Abstract
Cotton (Gossypium spp.) fiber cells are seed trichomes derived from the epidermal layer of the cotton seed coat. The molecular components responsible for regulating fiber cell differentiation have not been fully elucidated. A cotton PROTODERMAL FACTOR1 gene (GbPDF1) was found to be expressed preferentially during fiber initiation and early elongation, with highest accumulation in fiber cells 5 d post anthesis. PDF1 silencing caused retardation of fiber initiation and produced shorter fibers and lower lint percentage compared with the wild type, indicating that the gene is required for cotton fiber development. Further analysis showed that a higher accumulation of hydrogen peroxide occurred in the RNA interference transgenic cotton lines. Meanwhile, the expression of several genes related to ethylene and pectin synthesis or sugar transport during cotton fiber growth was found to be significantly reduced in the PDF1-suppressed cotton. Three proteins interacting with GbPDF1 in yeast and in planta might involve cellular signaling or metabolism. GbPDF1 promoter::GUS constructs in transgenic cotton were predominantly expressed in the epidermis of ovules and developing fibers. Progressive deletions of the GbPDF1 promoter showed that a 236-bp promoter fragment was sufficient for basal GbPDF1 transcription in cotton. Mutation of putative regulatory sequences showed that HDZIP2ATATHB2, an element within the fragment, was essential for PGbPDF1-1 expression. The binding activity between this cis-element and nuclear extracts from fiber-bearing cotton ovules at 5 d post anthesis was specific. We conclude that GbPDF1 plays a critical role together with interaction partners in hydrogen peroxide homeostasis and steady biosynthesis of ethylene and pectin during fiber development via the core cis-element HDZIP2ATATHB2.
Cotton (Gossypium spp.) fibers are highly elongated single-cell trichomes that arise from the outer epidermis of the ovules. Fiber growth involves four distinct but overlapping steps: fiber cell initiation, elongation, secondary wall biosynthesis, and maturation (Basra and Malik, 1984; Ruan and Chourey, 1998). The quality of fiber, which is based on its final length and strength, is determined mainly during the elongation and secondary cell wall deposition stage (Li et al., 2007). The elongation phase of cotton fiber development is the best-studied period. A recent review suggested that cotton fibers elongate according to a linear growth model (Qin and Zhu, 2011). However, the number of fiber cells of each ovule, which is another determinant of yield, is established at the initiation stage. Unfortunately, the molecular basis of fiber initiation remains largely unknown (Lee et al., 2007).
Cotton fiber initiation is first observed as the cell ballooning out from certain protodermal cells of the cotton ovule prior to or on the day of anthesis (Yang et al., 2006; Lee et al., 2007; Wu et al., 2007). The process of fiber initiation is rapid, making it difficult to visualize how the protodermal cells turn into fibers. Biochemical analyses revealed that fiber cell fate determination occurs prior to the formation of morphologically visible fiber initials (Graves and Stewart, 1988a, 1988b; Lee et al., 2006, 2007). In addition, numerous fiber-related ESTs were produced and characterized at the transcriptional level in efforts to explore the molecular basis of fiber cell initiation (Lee et al., 2006; Yang et al., 2006; Wu et al., 2007). Comparative analyses of gene expression profiles between the naked seed mutant (n1n1) and its isogenic wild-type line of cotton (TexasMarker-1 [TM-1]) revealed a model of temporal activation of regulatory networks during the early stages of fiber development (Lee et al., 2006). Among the molecular events of gene activation in fiber cell primordia, the first is activation of the “patterning” genes by signals for fiber differentiation, accompanied by sequential activation of proteins such as MYB2 and MYB25 (Lee et al., 2006). Increasing the expression of Gossypium arboreum (Ga)MYB2 in Arabidopsis (Arabidopsis thaliana) rescued the trichome formation of a gl1 mutant and induced the production of seed trichomes (Wang et al., 2004). Overexpression of Gossypium hirsutum GhMYB25 resulted in an increase of cotton fiber initiation and leaf trichome number in transgenic tobacco (Nicotiana tabacum; Wu et al., 2006). Correspondingly, suppression of GhMYB25 expression in cotton altered the timing of rapid fiber elongation, resulting in short fibers and a reduction in the number of trichomes (Machado et al., 2009). More recently, Walford et al. (2011) reported that another MYB protein, GhMYB25-like, plays a key role in the very early stages of fiber cell differentiation, with suppression of GhMYB25-like resulting in cotton plants with fiberless seeds.
Cotton PROTODERMAL FACTOR1 (GhPDF1) is considered to be a potential “patterning” gene working in fiber cell primordia in conjunction with other proteins. The expression of GhPDF1 is strongly induced in immature ovules, prior to the formation of fiber initials, and remains highly expressed until 5 d post anthesis (DPA; Lee et al., 2006). Our previous research in G. barbadense 3-79 demonstrated that the transcripts of GbPDF1 accumulate in a similar pattern, consistent with a possible role in cell fate determination as well as fiber development (Tu et al., 2007). Two protodermal factor-related transcripts were most abundant in the GH_TMO library, which was enriched in ESTs of ovules during the earliest stage of fiber development (Yang et al., 2006). AtPDF1, the homolog of GbPDF1 in Arabidopsis, is a protein involved in cell fate determination in the early stage of epidermal tissue development. The expression of AtPDF1 was exclusively limited in the L1 layer of shoot apices and was protoderm specific during embryogenesis (Abe et al., 1999). Nonetheless, the role of GhPDF1 in the cotton ovule and protodermal fiber cells remains unclear.
To unravel the role of PDF1 function in cotton fiber development, we used bioinformatics, molecular genetics, and interaction with other molecules during fiber initiation and early differentiation. Silencing GbPDF1 expression in cotton ovules around the day of anthesis revealed its role in the timing of fiber cell differentiation. A proposed functional mechanism of PDF1 in regulating the determination of fiber cell fate is discussed.
RESULTS
Isolation and Characterization of PDF1 in Cotton
GbPDF1 was isolated from a normalized cDNA library of G. barbadense 3-79 fiber (Tu et al., 2007). The full-length GbPDF1 cDNA consisted of 885 nucleotides, encoding a polypeptide of 294 amino acids.
Southern blotting of genomic DNA from diploid and tetraploid cotton showed that PDF1 is a low-copy-number gene in cotton (Supplemental Fig. S1A). Three and two PDF1-homologous genes were obtained from the genomic DNA of G. barbadense and G. hirsutum, respectively. The five sequences were classified into three types according to differences in the introns in their coding regions (Supplemental Fig. S1B). The two genes (GbPDF1.1 and GhPDF1.1) lacking introns were classified as type I. Type II contained only one sequence that was from G. barbadense (GbPDF1.2) with one 113-bp intron at the 54th amino acid. Each of these three cDNAs had an open reading frame of 885 bp. Five repeats of motif SY(S)GGGSPP were found between the two transmembrane regions. The remaining two sequences (type III) encoded proteins of 325 amino acids (GbPDF1.3) and 309 amino acids (GhPDF1.3), and each of them contained two introns. In addition, seven and nine repeats of the SY(S)GGGSP(P) motif were found in the deduced proteins of GhPDF1.3 and GbPDF1.3, respectively. As compared with GbPDF1.1, with a putative protein sequence identical to GbPDF1, a total of nine substitution sites were present in the other four proteins (Supplemental Fig. S1C).
GbPDF1 contains novel conserved domains. Eleven GbPDF1-homologous peptides from UniGenes of Gossypium raimondii, Citrus sinensis, and others were found by database searching. The proteins show high homology, especially at the C terminus (Supplemental Fig. S2A), suggesting that this region is important for PDF1 function. The proteins most similar to GbPDF1 were from Citrus species (Ccl.2324 and Csi.5600; Supplemental Fig. S2B).
Bioinformatics analyses predicted that GbPDF1 contains two transmembrane helices (Tusnády and Simon, 2001). The first is in the N terminus (residues 9–28), and the second is in residues 193 to 210 (Supplemental Fig. S1C). To determine the subcellular localization of GbPDF1, a translational fusion between GbPDF1 and G3GFP (GbPDF1:G3GFP), under the control of the cauliflower mosaic virus (CaMV) 35S promoter, was transiently expressed in tobacco (Nicotiana benthamiana). Confocal imaging revealed that GbPDF1:G3GFP was localized to the cell surface and cytoplasm (Supplemental Fig. S3A). To examine whether GbPDF1 was localized to the cell wall, a plasmolysis experiment was performed in transformed Arabidopsis root cells. GFP signal was internalized and associated with the plasma membrane and cytoplasm, but there was no signal present around the cell wall (Supplemental Fig. S3B). These data suggest that GbPDF1 is likely to be associated with the plasma membrane, cytoplasm, or both but is not secreted to the cell wall or localized to the nucleus. This is consistent with GbPDF1 having two transmembrane domains.
