Abstract
Progressive augmentation of behavioral response following repeated psychostimulant administrations is known as behavioral sensitization, and is an indicator of a drug’s liability for abuse. It is known that methylphenidate (MPD) (also known as Ritalin), a drug used to treat Attention-Deficit Hyperactivity Disorder (ADHD), induces sensitization in animals following repeated injections. It was recently reported that bilateral electric (non-specific) lesion of prefrontal cortex (PFC) prevented MPD elicited behavioral sensitization. Since PFC sends glutamatergic afferents to both ventral tegmental area (VTA) and nucleus accumbens (NAc), sites that are involved in induction and expression of behavioral sensitization respectively and glutamate from PFC is known to modulate dopamine cell activity in VTA and NAc, this study investigated the role of descending glutamate from PFC in MPD elicited behavioral sensitization. Locomotor activity of three groups of rats- control, sham operated and group with specific chemical lesion of glutamate neurons of PFC- was recorded using an open-field assay. On experimental day (ED) 1, the locomotor activity was recorded post a saline injection. The sham and lesion groups underwent respective surgeries on ED 2, and were allowed to recover for five days (from ED 3 to ED 7). The post-surgery baseline was recorded on ED 8 following a saline injection. On ED’s 9 through 14, 2.5 mg/kg MPD was given, followed by a four day washout period (ED 15 –18). All three groups received a rechallenge injection of 2.5 mg/kg on ED 19 and their locomotor activity on various days was analyzed. It was found that ibotenic acid lesion modulated the acute and chronic effects of MPD and hence suggests that PFC glutamatergic afferents are involved in the acute effect of MPD as well as in its chronic effects such as behavioral sensitization to MPD.
Keywords: Ritalin (Methylphenidate), PFC lesion, Locomotor activity, ibotenic acid
1. Introduction
The prefrontal cortex (PFC) is a multi-modal association area involved in working memory, directing actions towards goals, and personality expression (Goldman-Rakic, 1978). The function of PFC is compromised in Attention Deficit Hyperactivity Disorder (ADHD), a neuropsychiatric behavioral disorder characterized by developmentally inappropriate inattention, hyperactivity, and impulsivity (Beiderman et al., 2008; Faraone and Beiderman, 1998; Zuddas et. al., 2000). The neuropsychological deficits found in ADHD children involve issues with executive operations and working memory, which are functions of PFC (Faraone and Beiderman, 1998). Recently, Lee et. al. (2008) reported that the PFC is involved in behavioral sensitization to methylphenidate (MPD), the drug of choice for treating ADHD (Askenasy et. al., 2007; Swanson et. al., 1998, 1999). Behavioral sensitization is the long-lasting pharmacologically elicited hypersensitivity following repetitive psychostimulant administration (Dafny and Yang, 2006; Gaytan et. al., 1997; Kalivas and Stewart, 1991; Lee et. al., 2008; Robinson, 1984; Robinson & Berridge, 1993; Wolf, 1998; Yang et. al., 2003). It is considered to be an experimental model for compulsive drug seeking behavior and an indication of the liability potential of the drug (Dafny & Yang, 2006; Robinson & Berridge, 1993; Wolf, 1998).
The PFC sends glutamatergic afferents to both ventral tegmental area (VTA) and nucleus accumbens (NAc), sites that are involved in induction and expression of behavioral sensitization to psychostimulants (Kalivas et. al., 2000) and is one of the major source of excitatory amino acid (EAA) input to VTA and NAc (Sesack and Pickel, 1992). It was reported that PFC glutamatergic afferents modulate the NAc and VTA dopaminergic neurons (Imperato et. al., 1990; Kalivas and Stewart, 1991; Kalivas et. al., 1993). It is suggested that glutamate from PFC enhances dopamine cell activity in the VTA, which in turn synergizes with the psychostimulant-induced increase in DA transmission in NAc to promote psychostimulant-induced behavior (Kalivas, 2000). Thus, we hypothesize that eliminating glutamate from PFC might affect the acute and/or chronic effects of MPD.
Studies with other psychostimulants like amphetamine and cocaine, which share chemical and pharmacological properties with MPD (Askenasy et. al., 2007; Kollins et. al., 2001), have demonstrated the role of glutamatergic afferents of PFC in behavioral sensitization (Li et. al., 1999; Pierce et. al., 1998; Ramos et. al., 2005; Wolf et. al., 1995; Wolf, 1998). Glutamate receptor blockade prevents the development of behavioral sensitization to repetitive amphetamine and cocaine administration (Wolf, 1998). Glutamate antagonist injections or destroying glutamate cells of PFC with the neurotoxin ibotenic acid have prevented the expression of behavioral sensitization to methamphetamine, amphetamine and cocaine treatments (Li et. al., 1999; Pierce et. al., 1998; Ramos et. al., 2005; Wolf et. al., 1995). Additionally, alterations in glutamate transmission are associated with induction and expression of behavioral sensitization to amphetamine and cocaine (White et. al., 1995).
Previous dose response studies (Gaytan et. al., 1996, 1997,Gaytan et. al., 2000a, 2000b; Yang et. al., 2006, 2007) using open field assay demonstrated that 2.5mg/kg MPD (i.p.) elicits behavioral sensitization. Therefore, this dose was selected in this study. The objective of the study was to examine the role of PFC glutamatergic afferents in acute and chronic effects of MPD. Locomotor activity, measured with open field assay, of three male adult rat groups: control, sham-operated and ibotenic acid injected into the PFC groups was used to study the effects of bilateral selective glutamatergic neuronal lesions of PFC on MPD induced locomotion and behavioral sensitization.
2. Results
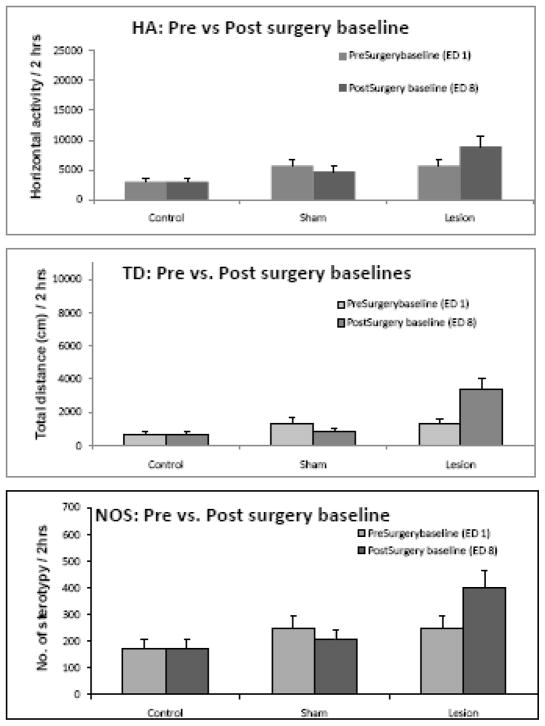
Effect of surgery on baseline activity – Comparing ED 8 with ED 1
Two sham operations were performed. In sham group 1, the rats were anesthetized, the skin and the scalp muscles were removed and the bregma was exposed, while for sham group 2, in addition to the incision under general anesthesia, holes were drilled above the PFC and saline was injected into the PFC in the same volume and speed as the ibotenic acid injection. It was observed that both the sham operations had no effect on baseline activity in all indices analyzed i.e., horizontal activity (HA), total distance (TD) and number of stereotypy (NOS). Since the two sham groups exhibited similar activity throughout the experiment, their data is presented as one group. (Fig. 2). The ibotenic acid lesion in PFC group exhibited higher locomotor activity in HA, TD and NOS, which however did not reach significance (Fig. 2). Therefore, the locomotor activity after surgical manipulations (post-surgery baseline – ED 8) was used as control to study the drug effects.
