Abstract
Various types of phagocytes mediate the clearance of apoptotic cells. We previously reported that human and murine high endothelial venule (HEV) cells ingest apoptotic cells. In this report, we examined endothelial cell fucoidin receptor-mediated phagocytosis using a murine endothelial cell model mHEV. mHEV cell recognition of apoptotic leukocytes was blocked by fucoidin but not by other phagocytic receptor inhibitors such as mannose, fucose, N-acetylglucosamine, phosphatidylserine (PS), or blocking anti-PS receptor antibodies. Thus, the mHEV cells used fucoidin receptors for recognition and phagocytosis of apoptotic leukocytes. The fucoidin receptor-mediated endothelial cell phagocytosis was specific for apoptotic leukocytes, as necrotic cells were not ingested. This is in contrast to macrophages, which ingest apoptotic and necrotic cells. Endothelial cell phagocytosis of apoptotic cells did not alter viable lymphocyte migration across these endothelial cells. Antibody blocking of CD44 and α4 integrin on the apoptotic leukocyte inhibited this endothelial cell phagocytosis, suggesting a novel function for these adhesion molecules in the removal of apoptotic targets. The removal of apoptotic leukocytes by endothelial cells may protect the microvasculature, thus ensuring that viable lymphocytes can successfully migrate into tissues.
Keywords: lectin, carbohydrate, cell adhesion molecules, phosphatidylserine, endothelial cells
INTRODUCTION
Clearance of apoptotic cells occurs in multiple biological processes, such as embryonic development and maintenance of tissue homeostasis, and during inflammatory processes. Professional phagocytes, including macrophages and microglial cells, remove apoptotic cells [1, 2]. If apoptotic cells are not rapidly removed by phagocytosis, tissue damage may result from the release of toxic cellular enzymes and auto antigens. For example, resolution of inflammation cannot occur when apoptotic neutrophils present in an inflamed arthritic joint are not removed by macrophages [3]. The ingestion of apoptotic cells can have effects other than the mere disposal of dead or dying cells. Macrophage ingestion of apoptotic cells appears to result in the suppression of inflammation, the modulation of cell killing, and the regulation of immune responses [4].
During apoptosis, cells undergo cell-surface changes that mediate recognition and ingestion by phagocytes. Multiple types of receptors on phagocytes that recognize apoptotic cells include lectin receptors, lipid receptors, complement receptors, CD14, and integrins. Different phagocytes use different receptor repertoires for recognition of apoptotic cells. Lectin receptors recognize apoptotic cell-surface carbohydrates with side-chain sugars that are no longer covered by more terminal residues, such as sialic acid [3]. Murine liver endothelial cells [5], human Kupffer cells [6], and macrophages [7] use these carbohydrate-specific receptors. Lectin recognition is classically characterized by inhibition of phagocytosis in the presence of excess exogenous sugars. Although some phagocytic receptors recognize carbohydrates on the surface of dying cells, others recognize surface lipid modifications. The lipid receptors, scavenger receptor (SR)-A, CD36, SR-BI, SR-BII, CD68, and lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1), have been demonstrated to bind oxidized and/or acetylated LDL-like sites as well as the anionic phospholipid phosphatidylserine (PS) on the outer membrane surface of apoptotic cells [8, 9]. Additionally, Fadok et al. [10] have recently described a PS receptor (PSR) that stereospecifically recognizes PS in the outer leaflet of apoptotic cell membranes. A wide variety of professional and amateur phagocytes, including human and murine macrophages [11], murine microglia [12], and rat vascular smooth muscle cells [13], use the PSR to ingest apoptotic cells.
Additional receptors are used by phagocytes to promote the recognition and ingestion of apoptotic cells. Apoptotic lymphocytes and neutrophils coated with the complement protein, iC3b, are rapidly phagocytosed by human macrophages via the complement receptors, CR3 and CR4 [14]. The lipopolysaccharide (LPS) receptor, CD14, on human macrophages also recognizes intercellular adhesion molecule-3 (ICAM-3) and/or PS on apoptotic cells [15, 16]. Integrins, including αVβ3, αVβ5, and β1, can also behave as phagocytic receptors. These integrins can act alone or in combination with the lipid receptors CD36 and PSR [11, 17, 18]. Whether integrins function in combination with phagocytic lectin receptors is not known. Thus, the use of different receptors by different phagocytes in different microenvironments [19] insures effective removal of apoptotic corpses.
We have previously demonstrated that endothelial cells phagocytose apoptotic leukocytes in vivo and in vitro [20]. High endothelial venule (HEV) cells line blood vessels that lead into peripheral lymphoid tissues and are primarily known for their promotion of resting lymphocyte migration from the blood into lymph nodes [21, 22]. The endothelial cell function of ingesting apoptotic leukocytes suggests that only viable, functional lymphocytes are permitted to migrate into lymph nodes, whereas the apoptotic cells are removed before entering the lymphoid tissues, thus preventing damage to the endothelial cells. In this report, we demonstrate that the fucoidin receptor can act independently of the PSR on endothelial cells. Further, we have identified that endothelial cells recognize CD44 and α4 integrin on apoptotic lymphocytes during phagocytosis.
MATERIALS AND METHODS
Reagents
All antibody stocks contained 0.1% azide except where stated azide-free. Antibodies were diluted and used at 0.004% azide. Affinity-purified goat anti-mouse immunoglobulin (Ig), F(ab′)2 (IgA, IgG, and IgM, H + L; azide-free), was obtained from ICN Pharmaceuticals (Aurora, OH). Fluorecein isothiocyanate (FITC)-conjugated rat anti-mouse CD45 (Ly5/T200), purified hamster anti-mouse CD61, FITC-conjugated mouse anti-hamster IgG, the isotype control hamster antitrinitrophenol (TNP) IgG (group 1, κ), phycoerythrin (PE)-conjugated goat anti-rat IgG, PE-conjugated goat anti-rabbit IgG, purified mouse IgM anti-TNP, purified rat anti-mouse CD51, purified rat anti-mouse CD29, purified rat anti-mouse CD49e, purified rat anti-mouse CD106, purified rat anti-mouse CD62L (azide-free), purified rat anti-mouse CD102 (azide-free), purified rat anti-mouse CD31, and the isotype-control, purified rat IgG anti-TNP were obtained from PharMingen (San Diego, CA). Purified FITC-conjugated goat anti-rat Ig (IgM + IgG, H + L, preadsorbed against pooled mouse serum) was from Southern Biotechnology Associates (Birmingham, AL). Polyclonal anti-SR-BI/-BII rabbit antiserum was obtained from Novus Biologicals (Littleton, CO). Purified rat anti-mouse SR-A was obtained from Serotec (Raleigh, NC). Purified rat anti-mouse CD44 and purified rat anti-mouse very late antigen-4 were from BioDesign (Saco, ME). Purified anti-human PSR antibody [monoclonal antibody (mAb) 217, azide-free] was a kind gift from Valerie Fadok (National Jewish Medical and Research Center, Denver, CO). Goat anti-mouse IgM–FITC, μ chain-specific, was obtained from Jackson ImmunoResearch (West Grove, PA). D-Mannose, D-fucose, L-fucose, D-galactose, N-acetylglucosamine (GlcNAc), D-glucose, mannan, fucoidin, phospho-L-serine, and phospho-D-serine were purchased from Sigma Chemical Co. (St. Louis, MO).
Cells and culture conditions
WEHI-231 cells (BALB/c mouse-derived, B cell lymphoma from American Tissue Culture Collection, Manassas, VA) were maintained in 50 –100 μM 2-mercaptoethanol in Dulbecco’s modified Eagle’s medium (DMEM) culture consisting of DMEM medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin. 32D cells (premyeloid stem-cell line from David Askew, University of Cincinnati, OH) were cultured in DMEM culture medium with 10% WEHI-3-conditioned medium added as a source of interleukin-3 (IL-3) [23]. Spleen cells were isolated from 4- to 6-week-old BALB/c male mice (bred under pathogen-free conditions at Harlan Industries, Indianapolis, IN), red blood cells (RBC) were removed by hypotonic shock with sterile, distilled water for 3 s, and the spleen lymphocytes (>90% viability) were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin [24]. The BALB/c mouse-derived, HEV-like cell lines (mHEVa and mHEVc) were described previously by this laboratory [24] and cultured in HEV medium consisting of RPMI-1640 medium supplemented with 20% heat-inactivated FCS, 1 mM HEPES buffer (pH 7.2), 10 mM sodium bicarbonate, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μg/ml gentamycin. All cells were incubated in a humidified atmosphere at 37°C in 6% CO2 air.
