Abstract
Defects in O-mannosylation of α-dystroglycan are thought to cause certain types of congenital muscular dystrophies with neuronal migration disorders. Among these muscular dystrophies, Walker–Warburg syndrome is caused by mutations in the gene encoding putative protein O-mannosyltransferase 1 (POMT1), which is homologous to yeast protein O-mannosyltransferases. However, there is no evidence that POMT1 has enzymatic activity. In this study, we first developed a method to detect protein O-mannosyltransferase activity in mammalian cells. Then, using this method, we showed that coexpression of both POMT1 and POMT2 (another gene homologous to yeast protein O-mannosyltransferases) was necessary for the enzyme activity, but expression of either POMT1 or POMT2 alone was insufficient. The requirement of an active enzyme complex of POMT1 and POMT2 suggests that the regulation of protein O-mannosylation is complex. Further, protein O-mannosylation appears to be required for normal structure and function of α-dystroglycan in muscle and brain. In view of the potential importance of this form of glycosylation for a number of developmental and neurobiological processes, the ability to assay mammalian protein O-mannosyltransferase activity should greatly facilitate progress in the identification and localization of O-mannosylated proteins and the elucidation of their functional roles.
Muscular dystrophies are genetic diseases that cause progressive muscle weakness and wasting (1, 2). The causative genes of several muscular dystrophies have been identified in the past 15 yr. The best known is the one described by Duchenne that results from mutations in the gene encoding a protein called dystrophin. Another subclass is congenital muscular dystrophies, where muscle weakness is apparent at birth or shortly afterward. Recent data suggest that aberrant protein glycosylation of a specific glycoprotein, α-dystroglycan (α-DG), is the primary cause of some forms of congenital muscular dystrophy (3, 4).
DG is encoded by a single gene and cleaved into two proteins, α- and β-DG, by posttranslational processing (5). In skeletal muscle, DG is a component of the dystrophin–glycoprotein complex. α-DG is an extracellular peripheral membrane glycoprotein anchored to the cell membrane by binding to β-DG, which is a transmembrane glycoprotein. The α-DG–β-DG complex is expressed in a broad array of tissues and is thought to act as a transmembrane linker between the extracellular matrix and intracellular cytoskeleton. This is because α-DG binds to laminin, and the intracellular domain of β-DG interacts with dystrophin in skeletal muscle (4, 6). α-DG is heavily glycosylated, and its sugars have a role in binding to laminin, neurexin, and agrin (4, 7, 8). We previously found that the glycans of α-DG include O-mannosyl oligosaccharides, and that a sialyl O-mannosyl glycan, Siaα2–3Galβ1–4GlcNAcβ1–2Man, is a laminin-binding ligand of α-DG (9). Mammalian O-mannosylation is an unusual type of protein modification that was first identified in chondroitin sulfate proteoglycans of brain (10–12) and is present in a limited number of glycoproteins of brain, nerve, and skeletal muscle (9, 13–17).
Muscle–eye–brain disease [MEB; Online Mendelian Inheritance in Man (OMIM) no. 253280] is an autosomal recessive disorder characterized by congenital muscular dystrophy, ocular abnormalities, and brain malformation (type II lissencephaly) (18). We previously reported that MEB is caused by mutations in the gene encoding POMGnT1 (UDP-N-acetylglucosamine: protein O-mannose β1,2-N-acetylglucosaminyltransferase 1). POMGnT1 is responsible for the formation of the GlcNAcβ1–2Man linkage of O-mannosyl glycan, and all mutations cause a loss of enzyme activity (19–21). Like MEB, Fukuyama-type congenital muscular dystrophy (FCMD; OMIM no. 253800) (22) and Walker–Warburg syndrome (WWS; OMIM no. 236670) (23) are autosomal recessive disorders characterized by congenital muscular dystrophy, lissencephaly, and eye anomalies. FCMD and WWS are caused by mutations in the genes fukutin (24) and protein O-mannosyltransferase 1 (POMT1) (25). POMT1 encodes a protein that is homologous to members of the family of protein O-mannosyltransferases (PMTs, in vertebrates named POMTs) in yeast (26). In yeast, PMTs catalyze the transfer of a mannosyl residue from dolichyl phosphate mannose (Dol-P-Man) to Ser/Thr residues of certain proteins (27). However, in mammals, there is no evidence that POMT1 has this activity, and its function remains unknown. The function of fukutin is also unknown, although an analysis of its sequence predicts that it is involved in modifying cell surface glycans (28).
Recently, 20% of WWS patients (6 of 30 unrelated WWS cases) were found to have mutations in POMT1. POMT1 is highly expressed in fetal brain, testis, and skeletal muscle (26), which are the tissues affected in WWS. It is noteworthy that none of the 30 cases studied had mutations in another homologue, POMT2, which is 33% identical to POMT1 (25, 29). POMT2 was found not to have POMT activity (29). Thus, POMT activity of POMTs has not yet been detected in vertebrates. Determining whether POMT1 is involved in the biosynthesis of O-mannosyl glycans will help in understanding the relationship between protein O-mannosylation and congenital muscular dystrophies with brain malformation.
In this study, we developed a method to detect the enzymatic activity of POMT in mammalian cells and tissues. Using this method, we examined whether POMT activity is encoded by the POMT1 gene and/or the POMT2 gene after expression of POMT1, POMT2, or both. We found that POMT activity was significantly increased in cells cotransfected with both POMT1 and POMT2 but not in cells transfected with POMT1 or POMT2 alone.