Figure 2.
summarizes the baseline locomotor activity for experimental day (ED) 1 (pre-surgery baseline) and ED 8 (post-surgery baseline, see Table 1) for control, sham and lesion groups. The surgery caused non-significant fluctuations in all three indices for the sham group, whereas the ibotenic acid lesions of PFC caused augmented activity in horizontal activity (HA), total distance (TD), and number of stereotypy (NOS) for the lesion group.
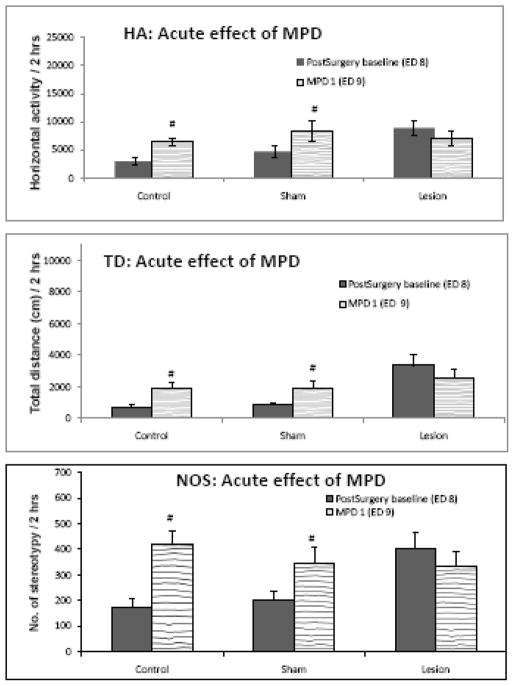
Effect of acute MPD administration – Comparing ED 9 to ED 8
Figure 3 summarizes the initial (acute) effect of administrating 2.5 mg/kg of MPD to all three groups. Comparing horizontal activity on ED 8 (post-surgery baseline; Table 1) to ED 9 (1st injection of MPD), indicated that 2.5 mg/kg MPD elicits a significant ( F = 4.632; p < 0.05) increase in locomotor activity in control and sham groups in all three locomotor indices studied. However, MPD in the ibotenic acid lesion group did not alter the locomotor activity (Fig. 3) of this group in any of the locomotor indices analyzed as compared to the initial pre-drug treatment recording (ED 8).
Figure 3.
displays the effect of acute injection of MPD on all three rat groups. It was observed that a single dose of 2.5mg/kg MPD elicits a significant increase in activity in all three locomotor indices (HA, TD, and NOS) in control and sham groups. This increase in activity following the MPD injection was not observed in the ibotenic acid in PFC (lesion) group. # indicates significance (p < 0.05) compared to ED 8.
Table 1.
Table 1 describes the experimental protocol. Pre and Post surgery baselines were recorded on experimental days (EDs) 1 and 8 after a saline injection. The lesion and sham groups underwent surgery on ED 2 and were given five days to recover. After establishing the post surgery baseline on ED 8, all three groups received 2.5mg/kg MPD injection once daily from ED 9 to ED 14. A washout period followed from EDs 15 to 18 where behavior was recorded at the time of previous day injections but no injections were administered. Rechallenge injections of 2.5 mg/kg MPD was given on ED 19.
| Group | ED 1 | ED 2 | EDs 3–7 | ED 8 | EDs 9–4 | EDs 15–18 | ED 19 |
|---|---|---|---|---|---|---|---|
| Control | Saline | - | - | Saline | 2.5mg/kg MPD | No treatment | 2.5mg/kg MPD |
| Shams 1 & 2 | Saline | Surgery | - | Saline | 2.5mg/kg MPD | No treatment | 2.5mg/kg MPD |
| Ibotenic Acid Lesion Group | Saline | Surgery | - | Saline | 2.5mg/kg MPD | No treatment | 2.5mg/kg MPD |
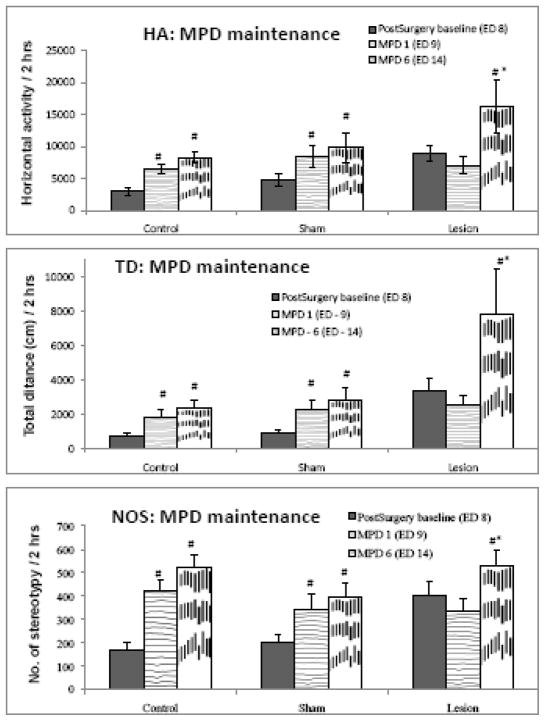
Effect of MPD administration in the induction phase (comparing ED 14 to ED 9)
Figure 4 compares the locomotor activity of the three groups after six daily injections of 2.5 mg/kg MPD to that after the initial injection of MPD (ED 9) and shows that MPD administration on ED14 elicits significant (F = 4.441; p < 0.05) increase on locomotion (HA, TD and NOS) of the ibotenic acid lesion group compared to ED9. The control and sham groups exhibited augmented activity that was significantly (F = 3.233; p < 0.05) greater than their post-surgery baseline.