Triggering of leukocyte cell death
As consistently reported in the literature for WEHI-231 cells, they (2 × 106/ml) were induced to undergo apoptosis by cross-linking cell-surface Igs by the addition of 4 μg/ml goat anti-mouse Ig, F(ab′)2, in DMEM culture medium [25]. After 24 h, the WEHI-231 cells were washed and placed in DMEM culture medium. To induce necrosis in WEHI-231 cells, they (6 × 106/ml) were incubated at 55°C for 15 min [26]. The necrotic cells were then incubated at 37°C for 5 min to equilibrate them to the temperature of the other cell treatments. Necrotic WEHI-231 cells were also examined by light microscopy for characteristic membrane distortions found in necrotic cells [26]. 32D cell apoptosis was induced by washing the cells to remove IL-3 and incubating in culture medium without IL-3 [23]. Splenic lymphocyte (1 × 106/ml) apoptosis was induced by 500 rad γ irradiation [27].
Apoptotic characteristics
MC540 flow cytometric assay [25]
Leukocytes (1 × 106 cells) were centrifuged for 5 min at 200 g, resuspended in 0.16 μg/ml MC540 (Sigma Chemical Co.) in phosphate-buffered saline (PBS) for 20 min in the dark at room temperature, washed twice in PBS, and analyzed by flow cytometry.
Annexin V-FITC flow cytometric assay (apoptotic cell system Annexin V-FITC, Trevigen, Gaithersburg, MD)
Leukocytes (1 × 106 cells) were washed with PBS (4°C) and suspended in 200 μl Annexin V incubation reagent (4°C) containing Annexin V-conjugate for 15 min in the dark at room temperature. Binding buffer (400 μl, 1×) was added, and then the cells were analyzed by flow cytometry.
Propidium iodide (PI)-labeled hypodiploid nuclei [28]
Leukocytes (2 × 106) were washed in PBS (4°C), fixed in 2 ml 70% ethanol (4°C) for 30 min, washed in 10 ml PBS (4°C), suspended in DNA-staining reagent containing 50 μg/ml PI (Sigma Chemical Co.) at room temperature for 15 min, and then analyzed by flow cytometry to determine percent of cells with hypodiploid nuclei.
DNA fragmentation assay [27]
This assay determines the percent of DNA in a population of cells that is fragmented rather than the percent of cells with fragmented DNA. WEHI-231 cells (6 × 106 anti-Ig-treated or control cells) were centrifuged at 200 g for 10 min. The cells were lysed at room temperature for 20 min with 400 μl lysing solution comprised of 0.2% Triton X-100, 1 mM EDTA, 10 mM Tris-HCl, pH 7.5. To pellet the intact DNA, the lysates were centrifuged at 13,000 g. The supernatant containing the fragmented DNA was transferred to a separate tube. Additional lysing solution (400 μl) was added to the pellet of intact DNA. To precipitate the DNA, 200 μl 25% trichloroacetic acid (TCA) was added, and the samples were incubated at 4°C overnight. The TCA was removed, and 80 μl 5% TCA was added to the DNA to facilitate hydrolysis at 90°C for 10 min. The diphenylamine reagent (160 μl), comprised of 1.5% sulfuric acid, 0.2% acetaldehyde, and 98% glacial acetic acid, was added to supernatant and pellet samples, and they were then incubated at room temperature overnight for color development by an unknown mechanism. The optical density (OD)600 was determined using a microtiter plate reader. The % fragmented DNA = ODsupernatant/(ODpellet + ODsupernatant).
Cell membrane permeability [29]
Leukocytes (1 × 106 cells) were suspended in 1 ml 0.2% glucose in PBS at room temperature. The DNA staining reagent [PBS; 0.1 mM EDTA, 0.05 mg/ml RNase A (50 U/mg, protease- and DNase-free, Boehringer-Mannheim, Indianapolis, IN), pH 7.4] containing 5 μg/ml PI was added to the cells immediately before flow cytometry analysis of each sample.
Endothelial cell phagocytic assay
Endothelial cell phagocytosis was determined as described previously by our laboratory [30]. Briefly, endothelial cells (mHEVa and mHEVc) were plated in 24-well microtiter plates and incubated for 2–3 days at 37°C to generate confluent cell monolayers. Leukocytes (30 × 106) were labeled with a viable dye by incubation with 50 μg 5-(and 6)-carboxytetramethylrhodamine, succinimidyl ester (TAMRA; Molecular Probes, Eugene, OR) in 2 ml HEV medium (without FCS) for 15 min at 37°C. This concentration of dye sufficiently labels the leukocytes without detectable cell leakage of the dye or acquisition of cell-free dye by endothelial cells during the assay, as determined by fluorescence microscopy. The TAMRA stock in dimethyl sulfoxide (25 mg/ml) expires within 6 months when stored at −20°C. The TAMRA-labeled leukocytes were triggered to undergo cell death as described above. mHEV cell monolayers or leukocytes were pretreated with receptor inhibitors as indicated in the figures for 30 min at 37°C. The receptor inhibitors were not washed out unless indicated. The inhibitors at the concentrations used were not toxic to the endothelial cells or the leukocytes as determined by trypan blue exclusion (≥80% viability). At the indicated time points, postinduction of cell death, TAMRA-labeled control and dying leukocytes (3 × 106 cells) were washed and added to the mHEV cell monolayers for an optimal 5 h at 37°C for phagocytosis [20]. Three million apoptotic leukocytes saturate the endothelial monolayer capacity for phagocytosis (data not shown). Culture medium was removed, and the cells were treated with 0.03% EDTA for 5 min to dissociate the mHEV cell monolayers and create a single-cell suspension. To label surface-bound, nonphagocytosed leukocytes, cells from the mHEV cell/leukocyte cocultures were incubated in 200 μl PBS– 0.3% bovine serum albumin (BSA)– 0.15% NaN3 with 2 μg FITC-conjugated rat anti-mouse CD45 (Ly5/T200) at 4°C for 30 min. The cells were washed and analyzed by flow cytometry using FACScan/Lysys II or FACSCalibur systems (Becton Dickinson, San Jose, CA). The mHEV cells were “gated on” in a forward- versus side-scatter (FSC, SSC, respectively) dot plot. These mHEV cells were analyzed for association with TAMRA-labeled (ingested) leukocytes (region R4, see Fig. 1, E–G) or TAMRA/CD45-labeled (external) leukocytes (region R5, see Fig. 1, E–G). The endothelial cells in regions R4 and R5 in a sample had similar levels of TAMRA fluorescence (see Fig. 1, E–G). The mHEV cells in region R4 contained ingested leukocytes as confirmed by cell sorting and confocal microscopy [30]. Cells at all time points were examined by fluorescence microscopy to confirm target cell ingestion (data not shown). This is important because when TAMRA is near expiration, or if too much dye is used, the dye can leak and diffusely label endothelial cells. On the rare occasion that this occurs, the experiment is discarded. Endothelial cells below region R4 and located in region R3 were nonlabeled or may have contained small debris that is not readily discernable by fluorescence microscopy. The data from flow cytometry are reported as the percent of mHEV cells that phagocytosed apoptotic cells, i.e., percent of endothelial cells in region R4.
