2 (ratio of 1:10:3). CYP154A1, CYP154C1, CYP158A1, and CYP158A2 were poorly reduced in similar experiments utilizing FR2, with maximum reduction <15% of the full reduction generated by Na2S2O4. Titrations with increasing fdx and/or FR2 did not significantly increase reduction of the CYPs. In contrast, FR3 efficiently reduced CYP154A1, CYP154C1, CYP158A1, and CYP158A2 in combination with different fdx isoforms (>85% of the full reduction) but poorly reduced CYP105D5 (<15%). Altering the amounts of fdx and/or FR3 did not significantly increase their reduction.
Fig. 5.
Absorbance changes induced on titration of an S. coelicolor A3(2) xenobiotic P450, CYP105D5, and an S. coelicolor A3(2) P450 involved in secondary metabolism, CYP158A2, with specific S. coelicolor A3(2) fpr, FR2, and FR3. (i) Heme-spin change associated with the interaction of FR2, with CYP105D5. (ii) Heme-spin change associated with the interaction of FR2 with CYP158A2. (iii) Heme-spin change associated with the interaction of FR3 with CYP105D5. (iv) Heme-spin change associated with the interaction of FR3 with CYP158A2.
Table 1. CO-based reduction of purified recombinant S. coelicolor A3(2) CYPs by fpr (FR2 and FR3).
| CYP | FR2, % | FR3, % |
|---|---|---|
| CYP105D5 | 90 ± 5 | <10 |
| CYP154A1 | 10 ± 3 | 85 ± 7 |
| CYP154C1 | 15 ± 6 | 75 ± 5 |
| CYP158A1 | 10 ± 3 | 90 ± 6 |
| CYP158A2 | 10 ± 3 | 90 ± 5 |
Reduction is compared with 100%, determined by sodium hydrosulfite. Combinations of P450/fdx/FR2 or -FR3 in a ratio of 1:10:3 were used. Experiments were performed in triplicate.
Oxidation of BAP by S. coelicolor A3(2). Oxidation of BAP was examined by using whole-cell S. coelicolor A3(2) cultures incubated with the compound for 6 and 24 h. S. coelicolor A3(2) metabolized BAP, with 3-OH BAP being the only product observed (data not shown). No 4,5-dihydrodiol or 7,8-dihydrodiol metabolites of BAP were detectable at either time point. The extent of metabolism was ≈1% of the total BAP added. In reconstitution experiments with purified enzymatic components, CYP105D5 was shown to metabolize BAP more efficiently with FR2 as the terminal component of the reconstitution system (0.15 nmol of 3-OH BAP produced per min per nmol of CYP105D5) in comparison to FR3, which was inefficient at driving CYP105D5 activity (0.01 nmol of 3-OH BAP produced per min per nmol of CYP105D5). Saturating amounts of FR3 did not significantly increase catalytic activity in experiments, and omission of fdx from reconstitution assays saw FR2 activity fall to 10% of that reported.
Discussion
S. coelicolor A3(2) is the most studied member of the bacterial genus Streptomyces, which is important in medicine because its members produce over two-thirds of microbially derived therapeutics in current medical use. CYPs are important in the biosynthetic pathways of many of these compounds. We have used a RT-PCR approach to follow CYP and reductase message patterns during the complex life cycle of this organism. Our results indicate that CYP activity is regulated in this organism by the appearance of their necessary reductase partners at a specific time point, before the organism undergoes the transition from primary to secondary metabolism. This finding is in direct contrast to CYP mRNA, which was detected earlier in development. Furthermore, a single fpr gene, FR2, was shown to be activated during xenobiotic challenge of the cells and was highly selective in the reduction of the CYP (CYP105D5) believed to be involved in xenobiotic metabolism.