Figure 4.
compares the locomotor activity after the 6th consecutive injection of MPD (ED 14) to the locomotor activity after the acute (1st) injection of MPD (ED 9) and post-surgery baseline (ED 8). The control and sham groups exhibited significantly higher activity following the 1st injection of MPD and this increase was maintained following the 6th injection of MPD in all three indices. The lesion group, however, did not exhibit increased activity following the 1st injection, but had significantly elevated activity following the 6th injection of MPD in HA, TD and NOS. # indicates significance (p < 0.05) compared to ED 8; * - indicates significance (p < 0.05) compared to ED 9.
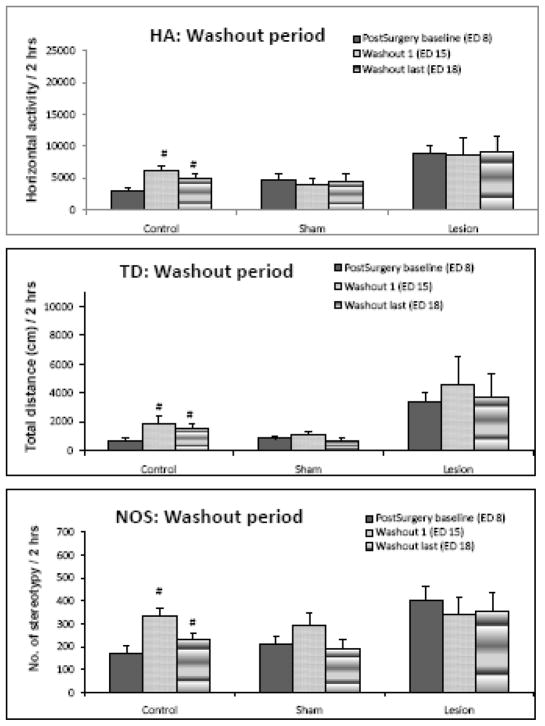
Locomotor activity during the washout phase (comparing ED’s 15 and 18 with ED 8)
Figure 5 summarizes the locomotor activity of ED 8, 15 and 18 for two hours at the time of previous injections i.e., 06:45 to 08:45. It was found that there was a significant (F = 10.562; p< 0.05) increase in all three indices of locomotor activity on the first and last days of washout in the control group. Sham and ibotenic acid lesion in PFC group did not display any significant increase in locomotor activity on any washout days in any of the three locomotor indices (Fig 5).
Figure 5.
summarizes the locomotor activity during the washout period. The control group had significantly (p < 0.05) elevated activity on both the first and last day of washout. The sham and lesion groups exhibited activity similar to the post-surgery baseline activity throughout the washout days. # indicates significance (p < 0.05) compared to ED 8.
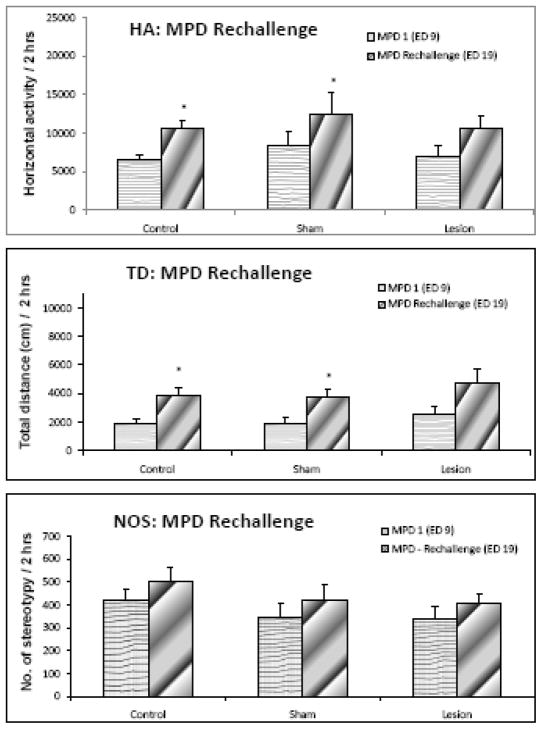
Effect of MPD rechallenge (comparing ED 19 to ED 9)
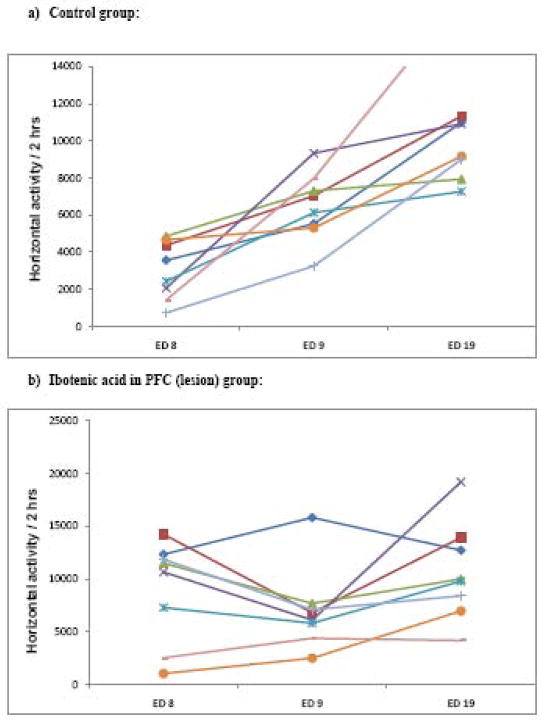
Figure 6 summarizes the observations noted on ED 19 (the last day of MPD injection; Table 1) compared to ED 9 and shows that MPD injection on ED 19 elicited significant (F = 5.823; p < 0.05) increases in horizontal activity and total distance in the control and sham groups. In the lesion group, the rechallenge injection of MPD (on ED 19) elicited an increase in horizontal activity and total distance travelled but was found to be statistically not significant. Number of stereotypy exhibited non-significant increases in all three groups. Figure 7a shows the individual animal responses of the control group to the acute (ED 9) and rechallenge injection (ED 19) of MPD, as compared to the post surgery baseline (ED 8) and indicates that the animals exhibited an increase in locomotion following the acute injection of MPD (ED 9) and that the locomotor response to the rechallenge injection (ED 19) was even higher than that observed on ED 9 (i.e., the animals were sensitized), while figure 7b indicates that the individual animal responses in the ibotenic acid lesion group 1) did not exhibit the increase in activity expected after an acute injection of MPD (ED 9) and 2) exhibited almost similar activity on both the acute as well as rechallenge injection of MPD (ED 19).