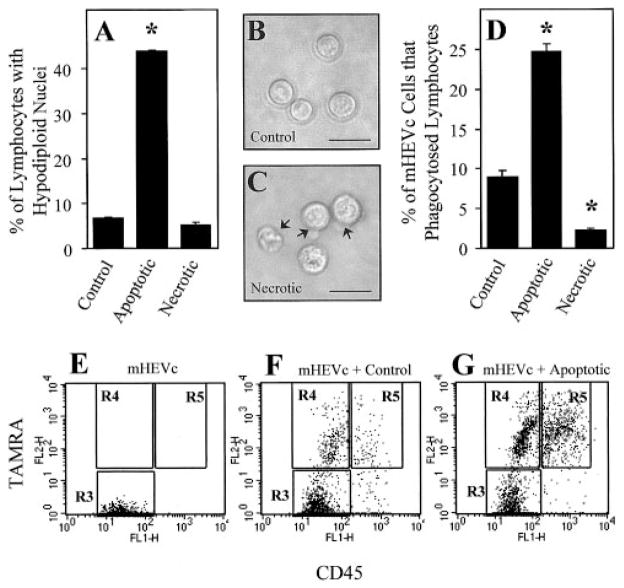
Fig. 1.
mHEVc cells phagocytose apoptotic cells but not necrotic cells. (A) PI-labeling of hypodiploid, ethanol-fixed control, apoptotic, and necrotic cells. (B) Light micrograph of control WEHI-231 cells. (C) Light micrograph of necrotic WEHI-231 cells with arrows indicating characteristic necrotic membrane perturbations. (D) Phagocytosis of control, apoptotic, and necrotic WEHI-231 cells by mHEVc cell monolayers. Apoptosis was induced in the WEHI-231 cells (2 × 106 cells/ml) by cross-linking their cell-surface Igs with goat anti-mouse Ig F(ab′)2 (4 mg/ml) for 24 h at 37°C [25], washed to remove excess antibody, and cultured for an additional 24 h. Necrotic WEHI-231 cells (6 × 106 cells/ml) were prepared by incubation at 55°C for 15 min [26]. Control, apoptotic, and necrotic WEHI-231 cells (3 × 106 cells) were then incubated with confluent mHEVc cell monolayers for 5 h to allow for optimal phagocytosis to occur [20]. The percent of mHEVc cells that engulfed target WEHI-231 cells was determined by a fluorescence-based phagocytosis assay [30] in which mHEVc cells were gated on in a FSC versus SSC dot plot (data not shown) and then analyzed for the TAMRA and CD45 labels. (E–G) Flow cytometry analysis of endothelial cell phagocytosis. Endothelial cells were gated on in a FSC versus SSC dot plot, and then endothelial cell-associated fluorescence was examined in a dot plot of FL1 versus FL2 (CD45 vs. TAMRA) of mHEVc cells alone, mHEVc cells associated with control WEHI-231 cells, and mHEVc cells associated with apoptotic WEHI-231 cells. (A and D) Data are presented as the mean of at least duplicate samples ± SD. Data are from a representative experiment of three experiments. Original bar = 50 μm. Arrow, Necrotic membrane perturbations. *, P < 0.05, as compared with control. R3, Region 3, mHEVc cells; R4, region 4, mHEVc cells that had phagocytosed TAMRA-labeled WEHI-231 cells; R5, region 5, mHEVc cells with externally bound TAMRA- and CD45-labeled WEHI-231 cells. Cell sorting and confocal microscopy confirmed regions, as we previously reported [30].
Lymphocyte migration assay [31] subsequent to endothelial cell phagocytosis of apoptotic cells
Briefly, mHEV cells were plated and grown to confluence in the upper chamber of Transwells with 12 μm pores (Costar, Cambridge, MA). The mHEVc cell monolayers were incubated with or without apoptotic 32D cells for 5 h to allow phagocytosis to occur. Where indicated, fucoidin was added to the upper chamber of the Transwell. The monolayers were washed to remove excess fucoidin and nonbound, apoptotic 32D cells. 32D cells do not migrate across the endothelial cell monolayer (unpublished results). Splenic lymphocytes (4 × 106) and 4 – 8 × 106 splenic RBC were then added to the upper chamber on top of the mHEV cell monolayer. The cells were incubated at 37°C under static conditions. The RBC served as an internal control for integrity of the monolayer, as they are smaller than the lymphocytes and do not migrate. Thus, if RBC were present in the bottom chamber, the monolayer was disrupted, and the Transwell was discarded. Lymphocytes were collected from the bottom of the chamber at the indicated times. Asynchronous lymphocyte migration occurs up to 48 h, whereas migration by a particular lymphocyte occurs within minutes [24].
Antibody labeling of cell-surface proteins
mHEV cells (dissociated from a monolayer in one well of a 24-well plate) or leukocytes (1 × 106) were incubated in 200 μl PBS– 0.3% BSA–0.15% NaN3 with 2 μg primary antibody at 4°C for 30 min (except 150 μg anti-PSR antibody was used). The cells were then washed and labeled with 2 μg fluorophore-conjugated secondary antibody. After washing the cells twice, they were analyzed by flow cytometry.
Data analysis
Data were analyzed by ANOVA and Tukey’s multiple comparison test (Sigma Stat, SPSS Inc., Chicago, IL).
RESULTS
Murine endothelial cell lines phagocytose apoptotic but not necrotic leukocytes
We have reported that murine and human lymph node endothelial cells phagocytose apoptotic cells in vivo and in vitro [20]. Two endothelial cell lines derived from murine axillary and cervical lymph nodes (mHEVa and mHEVc, respectively) also phagocytose apoptotic leukocytes [20]. Macrophages ingest apoptotic and necrotic targets [26]. To examine the specificity of the endothelial cell lines for apoptotic versus necrotic leukocytes, apoptosis was induced in murine B cell lymphoma WEHI-231 cells by cross-linking cell-surface Ig with goat anti-mouse Ig (Fab′2) for 24 h, followed by cell culture in the absence of the Ig for an additional 24 h [25]. WEHI-231 cells were made necrotic by incubating the cells at 55°C for 15 min [26]. PI labeling of hypodiploid nuclei and analysis by flow cytometry verified apoptotic cell death (Fig. 1A), and light microscopy verified necrotic cell death (Fig. 1, B and C). Compared with control or necrotic WEHI-231 cells, apoptotic WEHI-231 cells had a significantly higher percentage of hypodiploid nuclei (Fig. 1A). Heat-killing WEHI-231 cells resulted in cell swelling and loss of membrane integrity in the absence of DNA degradation (Fig. 1, A and C), as reported by Cocco and Ucker [26] for the T cell hybridoma DO11.10. The percentage of mHEVc cells that ingested target cells was then determined using a fluorescence-based phagocytosis assay [30]. Endothelial cells were gated on in FSC versus SSC and were examined for leukocyte-associated fluorescence (Fig. 1, E–G). A conservative gate (region R4) is placed on endothelial cells with TAMRA fluorescence intensities comparable with nonphagocytosed leukocytes (region R5). Control samples containing leukocytes without endothelial cells fall within region R5. Cells below region R4 on the FL2 axis (region R3) are nonfluorescent or may contain small debris. The gates are similar at all time points. The mHEVc cells specifically ingested apoptotic lymphocyte targets compared with control or necrotic targets (Fig. 1D). Endothelial cell phagocytosis of apoptotic leukocytes was confirmed by fluorescence microscopy (data not shown).
Maximum mHEV cell phagocytosis of apoptotic leukocytes occurs before 50% of apoptotic cells acquiring characteristic apoptotic membrane changes
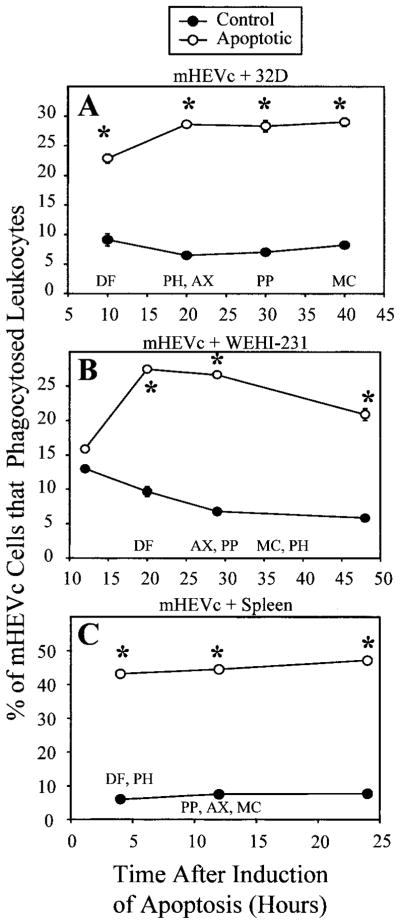
Our laboratory previously reported that leukocytes acquire apoptotic characteristics with an order that is dependent on the cell type and the mode of induction of apoptosis [32]. Therefore, we compared the time for recognition for phagocytosis with time for acquisition of membrane-associated apoptotic characteristics and DNA damage. MC540 incorporation, Annexin V-FITC binding, and PI permeability detected the membrane changes. DNA damage was detected by determining the percent of cells with PI labeling of hypodiploid nuclei and by determining the percent of DNA that was fragmented. The colorimetric DNA fragmentation assay detects small DNA breaks in a population of cells earlier than the detection of changes in PI labeling of hypodiploid nuclei in individual cells [32]. As the order and acquisition of these characteristics depend on the cell type [32], it is important to use multiple parameters to examine apoptosis. The time for 50% maximum number of leukocytes with these characteristics, as we previously reported [32], is indicated in Figure 2. Apoptosis was induced in 32D cells by depriving the cultures of IL-3, WEHI-231 cells were treated with anti-Ig as described above, and spleen cells were irradiated with 500 rad γ irradiation. Postinduction of leukocyte apoptosis, control, and apoptotic 32D cells (Fig. 2A), control and apoptotic WEHI-231 cells (Fig. 2B), and control and apoptotic spleen cells (Fig. 2C) was added to mHEVc cell monolayers at time points indicated. Less than 5 h before the 50% maximums for apoptotic characteristics, there was no significant detection of the apoptotic characteristic in the leukocyte population [32]. When the mHEVc cells were cocultured with apoptotic 32D cells, apoptotic WEHI-231 cells, or apoptotic spleen cells, there was maximum endothelial cell phagocytosis of the apoptotic cells before 50% maximum number of leukocytes with apoptotic membrane changes or labeling of hypodiploid nuclei (Fig. 2). This does not preclude that some of these apoptotic changes occurred in the leukocytes that were ingested. Thus, Figure 2 delineates the time course for recognition of phagocytosis versus apoptotic changes in the cell population. Similar results were obtained with mHEVa cells (data not shown).