CYP activities in Streptomyces spp. have been reported widely during the last few years, in particular, their involvement in antibiotic production. Specific functions have been ascribed to CYPs in the biosynthesis of many different classes of medicinal compounds produced by Streptomyces; in antibacterial agent biosynthesis, e.g., SanQ in nikkomycin production (29); in antiparasitic agent biosynthesis, e.g., AveE in avermectin biosynthesis (30); in antifungal agent biosynthesis, e.g., amphL and amphN in amphotericin biosynthesis (14); in anti-HIV peptide biosynthesis, e.g., comI and comJ in complestatin biosynthesis (31); and in antitumor drug biosynthesis in the myxobacterium Sorangium cellulosum, e.g., epoK in epothilone biosynthesis (15, 32). Most of these CYPs have not yet been classified into the P450 nomenclature. In most instances the antibiotic gene clusters containing the CYP do not contain the reductase partners (fdx and fpr) necessary for CYP activity. The completed genome sequence for S. coelicolor A3(2) has revealed that the number of annotated reductase partners is low compared with the CYP number, three fpr and six fdx compared with 18 CYPs (4). This is not a unique feature among Streptomyces spp. The recently completed genome of the related organism Streptomyces avermitilis revealed six putative fpr and nine fdx to support the activities of the 33 CYPs present in this organism (33). Hence, it is probable that for Streptomyces spp. in general, combinations of a relatively small number of fdx and fpr are able to interact with and support the whole diversity of CYPs in each species.
As noted earlier, Streptomyces spp. have a complex life cycle compared with most other bacteria. Spores germinate leading to vegetative growth and mycelium. This germination is followed by aerial growth and the formation of a specialized sporulating mycelium. Microarray technology indicated that all S. coelicolor A3(2) CYP genes are expressed throughout the developmental cycle (20). Our data confirm this finding and show that all 18 CYPs are observed by 3–6 h of growth. What was most interesting from our results was the delayed timing of fpr gene expression relative to CYP gene expression, which suggested that the expression of the reductase controlled CYP activity in S. coelicolor A3(2). Although not all 18 CYP proteins have been analyzed yet, a close coupling between the appearance of CYP mRNA and protein is observed, which further emphasizes that P450 activity in S. coelicolor A3(2) is regulated by the presence of reductase. In studies on other, predominantly mammalian, systems, regulation of CYP activity occurred at the level of gene transcription, mRNA processing, mRNA stabilization, translation, and enzyme stabilization (for reviews see refs. 7, 34, and 35). Reductase levels have not previously been suggested to regulate CYP activity, and it is unclear why this strategy has been adopted by S. coelicolor rather than having specific CYPs expressed in response to particular needs, with the cognate reductases generally available. The streptomycete secondary metabolic pathways are highly complex, involving many regulatory genes and sigma factors, as revealed from the completed genome sequence (4). Regulating CYP activity so that it becomes available for relevant secondary metabolic pathways might have been expected a priori to be via a transcriptional activation of expression. CYP activity produced too early might lead to structurally altered substrates for the earlier stages of metabolism. However, for the CYPs in operons, their polycistronic expression is activated simultaneously, and so regulating enzyme activity by means of the presence of the necessary reductase seems to have evolved as a mechanism of cellular metabolic control in such pathways. However, Streptomyces are soil-dwelling bacteria (36) and living in such environments they can be challenged by the chemical products generated by other organisms or from their decay (as well as by man-made chemical pollutants). CYPs are known to be involved in such xenobiotic metabolism, and S. coelicolor A3(2) has developed a unique answer if challenged by such poisons. Exposure leads to the expression of a specific fpr gene, FR2, which in combination with fdx can support xenobiotic metabolism such as CYP105D5, whereas FR3 cannot effectively do so. To date, all Streptomyces spp. contain at least one member of the CYP105D subfamily, which has the remarkable capacity for broad-based xenobiotic metabolism, utilizing compounds of diverse structure and chemistry (37, 38).
In conclusion, in Streptomyces CYPs have been harnessed in defense mechanisms involving different antibiotics and other antimicrobial compounds in addition to having the ability to detoxify organic poisons. We propose a process of regulation of CYP activity that is coordinated at the level of fpr expression. Furthermore, it is shown that individual CYPs have a specific preference for a fpr depending on its particular function, e.g., secondary or xenobiotic metabolism. This finding is very different from eukaryotic CYP systems where a single reductase drives numerous CYPs (6). Understanding this mechanism of regulating CYP activity will be crucial in manipulating secondary metabolic pathways for the production of new and potent antibiotics by using streptomycetes as well as in biotransformation and bioremediation programs where CYPs are involved.