Expression of GbPDF1 Is Correlated with Cotton Fiber Initiation and Early Elongation
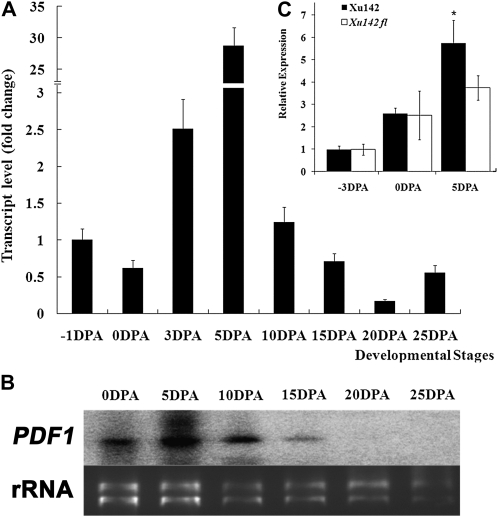
GbPDF1 transcripts were detected in the immature ovules of G. barbadense 3-79 as early as prior to the formation of fiber initials and reached the highest level in the 5-DPA fiber (Fig. 1A). The transcript abundance sharply decreased from 5- to 25-DPA fibers, which mimicked the results of northern blotting during fiber development (Fig. 1B). In root and leaf, expression of GbPDF1 was at a very low level (Tu et al., 2007). A much higher level of GbPDF1 transcripts was found in the 5-DPA fiber-bearing ovules of Xuzhou 142 (Xu142) than in the same stage ovules of the fuzzless-lintless mutant (Xu142 fl), which lacks fibers (Fig. 1C). These results indicate that GbPDF1 is highly expressed in cotton fibers during fiber initiation and the early stages of elongation.
Figure 1.
PDF1 was expressed predominantly in the early stage of cotton fiber development. A, The transcript of GbPDF1 reached the highest level in the 5-DPA fiber. The templates were the first-strand cDNA derived from total RNA of −1-, 0-, and 3-DPA fiber-bearing ovules or 5- to 25-DPA fibers. The expression level is relative to the transcripts in the ovules at −1 DPA. B, Northern blotting showed the highest PDF1 transcript accumulation in the 5-DPA fiber cells. In the top panel, lanes were loaded with 20 μg of total RNA from ovules with fibers attached (0 DPA) or fibers (5–25 DPA). The full-length cDNA of GbPDF1 was used as the probe. The bottom panel shows ethidium bromide staining of the RNA gel used to show equal loading. C, PDF1 transcripts were more abundant in 5-DPA ovules of Xu142 than its fl mutant (Xu142 fl). The accumulation of PDF1 in the fiber-bearing ovules at 5 DPA was significantly higher than that of the fl ovules (t test, * P < 0.05).
PDF1-Suppressed Cotton Showed Retarded Fiber Differentiation
The sequence of GbPDF1 was identical to the PDF1 homolog isolated from G. hirsutum cv TM-1 (GhPDF1.1). To identify the function of GbPDF1, one overexpression construct and two RNA interference (RNAi) constructs targeted to the coding region or 3′ untranslated region, respectively, were generated and introduced into cotton via Agrobacterium tumefaciens-mediated transformation. GbPDF1-overexpressing transgenic cotton was difficult to obtain because of poor plant regeneration (Supplemental Fig. S4). Only a few morphologically abnormal plantlets were obtained, and they failed to set seed. However, 28 independent PDF1-suppressed (RNAi) transgenic cotton lines (T0) were obtained and transplanted to soil for seed reproduction. All the transformed lines showed normal phenotypes, and the positive transformants were confirmed by Southern blotting (Supplemental Fig. S5).
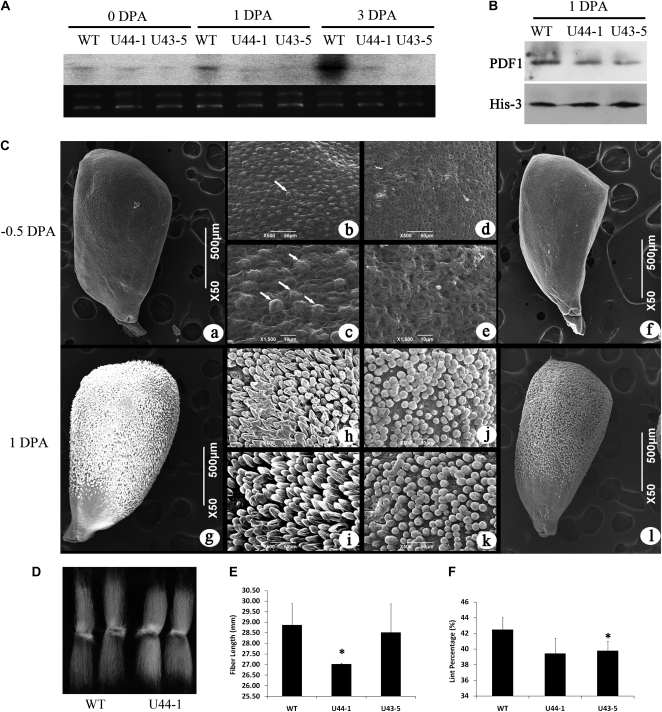
The expression level of PDF1 in 5-DPA fibers of T1 RNAi plants was investigated by quantitative reverse transcription (qRT)-PCR. The accumulation of PDF1 transcripts was significantly decreased in the ovules and fiber cells of the silenced plants compared with the wild-type plants (Supplemental Fig. S6A). The ovules at 1 DPA from two independent RNAi transgenic lines (U44 and C35) and a control were subjected to scanning electron microscopy. Fiber elongation in the RNAi transformants was retarded compared with both wild-type and control plants (Supplemental Fig. S6B). To further examine the effect of PDF1 silencing, we analyzed the transgenic plants of the T2 generation. The T2 progeny from RNAi line U44 were examined the following year, and the same results were obtained at 1 DPA (Fig. 2C), indicating that the delay in fiber development caused by PDF1 suppression could be stably inherited. To ascertain the time point at which the difference in fibers between the RNAi plants and the wild type appeared, ovules before anthesis were sampled for analysis. The wild-type fibers began to balloon out from the epidermal surface 12 h before flowering, whereas the PDF1-silenced plants showed no trichome protrusion on the surface of the ovules (Fig. 2C). Therefore, the retardation occurred at the fiber cell fate determination stage, suggesting that the suppressed PDF1 was effective in the initial protrusion of the fiber cells above the ovule surface. Comparison of the mature fiber length between the PDF1-RNAi transgenic plants and control plants revealed that the reduction of PDF1 in fiber resulted in shorter fibers in the RNAi transformants than in the wild type. The length of fiber in the T2 line U44-1 (27.03 ± 0.05 mm) was significantly shorter than that of the controls (28.86 ± 1.03 mm; t test, P < 0.05), and the fiber length of U43-5 was 28.34 ± 0.54 mm, a little shorter than the control (Fig. 2, D and E). The lint percentage of the transgenic lines was consistently lower than that for the control plants (Fig. 2F). However, other measures of agronomic performance, such as micronaire value, were not different between the transgenic lines and the control (data not shown). All the observations for RNAi plants were associated with a reduction of GbPDF1 transcript and protein (Fig. 2, A and B).
Figure 2.
The PDF1-suppressed T2 cotton further confirmed that fiber differentiation was later than that of controls, and the mature fiber was shorter or with a lower lint percentage than the wild type. A, Northern blotting showed the lower expression of PDF1 in the fiber-bearing ovules of RNAi transgenic plants compared with the wild type (WT). The signal of 32P-labeled PDF1 transcripts (top) and the ethidium bromide staining of the RNA gel showing equal loading (bottom) are shown. B, Protein abundance of PDF1 was decreased in the RNAi ovules according to western blotting with specific antibodies against GbPDF1. In the top panel, lanes were loaded with 50 μg of total protein extracted from 1-DPA cotton ovules and detected using PDF1-specific polyclonal antiserum. In the bottom panel, anti-histone 3 served as the loading control. C, The morphological differences of fiber cells between the wild type and PDF1-silenced T2 cotton plants. Fiber cells of the wild type ballooned out from the seed coat at about 12 h before anthesis (a–c). However, the PDF1-suppressed cotton ovules were smooth without fiber initials (d–f). The fiber cells were observed at 50× (a and f), 500× (b and d), and 1,500× (c and e). White arrows show the fiber cells (b and c). At 1 DPA, the fibers of the control began to elongate (g–i), but the PDF1-silenced cotton fibers seemed to stay at the initiation stage (j–l). Panels i and k show the middle of ovules, and panels h and j show zoomed images into ovules near the chalaza. D, The mature fibers from RNAi-transformed cotton plants were shorter than control fibers. E, Measurement of fiber lengths showed that fiber in the transgenic plants was shorter than that in the wild-type plant. Bars represent sd of three measurements. F, Lower lint percentage in the PDF1-RNAi transgenic plants than in wild-type cotton. Asterisks indicate statistically significant differences between transgenic lines and the wild type, as determined by Student’s t test (P < 0.05).
The Fiber Development Network Is Impaired Partially in the GbPDF1-Suppressed Transgenic Plants
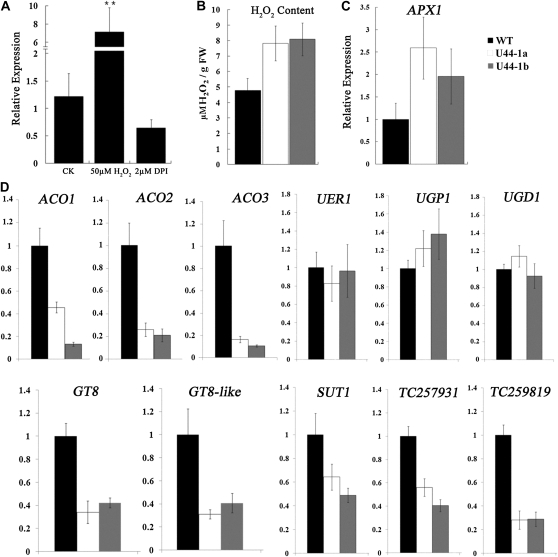
In order to identify the mechanism of GbPDF1, its expression profiles were analyzed when several phytohormones and chemicals were added into the ovule culture medium. The GbPDF1 transcripts in the wild-type ovules increased significantly with 50 μm hydrogen peroxide (H2O2) and decreased with diphenyleneiodonium (DPI; Fig. 3A). However, the H2O2 content in the knockdowns of GbPDF1 3-DPA in planta ovules was much higher than in the wild type (Fig. 3B). The expression level of the functional ASCORBATE PEROXIDASE1 (APX1) was consistently increased in the PDF1-suppressed 3-DPA ovules and fibers to maintain H2O2 homeostasis during cotton fiber development (Li et al., 2007; Fig. 3C). Therefore, GbPDF1 may play a role in the regulation of antioxidation metabolism.