Methods
Construction of Expression Plasmids. Human cDNAs encoding POMT1 (26) and POMT2 (29) were obtained by RT-PCR and 5′-RACE. RT-PCR was carried out by an Access RT-PCR kit (Promega) with brain total RNA (Clontech). 5′-RACE was carried out by nested PCR by using Human Brain and Kidney Marathon-Ready cDNAs (Clontech). The myc-tag sequence (GGPEQKLISEEDLNS) was fused to the C terminus of POMT1. DNA sequences were confirmed by an ABI PRISM 377 DNA sequencer (Perkin–Elmer Japan, Yokohama, Japan) with the Thermo Sequenase II Dye Terminator Cycle Sequence kit (Amersham Pharmacia Biosciences). The cDNAs encoding POMT1-myc and POMT2 were inserted into a mammalian expression vector, pcDNA3.1 Zeo(–) (Invitrogen) and pcDNA3.1 Hygro(+) (Invitrogen), respectively.
Using netoglyc 2.0 (www.cbs.dtu.dk/services/NetOGlyc), we predicted that potential O-glycosylation sites of α-DG are clustered in the region corresponding to amino acids 313–483 (30). This region does not have any potential N-glycosylation sites. We amplified this region from mouse genomic DNA by PCR by using the primer set 5′-GGGAATTCCACGCCACACCTACAC-3′ (sense) and 5′-GGGTCTAGAACTGGTGGTAGTACGGATTCG-3′ (antisense) and subcloned it into the pGEX-4T-3 vector to express the peptide as a GST-fusion protein (Amersham Pharmacia Biosciences). The correctness of the subcloned sequence was confirmed by DNA sequencing.
Preparation of GST-α-DG. BL21(DE3) Escherichia coli cells were transformed with the expression plasmid of GST-α-DG. Cultures were grown at 37°C to A620 = 0.5. At this point, 1 mM isopropyl-d-thiogalactopyranoside was added to the culture to induce GST-α-DG expression. The induced cells were grown in parallel for an additional4hat37°C, harvested by centrifugation at 6,000 × g for 15 min at 4°C, suspended in 10 ml of PBS, pH 7.4, and broken with a tip-type sonicator. The cell supernatant was recovered by ultracentrifugation at 100,000 × g for 1 h, loaded onto a glutathione-Sepharose column (GSTrap, 1 ml, Amersham Pharmacia Biosciences), and washed with PBS. The absorbed recombinant GST-α-DG proteins were eluted with 10 mM reduced glutathione in PBS at a flow rate of 1 ml/min and dialyzed with 50 mM (NH4)HCO3, pH 7.0. Protein concentration was determined by BCA assay (Pierce), and the purity of GST-α-DG was confirmed by SDS/PAGE visualized with Coomassie brilliant blue R-250 and by Western blotting with anti-GST mAb (GST-2) (Sigma–Aldrich).
Cell Culture and Preparation of Cell Extracts. Human embryonic kidney 293T (HEK293T) cells were maintained in DMEM (Invitrogen) supplemented with 10% FBS (Invitrogen)/2 mM l-Gln/100 units/ml penicillin/50 μg/ml streptomycin at 37°C with 5% CO2. The cells were homogenized in 10 mM Tris·HCl, pH 7.4/1 mM EDTA/250 mM sucrose/1 mM DTT, with a protease inhibitor mixture (3 μg/ml pepstatin A/1 μg/ml leu-peptin/1 mM benzamidine-HCl/1 mM PMSF). After centrifugation at 900 × g for 10 min, the supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h. The precipitate was used as the microsomal membrane fraction. Protein concentration was determined by BCA assay.
Expression of Human POMT1 and POMT2. The expression plasmids of human pcDNA3.1-POMT1-myc and pcDNA3.1-POMT2 were transfected into HEK293T cells by using LipofectAMINE PLUS reagent (Invitrogen), according to the manufacturer's instructions. The transfected cells were cultured for 3 days in complete medium, harvested, and homogenized.
Antibodies and Western Blot Analysis. Rabbit antiserum specific to the human POMT2 was produced by using a synthetic peptide corresponding to residues 390–403 (HNTNSDPLDPSFPV) of POMT2. A Cys residue was added to the N terminus of the POMT2 synthetic peptide (390–403 aa), so that the antigenic peptide could be conjugated to keyhole limpet hemocyanin (KLH). Rabbits were immunized with the antigenic peptide-KLH conjugate. Anti-myc mAb was purchased from Invitrogen. The microsomal fractions (20 μg) were separated by SDS/PAGE (10% gel), and proteins were transferred to a poly(vinylidene difluoride) membrane. The membrane, after blocking in PBS containing 5% skim milk and 0.5% Tween 20, was incubated with each Ab, and then the membrane was treated with anti-rabbit or anti-mouse IgG conjugated with horseradish peroxidase (Amersham Pharmacia Biosciences). Proteins bound to Ab were visualized with an enhanced chemiluminescence kit (Amersham Pharmacia Biosciences).