Acknowledgments
We thank Professor F. Peter Guenguerich (Vanderbilt University Medical School) for helpful discussions and advice and Professor David Hopwood (John Innes Centre, Norwich, United Kingdom) for continued interest in our work and critical reading of the manuscript. D.C.L. was a visiting fellow to Vanderbilt University Medical School sponsored by the Wellcome Trust. Support of the Biotechnology and Biological Sciences Research Council (D.C.L. and S.L.K.) and National Institutes of Health Grants GM37942 and ES00267 (to M.R.W.) is appreciated.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CYP, cytochrome P450; fdx, ferredoxin; fpr, ferredoxin reductase; BAP, benzo[a]pyrene; DMBA, dimethylbenzanthracene.
References
- 1.Chater, K. F. (1993) Annu. Rev. Microbiol. 47, 685–713. [DOI] [PubMed] [Google Scholar]
- 2.Hopwood, D. A. (1999) Microbiology 145, 2183–2203. [DOI] [PubMed] [Google Scholar]
- 3.Chater, K. F. & Merrick, M. J. (1979) in Developmental Biology of Prokaryotes, ed. Parish, J. H. (Blackwell Scientific, Oxford), pp. 93–114.
- 4.Bentley, S. D., Chater, K. F., Cerdeno-Tarraga, A. M., Challis, G. L., Thomson, N. R., James, K. D., Harris, D. E., Quail, M. A., Kieser, H., Harper, D., et al. (2002) Nature 417, 141–147. [DOI] [PubMed] [Google Scholar]
- 5.Carreras, C. W. & Santi, D. V. (1998) Curr. Opin. Biotechnol. 9, 403–411. [DOI] [PubMed] [Google Scholar]
- 6.Guengerich, F. P. (1991) J. Biol. Chem. 266, 10019–10022. [PubMed] [Google Scholar]
- 7.Ortiz de Montellano, P. R., ed. (1995) Cytochrome P450: Structure, Mechanism, and Biochemistry (Plenum, New York).
- 8.Guengerich, F. P. (2000) Drug Dev. Res. 49, 4–16. [Google Scholar]
- 9.Nelson, D. R., Koymans, L., Kamataki, T., Stegeman, J. J., Feyereisen, R., Waxman, D. J., Waterman, M. R., Gotoh, O., Coon, M. J., Estabrook, R. W., et al. (1996) Pharmacogenetics 6, 1–40. [DOI] [PubMed] [Google Scholar]
- 10.Nelson, D. R. (1999) Arch. Biochem. Biophys. 369, 1–10. [DOI] [PubMed] [Google Scholar]
- 11.Weber, J. M., Leung, J. O., Swanson, S. J., Idler, K. B. & McAlpine, J. B. (1991) Science 252, 114–117. [DOI] [PubMed] [Google Scholar]
- 12.Xue, Y., Wilson, D., Zhao, L., Liu, H. & Sherman, D. H. (1998) Chem. Biol. 5, 661–667. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez, A. M., Olano, C., Mendez, C., Hutchinson, C. R. & Salas, J. A. (1995) FEMS Microbiol. Lett. 127, 117–120. [DOI] [PubMed] [Google Scholar]
- 14.Caffrey, P., Lynch, S., Flood, E., Finnan, S. & Oliynyk, M. (2001) Chem. Biol. 8, 713–723. [DOI] [PubMed] [Google Scholar]
- 15.Tang, L., Shah, S., Chung, L., Carney, J., Katz, L., Khosla, C. & Julien, B. (2000) Science 287, 640–642. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda, H., Nonomiya, T., Usami, M., Ohta, T. & Omura, S. (1999) Proc. Natl. Acad. Sci. USA 96, 9509–9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar, I., Aparicio, J. F., Haydock, S. F., Khaw, L. E., Schwecke, T., Konig, A., Staunton, J. & Leadlay, P. F. (1996) Gene 169, 1–7. [DOI] [PubMed] [Google Scholar]
- 18.Lamb, D. C., Skaug, T., Song, H.-L., Jackson, C. J., Podust, L. M., Waterman, M. R., Kell, D. B., Kelly, D. E. & Kelly, S. L. (2002) J. Biol. Chem. 277, 24000–24005. [DOI] [PubMed] [Google Scholar]
- 19.Kieser, T., Bibb, M. J., Chater, K. F., Buttner, M. & Hopwood, D. A. (2000) Practical Streptomyces Genetics (John Innes Foundation, Norwich, U.K.).