Figure 6.
summarizes the locomotor activity of the rechallenge injection of MPD (ED 19) compared to the initial effect of MPD on ED 9 of the three rat groups and shows that the control and sham group had significantly (p < 0.05) elevated activity post rechallenge injection in HA and TD. The lesion group exhibited non-significant increase in activity. NOS was not sensitized for any of the groups. * - indicates significance compared to ED 9.
Figure 7.
Figure 7a) summarizes and displays the trends of each animal in the control group (n = 8) to acute (ED 9) and rechallenge injection of MPD (ED 19) compared to post surgery baseline (ED 8). A significant increase in activity was observed after the acute injection of MPD (ED 9), and even further increase in activity (sensitization) was noted after the rechallenge injection of MPD (ED 19) in the control group.
Figure 7b shows similar summary of the individual responses of the rats in the ibotenic acid lesion group (n = 8) to the acute injection of MPD (ED9) and the MPD rechallenge injection (ED 19) compared to their response to their post surgery baseline (ED 8). It was observed that majority of the animals showed an attenuated locomotor response to the acute injection of MPD (ED 9) and the locomotor response to the rechallenge injections of MPD (ED 19) was similar to the post surgery baseline (ED 8). (Most animals did not express sensitization.)
3. Discussion
With the abuse of MPD on the rise, it is of much concern that not much is known about its circuitry of its action and/or its chronic effects such as behavioral sensitization, which is used as an experimental model for studying the liability of chronic use of a drug (Dafny and Yang, 2006). Lee et. al. (2008) reported recently that electrolytic lesioning of the PFC prevented behavioral sensitization to MPD. Since PFC is a significant source of excitatory amino acid (EAA) afferents to both VTA and NAc (Kalivas et. al., 2000), and is known to modulate the dopaminergic transmission at VTA and NAc (Imperato et. al., 1990; Kalivas and Stewart, 1991; Kalivas et. al., 1993), the role of PFC glutamatergic afferents in acute and chronic effects of MPD administration was studied via bilateral ibotenic acid infusions into PFC that selectively destroys the local glutamatergic neurons without disturbing other axon terminals (Arneri et. al., 1988). The main finding of this study was that PFC glutamatergic afferents are essential for the acute and chronic effects of MPD as assessed by the open field assay.
Locomotor activity measured after 5 days of bilateral ibotenic acid injections into PFC (post-surgery baseline) was augmented than the pre-surgery baseline but this augmentation was not statistically significant. Similar observations were reported by Jaskiw et. al. (1990) and Braun et. al. (1993) who also noted hyperactivity post ibotenic acid lesions of PFC. Thus, the ibotenic acid lesion group exhibited an accelerated post surgery baseline compared to the other two groups. Therefore, each group was compared to its own baseline.
The acute effect of MPD, as assessed by open field assay, was a significant increase in locomotion in the control and sham groups. However, this increase of activity in all the three locomotor indices analyzed was prevented by ibotenic acid PFC lesions. This suggests that the glutamatergic afferents of PFC or its connections downstream are crucial for the acute effects of MPD. Similar observations using another psychostimulant, namely amphetamine, were reported by Lipska et. al. (1998), while Volkow et. al. (1999) reported that ibotenic acid lesions of PFC did not alter the acute amphetamine-induced locomotor activity. And yet others found that ibotenic acid lesions of PFC caused an increased in acute response to amphetamine administration (Cador et. al., 1999; Jaskiw et. al., 1990). Lacroix et. al. (2000) found that the lighting of the room (that caused stress to the rats) determined the presence or absence of amphetamine-induced hyperactivity. Lee et. al. (2008) noted that the size of the lesions might have also contributed to the differences mentioned above.
The lack of MPD-induced hyperactivity in the PFC ibotenic acid lesion group may be attributed to the destruction of glutamatergic innervation of NAc by PFC since it has been reported that the cortico-limbic glutamate afferents to the NAc are important for rat exploratory behavior (Koob and Swerdlow, 1988; Maldonado-Irizarry and Kelley, 1994), which translates to increased locomotion. Therefore, the data may be interpreted that the hyper-locomotor activity induced by MPD in control and sham groups might be due to the activation of the same cortico-limbic glutamate afferents to NAc, and the destruction of glutamatergic afferents to NAc from PFC is what contributes to the failure of MPD - induced hyperlocomotion. However, there have been different reports about the glutamatergic innervations of NAc by PFC: while many refer to these innervations (Christie et. al., 1985; Kalivas, 2000; Sesack and Pickel, 1992; Imperato et. al., 1990; Kalivas and Stewart, 1991; Kalivas et. al., 1993; Lacroix et. al., 2000; Pierce et. al., 1998 ), few have reported that NAc-projecting DA neurons are not directly innervated by PFC glutamate terminals (Carr and Sesack, 2000; Gao et. al., 2007; Sesack et al., 2003). Either way, if not directly, there are reports of indirect glutamatergic innervations of NAc through VTA neurons. Thus, the ibotenic acid lesion of PFC might be contributing to the MPD-induced hypoactivity due to glutamate-DA interactions in the VTA than a simple excitation of DA neurons of NAc (Lacroix et. al., 2000).
It was reported by Gao et. al. (2007) that 1) stimulation of PFC activates DA neurons in VTA, whereas PFC inactivation produces the opposite effect (Gariano and Groves, 1988; Murase et al., 1993; Overton et al., 1996; Svensson and Tung, 1989; Tong et al., 1995). 2) Stimulating the PFC is also known to cause an increase extracellular DA levels in the NAc (Karreman and Moghaddam, 1996; Murase et al., 1993; Taber and Fibiger, 1995; Taber et al., 1995; You et al., 1998) and 3) this increase of extracellular DA levels in NAc is mitigated by glutamate antagonists administered into the VTA, but not into the NAc (Gao et. al., 2007). Thus, activation of DA neurons projecting to the NAc seems to be under the control of glutamate release in the VTA. Based on these findings, the current observations may be interpreted as Glutamate from PFC excites VTA dopaminergic neurons, which causes an increase in the DA release in NAc. The DA released in NAc inhibits the NAc outcome, which is to inhibit locomotion (Kafetzopoulos, 1986; Kelly & Roberts, 1983; Kelsey and Wilmore, 2006). Thus, inhibition of inhibitory outcome of NAc via excitatory afferents from PFC might be how MPD induces hyperactivity. Therefore, the elimination of the MPD-induced hyperactivity in the ibotenic acid group can then be understood as the loss of disinhibition of NAc which is an indirect result of the loss of excitatory output of PFC.