Fig. 2.
mHEV cell phagocytosis of apoptotic cells at different times after induction of apoptosis. (A) mHEVc cell phagocytosis of 32D cells. (B) mHEVc cell phagocytosis of WEHI-231 cells. (C) mHEVc cell phagocytosis of spleen cells. Apoptosis was induced in the leukocytes using the following methods: 32D cells were deprived of IL-3 in their culture medium [23]; WEHI-231 cells were treated with anti-Ig as described in Figure 1; and spleen cells were irradiated with 500 rad γ irradiation [27]. Control and apoptotic leukocytes were incubated with mHEVc cell monolayers at the time points indicated. Also designated are the times for 50% of maximum number of the leukocytes that had obtained these apoptotic characteristics: MC, MC540-labeled; AX, Annexin V-FITC incorporation; PH, PI-labeled hypodiploid nuclei in ethanolfixed cells; DF, DNA fragmentation; PP, permeability to PI [32]. The cocultures were incubated for 5 h for phagocytosis to occur. The percent of mHEVc cells that engulfed target leukocytes was determined by a phagocytosis assay [30]. Data are presented as the mean of at least duplicate samples ± SD. Points without error bars indicate bars smaller than the symbol. Data in each panel are from a representative experiment of seven experiments (A and B) and four experiments (C). Similar results were obtained with the mHEVa cell line in four to seven experiments (data not shown). *, P < 0.05, as compared with the control at the same time point. Control, Noninduced leukocytes; Apoptotic, apoptosis-induced leukocytes.
Fucoidin-mediated phagocytosis of apoptotic leukocytes that is independent of the PSR
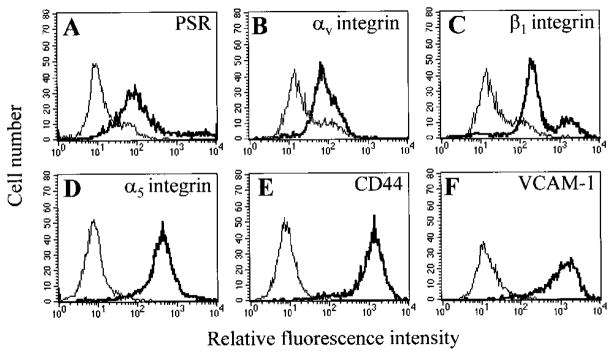
Phagocytes ingest apoptotic cells by using multiple types of phagocytic receptors, such as the PSR [10, 11], and carbohydrate-specific lectin receptors [33]. In addition, αVβ3, αVβ5, and β1 integrins have been reported as phagocytic receptors as well as coreceptors on the phagocyte in lipid receptor-mediated phagocytosis of apoptotic leukocytes [11, 17, 18]. Therefore, we examined receptor expression on mHEV cells and then determined whether ligands known to block these receptors inhibited mHEV cell phagocytosis of apoptotic leukocytes. It was determined whether the mHEVc cell line expresses the PSR [10], the scavenger receptors SR-A, SR-BI, and SR-BII [34], CD14 [15], and the integrins CD51 (αV chain) [11], CD49e (α5 chain) [35], CD29 (β1 chain) [36], and CD61 (β3 chain) [11]. mHEVc cells were indirectly immunofluorescent-labeled with antibodies to these receptors and then analyzed by flow cytometry. The mHEVc cells express the PSR and the integrins αV, β1, and α5 (Fig. 3). In addition to these phagocytic receptors, the mHEVc cells express the adhesion molecules CD44 and CD106 (VCAM-1; Fig. 3). The mHEVc cells do not express the scavenger receptors SR-A, SR-BI, or SR-BII, the LPS receptor CD14, or β3 integrin (data not shown). The antibodies were functional, as they bound to positive-control cells, including peritoneal macrophages for CD14 [15], J774.1 macrophages for SR-A, SR-BI, and SR-BII [37], and 32D cells for β3 integrin [23] (data not shown).
Fig. 3.
mHEVc cell expression of phagocytic receptors and cell adhesion molecules. Cells were labeled with mAb directed against the following cell-surface proteins, followed by a FITC-conjugated secondary antibody and flow cytometric analysis: (A) PSR; (B) αv integrin; (C) β1 integrin; (D) α5 integrin; (E) CD44; and (F) vascular cell adhesion molecule-1 (VCAM-1). Isotype-matched control antibodies are included in each panel and indicated as the thin lines. Nonlabeled cells had similar profiles as cells labeled with isotype antibodies (data not shown).
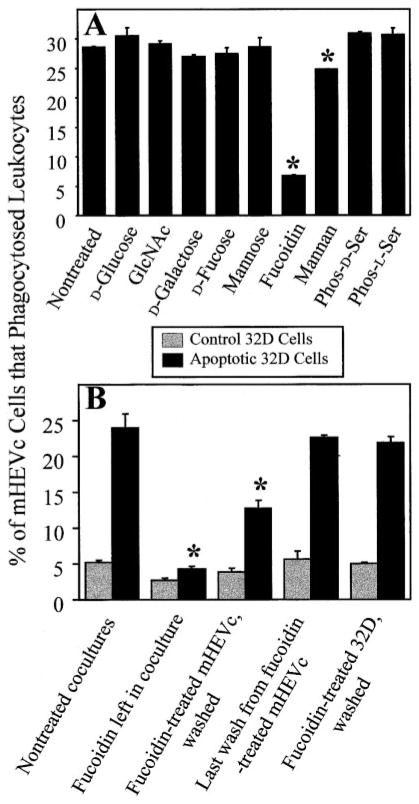
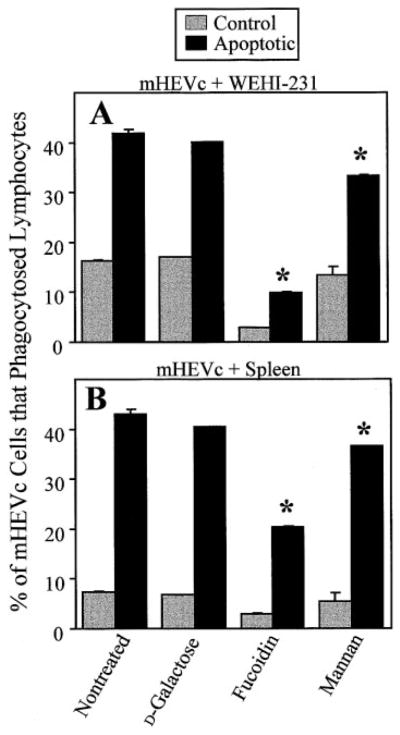
To determine whether mHEVc cell phagocytosis was mediated by the PSR or other lipid receptors, mHEVc cells were preincubated with phospho-L-serine [11], and phagocytosis was quantified using 32D cells after 20 –30 h of induction of apoptosis. Phagocytosis was not significantly inhibited by phospho-L-serine or the phospho-D-serine control (Fig. 4A). These lipids also did not inhibit phagocytosis at any time point or for any cell type described in Figure 2 (data not shown). Next, we determined whether lectin receptors were involved in mHEV cell phagocytosis by blocking with the carbohydrates mannose, fucose, GlcNAc, mannan, and fucoidin. Others have reported that these saccharides block macrophage and endothelial cell engulfment of apoptotic targets [5, 7]. GlcNAc, D-glucose, D-fucose, D-mannose, and D-galactose did not inhibit mHEVc cell phagocytosis of apoptotic 32D cells (Fig. 4A). Also, L-fucose had no effect on mHEVc cell phagocytosis (data not shown). These carbohydrates also did not block phagocytosis at any time point or for other leukocytes (Fig. 2; data not shown). Fucoidin, however, did significantly inhibit mHEVc cell ingestion of the apoptotic leukocytes (Fig. 4A), and this inhibition was dose-dependent (data not shown). There was also a consistent, small but significant inhibition by mannan (Fig. 4A). Furthermore, inhibition of phagocytosis by mannan and fucoidin, but not other carbohydrates, occurred when phagocytosis was tested at 10 – 48 h after the induction of apoptosis (data not shown). Similar results were obtained with the mHEVa cell line (data not shown). In contrast to these studies with endothelial cells, when the same lot of fucoidin and fucose was used in an assay that quantified human macrophage binding of Candida albicans, fucose but not fucoidin blocked macrophage attachment (Simon L. Newman, University of Cincinnati, OH, personal communication).