Assay for POMT Activity. Assays for POMT activity were carried out in a 20-μl reaction volume containing 20 mM Tris·HCl (pH 8.0), 100 nM Dol-P-[3H]Man (125,000 dpm/pmol) (American Radiolabeled Chemicals, St. Louis), 2 mM 2-mercaptoethanol, 10 mM EDTA, 0.5% n-octyl-β-d-thioglucoside (DOJINDO, Kumamoto, Japan), 10 μg of GST-α-DG, and 80 μg of microsomal membrane fraction. The reaction was initiated by adding the protein extract. After 1-h incubation at 28°C, the reaction was stopped by adding 200 μl of PBS containing 1% Triton X-100, and the reaction mixture was centrifugated at 10,000 ×g for 10 min. The supernatant was removed, mixed with 400 μl of PBS containing 1% Triton X-100 and 10 μl of glutathione-Sepharose 4B beads (Amersham Pharmacia Biosciences), rotated at 4°C for 1 h, and washed three times with 20 mM Tris·HCl (pH 7.4) containing 0.5% Triton X-100. The radioactivity adsorbed to the beads was measured by using a liquid scintillation counter. The incorporation of radioactive mannose into GST-α-DG was detected by SDS/PAGE and subsequent autoradiography.
To characterize the linkage of the mannosyl residue, the radioactive products absorbed to the glutathione-Sepharose beads were incubated with jack bean-α-mannosidase (0.8 units) (Seikagaku, Tokyo) in 50 μl of 0.1 M ammonium acetate buffer (pH 4.5) containing 1 mM ZnCl2 at 37°C. Jack bean-α-mannosidase (0.8 units) was added freshly every 24 h and incubated for up to 60 h. Inactivated jack bean-α-mannosidase, prepared by heating the enzyme (100°C, 5 min), was used a control. After incubation, the radioactivities of the supernatant and the beads were measured by using a liquid scintillation counter.
Results
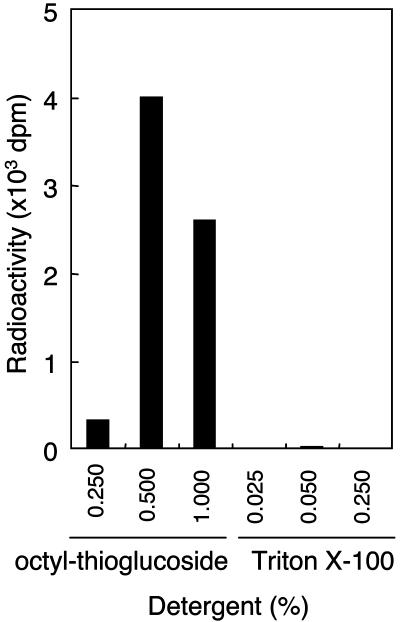
Detection of Transferase Activity of Mannose from Dol-P-Man to GST-α-DG. Initially, we attempted to detect mannose transferase activity based on an assay for POMT activity in yeast using several synthetic peptides and Triton X-100 as a detergent (31, 32). However, we did not detect any activity in several mammalian tissues and cells, possibly due to the specificity of the acceptor peptide sequence. If the enzyme recognizes a specific amino acid sequence, α-DG may be a suitable acceptor, because α-DG has O-mannosyl glycan (9, 17). Therefore, we prepared a GST fusion protein of α-DG (GST-α-DG) for a candidate acceptor by using an E. coli expression system. However, using the GST-α-DG as an acceptor and the Dol-P-Man as a donor substrate, we still did not observe any enzymatic activity. Next, we examined the effect of detergent because yeast PMTs were integral membrane proteins and thus hydrophobic proteins (27). Because Triton X-100 is a nonionic detergent, we examined several ionic and ampholytic detergents, including alkylglycosides. We found that n-octyl-β-d-thioglucoside was most effective, and its optimal concentration was 0.5% (wt/vol) (Fig. 1). Accordingly, 0.5% of n-octyl-β-d-thioglucoside was used in the following enzyme assays.
Fig. 1.
Effect of detergents on POMT activity. POMT activity was measured in a 20-μl reaction volume containing 20 mM Tris·HCl (pH 8.0), 100 nM Dol-P-[3H]Man (125,000 dpm/pmol), 2 mM 2-mercaptethanol, 10 mM EDTA, 10 μg of GST-α-DG, and 80 μg of HEK293T cell microsomal membrane fraction in the presence of n-octyl-β-d-thioglucoside or Triton X-100. The reaction was initiated by adding the protein extract and continued at 28°C for 1 h. After incubation, GST-α-DG was separated by glutathione-Sepharose beads and then the incorporated [3H]Man to GST-α-DG was measured with a liquid scintillation counter.
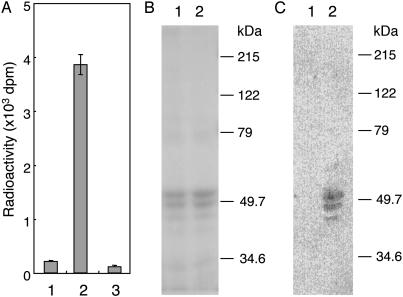
Next, we confirmed that a mannose residue was transferred from Dol-P-Man to GST-α-DG. As shown in Fig. 2A, the radioactivity of [3H]Man was incorporated into the glutathione-Sepharose beads in the presence of both the membrane fraction and an acceptor (lane 2), but the radioactivity was not incorporated without the membrane fraction (lane 1) or without the acceptor (lane 3). GST-α-DG eluted from the glutathione-Sepharose beads was detected as triplet bands at ≈50 kDa in the presence or absence of the membrane fraction by SDS/PAGE (Fig. 2B). Because all bands were stained with the anti-GST Ab, the largest molecular-weight band was thought to be the full-length GST-α-DG, and the smaller bands were probably fragments of degraded GST-α-DG. We observed significant incorporation of radioactive mannose into all GST-α-DGs in the presence of the membrane fraction (Fig. 2C, lane 2). No radioactivity was observed when GST-α-DG was incubated with GDP-[3H]Man instead of Dol-P-[3H]Man. Based on these results, we concluded that the mannose donor was Dol-P-Man and not GDP-Man.