These results are, however, slightly perplexing since Lee et. al. (2008) reported that non-selective electrolytic lesioning of PFC did not alter the acute effect of MPD, while selective destruction of glutamatergic afferents of PFC eliminated this effect of MPD. But as noted by Lee et. al. (2008), it is possible that the electrolytic lesions may have a different effect on locomotion than that caused by selective chemical lesion because electrolytic lesions eliminate numerous efferent and afferent neuronal pathways. Thus, it seems that destruction of both glutamatergic and dopaminergic afferents from PFC does not change the acute effect of MPD (Lee et. al., 2008). However, elimination of only glutamatergic afferents of PFC eliminates the hyperactivity induced by the 1st injection of MPD. This might be conjectured to be due to changes in neurotransmitter balances caused by electrolytic lesion that somehow counter affects the loss of glutamatergic afferents of PFC.
For control and sham groups, there was increased activity on ED 14 as compared to ED 9 even though it did not reach significance. Although a sensitized response to MPD has been reported in the past (Dafny and Yang, 2006; Gaytan et. al., 1997; Yang et. al., 2007), it is known that repeated administrations of psychostimulants though typically produce sensitized behavioral responses, this response is not always present (Martin-Iverson et. al., 1988, Todtenkopf and Carlezon, 2006). However, the increase in locomotor activity on ED 14 was significantly higher than baseline (ED 8), and hence this increase might suggest sensitization. The lack of hyperactivity following the initial (acute) injection of MPD, however, did not prevent the increase in locomotor activity (Fig. 3) following the 6th daily MPD injection (chronic treatment of MPD, Yang et. al., 2007). This result can be interpreted in several ways: 1) the pathway mediating the acute effect is different from the one mediating the chronic effects, which implies that the neural circuitry of acute action of MPD is different than the neural circuitry that underlies behavioral sensitization and dependence or 2) as suggested by Jaskiw et al. (1990) the loss of glutamatergic innervations of NAc from PFC may have been perhaps compensated for by excitatory input from other structures (Christie et. al., 1986; Robinson and Beart, 1988; Walaas, 1981). The latter explanation of the observation is contingent on the thought that the two phenomena observed (lack and presence of MPD-induced hyperactivity on experimental days 9 and 14 respectively) are due to loss and compensation of glutamatergic innervation to NAc. Interestingly, the increase in locomotor activity after the 6th daily MPD injection suggested that the lack of hyperactivity in the lesion group following the 1st injection was not due to some sort of a ‘ceiling effect’ i.e., the possibility of an increase in locomotor activity was present but did not occur on ED 9. Moreover, the significant increase in activity following the 6th injection of MPD seen in the lesion group may be attributed to the fact that there was no increase in activity following the 1st injection in the ibotenic lesion group, while the control and sham groups had significantly higher locomotor activity post 1st injection of MPD too.
During the washout period, increased locomotor activity was observed on both the first and last washout day in the intact control group. This was interpreted as anticipation of getting the drug and/or withdrawal effect (Algahim et al, 2009; Yang et al, 2007). The sham and the lesion group didn’t display any change in baseline locomotor activity on neither the first nor the last day of washout. Since stress is known to affect memory (Chen et. al., 2008), the lack of withdrawal or drug anticipation might be attributed to the stress induced by the anesthesia and surgery that sham and lesion groups underwent.
Upon administration of the MPD rechallenge injection (ED 19), it was found that horizontal activity and total distance was significantly (p < 0.05) augmented compared to ED 9 in control and sham groups, i.e., behavioral sensitization was expressed on ED 19. For the lesion group, non-significant increases in HA and TD were observed. However, the individual responses indicated that majority of the animals exhibited similar locomotor activity on ED 8 (post surgery baseline), ED 9 (1st injection of MPD), and ED 19 (rechallenge injection of MPD) (Figure 7). These results, taken in conjecture with Gaytan et. al.’s (2000b) observations using MK-801 (a NMDA antagonist), suggest that glutamatergic afferents of PFC might be involved in the expression of behavioral sensitization to MPD. It would be interesting to see if MK-801 administered directly into VTA or NAc brings about similar results. For number of stereotypy, sensitization was not observed in any of the three groups. This is in accordance with previous reports using similar dose that indicate that this dosage of MPD does not cause sensitization of number of stereotypy (Gaytan et. al., 1997).
Glutamate has been implicated in behavioral sensitization to other psychostimulants (Clark and Overton, 1998; Kalivas, 2000; Li et. al., 1999; Pierce et. al., 1998; Ramos et. al., 2005; Wolf et. al., 1995; Wolf, 1998). However, different roles for glutamate have been proposed in behavioral sensitization to psychostimulants: while Clark and Overton (1998) suggest that behavioral sensitization is due to the potentiation of excitatory amino acid (EAA) afferents on DA neurons (of VTA) i.e., psychostimulant induce an increase in activity in prefrontal cells which ultimately impact DA neurons in VTA, Kalivas (2000) believes it is the increased release of EAA to NAc that underlies the expression of behavioral sensitization to psychostimulants. Since our data exhibited increased locomotor activity in the ibotenic acid treated group in the induction phase (ED 14), but this increase was lost after the rechallenge injection with MPD on ED 19, this suggests that glutamate of PFC participates atleast in part in the expression phase and not in the induction phase. Therefore, the theory of Kalivas (2000) of increased EAA to NAc seems more applicable to our data.
Statistical analysis of data using two way ANOVA with Bonferroni post hoc contrasts was performed to check for interaction effect between experimental days and experimental groups. It indicated that there was no significant interaction between experimental days and experimental groups and that the activity of the lesion group was significantly different than control and sham groups, and that there was no significant difference between the activities of control and sham groups. In conclusion, this study demonstrated that the acute effect of methylphenidate as well as chronic effects of the drug such as behavioral sensitization may be dependent upon PFC glutamatergic afferents.
4. Experimental procedure
Animals
Thirty adult male Sprague-Dawley rats (Harlan, Madison, WI, USA) were housed, two per cage (home cage), with free access to food and water. The room was maintained at 21± 2 °C, and relative humidity of 50 ± 5%. The animals were kept on 12:12 light/dark cycle; light period from 6:00 am to 6:00pm. The animals weighed in between 180 to 200g at the beginning of the experiment. On experimental days, the rats were transferred to the recording chamber (test cage; 1 rat per cage) and allowed to acclimatize to the test cage for 15–20 minutes. Four groups of animals were used – intact control (n = 8), sham1 (n = 6), sham2 (n = 8) and ibotenic injected group (n = 8).