Fig. 4.
mHEVc cell phagocytosis of apoptotic 32D cells is fucoidin receptor-mediated. (A) mHEVc cell phagocytosis of apoptotic 32D cells in the presence of sugars and phospholipids. (B) Fucoidin effects on mHEVc cells versus 32D cells. Apoptosis was induced in 32D cells for 20–30 h as described in Figure 2, which corresponds to the time after induction of apoptosis when there is maximum mHEVc cell recognition. The cocultures were incubated for 5 h for phagocytosis, and the percent of mHEVc cells that engulfed target leukocytes was determined. (A) mHEVc phagocytosis was measured in the presence or absence of 20 mM D-glucose, 20 mM N-acetylglucosamine (GlcNAc), 20 mM D-galactose, 20 mM D-fucose, 20 mM D-mannose, 200 μM fucoidin, 375 μM mannan, 10 mM phospho-L-serine, or 10 mM phospho-D-serine. Also, 20 mM L-fucose was tested and had no effect on mHEVc cell phagocytosis (data not shown). (B) mHEVc cells or 32D cells were pretreated with 100 μM fucoidin for 30 min. Fucoidin was left in, or the cells were washed five times to remove excess fucoidin. The last wash from fucoidin-treated mHEVc cells was added to nontreated cocultures. Then, phagocytosis was examined. Dose curves for the inhibitors were done (data not shown). Shown are the optimal inhibitor doses. Data in all panels are presented as the mean of at least duplicate samples ± SD. (A) Data are from a representative experiment of seven experiments for nontreated fucoidin and galactose and two experiments for the other treatments at this time point. Similar effects of inhibitors were observed for all time points in Figure 2 (data not shown). (B) Data are from a representative experiment of three experiments. Similar results were obtained with the mHEVa cell line in two to seven experiments (data not shown). *, P < 0.05, as compared with controls (nontreated, glucose, galactose, or phospho-D-serine).
As fucoidin had the greatest inhibitory effect on mHEV cell phagocytosis of apoptotic leukocytes, we next sought to determine whether fucoidin blocked receptors on the mHEV cells or target leukocytes. Endothelial cell monolayers or the 32D cells were pretreated with fucoidin for 30 min and washed to remove nonbound fucoidin, and then phagocytosis was quantified. Fucoidin pretreatment of endothelial cells, but not 32D cells, inhibited phagocytosis (Fig. 4B), suggesting that endothelial cell fucoidin receptors, but not other carbohydrate receptors or PS receptors, were critical for recognition. Also, phagocytosis was not blocked when medium taken from the last wash of fucoidin-pretreated endothelial cells was added to nontreated mHEVc cell/32D cell cocultures, indicating that fucoidin was removed by the washing. In these studies, the optimal concentration of fucoidin that exhibited >90% inhibition and was not toxic to the cells was within the range of 50 –300 μM depending on the fucoidin lot tested (data not shown).
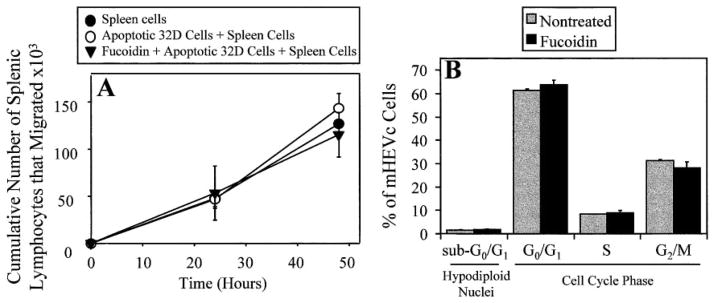
Phagocytosis of apoptotic cells may protect endothelial cells from toxic cellular products released by dying cells. Therefore, we determined whether endothelial cell phagocytosis of apoptotic cells allowed for other endothelial cell monolayer functions, such as the promotion of lymphocyte migration [31]. mHEVc cell line monolayers were incubated with apoptotic 32D cells for 5 h during which phagocytosis occurred. Apoptotic 32D cells were used for phagocytosis since viable 32D cells do not migrate across the endothelial cell monolayers, whereas viable WEHI-231 cells do migrate across the mHEVc cell line monolayers (unpublished observation). After phagocytosis, the monolayers were washed, and the spleen cell migration was examined. The number of spleen cells that migrated across the mHEVc cell line monolayers, which had phagocytosed apoptotic 32D cells, was not significantly different from the number of spleen cells that migrated across control mHEVc cell line monolayers, which were not exposed to apoptotic 32D cells (Fig. 5A). As a control, phagocytosis was blocked by pretreating mHEV cell monolayers with 100 μM fucoidin for 30 min. For these samples, 32D cells were added for 5 h, and then excess fucoidin and nonbound 32D cells were removed by washing before the addition of spleen cells. Spleen cell migration was not affected when the endothelial cell monolayers were pretreated with fucoidin, demonstrating that migration occurred even though phagocytosis had been blocked, and that fucoidin does not affect viable lymphocyte migration. In addition, the presence of apoptotic 32D cells bound to the monolayers did not affect spleen cell migration. Similar results were obtained with the mHEVa cell line (data not shown). These data suggest that transendothelial cell migration can proceed when 25–30% of the endothelial cells had phagocytosed apoptotic cells.
Fig. 5.
Fucoidin does not alter other functions of endothelial cells. (A) Spleen cell migration across mHEVc cell monolayers after ingestion of apoptotic 32D cells. (B) Endothelial cells containing hypodiploid nuclei (sub-G0/G1) or in G0/G1, S, or G2/M. Apoptosis was induced in 32D cells for 20 –30 h as described in Figure 2. (A) mHEV cell monolayers were incubated with apoptotic 32D cells in the presence or absence of 100 μM fucoidin for 5 h for phagocytosis to occur. Monolayers were washed to remove excess fucoidin and nonbound, apoptotic 32D cells. Viable spleen cells were then added to the monolayers, and the number of spleen cells that had migrated to the bottom chamber of the transwells was counted at 24 and 48 h [31]. Spleen cells, Splenic lymphocytes were added to the mHEV cell monolayers. Apoptotic 32D Cells + Spleen Cells, Apoptotic 32D cells were added for 5 h and washed before the addition of splenic lymphocytes to the mHEV cell monolayers. Fucoidin + Apoptotic 32D Cells + Spleen Cells, mHEV cell monolayers were pretreated with 100 μM fucoidin for 30 min, apoptotic 32D cells were added for 5 h, mHEVc monolayers were washed, and then, splenic lymphocytes were added to the mHEVc cells. (B) mHEVc cell monolayers were pretreated with and without 50 μM fucoidin for 30 min, and then, the monolayers were cultured for 5 h (the length of time used for the phagocytic assay). The endothelial cell monolayers were disrupted with PBS/0.03% EDTA, washed one time with PBS, and fixed in 70% ethanol for at least 30 min. The DNA was labeled with DNA-staining reagent containing 50 μg/ml PI, and the fluorescence was examined by flow cytometry. Data in all panels are presented as the mean of at least duplicate samples ± SD. Data are from a representative experiment of two experiments.
It is unlikely that fucoidin was a general metabolic inhibitor of endothelial cells. The lack of effect of fucoidin on migration (Fig. 5A) indicates that fucoidin does not have a general metabolic effect on endothelial actin because actin changes occur when endothelial cells retract during leukocyte migration [31]. In addition, this suggests that it is unlikely that fucoidin affects the actin changes involved in cell phagocytosis. Fucoidin (50 μM) also did not affect endothelial cell-cycle progression or cause apoptosis, as determined by PI labeling of DNA and flow cytometry (Fig. 5B). Furthermore, fucoidin did not affect endothelial cell viability, as determined by trypan blue exclusion (data not shown).