Figure 3.
The knockdowns of GbPDF1 impaired the fiber development gene network. A, qRT-PCR analysis of PDF1 transcripts from wild-type ovules after 2 d of control (CK), H2O2, or DPI treatment. RNA samples from ovules cultured for the same period of time without the addition of H2O2 or DPI were used as controls. B, Higher concentrations of H2O2 accumulated in GbPDF1-suppressed cotton ovules at 3 DPA. FW, Fresh weight. C and D, Expression analysis of several fiber-related genes in ovules from GbPDF1-suppressed cotton lines and the control at 3 or 0 DPA. The y axis shows the relative expression level normalized to the wild type (WT).
H2O2 concentration and GhAPX1 transcription can be promoted by exogenous ethylene treatments in cotton ovules (Li et al., 2007). Three ethylene synthesis-related genes (GhACO1, GhACO2, and GhACO3; Shi et al., 2006) active during fiber development were selected for a comparative analysis between the transgenic and wild-type fiber and ovules at 0 DPA using qRT-PCR. GbPDF1 suppression led to a substantial reduction of all three GhACO genes (Fig. 3D). These data indicated that the ACO genes were likely regulated by PDF1 directly or indirectly.
Pectin biosynthesis is an important biochemical reaction downstream of ethylene signaling that promotes fiber elongation (Pang et al., 2010). We selected several known pectin-related genes, UER1, UGP1, and UGD1 (Pang et al., 2010), and two putative glycosyltransferases (GT8 and GT8-like) for analysis in 0-DPA ovules. GT8 was preferentially expressed in ovules at the day of anthesis, but GT8-like showed no significant expression difference during fiber development (Supplemental Fig. S7B). The transcripts of UER1, UGP1, and UGD1 were unchanged in the GbPDF1-silenced plants; however, the GT8 and GT8-like transcripts were clearly decreased by half or more (Fig. 3D). In addition, our results revealed that reduced GbPDF1 expression led to a substantial decrease of SUT1 (TC192320) and two putative sugar transporters (TC259819 and TC257931; Fig. 3D), which indicated that GbPDF1 potentially influences the supply of carbon during fiber development.
Interacting Proteins of GbPDF1 in Yeast and in Planta
Identifying the proteins interacting with GbPDF1 would improve our understanding of the role of GbPDF1 during cotton fiber differentiation. The full-length GbPDF1 fused with the GAL4-DNA-binding domain was used to screen a yeast two-hybrid library, using the cDNAs from cotton ovules and fibers to construct prey vectors. Thirteen candidate GbPDF1-interacting proteins were identified by screening 1.18 × 105 colonies (Supplemental Table S1). The open reading frame of each candidate was fused with the activation domain for retransformation.
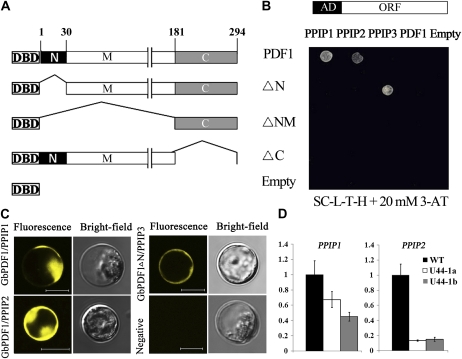
For further analyses, new baits containing specific fragments of GbPDF1 were constructed, designated as ΔN (residues 31–294), ΔNM (residues 182–294), and ΔC (residues 1–182; Fig. 4A). Three putative proteins were obtained after being retransformed into the bait strains that contained full-length or domain-deleted GbPDF1 fragments; these proteins were named PPIP1 (for putative GbPDF1 interaction protein), PPIP2, and PPIP3, respectively (Fig. 4B; Table I). These three genes could be detected in fiber and ovules (Supplemental Fig. S7, A and B).
Figure 4.
The native or truncated GbPDF1 interacted with PPIPs in yeast and in planta. A, Structure of the GbPDF1 protein and schematic diagrams of truncated fragments for yeast two-hybrid analysis. GbPDF1 was divided into three fragments as N (amino acids 1–30), M (amino acids 31–180), and C (amino acids 181–294). ΔN was the GbPDF1 protein with the N fragment deleted, ΔNM was the protein with only the C fragment, and ΔC was the protein without fragment C. The native and different fragments were fused with the DNA-binding domain of GAL4 as bait for yeast two-hybrid analysis. The plasmid of pDEST32 was empty. B, PPIP1 and PPIP2 interacted with GbPDF1, but PPIP3 bound with the N-domain-deleted protein in yeast. The GAL4-activation domain (AD)-fused interaction partners were retransformed into the yeast strains carrying different baits. The pDEST22 plasmid was empty. Transformants were assayed for growth on 20 mm 3-amino-1,2,4-triazole (3-AT) synthetic complete medium without Leu, Trp, and His (SC-L-T-H). ORF, Open reading frame. C, The interactions were confirmed by transient expression in planta. Fluorescence signal could be detected in the transformed protoplasts, except for the negative control. D, PPIP1 and PPIP2 were down-regulated in the RNAi transgenic cotton plants compared with the wild type.
Table I. Proteins that interacted with GbPDF1, as identified from retransformation after library screening.
| Protein | Length | Conserved Domains | Homologous Protein | Accession No. | E Value | Homologya | Score | Reference |
| PPIP1 | 550 Amino acids | Ubiquitin, ubiquitin-like domain, ubiquitin-associated domain | AtDSK2 | AAN13037 | 0 | Id = 66%, Po = 75% | 634 | |
| PPIP2 | 371 Amino acids | AKRs | NtTIP2 | AAO91861 | 1E-159 | Id = 83%, Po = 90% | 545 | Fridborg et al. (2003) |
| NtTIP3 | AAO91862 | 1E-158 | Id = 81%, Po = 89% | 541 | Fridborg et al. (2003) | |||
| AtAKR2A | NP195270 | 8E-127 | Id = 63%, Po = 76% | 435 | Shen et al. (2010) | |||
| PPIP3 | 232 Amino acids | GRAM | AtGEM-L5 | AAR20771 | 2E-107 | Id = 76%, Po = 88% | 370 |
Id, Identities; Po, positives.
As shown in Table I, PPIP1 encodes a ubiquitin protein with a ubiquitin-like domain in the N terminus followed by a ubiquitin-associated domain. However, no interaction was detected between the truncated GbPDF1 fragments and PPIP1. PPIP2, a protein containing an ankyrin repeat (AKR) with high homology to NtTIP2 (Fridborg et al., 2003), interacted only with the full-length GbPDF1. A GRAM (for glucosyltransferases, Rab-like GTPase activators, and myotubularins) domain was found in PPIP3. Interestingly, PPIP3 could not bind with GbPDF1 but could bind to the fragment lacking the N domain (residues 1–30). All of these results were confirmed by β-galactosidase assay (Supplemental Fig. S8). The GbPDF1 protein could not form a dimer in yeast, because no interaction was found between GbPDF1 and the four baits (Fig. 4B). Therefore, it was less likely that GbPDF1 works as a homodimer.
The bimolecular fluorescence complementation (Waadt et al., 2008) and transient gene expression (Yoo et al., 2007) systems verified the protein interactions in planta. The corresponding constructs (VenusN:GbPDF1 with VenusC:PPIP1/2 and VenusN:GbPDF1 ΔN with VenusC:PPIP3, respectively) were cotransformed into protoplasts, and strong yellow fluorescence was expressed in the plasma membrane and cytoplasm (Fig. 4C). These results suggest that GbPDF1 (GbPDF1 ΔN) interacts with PPIP1, PPIP2, or PPIP3 in living plant cells. Additionally, in the RNAi transgenic cotton ovule, PPIP1 and PPIP2 expression levels were decreased (Fig. 4D).
The 236-bp Promoter Fragment Was Sufficient to Drive GUS Expression in the Cotton Ovule and Fiber
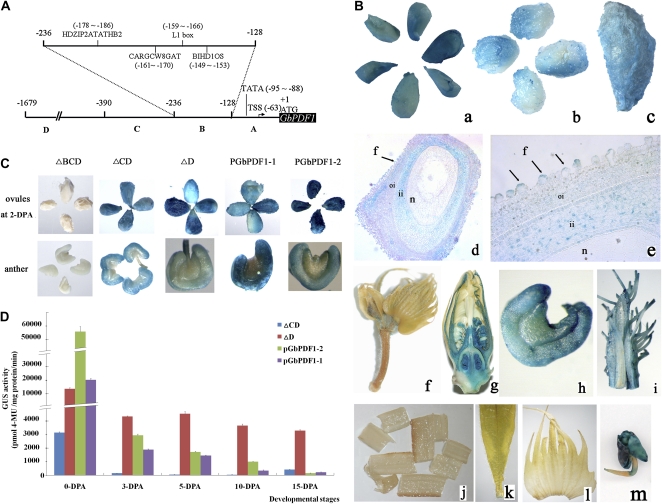
Two GbPDF1 gene promoters, differing in the lengths of the 5′ flanking regions (PGbPDF1-1, 1,679 bp; PGbPDF1-2, 1,285 bp), were cloned from G. barbadense 3-79 by genome walking. The two promoters shared a nearly identical 390-bp sequence upstream of the translational initiation codon. The promoter sequences were classified into four distinct regions according to the locations of known cis-elements (Fig. 5A). The regions from −391 to −1,679 bp in the sequence of PGbPDF1-1 and from −391 to −1,285 bp in PGbPDF1-2 were designated as regions D and D′, respectively. The common 390-bp fragment comprised regions A (from −1 to −128), B (−129 to −236), and C (−237 to −390). Region A contained only the elements for basal transcription. A putative TATA box (from −95 to −88) that provided the binding site for RNA polymerase was present in the two promoters. The transcriptional start site of GbPDF1 was found at position −63 from the initial ATG according to the TSSP prediction result (Fig. 5A). Region B contained a binding site (CARGCW8GAT) for the MADS-domain protein AGAMOUS-like15 (Tang and Perry, 2003), and three cis-elements were identified that were predicted to bind with homeodomain transcription factors. BIHD1OS is the binding site for a rice (Oryza sativa) BELL homeodomain transcription factor (Luo et al., 2005). HDZIP2ATATHB2 was found to interact with ATHB2 (Ohgishi et al., 2001). The L1 box is a conserved cis-element located in the promoters of L1 layer-specific genes in Arabidopsis (Abe et al., 2001).