Fig. 2.
POMT activity in HEK293T cells. (A) Incorporation of [3H]Man into GST-α-DG. Lane 1, incubation with GST-α-DG but without membrane fraction; lane 2, incubation with membrane fraction and GST-α-DG; lane 3, incubation with membrane fraction but without GST-α-DG. (B) After incubation, a reaction mixture oflane1or2in A was subjected to SDS/PAGE (10% gel) and was stained by Coomassie. Lane 1, corresponds to lane 1 of A, and lane 2 corresponds to lane 2 of A. (C) Autoradiography of B. POMT activity was based on the rate of mannose transfer to GST-α-DG using the membrane fractions from HEK293T cells. The products were recovered by the glutathione-Sepharose beads. Incorporation of [3H]Man into GST-α-DG was measured with a liquid scintillation counter. Molecular mass standards are shown to the right of B and C.
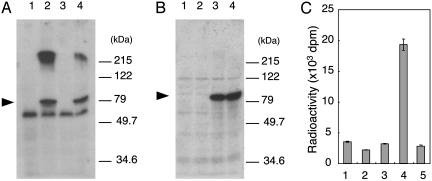
POMT Activity of Human POMT1 and POMT2. The mammalian POMT1 and POMT2 genes are thought to encode POMTs, because they are homologs of yeast O-mannosyltransferases (PMTs) (26, 29). However, attempts to show that mammalian POMT1 and/or POMT2 has POMT activity have so far been unsuccessful. To demonstrate the POMT activities of POMT1 and POMT2, we constructed the expression plasmids pcDNA3.1-POMT1myc and pcDNA3.1-POMT2, which express POMT1 tagged with the myc epitope (POMT1myc) and POMT2, respectively. These two plasmids were transfected individually or simultaneously into HEK293T cells, and then we recovered the microsomal membrane fraction from the transfected cells. A Western blot analysis using an anti-myc Ab and anti-POMT2 antiserum revealed POMT1myc and the POMT2 at ≈75 kDa in the membrane fraction of each type of transfected cells (Fig. 3 A and B). The band at ≈60 kDa in Fig. 3A was a nonspecific band. We assessed the POMT activity by using these membrane fractions as an enzyme source. Because HEK293T cells had endogenous POMT activity (Fig. 2 A, lane 2), we assumed that the activity in the mock-transfected cells (Fig. 3C, lane 1, transfected with a vector alone) was the background of this assay. The POMT activity was significantly increased in the cells cotransfected with POMT1 and POMT2 (Fig. 3C, lane 4) but was not increased in the cells transfected with either POMT1 or POMT2 alone (Fig. 3C, lanes 2 and 3). These results clearly show that the human POMT1 and/or POMT2 have POMT activity, and the expression of enzymatic activity requires both POMT1 and POMT2. It is probable that a heterophilic interaction between POMT1 and POMT2 is involved in the formation of the active enzyme. It is noteworthy that the POMT activity in a mixture of the membrane fractions from the POMT1- and POMT2-transfected cells was similar to the background level (Fig. 3C, lane 5). This suggests that the complex between POMT1 and POMT2 is formed during the synthesis of POMT1 and POMT2.
Fig. 3.
POMT activity of human POMT1 and POMT2. Western blot analyses of myc-tagged POMT1 (A) and POMT2 (B) expressed in HEK293T cells. Lanes 1, cells transfected with vector alone; lanes 2, cells transfected with human POMT1; lanes 3, cells transfected with human POMT2; lanes 4, cells cotransfected with POMT1 and POMT2. The proteins (20 μg of HEK293T cell microsomal membrane fraction) were subjected to SDS/PAGE (10% gel), and the separated proteins were transferred to a poly(vinylidene difluoride) (PVDF) membrane. The PVDF membrane was stained with anti-myc (A) or anti-POMT2 Ab (B). Molecular mass standards are shown to the right of A and B.(C) POMT activity of membrane fractions from each of these four types (lanes 1–4) plus a mixture of the membrane fractions from the POMT1-transfected cells and POMT2-transfected cells (lane 5).
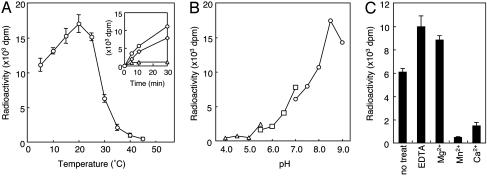
Temperature had a dramatic effect on the rate of the reaction, and the enzyme had an optimal temperature ≈22°C (Fig. 4A). The lower reaction rate above 30°C is probably due to lability of the enzyme at higher temperatures. At this point, it is unclear why the enzyme activity shows this temperature lability. Degradation of enzymes and acceptor proteins during incubation is probably not the cause, because POMT1, POMT2, and GST-α-DG were not degraded significantly during incubation, and the same temperature–activity relationships were seen even at short incubation times of 5–30 min (Fig. 4A Inset). It is possible that the observed temperature lability is due to dissociation of a stabilizing protein from the enzyme–acceptor complex during incubation, or to a temperature-dependent change in the detergent effects. The optimal pH of the POMT activity of POMT1 and POMT2 was ≈8.5 (Fig. 4B). Most of the glycosyltransferases studied to date require divalent cations as activators (33). In previous yeast PMT assays (31, 32), Mg2+ (7∼8 mM) was present in the reaction mixture. Among the divalent metal ions examined in the present study, Mg2+ showed slightly to activate POMT (Fig. 4C). The enzyme was fully active in the presence of 10 mM EDTA. Both Ca2+ and Mn2+ suppressed the enzyme activity. Taken together, human POMT did not require a divalent cation for its activity.