- 20.Huang, J., Lih, C.-J., Pan, K.-H. & Cohen, S. N. (2001) Genes. Dev. 15, 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough, N. M. & Adams, J. M. (1978) Biochemistry 17, 5560–5566. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook, J., Fritsch, E. F. & Maniatis, T. (1989) Molecular Cloning: A Laboratory Manual (Cold Spring Harbor Lab. Press, Plainview, NY), 2nd Ed.
- 23.Jenkins, C. M. & Waterman, M. R. (1994) J. Biol. Chem. 269, 27401–27408. [PubMed] [Google Scholar]
- 24.Jenkins, C. M. & Waterman, M. R. (1998) Biochemistry 37, 6106–6113. [DOI] [PubMed] [Google Scholar]
- 25.Bellamine, A., Mangla, A. T., Nes, W. D. & Waterman, M. R. (1999) Proc. Natl. Acad. Sci. USA 96, 8937–8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer, E., Guo, Z., Ueng, Y.-F., Bell, L. C., Zeldin, D. & Guengerich, F. P. (1995) Chem. Res. Toxicol. 8, 136–142. [DOI] [PubMed] [Google Scholar]
- 27.Omura, T. & Sato, R. (1964) J. Biol. Chem. 239, 2379–2385. [PubMed] [Google Scholar]
- 28.Hanukoglu, L., Spitsberg, V., Bumpus, J. A., Dus, K. M. & Jefcoate, C. R. (1981) J. Biol. Chem. 256, 4321–4328. [DOI] [PubMed] [Google Scholar]
- 29.Zeng, H., Tan, H. & Li, J. (2002) Curr. Microbiol. 45, 175–179. [DOI] [PubMed] [Google Scholar]
- 30.Pang, C. H., Matsuzaki, K., Ikeda, H., Tanaka, H. & Omura, S. (1995) J. Antibiot. 48, 59–66. [DOI] [PubMed] [Google Scholar]
- 31.Chiu, H. T., Hubbard, B. K., Shah, A. N., Eide, J., Fredenburg, R. A., Walsh, C. T. & Khosla, C. (2001) Proc. Natl. Acad. Sci. USA 98, 8548–8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Julien, B. & Shah, S. (2002) Antimicrob. Agents Chemother. 46, 2772–2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb, D. C., Ikeda, H., Nelson, D. R., Ishikawa, J., Skaug, T., Jackson, C. J., Omura, S., Waterman, M. R. & Kelly, S. L. (2003) Biochem. Biophys. Res. Commun. 307, 610–619. [DOI] [PubMed] [Google Scholar]
- 34.Waxman, D. J. (1999) Arch. Biochem. Biophys. 369, 11–23. [DOI] [PubMed] [Google Scholar]
- 35.Honkakoski, P. & Negishi, M. (2000) Biochem. J. 347, 321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodgson, D. A. (2000) Adv. Microb. Physiol. 42, 47–238. [DOI] [PubMed] [Google Scholar]
- 37.Trower, M. K., Lenstra, R., Omer, C., Buchholz, S. E. & Sariaslani, F. S. (1992) Mol. Microbiol. 6, 2125–2134. [DOI] [PubMed] [Google Scholar]
- 38.Taylor, M., Lamb, D. C., Cannell, R., Dawson, M. & Kelly, S. L. (1999) Biochem. Biophys. Res. Commun. 263, 838–842. [DOI] [PubMed] [Google Scholar]