Figure 5.
Diagram of the GbPDF1 promoter, and expression patterns of GUS driven by the native and truncated promoter in transgenic cotton plants. A, The structure of the GbPDF1 promoter, PGbPDF1-1. Regions A, B, C, and D ranged from −1 to −128, −129 to −236, −237 to −390, and −391 to −1,679 bp, respectively. The transcription start site was at −63 bp upstream of ATG. The TATA box was located from −95 to −88 bp. The predicted cis-regulatory elements in region B are labeled in detail. B, PGbPDF1 preferentially drives GUS expression in the cotton ovule and fiber. Staining was observed on the surface of the ovules and fiber at –1 DPA (a), 2 DPA (b), and 5 DPA (c). Reproductive tissues such as flower (g), anther (h), and filament (i) were stained. No GUS activity was detected in bud (f), stem (j), leaf (k), or bract (l) of transgenic cotton. GUS was expressed moderately in the cotyledons and root tip of the 2-d-old seedlings (m). A high level of GUS activity was located in the fiber cells and inner integument according to longitudinal sections of the stained 1-DPA ovules carrying PGbPDF1-1::GUS viewed with a light microscope at 100× (d) and 400× (e). f, Fiber cell; ii, inner integument; n, nucellus; oi, outer integument. Black arrows show the fiber initials. C, Comparison of GUS expression in transformed cotton among different constructs. The plants carrying the ΔBCD vector showed no GUS expression in anther and 2-DPA ovules. The ΔCD- and ΔD-carrying plants showed a similar expression pattern to the native promoters PGbPDF1-1 and PGbPDF1-2. D, The deleted promoters resulted in different accumulation patterns of GUS. GUS activity in the ovules and fibers of transformed cotton was determined. Plants carrying each of the four constructs showed the most accumulation of GUS in 0-DPA ovules. Cotton with ΔD retained a high level of GUS from 0 to 15 DPA, while GUS in the samples with the other three constructs decreased. Error bars represent sd of three replicates. 4-MU, 4-Methylumbelliferone.
Expression of the GUS gene, under the control of the GbPDF1 promoters, was tested using stable transformation of cotton. Histochemical analysis of GUS activity was carried out in ovules and fibers at various stages of development. In the case of PGbPDF1-1, GUS activity was observed in the ovule and fiber from 0 to 5 DPA (Fig. 5B). GUS activity was found in the ballooned fibers, the inner integument, and some cells of the outer integument adjacent to the fibers (Fig. 5B). GUS expression was also visible in the reproductive tissues such as anther and style, but the GUS activity was much less than that of fiber-bearing ovules (Fig. 5B; Supplemental Fig. S9). However, the signals were barely detectable in vegetative organs such as leaf, bract, and stem.
To define the core regulatory region responsible for the transcriptional regulation of GbPDF1, three progressive 5′ deletions of PGbPDF1-1 fused with GUS were also introduced into cotton. Region A alone could not activate GUS in the ovules or other tissues (Fig. 5C). However, no significant difference in GUS expression pattern was observed in the transgenic cotton containing ΔCD and ΔD as compared with the full-length promoter. Accordingly, the minimal region necessary for the expression of PDF1 in cotton was confined within the 236-bp fragment (regions A and B).
In addition, we also determined GUS protein activity in the ovule and fiber at different developmental stages. The highest GUS activity was in the ovule on the day of anthesis, even though the transgenic cotton carried different lengths of promoter fragments. However, the promoter activity decreased gradually during fiber development, and a very low accumulation of expression was detected in the 15-DPA fiber cells, except in the lines that carried the ΔD construct (Fig. 5D). Clearly, region B was important for the basal transcription of GbPDF1 but was not responsible for its high accumulation in the fibers, according to the different GUS activity between plants that carried ΔCD and ΔD.
Mutational Analysis within Region B Revealed That HDZIP2ATATHB2 Was the Key Regulatory Element
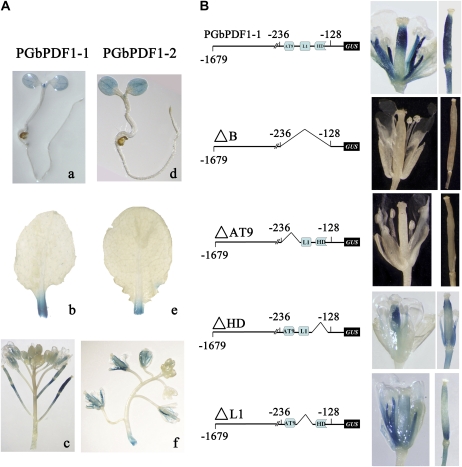
We deleted region B to generate the constructs ΔB, ΔAT9 (HDZIP2ATATHB2), ΔL1 (L1 box and CARGCW8GAT), and ΔHD (BIHD1OS). To elucidate the role of the putative regulatory sequences, T1 and T2 generations of Arabidopsis transformants with PGbPDF1::GUS were used for histochemical analyses. GUS activity was detected in the vegetative shoot meristem, cotyledons of a 5-d-old seedling, and the cutting site of older leaves. After flowering, the styles, stamens, and immature siliques were stained (Fig. 6A).
Figure 6.
Critical regulatory element HDZIP2ATATHB2 was determined by GUS assays in stably transformed Arabidopsis plants. A, Localization of PGbPDF1-1::GUS (a–c) and PGbPDF1-2::GUS (d–f) in transformed Arabidopsis. Both of the promoters could drive GUS expression in 5-d-old seedlings (a and d), leaves (b and e), and inflorescences (c and f). B, The GUS activity in the inflorescences and siliques of the transformed Arabidopsis plants was abolished once region B or the regulatory element HDZIP2ATATHB2 (AT9) was deleted in the sequence of PGbPDF1-1.
As expected, the region B-deleted promoter was unable to drive the reporter gene. GUS activity in the plants that carried ΔL1 and ΔHD showed a similar pattern as those containing the full-length promoter. However, GUS activity was undetectable in the inflorescence and immature siliques of transgenic plants carrying the construct without HDZIP2ATATHB2 (5′-TAATAATTA-3′; Fig. 6B).
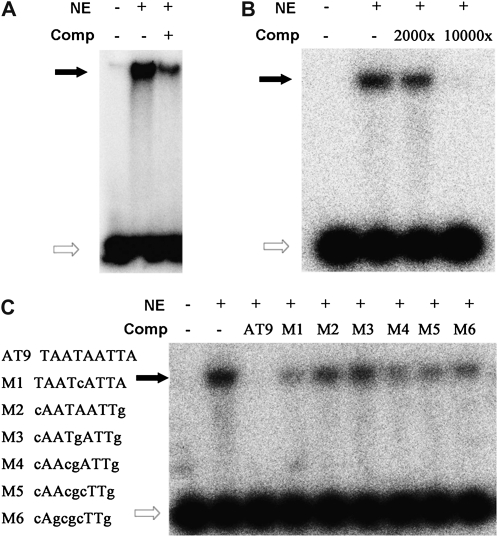
To confirm if there were any proteins binding with HDZIP2ATATHB2, several probes were designed for gel-shift assays with the nuclear extracts of the reproductive tissues of Arabidopsis and fiber-bearing cotton ovules. The first probe was HDZIP2ATATHB2 itself; the second and the third ones were P1 (from −229 to −180) and P2 (from −179 to −130); in addition, SP1 (5′-TAAT-3′) and SP2 (5′-AATTA-3′) split the HDZIP2ATATHB2 element. No matter whether the nuclear extracts were from Arabidopsis or cotton, the 32P-labeled HDZIP2ATATHB2 was retained during the separation on polyacrylamide gels (Fig. 7, A and B). The competitive probes unrelated to HDZIP2ATATHB2 did not efficiently reduce the intensity of the band shifts compared with HDZIP2ATATHB2 itself as the competitor (Fig. 7C). These results showed that the observed HDZIP2ATATHB2-binding activity was sequence specific. However, no interaction was found between P1 or P2 and the nuclear extract from Arabidopsis (Supplemental Fig. S10, A and B), which agreed with the GUS staining result. The loss activity of probes binding with cotton nuclear extracts was observed once the HDZIP2ATATHB2 was split (Supplemental Fig. S10C). These data together suggest the direct involvement of the HDZIP2ATATHB2 motif in regulating the basal expression of GbPDF1 in Arabidopsis as well as in cotton fiber.
Figure 7.