Fig. 4.
Dependence of POMT activity of human POMT1 and POMT2 on temperature (A), pH (B), and various divalent cations and EDTA (C). (A) The temperature–activity relationships at short incubation times (5–30 min) are shown (Inset). Squares, 15°C; circles, 25°C; triangles, 35°C. (B) Triangles, squares, and circles indicate sodium acetate buffer (pH 4.0∼5.5), Mes buffer (pH 5.5∼7.0), and Tris·HCl buffer (pH 7.0∼9.0), respectively. (C) The enzyme was assayed with various divalent cations at 10 mM or EDTA at 10 mM. The presence of Mg2+ had a slight enhanced effect on the activity. The enzyme was fully active in the presence of EDTA. Mn2+ and Ca2+ suppressed the enzyme activity. Eighty micrograms of HEK293T cell microsomal membrane protein was used for each assay.
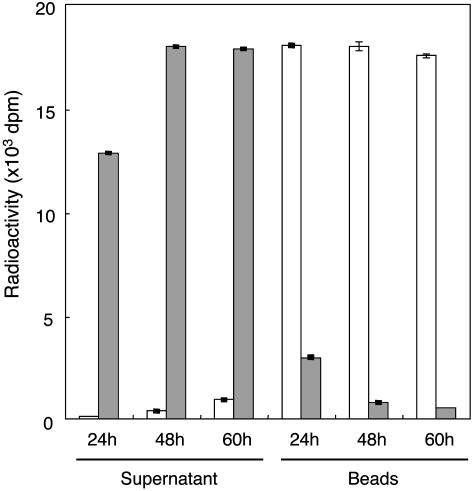
Identification of Product by POMT Products. Digestion of [3H]mannosyl-GST-α-DG by jack bean α-mannosidase, which cleaves the Manα1-Ser/Thr linkage (32), released the radioactivity from the GST-α-DG and decreased the radioactivity remaining in the GST-α-DG (on the beads) (Fig. 5). The release of radioactivity increased with increasing concentration of α-mannosidase. The radioactivity was not released by treatment with heat-inactivated α-mannosidase (Fig. 5). Heat-inactivated α-mannosidase treatment confirmed that the GST-α-DG was not eluted spontaneously from the glutathione-Sepharose beads during treatment with the α-mannosidase (data not shown). These results indicate that a mannose residue is linked to a Ser or a Thr in the GST-α-DG by an α-O-glycosidic linkage and not by a β-O-glycosidic linkage.
Fig. 5.
α-Mannosidase digestion of mannosyl-GST-α-DG. Glutathione-Sepharose beads bearing [3H]mannosyl-GST-α-DG were incubated with jack bean α-mannosidase. At 24-h incubation, 0.8 unit of enzyme was added at 0 h; at 48-h incubation, 0.8 unit of enzyme was added at 0 and 24 h; and at 60-h incubation, 0.8 unit of enzyme was added at 0, 24, and 48 h. After incubation and centrifugation, the radioactivities of the supernatant and the beads were measured with a liquid scintillation counter. Filled bars, active α-mannosidase; open bars, inactive (heat-treated) α-mannosidase. The radioactivity released to the supernatant increased with increasing amount of α-mannosidase used, and the radioactivity remained on the beads decreased correspondingly.
Discussion
Protein O-mannosylation is catalyzed by a family of POMTs (PMTs), which were first characterized in the yeast Saccharomyces cerevisiae (27). Two human homologues of PMT (POMT1 and POMT2) were found (26, 29), but their gene products did not show any POMT activity.
We first tried to develop an assay method to detect POMT activity in mammalian tissues and cells using the method for assaying yeast-like POMT activity in vitro. We did not detect any enzymatic activity when we used different organs, tissues, and cultured cells as an enzyme source; Dol-P-Man or GDP-Man as the sugar donor; Triton X-100 as a detergent; and various synthetic peptides as the acceptor. One possible reason for the lack of activity is that mammalian POMT might recognize an amino acid sequence different from the one recognized by the yeast enzyme. Another possible reason is that the hydrophobicity of the mammalian enzyme might be different from that of the yeast enzyme. Therefore, we thought that α-DG might be a suitable acceptor, because it is an O-mannosylated protein in mammals (9, 14, 15, 17). Therefore, we used a GST fusion protein of α-DG (GST-α-DG) instead of synthetic peptides for the acceptor and detergents other than Triton X-100. With these changes, we succeeded in detecting mammalian POMT activity. The best detergent under our experimental conditions was n-octyl-β-d-thioglucoside (Fig. 1).