Procedure
On experimental day (ED) 1, all animals were injected 0.8ml of saline between 06:30 and 07:00 and their locomotor activity was recorded for two hours. The next day (ED 2), the animals were divided into four groups: control (n = 8), sham1 (n = 8), sham2 (n = 6), and lesion group (n = 8). The lesion and sham groups underwent surgery on ED 2, followed by five days of recovery (ED 3 – 7). On ED 8, the locomotor activity of the animals was recorded 2 hours post saline injection to obtain the post surgery baseline. The next six days (ED 9 – 14) the animals were given single daily injections of 2.5mg/kg MPD intraperitoneally (i.p.) and locomotor activity was recorded immediately after injections on ED 9 and ED 14. No injections were given on EDs 15–18 (Washout period) and locomotor activity was recorded on first (ED 15) and last washout day (ED 18). The animals were rechallenged with 2.5mg/kg MPD dose on ED 19 (Table 1) and recordings were resumed for 2 hours post injection.
Surgeries
The rats were divided into three groups: sham 1, sham 2 (where saline was injected into PFC), and the bilateral ibotenic acid injected group. Two sham groups were used to see if injecting saline into the PFC caused any alterations on MPD effects or response.
For sham 1 group, under general anesthesia, an incision was made and the skull muscles removed to expose the bregma and the wound was stapled, without drilling the holes in the skull or inserting the needle into the PFC. For sham 2, an incision was made in the scalp and two holes (1 mm in diameter) were drilled above the PFC at 3.2 mm anterior to the bregma, 0.6mm lateral to each side of the midline. The co-ordinates were derived from the atlas of Paxinos and Watson (1986). 2.5μg of 0.9% isotonic saline was injected at a depth of 3 and 4 mm using a 27G needle. At each depth, the needle was kept for six minutes to allow the saline to diffuse.
Ibotenic acid injection (lesion)
Similar procedure as the one described for sham2 was performed. A 27G needle was inserted into the holes and lowered to depths of 4mm (Paxinos and Watson, 1986) and 2.5μg/μl of ibotenic acid solution was injected. The needle was kept for additional six minutes to allow the drug at the tip of the needle to diffuse (Braun et. al., 1993; Jaskiw et. al., 1990; Li and Wolf, 1997; Li. et. al., 1999) before lifting it up by 1mm (i.e., at a depth of 3 mm from the skull) and following the same ibotenic acid administration and the wait period. Identical procedure was repeated on the other side. A total of 5μg/5μl of ibotenic acid per hemisphere was injected (Braun et. al., 1993; Jaskiw et. al., 1990; Li and Wolf, 1997; Li et. al., 1999). After ibotenic acid injection, the muscle and skull skin were closed using wound closing staples. The rats were then allowed to recover for five days.
Drugs
Methylphenidate hydrochloride (MPD) was dissolved in 0.9% isotonic saline solution to make 2.5mg/mL MPD solution. The animals were injected with 2.5 mg/kg of MPD doses (See Table 1). Isotonic saline (0.9%) was added to equalize the volume of the injections to 0.8mL so that the volume of the injections was consistent between days and animals. The injections were given intraperitoneally (i.p.) between 6:30 and 7:00am. The dosage was chosen based on previous dose-response experiments to elicit behavioral sensitization (Amini et. al., 2004; Gaytan et. al., 1996, 1997; Lee et. al., 2008; Yang et. al., 2006). The time of administration was so decided because it was observed that behavioral sensitization following repetitive MPD administrations is best elicited if the drug is administered at the beginning of the light cycle (Dafny and Yang, 2006; Gaytan et. al., 2000).
Open field Apparatus
Open field cages (40.5cm × 40.5cm × 31.5cm) were used to record the animals’ locomotor activity (Algahim et. al., 2009; Gaytan et. al., 1996, 1997, 2000a, 2000b; Lee et. al., 2008; Yang et. al., 2003, 2006, 2007). Computerized animal activity monitoring (CAAM; AccuScan Instruments, Inc., Columbus, OH) system, having two levels of 16 infrared beams and sensors, monitored the animals’ activities by noting the number and sequence of beams interrupted. AccuScan Analyzer counted the number of beams interrupted every ten minutes and downloaded the data into OASIS program. The OASIS program further sorted the data into different locomotor indices.
Histology
Upon finishing the experiment, the animals were injected with excessive sodium barbital and perfused with 10% formaldehyde. The brains were removed and allowed to soak in 10% formaldehyde for at least 48 hours. Brain sections were cut at 40 μm using a Microm HM 505E cryostat (Microm GmbH, Germany) and allowed to dry for at least 24 hours, and then stained with Cresyl Violet. The accuracy and size of lesions was determined using the atlas of Paxinos and Watson (1986).
Data Analysis
The locomotor activity, recorded in 10 minute activity bins, was analyzed by comparing histograms (sum of all activity in 2 hours) of each of the different indices between the experimental days. The interaction effect for experimental groups and experimental days was analyzed for significance using a two way ANOVA followed by Bonferroni post hoc contrasts. Significance of differences between experimental days within a group was judged using ANOVA and Post hoc analysis with LSD test (Gaytan et. al., 1996, 2000 (a); Lee et. al., 2008; Yang et. al., 2003, 2007). Significance was set at p < 0.05. The following comparisons were analyzed: a) ED 8 was compared with ED 1 to see if surgery had an effect on the animal’s behavior. b) ED 9 was compared to ED 8 to study the acute effects of MPD. c) ED 9 was compared to ED 14 to check for induction of sensitization. d) ED 8 was compared with ED’s 15 and 18 to study the activity in the washout period. Finally, e) ED 19 was compared to ED 9 to see if sensitization was expressed after the washout period that followed chronic treatment (Table 1).
Three indices of locomotor activity were analyzed, namely total distance (TD), which records forward ambulation in centimeters, number of stereotypic movements (NOS) which summarizes the number of repetitive episodes, and horizontal activity (HA), which is the overall locomotor activity (Askenasy et. al., 2007; Gaytan et. al., 1996, 1997, 2000a, 2000b; Lee et. al., 2008; Yang et. al., 2003, 2006, 2007).
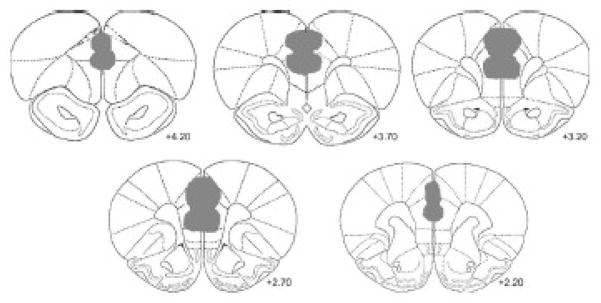
Figure 1.
shows a histological reconstruction map of the location and size of the lesion of the ibotenic acid in PFC group (n = 8).