We tested the ability of carbohydrates to block mHEV cell recognition of other apoptotic targets. Apoptotic WEHI-231 cell and apoptotic spleen cell ingestion by mHEVc cells (Fig. 6) and mHEVa cells (data not shown) was inhibited by fucoidin, and there was a consistent, albeit small, inhibition by mannan. The control sugar galactose had no effect on phagocytosis (Fig. 6). The other carbohydrates and phosphoserines also did not block mHEVc and mHEVa cell recognition of these targets (data not shown). Thus, fucoidin, but not the other receptor inhibitors tested, blocked mHEV cell recognition of apoptotic 32D cells, WEHI-231 cells, and spleen cells.
Fig. 6.
Fucoidin and mannan block mHEVc cell phagocytosis of apoptotic WEHI-231 cells and spleen cells. (A) Phagocytosis of apoptotic WEHI-231 cells by mHEVc cells. (B) Phagocytosis of apoptotic spleen cells by mHEVc cells. Apoptosis was induced in WEHI-231 cells and spleen cells for 29 and 12 h, respectively, and was as described in Figures 1 and 2. mHEVc cell monolayers were pretreated with 20 mM D-galactose, 200 μM fucoidin, or 375 μM mannan or were not treated for 30 min before the addition of the control or apoptotic cells to the endothelial cell monolayers. The excess carbohydrates were not washed out. Control and apoptotic leukocytes were added to mHEVc cell monolayers for 5 h to allow for phagocytosis. The percent of mHEVc cells that engulfed target leukocytes was determined. Data in all panels are presented as the mean of at least duplicate samples ± SD. Data are from a representative experiment of three experiments (A) and four experiments (B). Similar results were obtained with the mHEVa cell line (data not shown). *, P < 0.05, as compared with nontreated and D-galactose-treated controls. Control, Noninduced leukocytes. Apoptotic, Apoptosis-induced leukocytes.
Apoptotic cell α4 integrin and CD44 are recognized by mHEVc cells during phagocytosis
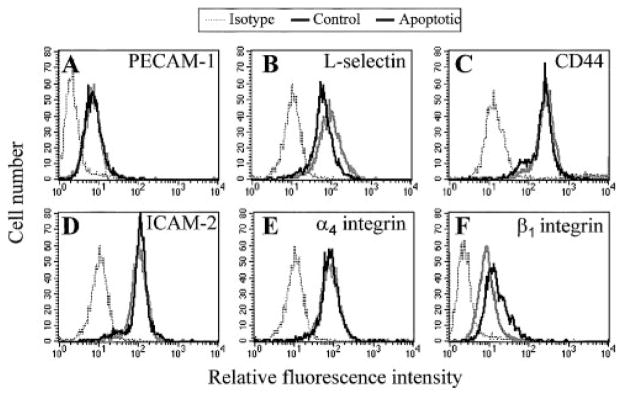
The αV integrins on phagocytic cells have been demonstrated to act alone or in concert with lipid receptors to facilitate engulfment of apoptotic cells by macrophages and dendritic cells [11, 17, 18]. Altered CD31 coreceptor interactions have also been implicated in the recognition phase of the phagocytic process [38]. Thus, we examined mHEV cell and target cell-adhesion molecule expression by indirect immunofluorescent labeling and flow cytometry. The mHEV cells express CD44 and VCAM-1 (CD106; Fig. 3) but not other adhesion molecules [24]. Multiple adhesion molecules are expressed by WEHI-231 cells (Fig. 7), including platelet-endothelial cell adhesion molecule-1 (PECAM-1; CD31), L-selectin (CD62L), CD44, ICAM-2 (CD102), α4 integrin, and β1 integrin. In addition, the expression of these molecules on WEHI-231 cells was not altered by anti-Ig induction of apoptosis (Fig. 7).
Fig. 7.
Apoptotic WEHI-231 cell-adhesion molecule expression. Apoptosis was induced in WEHI-231 cells as described in Figures 1 and 2. At 48 h postinduction of apoptosis, control and apoptotic WEHI-231 cells were labeled with mAb directed against the following cell-surface proteins, followed by a FITC-conjugated secondary antibody and flow cytometric analysis: (A) PE-CAM-1; (B) L-selectin; (C) CD44; (D) ICAM-2; (E) α4 integrin; and (F) β1 integrin. Nonlabeled cells had similar profiles as cells labeled with isotype antibodies (data not shown). Isotype, Isotype-antibody-labeled WEHI-231 cells. Control, Noninduced WEHI-231 cells. Apoptotic, Anti-Ig-induced WEHI-231 cells.
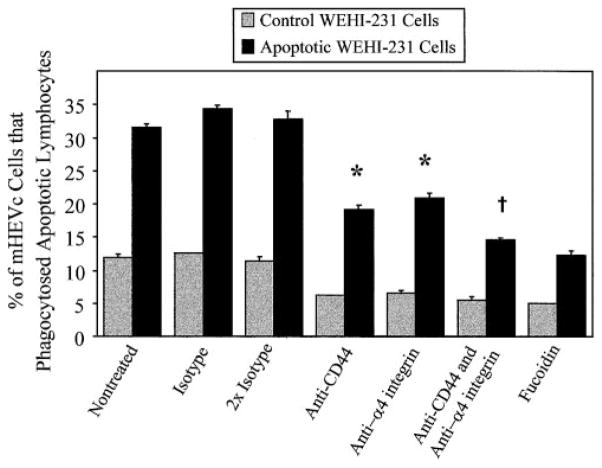
To determine whether cell-adhesion molecules function in mHEV cell recognition of apoptotic lymphocytes, endothelial cell monolayers or the WEHI-231 cells were incubated with blocking antibodies (20 μg/ml) for 30 min before coculturing the cells. The antibody remained in the coculture for the duration of the assay. It is interesting that blocking antibodies directed against CD44 and α4 integrin significantly inhibited mHEVc cell phagocytosis of apoptotic WEHI-231 cells (Fig. 8). These antibody effects were dose-dependent (data not shown). Anti-α4 integrin affected the WEHI-231 cells but not the mHEVc cells because α4 integrin is not expressed by the mHEVc cells (data not shown). However, WEHI-231 cells and mHEVc cells express CD44. The blocking effect of the anti-CD44 antibody was on the WEHI-231 cells and not the mHEVc cells because after pretreatment and washing of the WEHI-231 cells or mHEVc cells to remove nonbound antibody, only the pretreatment of the WEHI-231 cells exhibited inhibition (data not shown). Furthermore, pretreatment of the WEHI-231 cells with a combination of anti-CD44 and anti-α4 integrin antibodies caused a small, significant increase in inhibition compared with effects of individual antibodies (Fig. 8). These antibodies contained azide, yielding a final concentration for each antibody of 0.004% azide. There was no effect of isotype antibodies that contained azide, indicating that this concentration of azide did not affect phagocytosis. α4 integrin can associate with β1 integrin. However, the addition of anti-β1 integrin antibody to the combination of anti-CD44 and anti-α4 integrin provided no further inhibitory effect (data not shown). Addition of fucoidin with these antibodies also exhibited no greater inhibition than the fucoidin treatment alone (data not shown). Inhibition of WEHI-231 cell β1 integrin, PECAM-1, L-selectin, and ICAM-2 had no effect (data not shown). Blocking antibodies against the endothelial cell receptors PSR, αV integrin, α5 integrin, β1 integrin, CD44, and VCAM-1 had no inhibitory effect on mHEVc cell phagocytosis of WEHI-231 cells (data not shown). In summary, apoptotic cell CD44 and α4 integrin are recognized by endothelial cells for phagocytosis.
Fig. 8.