Using the assay method, mammalian POMT was found to catalyze a yeast-like O-mannosyl transfer reaction. It transfers a mannosyl residue from Dol-P-Man (not from GDP-Man) to Ser/Thr residues of target proteins, and it is localized in endoplasmic reticulum (27). Mammalian POMT may require a specific amino acid sequence or protein conformation for activity, because synthetic peptides ranging in length from 3 to 10 aa [e.g., 316–325(ATPTPVTAIG), 366–368(PTV)] from potential O-glycosylation sites of human α-DG, 313–483 did not serve as acceptors even using n-octyl-β-d-thioglucoside. It may be relevant that O-mannosylated glycoprotein is abundant in yeast cell walls but is unusual and limited in mammals (17, 27, 34). The requirement for a different detergent for the mammalian enzyme may be due to the different lipid composition in yeast and mammalian membranes.
Using this method, we demonstrated that human POMT1 and/or POMT2 have POMT activity, but only when they are coexpressed, suggesting that POMT1 and POMT2 form a heterocomplex to express enzymatic activity. Because yeast PMT1 and PMT2 form a heterocomplex, and this complex formation is essential for maximal POMT activity (35, 36), the complex in mammals may be similar to the complex in yeast. However, attempts to detect this complex after solubilization of membrane proteins using various detergents or a mixture of detergents, followed by immunoprecipitation with Abs to myc, POMT1, and POMT2 were unsuccessful. Why expression of POMT1 or POMT2 alone did not result in POMT activity is unclear. One possibility is that POMT1 and POMT2 cannot form a homocomplex. Another possibility is that a homocomplex of POMT1 or POMT2 does not have enzymatic activity. In HEK293T cells transfected with the POMT1 gene, the broad band seen above 215 kDa (Fig. 3A, lanes 2 and 4) probably represents POMT1 protein aggregates resulting from its overexpression, because there is no evidence for the presence of POMT2 protein migrating at this position (see Fig. 3B, lanes 3 and 4). POMT1 and POMT2 are expressed in all human tissues, but POMT1 is highly expressed in fetal brain, testis, and skeletal muscle, and POMT2 is predominantly expressed in testis (26, 29). O-Mannosylation seems to be uncommon in mammals, and only a few O-mannosylated proteins have been identified (17). It will be of interest to determine the regulatory mechanisms for protein O-mannosylation in each tissue. Future studies are needed to clarify the distribution of O-mannosyl glycans and of O-mannosylated glycoproteins in various mammalian tissues.
WWS and MEB are clinically similar autosomal recessive disorders characterized by congenital muscular dystrophy, lissencephaly, and eye anomalies, but WWS is a more severe syndrome than MEB (23, 37). Patients with WWS are severely affected from birth (brain malformation is particularly common), and few live beyond infancy. In MEB, the cerebral and ocular anomalies are also severe, but some patients reach adulthood (18, 37). The present results may explain the difference of severity between the two diseases. If POMGnT1, which is responsible for the formation of the GlcNAcβ1–2Man linkage of O-mannosyl glycans (19), is nonfunctional, only O-mannose residues may be present on α-DG in MEB. On the other hand, POMT1 mutations cause complete loss of O-mannosyl glycans in WWS. Because O-mannosyl glycans have several peripheral structures [Siaα2–3Galβ1–4GlcNAcβ1–2Man, Galβ1–4(Fucα1–3)GlcNAcβ1–2Man, and HSO3-3GlcAβ1–3Galβ1–4GlcNAcβ1–2Man] (17), none of these O-mannosyl glycans can be formed if either POMGnT1 or POMT1 does not function. Because these structures play an important role in adhesive processes, a defect in the biosynthesis of O-mannosyl glycans may have a severe effect on cell migration and cell adhesion. It is possible that attachment of a single mannose residue on α-DG in MEB is responsible for the difference in clinical severity of WWS and MEB.
Recently, 6 of 30 WWS patients were found to have mutations in POMT1, whereas none had mutations in POMT2 (25). A possible explanation for the absence of POMT2 mutations in human subjects is that POMT2 may be essential for normal development; i.e., POMT2 mutations may be embryonic-lethal. Another possibility is that patients with POMT2 mutations were simply not included in the 30 WWS patients. A worldwide survey of the occurrence of POMT2 mutations is needed to determine whether WWS is caused by POMT mutations. The rt mutation in Drosophila, which causes defects of myogenesis, was found to be due to a mutation in a homologue of POMT1 (29, 38). The mutation also causes reduced fertility and reduced viability. Although the rt gene product is not known to initiate the biosynthesis of O-mannosyl glycans, O-mannosylation is an evolutionarily conserved protein modification and may be essential for muscle development in both vertebrates and invertebrates.