The binding ability of the HDZIP2ATATHB2 box and nuclear proteins. A, Interactions of HDZIP2ATATHB2 with nuclear protein extracted from inflorescences and siliques of Arabidopsis Columbia plants. The positions of the DNA-protein complexes are shown as black arrows, and the white arrows show the free probes. Comp, Unlabeled competitor probe; NE, nuclear extract. B, In vitro interaction between HDZIP2ATATHB2 and nuclear extracts from 5-DPA fiber-bearing cotton ovules. C, The interaction between HDZIP2ATATHB2 and cotton nuclear extracts was specific. Six competitive probes with HDZIP2ATATHB2 mutant (M1–M6, listed at left) showed lower affinity with the cotton nuclear extracts than the native one (AT9).
DISCUSSION
GbPDF1 Contained Conserved Domains
GbPDF1 is a Pro- and Gly-rich protein with two putative transmembrane helices and several repeating motifs. The motif SY(S)GGGSPP found in the cotton PDF1 proteins was novel, and the repeat number was variable within the cotton homologs (Supplemental Fig. S1C). Domains conserved among the PDF1 proteins were assumed to be critical for their functions. First, we showed that the N terminus was important for the interaction between GbPDF1 and PPIPs, because the interaction patterns changed when the N region was deleted. Second, the highly homologous C terminus suggested its potential role in the protein’s function. The loss-of-function interaction between GbPDF1 and PPIPs after fragment C was deleted in yeast proved this deduction. Notably, the C region alone was not sufficient, because the PPIPs could not interact with the GbPDF1 fragment without the N and M domains (Figs. 4, A and B).
The Potential Role of GbPDF1 in Cotton Fiber Development
Previous studies suggested that PDF1 plays a critical role in the early stage of the development of epidermal tissues under the regulation of AtPDF2 and ATML1 (Abe et al., 1999, 2003). An intact epidermis is crucial for certain key processes in plant development, shoot growth, and plant defense (Javelle et al., 2011). For instance, the double L1-specific gene mutant pdf2-1 atml1-1 resulted in a disorganized shoot apex during embryogenesis and had problems in leaf initiation and development (Abe et al., 1999, 2003). Trichomes are the specialized structures that differentiate from epidermal cells (Ishida et al., 2008). Cotton fibers are trichomes, but they are derived from individual cells of the epidermal layer of the seed coat. Studies have also shown that cotton fibers share a similar model of cell fate determination with the leaf trichomes in Arabidopsis (Wang et al., 2004; Lee et al., 2007).
In our studies, we found that the transcripts of GbPDF1 accumulated predominantly in the fibers, especially at the early stage, suggesting its potential role in fiber development (Fig. 1, A and B). PDF1-silenced cotton showed retarded fiber initiation and had shorter fibers or lower lint percentage, and a field trial lasting for more years will be done to confirm the traits (Fig. 2; Supplemental Fig. S6). Crucially, scanning electron micrographs of the RNAi-transformed cotton ovules indicated that the delay in fiber development occurred before the outgrowth of fiber cells specialized from seed epidermal cells (Fig. 2C; Supplemental Fig. S6B). These results provided evidence for the role of PDF1 in regulating the initial protrusion of the fiber cells above the ovule surface, which is in agreement with the report that PDF1 may play important roles in the differentiation of fiber initials in the ovule protodermal layer (Yang et al., 2006). The timing of fiber initiation has a great impact on the number of lint fibers, because the later initials always develop as fuzz fibers (Lee et al., 2006). Both cotton fiber yield and quality are greatly improved by increasing the number of lint fibers, which is the result of increasing auxin levels in the epidermis of cotton ovules at the fiber initiation stage that transforms more fuzz cells into lint cells (Zhang et al., 2011). The shorter fibers and the decrease of fiber lint percentage in the PDF1-RNAi transgenic lines were consistent with the delay of fiber initiation.
Three important genes for ethylene biogenesis were reduced compared with the wild type. Previous studies demonstrated that ethylene and H2O2 interact to regulate cotton fiber elongation, and they can be induced by each other (Li et al., 2007; Qin et al., 2008). However, the increase in H2O2 content is accompanied by down-regulation of the three ACO genes in the PDF1-RNAi cotton (Fig. 3, B and D). The expression levels of the three ethylene-responsive genes (UER1, UGP1, and UGD1; Pang et al., 2010) showed no differences between wild-type and transgenic ovules (Fig. 3D), proving that no more ethylene was induced by the high accumulation of H2O2 in the knockdowns of GbPDF1. So, it is likely that GbPDF1 plays a role in the feedback regulatory mechanism from H2O2 to ethylene biosynthesis in modulating cotton fiber development.
The pectin biosynthesis of the PDF1-suppressed cotton fibers and ovules might be disturbed because of the down-regulation of GT8 and GT8-like together with the reduced carbon source (Fig. 3D). Pectin is a major polysaccharide component of the primary cell wall (Gou et al., 2007); it is important for fiber elongation and is regulated by ethylene signaling (Pang et al., 2010). Cotton GT8, which encodes a putative glycosyltransferase, might be involved in the synthesis of the pectic polysaccharides (Scheible and Pauly, 2004). The cotton GT8 is highly expressed in the 0-DPA ovules (Supplemental Fig. S7B) and shares 78% identities and 88% positives (number and fraction of residues for which the alignment scores have positive values; http://searchlauncher.bcm.tmc.edu/help/BLASToutput.html) at the amino acid level with AtGATL1, which plays a potential role in the synthesis of the structure to which the reducing end xylan oligosaccharide is attached (e.g. a specific fraction of pectin; Mohnen, 2008; Kong et al., 2011). Cotton GT8 may function similarly to AtGATL1, owing to the largely conserved roles of GATL genes between different species (Kong et al., 2011).
Our yeast two-hybrid data suggest that the interaction partners of GbPDF1 may involve cellular signaling or metabolism. PPIP1 is a well-conserved ubiquitin protein that functions in the regulation of protein degradation (Rothenberg and Monteiro, 2010). Dsk2p, a homolog of PPIP1 in Saccharomyces cerevisiae, is an adaptor that facilitates the delivery of polyubiquitin chains or proteins to the proteasome (Funakoshi et al., 2002). One homolog of PPIP2 is Arabidopsis ANKYRIN REPEAT-CONTAINING PROTEIN2A (AKR2A), which functions as a molecular chaperone for proteins that are targeted to membranes of different organelles in plant cells (Shen et al., 2010). Decreased AKR2A expression leads to reduced steady-state levels of peroxisomal membrane-bound APX3 and reduced targeting of APX3 to peroxisomes in plant cells, indicating that AKR2A plays an important role in controlling antioxidation metabolism (Shen et al., 2010). The GRAM domain in PPIP3 has been reported to be involved in membrane-associated processes such as intracellular protein or lipid-binding signaling pathways (Doerks et al., 2000). Oku et al. (2003) found that GRAM was essential for the fungal UDP-Glc:sterol glucosyltransferase in peroxisome degradation. The expression levels of PPIP1 and PPIP2 were down-regulated in PDF1-silenced cotton ovules, which suggested that the expression of these genes was also influenced by GbPDF1, although the mechanism of signal transduction could not be elucidated in this study.
A proposed model could be constructed according to the results above. PDF1 may influence ethylene and H2O2 production in cotton ovules, which would lead to a rate-limiting step in pectin biosynthesis and ultimately result in retarded fiber initiation and shorter fibers. GbPDF1 is synthesized on free ribosomes and moved to the right destination with the help of PPIP2. It then functions with PPIP3, and PPIP1 responds to the degradation of GbPDF1.
The Basal Expression of GbPDF1 Is Regulated via a cis-Element, HDZIP2ATATHB2, in the Promoter
According to the expression profile of 5′ deletions of PGbPDF1-1, we found that regions A and B were sufficient for the expression of GbPDF1 in cotton. Region B was thought to be critical for the basal expression of GUS, which was confirmed by GUS being undetectable in the plants carrying the ΔB construct (Fig. 6B). The expression pattern of PGbPDF1-1::GUS in Arabidopsis was similar to that driven by the promoter of AtPDF1 (Fig. 6A; Abe et al., 1999). The transcription of AtPDF1 was mainly regulated through the DNA-protein interaction between a pair of functionally redundant HD-ZIP class IV transcriptional factors (AtPDF2 and ATML1) and the L1 box. Previous studies showed that the L1 box was an 8-bp motif required not only for epidermis-specific gene expression in Arabidopsis but also for the expression of a fiber-specific gene, GaRDL1 (Abe et al., 2001; Wang et al., 2004). Two HD-ZIP class IV family members in cotton, GhHOX3 and GbML1, were found to interact with the L1 box (Wang et al., 2004; Zhang et al., 2010). Unexpectedly, the promoter fragment without L1 was able to drive GUS expression in the flowers and siliques (Fig. 6B). Moreover, the binding site for OsBIHD1 might not be the important element for PGbPDF1-1, because there was no significant difference between the transformed plants carrying ΔHD and the full-length GUS-fused plants. Ultimately, the plants with ΔAT9 abolished the ability of transcriptional activation completely. In addition, HDZIP2ATATHB2 could bind to proteins from 5-DPA fiber-bearing ovules in vitro specifically (Fig. 7). These observations suggest that HDZIP2ATATHB2 plays a critical role in the basal transcriptional control of PDF1 in cotton. However, region B as well as HDZIP2ATATHB2 is not responsible for the high accumulation of PDF1 transcripts in fibers, because very low GUS activity can be detected, except in ovules at 0 DPA in ΔCD-carrying cotton (Fig. 5D). Based on these results, we proposed that interaction between HDZIP2ATATHB2 and the binding protein was also activated in the fl mutant to keep a moderate expression level in ovules. The positive regulatory elements involved in increasing the transcriptional level of PDF1 might be located in region C, according to the GUS activity comparison between ΔCD and ΔD. In addition, the observed GUS activity in the cotton ovules and fibers at different developmental stages suggests that region D (D′) containing cis-elements was responsible for preserving the specificity of GbPDF1 transcripts (Fig. 5D).