CD44 and α4 integrin on apoptotic WEHI-231 cells interact with mHEVc cells to promote PSR-independent phagocytosis. Apoptosis was induced in WEHI-231 cells as described in Figures 1 and 2. At 48 h postinduction of apoptosis, monolayers of mHEVc cells in 24-well plates were treated with 50 μM fucoidin or not treated for 30 min, and target WEHI-231 cells (3 × 106 cells) were incubated with 20 μg/ml each blocking mAb or isotype antibody for 30 min at 37°C. As a control for antibody combinations, WEHI-231 cells were also incubated with two times the amount of isotype antibody (40 μg/ml). After the treatment, control and apoptotic WEHI-231 cells were added to the mHEVc monolayers for 5 h to allow for phagocytosis, with the respective antibody(s) remaining in the coculture. The percent of mHEVc cells that engulfed target WEHI-231 cells was determined [30]. The mHEVc cells do not express α4 integrin (data not shown); however, CD44 is expressed by the mHEVc cells, and anti-CD44 pretreatment of mHEVc cells followed by five washes did not inhibit phagocytosis, but WEHI-231 pretreatment with this antibody followed by five washes inhibited phagocytosis (data not shown). This indicates that CD44 expressed by WEHI-231 cells functions in recognition for endothelial cell phagocytosis. Data are presented as the mean of triplicate samples ± SD. Data are from a representative experiment of seven experiments. Similar inhibitory results were obtained at all time points shown in Figure 2 (data not shown). Anti-CD44 and anti-α4 integrin exhibited dose-dependent inhibition (data not shown). *, P < 0.05, as compared with isotype samples; †, P < 0.05, as compared with single pretreatment samples and with isotype controls. Control, Noninduced WEHI-231 cells. Apoptotic, Anti-Ig-induced WEHI-231 cells.
DISCUSSION
Rapid removal of apoptotic corpses in vivo is required for the resolution of inflammation and the retardation of various disease processes [3]. We report that the fucoidin receptor can mediate phagocytosis independent of the PSR or scavenger receptors. The fucoidin receptor recognizes apoptotic but not necrotic cells. In addition, apoptotic leukocyte α4 integrin and CD44 assist in PSR-independent phagocytosis. This is a novel function for CD44 and the α4 integrin. CD44 and α4 integrin likely bind to extracellular matrix (ECM) components on the endothelial cells since the α4 integrin ligand VCAM-1 was not required for phagocytosis.
Our previous in vivo studies show that HEV cells in the lymph nodes of corticosteroid-treated mice internalize apoptotic lymphocytes without causing any visible vessel damage within the lymph node tissue [20]. The recognition of apoptotic cells by endothelial cells probably does not limit lymphocyte migration because phagocytosis of apoptotic targets by mHEV cell monolayers does not affect subsequent viable lymphocyte migration across these monolayers. As HEV cells regulate lymphocyte recirculation into lymph nodes via migration, the clearance of apoptotic cells likely ensures protection of the microvasculature and thus maintains migration of viable lymphocytes into tissues. There are several disease states that could generate high levels of circulating apoptotic leukocytes, which come into contact with endothelial cells. For example, during HIV infection, circulating, virally infected CD4+ T cells have been shown to undergo apoptosis [39], and diabetic rats were observed to have increased apoptosis in peripheral lymphocytes [40]. Individuals with systemic lupus erythematosus are also known to have elevated levels of apoptotic lymphocytes in their systemic circulation [41]. Identification of mechanisms involved in endothelial cell recognition and phagocytosis of apoptotic cells would provide a better understanding of this clearance mechanism during tissue homeostasis and disease. This could then lead to proposing ways to manipulate phagocytosis in disease processes.
Multiple receptor use by phagocytes most likely evolved as a protective mechanism to ensure that clearance of apoptotic cells is successfully achieved. The type(s) of phagocytic receptor used depends on the phagocytic cell type [11], the body location of a phagocytic cell [19], and how the phagocytes are activated [42]. The mHEV cell lines used fucoidin-binding receptors for phagocytosis at all time points tested postinduction of apoptosis. The WEHI-231 cell ligand for the fucoidin receptor is likely a carbohydrate moiety because the carbohydrate fucoidin blocks recognition. Fucoidin is not likely a metabolic inhibitor of endothelial cells, as it did not affect endothelial cell-cycle progression, and it did not affect viable lymphocyte migration across endothelial cells, which requires endothelial cell-actin structural changes. The ligand for the fucoidin receptor has not been defined and is an area of current research. Maximum fucoidin receptor-dependent phagocytosis of apoptotic cells occurred before 50% maximum accumulation of cells with apoptotic outer-cell membrane PS or PI-labeled hypodiploid nuclei. However, excess PS and anti-PSR-blocking antibodies did not inhibit mHEV cell phagocytosis of the apoptotic leukocytes (even in combination with anti-CD44, anti-α4 integrin, and anti-β1 integrin antibodies, data not shown). Therefore, although the apoptotic targets displayed PS, and the mHEV cells expressed PSR, the mHEV cell lines do not require the PSR for the phagocytosis of apoptotic cells. Necrotic and apoptotic cells have been reported to accumulate PS, and macrophages ingest necrotic and apoptotic cells [26]. Thus, the specificity of mHEV cells for recognition of apoptotic but not necrotic cells is consistent with mHEV cell phagocytosis, which does not involve the PSR. Furthermore, it indicates that the fucoidin receptor process is specific for apoptotic cells. Thus, mHEV cells are a model for examining fucoidin receptor-mediated phagocytosis, independent of recognition by other known phagocytic cell receptors.
Fucoidin and other soluble carbohydrates have been shown to inhibit clearance of apoptotic cells in several systems. For example, monosaccharide solutions of mannose, fucose, mannan, and fucoidin inhibit (60% inhibition) fibroblast engulfment of aged neutrophils in vitro [33]. A cocktail of galactose, N-acetylglucosamine, and mannose blocks liver endothelial cell phagocytosis of apoptotic bodies in situ [43]. Fadok et al. [11] reported that N-acetylglucosamine-specific lectin receptors play a minor role in human macrophage ingestion of apoptotic neutrophils in vitro. In contrast, fucoidin and mannan, but not fucose, mannose, or N-acetylglucosamine inhibited mHEV cell-line phagocytosis of apoptotic leukocytes. The concentrations of mannose tested were up to 10 times greater than that for the inhibitor constant Ki for the mannose receptor [44, 45], indicating that it is unlikely that the mannose receptor is involved in mHEV cell recognition of apoptotic targets.
In addition to blocking the fucoidin receptor, fucoidin, which is a sulfated polymer of fucose, has been reported to block some of the scavenger receptors. Fucoidin blocks the scavenger receptors SR-A [46] and LOX-1 [9] but not the class B-type scavenger receptors CD36 and SR-BI [47]. CD36 can recognize externalized PS on the outer membrane of apoptotic cells for phagocytosis [3]. CD36, in concert with αVβ3, and thrombospondin also form a complex that recognizes unknown sites on apoptotic targets. Oka et al. [9] demonstrated that ingestion of aged/apoptotic RBC by Chinese hamster ovary cells expressing the bovine scavenger receptor LOX-1 was partially blocked by fucoidin. Furthermore, Sambrano et al. [48] demonstrated that the binding and phagocytosis of oxidized RBC by murine peritoneal macrophages were partially inhibited by fucoidin via an unidentified receptor. Studies by Platt et al. [46] suggest that fucoidin inhibits thymic macrophage engulfment of apoptotic thymocytes and that SR-A null macrophages exhibit a 50% reduction in phagocytosis of apoptotic thymocytes. However, in their study [46], they did not determine if fucoidin blocked SR-A or whether fucoidin blocked other receptors, as they did not test the inhibitory effect of fucoidin on phagocytosis by the SR-A null thymic macrophages. Since the mHEVa and mHEVc cells do not express SR-A, SR-BI, or SR-BII, as determined by immunofluorescent labeling and flow cytometry analysis, mHEVa and mHEVc cell recognition and ingestion of apoptotic cells cannot occur by means of these scavenger receptors. In addition, LOX-1 is an unlikely candidate for the mHEV cell lines, as PS blocks LOX-1 but not mHEV cell recognition of apoptotic leukocytes. The absence of β3 integrin on mHEV cells and the lack of effect of anti-αV integrin-blocking antibodies on mHEVc cell phagocytosis of apoptotic WEHI-231 cells suggest that they do not ingest apoptotic cells via the αVβ3/CD36/thrombospondin recognition complex.
Complement proteins in our studies did not mediate fucoidin receptor-mediated phagocytosis. Apoptotic cells coated with the complement protein iC3b are recognized by macrophage complement receptors CR3 and CR4 [14]. Coating the apoptotic leukocytes with iC3b and subsequent use of CR3 and CR4 by mHEV cells for phagocytosis are unlikely in these studies, because all experiments were performed in medium containing heat-inactivated serum, which prevents activation of the complement cascade [49].