Acknowledgments
This study was supported in part by NIH DA R01 027222. We thank Mallinckrodt, Inc. for its gift of methylphenidate.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague-Dawley rats. Pharmacology Biochemistry and Behavior. 2009;92:93– 99. doi: 10.1016/j.pbb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Amini B, Yang PB, Swann AC, Dafny N. Differential locomotor responses in male rats from three strains to acute methylphenidate. Intern J Neuroscience. 2004;114:1063–1084. doi: 10.1080/00207450490475526. [DOI] [PubMed] [Google Scholar]
- 3.Arneri SP, Woo JI, Meeley MP, Reis DJ. Spontaneous Release of Endogenous Aspartate and Glutamate From Rat Striatal Slices is Increased Following Destruction of Local Neurons by Ibotenic Acid. Neurochemical Research. 1988;13:423–428. doi: 10.1007/BF01268876. [DOI] [PubMed] [Google Scholar]
- 4.Askenasy EP, Taber KH, Yang PB, Dafny N. Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci. 2007;117:757–794. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Monuteaux MC, Spencer T, Wilens TE, MacPherson HA, Faraone SV. Stimulant Therapy and Risk for Subsequent Substance Use Disorders in Male Adults With ADHD: A Naturalistic Controlled 10-Year Follow-Up Study. American Journal of Psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- 6.Braun AR, Jaskiw GE, Vladar IK, Sexton RH, Kolachana BS, Weinberger DR. Effects of Ibotenic Acid Lesion of the Medial Prefrontal Cortex on Dopamine Agonist-Related Behaviors in the Rat. Pharmacology Biochemtstry and Behavior. 1993;46:51–60. doi: 10.1016/0091-3057(93)90316-l. [DOI] [PubMed] [Google Scholar]
- 7.Cador M, Bjijou Y, Cailhol S, Stinus L. d-amphetamine-induced behavioral sensitization: implication of a glutamatergic medial prefrontal cortex-ventral tegmental area innervation. Neuroscience. 1999;94:705–721. doi: 10.1016/s0306-4522(99)00361-9. [DOI] [PubMed] [Google Scholar]
- 8.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Dubé CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–11. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie MJ, James LB, Beart PM. An Excitant Amino Acid Projection from the Medial Prefrontal Cortex to the Anterior Part of Nucleus Accumbens in the Rat. J Neurochem. 1985;45:477–482. doi: 10.1111/j.1471-4159.1985.tb04013.x. [DOI] [PubMed] [Google Scholar]
- 11.Christie MJ, Rowe PJ, Beart PM. Effect of Excitotoxin Lesions in the Medial Prefrontal Cortex on Cortical and Subcortical Catecholamine Turnover in the Rat. J Neurochem. 1986;47:1593–1597. doi: 10.1111/j.1471-4159.1986.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 12.Clark D, Overton PG. Alterations in excitatory amino acid-mediated regulation of midbrain dopaminergic neurons induced by chronic psychostimulant administration and stress: relevance to behavioral sensitization and drug addiction. Addiction Biology. 1998;3:109– 135. doi: 10.1080/13556219872191. [DOI] [PubMed] [Google Scholar]
- 13.Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bul. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Faraone SV, Biederman J. Neurobiology of attention-deficit hyperactivity disorder. Biological Psychiatry. 1998;44:951–958. doi: 10.1016/s0006-3223(98)00240-6. [DOI] [PubMed] [Google Scholar]
- 15.Gao M, Liu C-L, Yang S, Jin GZ, Bunney BS, Shi WX. Functional coupling between the prefrontal cortex and dopamine neurons in the Ventral Tegmental Area. Journal of Neuroscience. 2007;27:5414– 5421. doi: 10.1523/JNEUROSCI.5347-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gariano RF, Groves PM. Burst firing induced in midbrain dopamine neurons by stimulation of the medial prefrontal and anterior cingulated cortices. Brain Res. 1988;462:194–198. doi: 10.1016/0006-8993(88)90606-3. [DOI] [PubMed] [Google Scholar]
- 17.Gaytan O, al-Rahim S, Swann A, Dafny N. Dose response characteristics of methylphenidate on different indices of rats’ locomotor activity at the beginning of the dark cycle. Brain Research. 1996;727:13–21. doi: 10.1016/0006-8993(96)00296-x. [DOI] [PubMed] [Google Scholar]
- 18.Gaytan O, Ghelani D, Martin S, Swann AC, Dafny N. Sensitization to locomotor effects of methylphenidate in rat. LifeSci. 1997;61:101–107. doi: 10.1016/s0024-3205(97)00598-5. [DOI] [PubMed] [Google Scholar]
- 19.Gaytan O, Yang PB, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Research. 2000;864:24– 39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- 20.Gaytan O, Nason R, Alagugurusamy R, Swann A, Dafny N. MK-801 blocks the development of sensitization to the locomotor effects of methylphenidate. Brain Research Bulletin. 2000b;51:485–492. doi: 10.1016/s0361-9230(99)00268-3. [DOI] [PubMed] [Google Scholar]
- 21.Goldman-Rakic PS. Handbook of Physiology The Nervous System Higher Functions of the Brain. 1. V. Bethesda, MD: Am. Physiol. Soc; 1978. Circuitry of primate prefrontal cortex and regulation of behavior by representational memory; pp. 373–417. sect. 1. chapt 9. [Google Scholar]
- 22.Imperato A, Honroe T, Jensen LH. Dopamine release in the nucleus accumbens is under glutamatergic control through non-NMDA receptors: a study in freely moving rats. Brain Research. 1990;530:223– 228. doi: 10.1016/0006-8993(90)91286-p. [DOI] [PubMed] [Google Scholar]
- 23.Jaskiw GE, Karoum F, Freed WJ, Philips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial prefrontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Research. 1990;534:263–272. doi: 10.1016/0006-8993(90)90138-2. [DOI] [PubMed] [Google Scholar]
- 24.Kafetzopoulos E. Effects of amphetamine and apomorphine on locomotor activity after kainic acid lesion of the nucleus accumbens septi in the rat. Psychopharmacology. 1986;88:271–274. doi: 10.1007/BF00180823. [DOI] [PubMed] [Google Scholar]
- 25.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Review. 1991;16:223– 244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW, Sorg BA, Hooks MS. The pharmacology and neural circuitry of sensitization to psychostimulants. Behav Pharmac. 1993;4:315–334. [PubMed] [Google Scholar]
- 27.Kalivas PW. A role of glutamate transmission in addiction to psychostimulants. Addiction Biology. 2000;5:325– 329. doi: 10.1111/j.1369-1600.2000.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 28.Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- 29.Kelly PH, Roberts DC. Effects of amphetamine and apomorphine on locomotor activity after 6-OHDA and electrolytic lesions of the nucleus accumbens septi. Pharmacology Biochemistry and Behavior. 1983;19:137–143. doi: 10.1016/0091-3057(83)90322-2. [DOI] [PubMed] [Google Scholar]