HDZIP2ATATHB2 is a 9-bp pseudopalindromic motif interacting with HD-ZIP class II proteins (Ohgishi et al., 2001). The Arabidopsis genome contains 10 genes belonging to the HD-ZIP II family, including ATHB2 and HAT2 (Ciarbelli et al., 2008). In Arabidopsis, overexpression of ATHB2 and HAT2 resulted in longer hypocotyls and petioles, respectively, but smaller and fewer leaves, with reduced formation of lateral roots. These phenotypes were regulated by auxin redistribution (Steindler et al., 1999; Sawa et al., 2002). These findings could help us to understand the role of the HD-ZIP II transcription factors in cotton fiber elongation through the interaction with HDZIP2ATATHB2. Future research should attempt to identify the target proteins that interact with the elements as well as their roles in cotton fiber differentiation.
CONCLUSION
In summary, our findings indicate that GbPDF1 is required for the fiber cell initiation process, possibly by keeping the normal concentrations of H2O2 and ethylene. According to promoter-truncated analysis, the 236-bp promoter fragment was sufficient for the gene expression in cotton. Moreover, we show that the regulatory element HDZIP2ATATHB2 supplied a binding site for the basic transcriptional machinery of GbPDF1.
MATERIALS AND METHODS
Plant Materials
The cotton plants Gossypium barbadense 3-79 and Gossypium hirsutum Xu142, Xu142 fl, and YZ1 used in these experiments were cultivated in the field in Wuhan, China, using normal farming practices. The bolls were tagged on the day of anthesis. The fiber cells at different developmental stages were removed carefully from ovules and immediately immersed in liquid nitrogen. Tissues such as petals and ovules were harvested from plants in the field and stored in liquid nitrogen. Roots and leaves were collected from 15-d-old seedlings cultured in a growth chamber. All frozen materials were preserved at −70°C. Arabidopsis (Arabidopsis thaliana) plants were grown in long-day conditions (16 h of light, 8 h of dark) under white fluorescent light at 20°C.
Cotton Ovule Culture and Treatment with H2O2 or NADPH Oxidase Inhibitor
Cotton ovules from wild-type plants were collected immediately after flower opening. They were sterilized and then placed in liquid BT medium supplemented with 50 μm H2O2 or 2 μm of the NADPH oxidase inhibitor DPI (Sigma; Li et al., 2010). The samples were collected for RNA extraction after 48 h of culture.
Southern and Northern Blotting and qRT-PCR
The methods of genomic DNA isolation and Southern blotting were according to Li et al. (2010). The probe for PDF1 gene detection was the small fragment digested from the cDNA sequence inserted in pSPORT with HindIII (Tu et al., 2007). For the detection of transgenic cotton plants, the probe was the NPTII fragment, and genomic DNA was digested with HindIII.
To determine the expression level of PDF1 in wild-type and transgenic cotton, RNA was isolated from the collected tissues using the method described by Zhu et al. (2005). Twenty micrograms of total RNA per lane was transferred onto nylon membranes. The blots were hybridized with 32P-labeled full-length GbPDF1. Hybridization and detection of the signal on the filter were as reported previously (Tu et al., 2007). The first cDNA strand was synthesized, and qRT-PCR was performed as described by Munis et al. (2010). The expression level of UBQ7 was used as the internal control to standardize the RNA samples for each reaction. Error bars indicate the sd of three sample replicates. The primers used in the study are listed in Supplemental Table S2.
Gene Cloning and Sequence Analysis
To obtain the genomic sequences of the PDF1 gene in TM-1 and 3-79, gene-specific primers (PDF1-full-F/R) were designed to amplify the full-length open reading frame using the genomic DNA as template. All the fragments were sequenced after being linked to T-vectors. The accession numbers of these genes can be found in Supplemental Table S3. The conserved domains of proteins were searched in the National Center for Biotechnology Information database (Marchler-Bauer et al., 2009). Transmembrane regions of GbPDF1 were predicted using the Web-based program HMMTOP (http://www.enzim.hu/hmmtop/).
To isolate the GbPDF1 promoter, two nested gene-specific primers (PDF1-GSP1 and PDF1-GSP2) were designed, and GenomeWalker technology (Clontech) was employed. According to the manufacturer’s protocol, eight fragments amplified in the secondary PCR were cloned in the pMD18-T plasmid (Takara) after purification (Qiagen) and then sequenced. The promoters of GbPDF1 were predicted with the computer program TSSP (http://www.softberry.com), and the putative cis-elements were identified from the PLACE database (Higo et al., 1999).
For phylogenetic analysis, the GbPDF1-homologous peptides were aligned with the ClustalX program (Thompson et al., 1997), and then maximum parsimony analysis was performed with MEGA4 (Tamura et al., 2007) using the neighbor-joining method with default parameters.
Subcellular Location of GbPDF1 in Arabidopsis Root Cells and Tobacco
The full-length cDNA of GbPDF1 without a stop codon was constructed to pGWB451 through recombination reaction (Nakagawa et al., 2007). The resulting construct consisted of GbPDF1 fused to the N terminus of G3GFP under the control of the CaMV 35S promoter. The construct was transferred into Agrobacterium tumefaciens strain GV3101 by electroporation for future Arabidopsis transformation (Clough and Bent, 1998) or transient expression in tobacco (Nicotiana benthamiana) via agroinfiltration (Sainsbury and Lomonossoff, 2008).
Green fluorescence of GbPDF1:G3GFP was detected in the root cells of 10-d-old Arabidopsis after plasmolysis with 4% (w/v) NaCl for 10 min as well as in the epidermal cell layer of tobacco leaves. Images were observed using a Leica Microsystems TCS SP2 AOBS confocal microscope.
Plasmid Construction and Plant Transformation
To construct the overexpression vector, a pair of primers (OE-PDF1-F/R) with added XbaI and SacI restriction sites, respectively, was used to amplify GbPDF1. The amplified product was ligated into pCAMBIA 2300S vector (Munis et al., 2010).
Two RNAi vectors were constructed to pHellsgate 4 through recombination reaction and checked by digestion with XhoI and XbaI, respectively (Helliwell et al., 2002). PDF1-RNAi-C was designed from the 24th to 621st nucleotides after the transcription start site ATG of GbPDF1. PDF1-RNAi-U carried the 3′ untranslated region-containing fragment, which consisted of the 875th to 1,008th nucleotides of GbPDF1 cDNA.
The 5′ flanking fragments were prepared by PCR with specific forward primers containing a HindIII site and reverse primers containing an XbaI site and then moved to pBI121 (Clontech) at HindIII-XbaI sites by replacing the CaMV 35S to produce PGbPDF1-1::GUS and PGbPDF1-2::GUS. The PGbPDF1-1 fragment was used to replace the CaMV 35S of pBINm-gfp5-ER to generate PGbPDF1-1::GFP (Haseloff et al., 1997). The cloned fragments were verified by sequencing. ΔBCD, ΔCD, and ΔD were constructed in the same manner as PGbPDF1-1::GUS. The locations of regions A, B, C, and D are shown in Figure 5A.
For further analysis of the cis-elements located within region B, the whole of region B and regions spanning from −186 to −178, −166 to −159, and −153 to −149 bp were deleted from PGbPDF1-1 to produce ΔB, ΔAT9, ΔL1, and ΔHD promoters, respectively. The upstream region of the deleted sites was amplified with HindIII (forward primer)-PstI (reverse primer) and cloned to the pUC19 vector after being digested, then the PstI-XbaI-containing downstream region of the deleted elements was inserted at the corresponding site. The modified promoters were fused with GUS on the base of pBI121.
The overexpression vector, RNAi vectors, PGbPDF1::GUS, ΔBCD, ΔCD, ΔD, and PGbPDF1-1::GFP were introduced into cotton (YZ1) plants by A. tumefaciens-mediated (EHA105 and LBA4404) transformation as described by Jin et al. (2006) and Li et al. (2010). After regeneration, transformed cotton lines were checked by PCR (V-RNAi-F/R for RNAi constructs and V-gus-F/R for GUS-fused vectors) and Southern blotting.
PGbPDF1-1::GUS, PGbPDF1-2::GUS, ΔB, ΔAT9, ΔL1, ΔHD, and pBI121 were transformed to Arabidopsis ecotype Columbia plants by the floral dip method (Clough and Bent, 1998). The transgenic Arabidopsis was selected on half-agar MS plates (Murashige and Skoog, 1962) with 30 mg L−1 kanamycin and then transplanted to soil. The number of transgenic plants obtained is listed in Supplemental Table S4.
Western Blotting
A polyclonal antiserum was raised in rabbit against a synthetic peptide corresponding to residues 1 to 13 (MERQRSKQVCLLM) of GbPDF1 (Neweast Bioscience). Proteins were extracted according to Li et al. (2005) with slight modification. The proteins were collected from the ovules and fibers in extraction buffer containing 50 mm Tris-HCl (pH 8.0), 0.5 mm CaCl2, 0.1% β-mercaptoethanol, 0.5% Triton X-100, and 1 mm phenylmethylsulfonyl fluoride. Western-blot experiments were performed as reported by Hu et al. (2011).