The skeletal muscle of patients with WWS, MEB, and FCMD was found to be deficient in highly glycosylated α-DG, whereas β-DG and laminin α2 were normally expressed (3, 7, 25, 39, 40). Additionally, defective glycosylation of α-DG has been implicated in congenital muscular dystrophy type 1C (MDC1C). The defective glycosylation is caused by mutations in a gene encoding a putative glycosyltransferase (FKRP, fukutin-related protein) (41). The gene large, which is mutated in the myodystrophy (myd) mouse, encodes a putative glycosyltransferase (42). α-DG in the muscle and brain of myd mice, like that of WWS, MEB, and FCMD patients, was found to be hypoglycosylated. Moreover, hypoglycosylated α-DG in the muscle membranes of MEB and FCMD patients and the myd mouse has greatly reduced affinities for laminin, neurexin, and agrin (8). In other words, interference in O-mannosylation of α-DG may lead to a combination of muscle, eye, and brain abnormalities and is a previously undescribed pathomechanism for muscular dystrophy as well as neuronal migration disorder. Some forms of muscular dystrophy may be due to defects of glycosyltransferases, but the substrates of these enzymes, with the exception of POMGnT1 (19) and POMT1 (this study), are largely unknown. Because FKRP and fukutin are thought to be Golgi-resident proteins (43), it is possible that defects of these proteins cause abnormal processing of α-DG. Identification and characterization of each enzyme will help reveal the molecular pathomechanisms of congenital muscular dystrophies that are accompanied by brain malformation. Because only 20% of WWS patients were found to have mutations in POMT1 (25), other as-yet-unidentified genes may be responsible for this syndrome. It will be important to determine whether other uncharacterized forms of muscular dystrophy are caused by defects in other glycosyltransferases, such as galactosyltransferase and sialyltransferase. In the future, α-DG may be a target of new glycotherapeutic strategies for muscular dystrophy as well as for neuronal migration disorder.
In view of the potential importance of this form of glycosylation for a number of developmental and neurobiological processes, the ability to assay vertebrate O-mannosyltransferase activity and knowledge of the requirement of a heterodimeric complex for enzyme activity should greatly facilitate progress in the identification and localization of O-mannosylated proteins and the elucidation of their functional roles.
Acknowledgments
This study was supported by Research Grants for Nervous and Mental Disorders (14B-4) and Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health, Labour, and Welfare of Japan; by a Grant-in-Aid for Scientific Research on Priority Area (no. 14082209) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by grants from the Mitsubishi Foundation and the Yamanouchi Foundation for Research on Metabolic Disorders.
Abbreviations: DG, dystroglycan; Dol-P-Man, dolichyl phosphate mannose; FCMD, Fukuyama-type congenital muscular dystrophy; MEB, muscle–eye–brain disease; PMT and POMT, protein O-mannosyltransferase; WWS, Walker–Warburg syndrome.
References
- 1.Burton, E. A. & Davies, K. E. (2002) Cell 108, 5–8. [DOI] [PubMed] [Google Scholar]
- 2.Emery, A. E. (2002) Lancet 359, 687–695. [DOI] [PubMed] [Google Scholar]
- 3.Muntoni, F., Brockington, M., Blake, D. J., Torelli, S. & Brown, S. C. (2002) Lancet 360, 1419–1421. [DOI] [PubMed] [Google Scholar]
- 4.Michele, D. E. & Campbell, K. P. (2003) J. Biol. Chem. 278, 15457–15460. [DOI] [PubMed] [Google Scholar]
- 5.Holt, K. H., Crosbie, R. H., Venzke, D. P. & Campbell, K. P. (2000) FEBS Lett. 468, 79–83. [DOI] [PubMed] [Google Scholar]
- 6.Winder, S. J. (2001) Trends. Biochem. Sci. 26, 118–124. [DOI] [PubMed] [Google Scholar]
- 7.Montanaro, F. & Carbonetto, S. (2003) Neuron 37, 193–196. [DOI] [PubMed] [Google Scholar]
- 8.Michele, D. E., Barresi, R., Kanagawa, M., Saito, F., Cohn, R. D., Satz, J. S., Dollar, J., Nishino, I., Kelley, R. I., Somer, H., et al. (2002) Nature 418, 417–422. [DOI] [PubMed] [Google Scholar]
- 9.Chiba, A., Matsumura, K., Yamada, H., Inazu, T., Shimizu, T., Kusunoki, S., Kanazawa, I., Kobata, A. & Endo, T. (1997) J. Biol. Chem. 272, 2156–2162. [DOI] [PubMed] [Google Scholar]
- 10.Finne, J., Krusius, T., Margolis, R. K. & Margolis, R. U. (1979) J. Biol. Chem. 254, 10295–10300. [PubMed] [Google Scholar]
- 11.Krusius, T., Finne, J., Margolis, R. K. & Margolis, R. U. (1986) J. Biol. Chem. 261, 8237–8242. [PubMed] [Google Scholar]
- 12.Krusius, T., Reinhold, V. N., Margolis, R. K. & Margolis, R. U. (1987) Biochem. J. 245, 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen, C. T., Chai, W., Loveless, R. W., Lawson, A. M., Margolis, R. U. & Feizi, T. (1997) J. Biol. Chem. 272, 8924–8931. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki, T., Yamada, H., Matsumura, K., Shimizu, T., Kobata, A. & Endo, T. (1998) Biochim. Biophys. Acta 1425, 599–606. [DOI] [PubMed] [Google Scholar]