- 30.Kelsey JE, Willmore EJ. Electrolytic lesions of the Nucleus Accumbens Enhance Locomotor Sensitization to Nicotine in Rats. Behavioral Neuroscience. 2006;120:600– 611. doi: 10.1037/0735-7044.120.3.600. [DOI] [PubMed] [Google Scholar]
- 31.Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Bheav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Swerdlow NR. The functional output of the mesolimbic dopamine system. Ann NY Acad Sci. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42108.x. [DOI] [PubMed] [Google Scholar]
- 33.Lacroix L, Spinelli S, White W, Feldon J. The effects of ibotenic acid lesions of the medial and lateral prefrontal cortex on latent inhibiton, prepusle inhibiton and amphetamine-induced hyperlocomotion. Neuroscience. 2000;97:459–468. doi: 10.1016/s0306-4522(00)00013-0. [DOI] [PubMed] [Google Scholar]
- 34.Lee MJ, Swann AC, Dafny N. Methylphenidate sensitization is prevented by prefrontal cortex lesion. Brain Res. 2008;76:131–140. doi: 10.1016/j.brainresbull.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Wolf ME. Ibotenic acid lesions of prefrontal cortex do not prevent expression of behavioral sensitization to amphetamine. Behavioral Brain Research. 1997;84:285– 289. doi: 10.1016/s0166-4328(96)00158-1. [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Hu XT, Berney TG, Vartanian AJ, Stine CD, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Lipska BK, Al-Amin HA, Weinberger DR. Excitotoxic Lesions of the Rat Medial Prefrontal Cortex. Neuropsychopharmacology. 1998;19:451– 464. doi: 10.1016/S0893-133X(98)00045-1. [DOI] [PubMed] [Google Scholar]
- 38.Maldonado-Irizarry CS, Kelley AE. Differential behavioral effects following microinjection of an NMDA antagonist into nucleus accumbens subregions. Psychopharmacology. 1994;116:65–72. doi: 10.1007/BF02244872. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Iverson MT, Stahl SM, Iversen SD. Chronic administration of a selective dopamine D-2 agonist: factors determining behavioral tolerance and sensitization. Psychopharmacology. 1988;95:534–539. doi: 10.1007/BF00172969. [DOI] [PubMed] [Google Scholar]
- 40.Murase S, Grenhoff J, Chouvet G, Gonon FG, Svensson TH. Prefrontal cortex regulates burst firing and transmitter release in rat mesolimbic dopamine neurons studied in vivo. Neurosci Lett. 1993;157:53–56. doi: 10.1016/0304-3940(93)90641-w. [DOI] [PubMed] [Google Scholar]
- 41.Overton PG, Tong ZY, Clark D. A pharmacological analysis of the burst events induced in midbrain dopaminergic neurons by electrical stimulation of the prefrontal cortex in the rat. J Neural Transm. 1996;103:523–540. doi: 10.1007/BF01273151. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; Orlando: 1986. [Google Scholar]
- 43.Pierce RC, Reeder DC, Hicks J, Morgan ZR, Kalivas PW. Ibotenic acid lesions of the dorsal prefrontal cortex disrupt the expression of behavioral sensitization to cocaine. Neurosc. 1998;82:1103–1114. doi: 10.1016/s0306-4522(97)00366-7. [DOI] [PubMed] [Google Scholar]
- 44.Ramos M, Goñi-Allo B, Aguirre N. Ibotenic acid lesions of the medial prefrontal cortex block the development and expression of 3,4-methylenedioxymethamphetamine-induced behavioral sensitization in rats. Behav Brain Res. 2005;160:304–311. doi: 10.1016/j.bbr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 45.Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology. 1984;84:466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- 46.Robinson TG, Beart PM. Excitatory amino acid projections from rat amygdala and thalamus to nucleus accumbens. Brain Res Bull. 1988;20:467–471. doi: 10.1016/0361-9230(88)90136-0. [DOI] [PubMed] [Google Scholar]
- 47.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 48.Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–60. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- 49.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 50.Svensson TH, Tung CS. Local cooling of pre-frontal cortex induces pacemaker-like firing of dopamine neurons in rat ventral tegmental area in vivo. Acta Physiol Scand. 1989;136:135–136. doi: 10.1111/j.1748-1716.1989.tb08640.x. [DOI] [PubMed] [Google Scholar]
- 51.Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429–433. [PubMed] [Google Scholar]
- 52.Swanson JM, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- 53.Taber M, Fibiger H. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- 55.Todtenkopf MS, Carlezon WA. Contribution of drug doses and conditioning periods to psychostimulant sensitization. Psychopharmacology. 2006;185:451– 458. doi: 10.1007/s00213-005-0259-1. [DOI] [PubMed] [Google Scholar]
- 56.Tong ZY, Overton PG, Clark D. Chronic administration of (1)-amphetamine alters the reactivity of midbrain dopaminergic neurons to prefrontal cortex stimulation in the rat. Brain Res. 1995;674:63–74. doi: 10.1016/0006-8993(94)01439-o. [DOI] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Fowler JS, Logan J, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D2 receptors. J Pharm Exp Ther. 1999;291:409–415. [PubMed] [Google Scholar]
- 58.Walaas I. Biochemical evidence for overlapping neocortical and allocortical glutamate projections to the nucleus accumbens and rostral caudatoputamen in the rat brain. Neuroscience. 1981;6:399–405. doi: 10.1016/0306-4522(81)90132-9. [DOI] [PubMed] [Google Scholar]
- 59.White FJ, Hu X-T, Zhang X-F, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmac Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- 60.Wolf ME, Dahlin SL, Hu X-T, Xue C-J, White K. Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with N-methyl D-aspartate antagonists. Neurosc. 1995;69:417–439. doi: 10.1016/0306-4522(95)00248-h. [DOI] [PubMed] [Google Scholar]
- 61.Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- 62.Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- 63.Yang PB, Swann AC, Dafny N. Dose-response characteristics of methylphenidate on locomotor behavior and on sensory evoked potentials recorded from the VTA, NAc, and PFC in freely behaving rats. Behavioral and Brain Functions. 2006;2:3. doi: 10.1186/1744-9081-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang PB, Swann AC, Dafny N. Chronic administration of methylphenidate produces neurophysiological and behavioral sensitization. Brain Res. 2007;1145:66–80. doi: 10.1016/j.brainres.2007.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. 1998;18:6492– 6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuddas A, Ancilletta B, Muglia P, Cianchetti C. Attention-deficit/hyperactivity disorder: a neuropsychiatric disorder with childhood onset. European Journal of Paediatric Neurology. 2000;4:53–62. doi: 10.1053/ejpn.1999.0263. [DOI] [PubMed] [Google Scholar]