Scanning Electron Microscopy and Fiber Quality Measurement
To compare the fiber initiation difference between PDF1-suppressed and wild-type plants, the cotton ovules around the day of anthesis were collected simultaneously from similar positions on cotton plants and fixed in 2.5% (v/v) glutaraldehyde. After dehydration in an ethanol series, the samples were transferred into isoamyl acetate and dried at the critical point. The cotton ovules were viewed and photographed with a JSM-6390/LV scanning electron microscope (JEOL). The transformed plants that carried the PGbPDF1-1::GFP were used as the transgenic control. After ginning, three independent fiber samples and cotton seeds from each T2 generation of PDF1-RNAi transgenic lines and wild-type cotton were weighed to determine the lint percentage (fiber weight/seed cotton weight), and the fibers were then sent to the Center of Cotton Fiber Quality Inspection and Testing, Chinese Ministry of Agriculture, for quality measurement. Data were processed with Student’s t test in Microsoft Excel.
Quantification of H2O2 Levels
The fiber-bearing ovules (0.5 g fresh weight) at 3 DPA from wild-type and PDF1-silenced plants were sampled for H2O2 measurement. H2O2 content was determined according to the production of H2TiO4 using TiCl4 as the substrate (Li et al., 2007). The concentration was expressed as μm g−1 fresh ovules.
Yeast Two-Hybrid Screening
The yeast two-hybrid assay was performed using the ProQuest Two-Hybrid System (Invitrogen). The bait vectors with full-length or partial cDNA of GbPDF1 fused to the GAL4-DNA-binding domain in the pDEST32 vector were generated. A prey library of cotton ovule and fiber (0-DPA ovule; 5-, 10-, and 15-DPA fiber) was constructed by fusing cDNAs with the GAL4 activation domain in the pDEST22 vector according to the manufacturer’s instructions. The yeast strain MaV203 carrying the full-length GbPDF1 bait vector was transformed with the plasmid DNA of the prey cDNA library. The transformants were selected on synthetic complete selection medium containing 20 mm 3-amino-1,2,4-triazole (Sigma) but lacking Leu, Trp, and His. The positive clones were isolated and retransformed to different bait strains to test their interaction, using empty vector as the control.
Bimolecular Fluorescence Complementation
The native and N domain (residues 1–30) deleted GbPDF1 was cloned into the SpeI-KpnI site of pVYNE(R) vector (Waadt et al., 2008) to obtain VenusN:GbPDF1 and VenusN:GbPDF1 ΔN fusion. The cDNAs of PPIP1, PPIP2, and PPIP3 were cloned into the SpeI-XhoI or SpeI-KpnI site of pVYCE(R) to obtain VenusC fusion. The interactions were detected through polyethylene glycol-calcium transfection of plasmid DNA in the protoplast of rice (Oryza sativa; Yoo et al., 2007). The fluorescence was assayed using a Leica Microsystems laser-scanning microscope.
GUS Assay of the Transgenic Plant Tissues
Histochemical localization of GUS activity was performed according to the protocol reported by Sessions et al. (1999). Tissues were prefixed in prechilled 80% (v/v) acetone for 30 min and infiltrated into staining solution (0.9 g L–1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid, 50 mm sodium phosphate buffer [pH 7.0], 20% [v/v] methanol, and 100 mg L–1 chloromycetin) under vacuum for 15 min and then moved to 37°C for 1 to 12 h. The chlorophyll was decolorized at 37°C with 75% to 95% (v/v) ethanol and fixed in 50% ethanol, 5% acetic acid, and 3.7% formaldehyde. Photographs were captured with an anatomy microscope (Leica Microsystems) or a Nikon D40 camera. The stained samples were cut into 8-μm-thick sections and examined using the protocol reported by Zhu et al. (2008). The quantitative analyses of GUS activity was expressed as pmol 4-methylumbelliferone mg−1 protein min−1, according to Cai et al. (2008). Fluorescence was measured at an excitation wavelength of 365 nm and an emission wavelength of 455 nm using the Infinite 200 PRO multimode reader (Tecan).
Electrophoretic Mobility Shift Assay
The isolation and quantification of nuclear extract from reproductive tissues of Arabidopsis and 5-DPA fiber-bearing cotton ovules was applied as described previously (Qiu et al., 2007). Gel mobility shift assay was applied using the protocol reported by Dai et al. (2007). The synthetic DNA probes were generated by mixing the two complementary oligonucleotides and labeled with [32P]dCTP using the Klenow fragment (New England Biolabs). DNA-binding reactions were performed in a 30-μL mixture. The mixture contained 10 mm Tris-HCl (pH 7.5), 50 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 1 mm MgCl2, 5% (v/v) glycerol, 3.5 μg of poly(dI-dC)-poly(dI-dC) (Sigma), 10 μg of the nuclear extract, and 1 ng of the probes. After incubation at room temperature for 20 min, the mixture was separated on 12% polyacrylamide gels in Tris-Gly buffer (25 mm Tris, 2 mm EDTA, and 380 mm Gly).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Genomic organization of PDF1 genes in cotton.
Supplemental Figure S2. Conserved C terminus was found among the PDF1 proteins from different sources of plants by sequence alignment.
Supplemental Figure S3. Subcellular localization of GbPDF1 through the transient and stable expression of the GbPDF1:G3GFP reporter construct.
Supplemental Figure S4. Fast-proliferated nonembryogenic callus with GbPDF1 overexpression.
Supplemental Figure S5. Southern blotting of transformed cotton plants.
Supplemental Figure S6. Reduced expression of PDF1 in T1 transformed cotton resulted in retarded initiation.
Supplemental Figure S7. Expression patterns of GT8, GT8-like, and PPIPs during fiber development.
Supplemental Figure S8. The control transformants for yeast two-hybrid and β-Gal assay results.
Supplemental Figure S9. The GUS activity in anther and style driven by the GbPDF1 promoters.
Supplemental Figure S10. Electrophoretic mobility shift assay results showed no interaction between P1 or P2 and extracts from Arabidopsis.
Supplemental Table S1. The proteins identified by yeast two-hybrid library screening.
Supplemental Table S2. Oligonucleotides used in this study.
Supplemental Table S3. Accession numbers of the sequences in this article.
Supplemental Table S4. The number of transgenic plants obtained in this study.
Supplementary Material
Acknowledgments
We are grateful to Dr. Jörg Kudla (Universität Münster) and Nakagawa Tsuyoshi (Shimane University) for kindly providing plasmids. Critical reviews and comments from Prof. Keith Lindsey (Durham University) and Qifa Zhang (National Key Laboratory of Crop Genetic Improvement) are appreciated. The technical assistance from Lihong Qin (Huazhong Agricultural University) for scanning electron microscopy and Xiaoyun Liu (National Key Laboratory of Crop Genetic Improvement) for bimolecular fluorescence complementation is especially appreciated.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T. (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Abe M, Takahashi T, Komeda Y. (1999) Cloning and characterization of an L1 layer-specific gene in Arabidopsis thaliana. Plant Cell Physiol 40: 571–580 [DOI] [PubMed] [Google Scholar]
- Abe M, Takahashi T, Komeda Y. (2001) Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J 26: 487–494 [DOI] [PubMed] [Google Scholar]
- Basra A, Malik CP. (1984) Development of the cotton fiber. Int Rev Cytol 89: 65–113 [Google Scholar]
- Cai M, Qiu D, Yuan T, Ding X, Li H, Duan L, Xu C, Li X, Wang S. (2008) Identification of novel pathogen-responsive cis-elements and their binding proteins in the promoter of OsWRKY13, a gene regulating rice disease resistance. Plant Cell Environ 31: 86–96 [DOI] [PubMed] [Google Scholar]
- Ciarbelli AR, Ciolfi A, Salvucci S, Ruzza V, Possenti M, Carabelli M, Fruscalzo A, Sessa G, Morelli G, Ruberti I. (2008) The Arabidopsis homeodomain-leucine zipper II gene family: diversity and redundancy. Plant Mol Biol 68: 465–478 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dai M, Zhao Y, Ma Q, Hu Y, Hedden P, Zhang Q, Zhou DX. (2007) The rice YABBY1 gene is involved in the feedback regulation of gibberellin metabolism. Plant Physiol 144: 121–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerks T, Strauss M, Brendel M, Bork P. (2000) GRAM, a novel domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Trends Biochem Sci 25: 483–485 [DOI] [PubMed] [Google Scholar]
- Fridborg I, Grainger J, Page A, Coleman M, Findlay K, Angell S. (2003) TIP, a novel host factor linking callose degradation with the cell-to-cell movement of Potato virus X. Mol Plant Microbe Interact 16: 132–140 [DOI] [PubMed] [Google Scholar]
- Funakoshi M, Sasaki T, Nishimoto T, Kobayashi H. (2002) Budding yeast Dsk2p is a polyubiquitin-binding protein that can interact with the proteasome. Proc Natl Acad Sci USA 99: 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou JY, Wang LJ, Chen SP, Hu WL, Chen XY. (2007) Gene expression and metabolite profiles of cotton fiber during cell elongation and secondary cell wall synthesis. Cell Res 17: 422–434 [DOI] [PubMed] [Google Scholar]
- Graves DA, Stewart JM. (1988a) Analysis of the protein constituency of developing cotton fibres. J Exp Bot 39: 59–69 [Google Scholar]
- Graves DA, Stewart JM. (1988b) Chronology of the differentiation of cotton (Gossypium hirsutum L.) fiber cells. Planta 175: 254–258 [DOI] [PubMed] [Google Scholar]