- 15.Smalheiser, N. R., Haslam, S. M., Sutton-Smith, M., Morris, H. R. & Dell, A. (1998) J. Biol. Chem. 273, 23698–23703. [DOI] [PubMed] [Google Scholar]
- 16.Chai, W., Yuen, C. T., Kogelberg, H., Carruthers, R. A., Margolis, R. U., Feizi, T. & Lawson, A. M. (1999) Eur. J. Biochem. 263, 879–888. [DOI] [PubMed] [Google Scholar]
- 17.Endo, T. (1999) Biochim. Biophys. Acta 1473, 237–246. [DOI] [PubMed] [Google Scholar]
- 18.Santavuori, P., Somer, H., Sainio, K., Rapola, J., Kruus, S., Nikitin, T., Ketonen, L. & Leisti, J. (1989) Brain Dev. 11, 147–153. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida, A., Kobayashi, K., Manya, H., Taniguchi, K., Kano, H., Mizuno, M., Inazu, T., Mitsuhashi, H., Takahashi, S., Takeuchi, M., et al. (2001) Dev. Cell 1, 717–724. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi, K., Kobayashi, K., Saito, K., Yamanouchi, H., Ohnuma, A., Hayashi, Y. K., Manya, H., Jin, D. K., Lee, M., Parano, E., et al. (2003) Hum. Mol. Genet. 12, 527–534. [DOI] [PubMed] [Google Scholar]
- 21.Manya, H., Sakai, K., Kobayashi, K., Taniguchi, K., Kawakita, M., Toda, T. & Endo, T. (2003) Biochem. Biophys. Res. Commun. 306, 93–97. [DOI] [PubMed] [Google Scholar]
- 22.Fukuyama, Y., Osawa, M. & Suzuki, H. (1981) Brain Dev. 3, 1–29. [DOI] [PubMed] [Google Scholar]
- 23.Dobyns, W. B., Pagon, R. A., Armstrong, D., Curry, C. J., Greenberg, F., Grix, A., Holmes, L. B., Laxova, R., Michels, V. V., Robinow, M., et al. (1989) Am. J. Med. Genet. 32, 195–210. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, K., Nakahori, Y., Miyake, M., Matsumura, K., Kondo-Iida, E., Nomura, Y., Segawa, M., Yoshioka, M., Saito, K., Osawa, M., et al. (1998) Nature 394, 388–392. [DOI] [PubMed] [Google Scholar]
- 25.Beltran-Valero De Bernabe, D., Currier, S., Steinbrecher, A., Celli, J., Van Beusekom, E., Van Der Zwaag, B., Kayserili, H., Merlini, L., Chitayat, D., Dobyns, W. B., et al. (2002) Am. J. Hum. Genet. 71, 1033–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jurado, L. A., Coloma, A. & Cruces, J. (1999) Genomics 58, 171–180. [DOI] [PubMed] [Google Scholar]
- 27.Strahl-Bolsinger, S., Gentzsch, M. & Tanner, W. (1999) Biochim. Biophys. Acta 1426, 297–307. [DOI] [PubMed] [Google Scholar]
- 28.Aravind, L. & Koonin, E. V. (1999) Curr. Biol. 9, R836–R837. [DOI] [PubMed] [Google Scholar]
- 29.Willer, T., Amselgruber, W., Deutzmann, R. & Strahl, S. (2002) Glycobiology 12, 771–783. [DOI] [PubMed] [Google Scholar]
- 30.Ibraghimov-Beskrovnaya, O., Ervasti, J. M., Leveille, C. J., Slaughter, C. A., Sernett, S. W. & Campbell, K. P. (1992) Nature 355, 696–702. [DOI] [PubMed] [Google Scholar]
- 31.Gentzsch, M. & Tanner, W. (1997) Glycobiology 7, 481–486. [DOI] [PubMed] [Google Scholar]
- 32.Bause, E. & Lehle, L. (1979) Eur. J. Biochem. 101, 531–540. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi, N., Honke, K. & Fukuda, M. (2002) Handbook of Glycosyltransferases and Related Genes (Springer, Tokyo).
- 34.Gentzsch, M. & Tanner, W. (1996) EMBO J. 15, 5752–5759. [PMC free article] [PubMed] [Google Scholar]
- 35.Gentzsch, M., Immervoll, T. & Tanner, W. (1995) FEBS Lett. 377, 128–130. [DOI] [PubMed] [Google Scholar]
- 36.Girrbach, V. & Strahl, S. (2003) J. Biol. Chem. 278, 12554–12562. [DOI] [PubMed] [Google Scholar]
- 37.Cormand, B., Pihko, H., Bayes, M., Valanne, L., Santavuori, P., Talim, B., Gershoni-Baruch, R., Ahmad, A., van Bokhoven, H., Brunner, H. G., et al. (2001) Neurology 56, 1059–1069. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Blanco, E. & Garcia-Bellido, A. (1996) Proc. Natl. Acad. Sci. USA 93, 6048–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kano, H., Kobayashi, K., Herrmann, R., Tachikawa, M., Manya, H., Nishino, I., Nonaka, I., Straub, V., Talim, B., Voit, T., et al. (2002) Biochem. Biophys. Res. Commun. 291, 1283–1286. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi, Y. K., Ogawa, M., Tagawa, K., Noguchi, S., Ishihara, T., Nonaka, I. & Arahata, K. (2001) Neurology 57, 115–121. [DOI] [PubMed] [Google Scholar]
- 41.Brockington, M., Blake, D. J., Prandini, P., Brown, S. C., Torelli, S., Benson, M. A., Ponting, C. P., Estournet, B., Romero, N. B., Mercuri, E., et al. (2001) Am. J. Hum. Genet. 69, 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grewal, P. K., Holzfeind, P. J., Bittner, R. E. & Hewitt, J. E. (2001) Nat. Genet. 28, 151–154. [DOI] [PubMed] [Google Scholar]
- 43.Esapa, C. T., Benson, M. A., Schroder, J. E., Martin-Rendon, E., Brockington, M., Brown, S. C., Muntoni, F., Kroger, S. & Blake, D. J. (2002) Hum. Mol. Genet. 11, 3319–3331. [DOI] [PubMed] [Google Scholar]