- Haseloff J, Siemering KR, Prasher DC, Hodge S. (1997) Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc Natl Acad Sci USA 94: 2122–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Wesley SV, Wielopolska AJ, Waterhouse PM. (2002) High-throughput vectors for efficient gene silencing in plants. Funct Plant Biol 29: 1217–1225 [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Yang X, Yuan D, Zeng F, Zhang X. (2011) GhHmgB3 deficiency deregulates proliferation and differentiation of cells during somatic embryogenesis in cotton. Plant Biotechnol J 9: 1038–1048 [DOI] [PubMed] [Google Scholar]
- Ishida T, Kurata T, Okada K, Wada T. (2008) A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59: 365–386 [DOI] [PubMed] [Google Scholar]
- Javelle M, Vernoud V, Rogowsky PM, Ingram GC. (2011) Epidermis: the formation and functions of a fundamental plant tissue. New Phytol 189: 17–39 [DOI] [PubMed] [Google Scholar]
- Jin S, Zhang X, Nie Y, Guo X, Liang S, Zhu H. (2006) Identification of a novel elite genotype for in vitro culture and genetic transformation of cotton. Biol Plant 50: 519–524 [Google Scholar]
- Kong Y, Zhou G, Yin Y, Xu Y, Pattathil S, Hahn MG. (2011) Molecular analysis of a family of Arabidopsis genes related to galacturonosyltransferases. Plant Physiol 155: 1791–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JJ, Hassan OS, Gao W, Wei NE, Kohel RJ, Chen XY, Payton P, Sze SH, Stelly DM, Chen ZJ. (2006) Developmental and gene expression analyses of a cotton naked seed mutant. Planta 223: 418–432 [DOI] [PubMed] [Google Scholar]
- Lee JJ, Woodward AW, Chen ZJ. (2007) Gene expression changes and early events in cotton fibre development. Ann Bot (Lond) 100: 1391–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Qin YM, Pang Y, Song WQ, Mei WQ, Zhu YX. (2007) A cotton ascorbate peroxidase is involved in hydrogen peroxide homeostasis during fibre cell development. New Phytol 175: 462–471 [DOI] [PubMed] [Google Scholar]
- Li XB, Fan XP, Wang XL, Cai L, Yang WC. (2005) The cotton ACTIN1 gene is functionally expressed in fibers and participates in fiber elongation. Plant Cell 17: 859–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu D, Tu L, Zhang X, Wang L, Zhu L, Tan J, Deng F. (2010) Suppression of GhAGP4 gene expression repressed the initiation and elongation of cotton fiber. Plant Cell Rep 29: 193–202 [DOI] [PubMed] [Google Scholar]
- Luo H, Song F, Goodman RM, Zheng Z. (2005) Up-regulation of OsBIHD1, a rice gene encoding BELL homeodomain transcriptional factor, in disease resistance responses. Plant Biol (Stuttg) 7: 459–468 [DOI] [PubMed] [Google Scholar]
- Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES. (2009) The MYB transcription factor GhMYB25 regulates early fibre and trichome development. Plant J 59: 52–62 [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C, Fong JH, Geer LY, Geer RC, Gonzales NR, Gwadz M, et al. (2009) CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res 37: D205–D210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohnen D. (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11: 266–277 [DOI] [PubMed] [Google Scholar]
- Munis MF, Tu L, Deng F, Tan J, Xu L, Xu S, Long L, Zhang X. (2010) A thaumatin-like protein gene involved in cotton fiber secondary cell wall development enhances resistance against Verticillium dahliae and other stresses in transgenic tobacco. Biochem Biophys Res Commun 393: 38–44 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15: 473–497 [Google Scholar]
- Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, et al. (2007) Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71: 2095–2100 [DOI] [PubMed] [Google Scholar]
- Ohgishi M, Oka A, Morelli G, Ruberti I, Aoyama T. (2001) Negative autoregulation of the Arabidopsis homeobox gene ATHB-2. Plant J 25: 389–398 [DOI] [PubMed] [Google Scholar]
- Oku M, Warnecke D, Noda T, Müller F, Heinz E, Mukaiyama H, Kato N, Sakai Y. (2003) Peroxisome degradation requires catalytically active sterol glucosyltransferase with a GRAM domain. EMBO J 22: 3231–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang CY, Wang H, Pang Y, Xu C, Jiao Y, Qin YM, Western TL, Yu SX, Zhu YX. (2010) Comparative proteomics indicates that biosynthesis of pectic precursors is important for cotton fiber and Arabidopsis root hair elongation. Mol Cell Proteomics 9: 2019–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YM, Hu CY, Zhu YX. (2008) The ascorbate peroxidase regulated by H2O2 and ethylene is involved in cotton fiber cell elongation by modulating ROS homeostasis. Plant Signal Behav 3: 194–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin YM, Zhu YX. (2011) How cotton fibers elongate: a tale of linear cell-growth mode. Curr Opin Plant Biol 14: 106–111 [DOI] [PubMed] [Google Scholar]
- Qiu D, Xiao J, Ding X, Xiong M, Cai M, Cao Y, Li X, Xu C, Wang S. (2007) OsWRKY13 mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20: 492–499 [DOI] [PubMed] [Google Scholar]
- Rothenberg C, Monteiro MJ. (2010) Ubiquilin at a crossroads in protein degradation pathways. Autophagy 6: 979–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan YL, Chourey PS. (1998) A fiberless seed mutation in cotton is associated with lack of fiber cell initiation in ovule epidermis and alterations in sucrose synthase expression and carbon partitioning in developing seeds. Plant Physiol 118: 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury F, Lomonossoff GP. (2008) Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol 148: 1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa S, Ohgishi M, Goda H, Higuchi K, Shimada Y, Yoshida S, Koshiba T. (2002) The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J 32: 1011–1022 [DOI] [PubMed] [Google Scholar]
- Scheible WR, Pauly M. (2004) Glycosyltransferases and cell wall biosynthesis: novel players and insights. Curr Opin Plant Biol 7: 285–295 [DOI] [PubMed] [Google Scholar]
- Sessions A, Weigel D, Yanofsky MF. (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J 20: 259–263 [DOI] [PubMed] [Google Scholar]
- Shen G, Kuppu S, Venkataramani S, Wang J, Yan J, Qiu X, Zhang H. (2010) ANKYRIN REPEAT-CONTAINING PROTEIN 2A is an essential molecular chaperone for peroxisomal membrane-bound ASCORBATE PEROXIDASE3 in Arabidopsis. Plant Cell 22: 811–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YH, Zhu SW, Mao XZ, Feng JX, Qin YM, Zhang L, Cheng J, Wei LP, Wang ZY, Zhu YX. (2006) Transcriptome profiling, molecular biological, and physiological studies reveal a major role for ethylene in cotton fiber cell elongation. Plant Cell 18: 651–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindler C, Matteucci A, Sessa G, Weimar T, Ohgishi M, Aoyama T, Morelli G, Ruberti I. (1999) Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development 126: 4235–4245 [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24: 1596–1599 [DOI] [PubMed] [Google Scholar]
- Tang W, Perry SE. (2003) Binding site selection for the plant MADS domain protein AGL15: an in vitro and in vivo study. J Biol Chem 278: 28154–28159 [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu LL, Zhang XL, Liang SG, Liu DQ, Zhu LF, Zeng FC, Nie YC, Guo XP, Deng FL, Tan JF, et al. (2007) Genes expression analyses of sea-island cotton (Gossypium barbadense L.) during fiber development. Plant Cell Rep 26: 1309–1320 [DOI] [PubMed] [Google Scholar]
- Tusnády GE, Simon I. (2001) The HMMTOP transmembrane topology prediction server. Bioinformatics 17: 849–850 [DOI] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J. (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Walford SA, Wu Y, Llewellyn DJ, Dennis ES. (2011) GhMYB25-like: a key factor in early cotton fibre development. Plant J 65: 785–797 [DOI] [PubMed] [Google Scholar]
- Wang S, Wang JW, Yu N, Li CH, Luo B, Gou JY, Wang LJ, Chen XY. (2004) Control of plant trichome development by a cotton fiber MYB gene. Plant Cell 16: 2323–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Llewellyn DJ, White R, Ruggiero K, Al-Ghazi Y, Dennis ES. (2007) Laser capture microdissection and cDNA microarrays used to generate gene expression profiles of the rapidly expanding fibre initial cells on the surface of cotton ovules. Planta 226: 1475–1490 [DOI] [PubMed] [Google Scholar]
- Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES. (2006) Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant Cell Physiol 47: 107–127 [DOI] [PubMed] [Google Scholar]
- Yang S, Cheung F, Lee JJ, Ha M, Wei NE, Sze SH, Stelly DM, Thaxton P, Triplett B, Town CD, et al. (2006) Accumulation of genome-specific transcripts, transcription factors and phytohormonal regulators during early stages of fiber cell development in allotetraploid cotton. Plant J 47: 761–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zhang F, Zuo K, Zhang J, Liu X, Zhang L, Sun X, Tang K. (2010) An L1 box binding protein, GbML1, interacts with GbMYB25 to control cotton fibre development. J Exp Bot 61: 3599–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zheng X, Song S, Zeng Q, Hou L, Li D, Zhao J, Wei Y, Li X, Luo M, et al. (2011) Spatiotemporal manipulation of auxin biosynthesis in cotton ovule epidermal cells enhances fiber yield and quality. Nat Biotechnol 29: 453–458 [DOI] [PubMed] [Google Scholar]
- Zhu H, Tu L, Jin S, Xu L, Tan J, Deng F, Zhang X. (2008) Analysis of genes differentially expressed during initial cellular dedifferentiation in cotton. Chin Sci Bull 53: 3666–3676 [Google Scholar]
- Zhu L, Tu L, Zeng F, Liu D, Zhang X. (2005) An improved simple protocol for isolation of high quality RNA from Gossypium spp. suitable for cDNA library construction. Acta Agron Sin 31: 1657–1659 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.