ICAM-3, a cell-adhesion molecule present on apoptotic lymphocytes, has been described as the ligand for the phagocytic receptor CD14 [15, 16]. As mHEV cells do not express CD14, this recognition system does not play a role in mHEV cell engulfment of apoptotic leukocytes. Recently, Brown et al. [38] reported a mechanism involving homophilic CD31-binding interactions between apoptotic cells and macrophages, in which the consequence is apoptotic cell ingestion and the active disengagement of viable cells. Blocking with anti-CD31 did not have an effect on mHEVc cell fucoidin receptor-mediated phagocytosis.
We demonstrated a novel role for CD44 and α4 integrin in phagocytosis. CD44 and α4 integrin on the WEHI-231 cells participated in the PSR-independent phagocytosis, as blocking these adhesion molecules inhibited phagocytosis. To our knowledge, α4 integrin and CD44 on the target cell have not previously been reported to participate in phagocytosis of apoptotic cells. In contrast, a role for CD44 on phagocytes has been described. Hart et al. [50] has demonstrated that antibody cross-linking of CD44 on macrophages augments phagocytosis of apoptotic neutrophils. In our studies, apoptotic cell α4 integrin likely binds to ECM ligands on the endothelial cell since inhibition of its endothelial cell ligand VCAM-1 had no effect on phagocytosis. Blocking ECM with arginine-glycineaspartame (RGD) peptides cannot be done, as RGD dissociates the endothelial cell monolayer (unpublished observation). Although VCAM-1 did not participate in phagocytosis, VCAM-1 is functional on the endothelial cells since it promotes viable lymphocyte migration [31]. It has been reported that an initial phagocytic cell–integrin-binding supports PSR function [17]. In contrast to this integrin function on the phagocytic cells, we demonstrated that the integrins on the apoptotic target cell participated in recognition during PSR-independent phagocytosis.
In summary, the endothelial cell lines used fucoidin receptor(s) to specifically recognize and phagocytose apoptotic but not necrotic leukocytes. The fucoidin receptor(s) is distinct from fucose, mannose, and N-acetylglucosamine lectin receptors, scavenger receptors, and the PSR. In addition, CD44 and α4 integrin on apoptotic targets promote ingestion. Endothelial cell phagocytosis of apoptotic cells may limit damage to endothelial cells that may otherwise occur by apoptotic leukocyte-binding to endothelial cells.
Acknowledgments
We thank Dr. Simon Newman for his discussion and review of this manuscript. We also thank Dr. Valerie Fadok (National Jewish Medical and Research Center, Denver, CO) for generously providing purified anti-human PSR antibody (mAb 217). J. D. J. and K. L. H. had equal contributions to this manuscript.
References
- 1.Schlegel RA, Williamson P. Phosphatidylserine, a death knell. Cell Death Differ. 2001;8:551–563. doi: 10.1038/sj.cdd.4400817. [DOI] [PubMed] [Google Scholar]
- 2.Magnus T, Chan A, Savill J, Toyka K, Gold R. Phagocytic removal of apoptotic, inflammatory lymphocytes in the central nervous system by microglia and its functional implications. J Neuroimmunol. 2002;130:1–9. doi: 10.1016/s0165-5728(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- 4.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 5.Dini L, Lentini A, Diez GD, Rocha M, Falasca L, Serafino L, Vidal-Vanaclocha F. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108:967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 6.Falasca L, Bergamini A, Serafino A, Balabaud C, Dini L. Human Kupffer cell recognition and phagocytosis of apoptotic peripheral blood lymphocytes. Exp Cell Res. 1996;224:152–162. doi: 10.1006/excr.1996.0123. [DOI] [PubMed] [Google Scholar]
- 7.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–358. [PMC free article] [PubMed] [Google Scholar]
- 8.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 9.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 11.Fadok VA, Warner ML, Bratton DL, Henson PM. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (alpha v beta 3) J Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- 12.Adayev T, Estephan R, Meserole S, Mazza B, Yurkow EJ, Banerjee P. Externalization of phosphatidylserine may not be an early signal of apoptosis in neuronal cells, but only the phosphatidylserine-displaying apoptotic cells are phagocytosed by microglia. J Neurochem. 1998;71:1854–1864. doi: 10.1046/j.1471-4159.1998.71051854.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennett MR, Gibson DF, Schwartz SM, Tait JF. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res. 1995;77:1136–1142. doi: 10.1161/01.res.77.6.1136. [DOI] [PubMed] [Google Scholar]
- 14.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL, Gregory CD. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 16.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol. 1999;162:6800–6810. [PubMed] [Google Scholar]
- 17.Hoffmann PR, deCathelineau AM, Ogden CA, Leverrier Y, Bratton DL, Daleke DL, Ridley AJ, Fadok VA, Henson PM. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J Cell Biol. 2001;155:649–659. doi: 10.1083/jcb.200108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albert ML, Pearce SF, Francisco LM, Sauter B, Roy P, Silverstein RL, Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J Exp Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meszaros AJ, Reichner JS, Albina JE. Macrophage phagocytosis of wound neutrophils. J Leukoc Biol. 1999;65:35–42. doi: 10.1002/jlb.65.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Hess KL, Tudor KS, Johnson JD, Osati-Ashtiani F, Askew DS, Cook-Mills JM. Human and murine high endothelial venule cells phagocytose apoptotic leukocytes. Exp Cell Res. 1997;236:404–411. doi: 10.1006/excr.1997.3745. [DOI] [PubMed] [Google Scholar]
- 21.Duijvestijn A, Hamann A. Mechanisms and regulation of lymphocyte migration. Immunol Today. 1989;10:23–28. doi: 10.1016/0167-5699(89)90061-3. [DOI] [PubMed] [Google Scholar]
- 22.Butcher EC. The regulation of lymphocyte traffic. Curr Top Microbiol Immunol. 1986;128:85–122. doi: 10.1007/978-3-642-71272-2_3. [DOI] [PubMed] [Google Scholar]
- 23.Askew DS, Ashmun RA, Simmons BC, Cleveland JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. [PubMed] [Google Scholar]
- 24.Cook-Mills JM, Gallagher JS, Feldbush TL. Isolation and characterization of high endothelial cell lines derived from mouse lymph nodes. In Vitro Cell Dev Biol Anim. 1996;32:167–177. doi: 10.1007/BF02723682. [DOI] [PubMed] [Google Scholar]
- 25.Reid S, Cross R, Snow EC. Combined Hoechst 33342 and merocyanine 540 staining to examine murine B cell cycle stage, viability and apoptosis. J Immunol Methods. 1996;192:43–54. doi: 10.1016/0022-1759(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 26.Cocco RE, Ucker DS. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol Biol Cell. 2001;12:919–930. doi: 10.1091/mbc.12.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen JJ, Duke RC, Fadok VA, Sellins KS. Apoptosis and programmed cell death in immunity. Annu Rev Immunol. 1992;10:267–293. doi: 10.1146/annurev.iy.10.040192.001411. [DOI] [PubMed] [Google Scholar]
- 28.Fraker PJ, King LE, Lill-Elghanian D, Telford WG. Quantification of apoptotic events in pure and heterogeneous populations of cells using the flow cytometer. In: Schwartz LM, Osborne BA, editors. Methods in Cell Biology. San Diego, CA: Academic; 1995. pp. 57–76. [DOI] [PubMed] [Google Scholar]
- 29.Mower DA, Jr, Peckham DW, Illera VA, Fishbaugh JK, Stunz LL, Ashman RF. Decreased membrane phospholipid packing and decreased cell size precede DNA cleavage in mature mouse B cell apoptosis. J Immunol. 1994;152:4832–4842. [PubMed] [Google Scholar]
- 30.Hess KL, Babcock GF, Askew DS, Cook-Mills JM. A novel flow cytometric method for quantifying phagocytosis of apoptotic cells. Cytometry. 1997;27:145–152. [PMC free article] [PubMed] [Google Scholar]
- 31.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 32.Hess KL, Johnson JD, Cook-Mills JM. Different orders for acquisition of apoptotic characteristics by leukocytes. J Leukoc Biol. 2001;70:405–412. [PubMed] [Google Scholar]
- 33.Hall SE, Savill JS, Henson PM, Haslett C. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J Immunol. 1994;153:3218–3227. [PubMed] [Google Scholar]
- 34.Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gildea LA, Morris RE, Newman SL. Histoplasma capsulatum yeasts are phagocytosed via very late antigen-5, killed, and processed for antigen presentation by human dendritic cells. J Immunol. 2001;166:1049–1056. doi: 10.4049/jimmunol.166.2.1049. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz BR, Karsan A, Bombeli T, Harlan JM. A novel beta 1 integrin-dependent mechanism of leukocyte adherence to apoptotic cells. J Immunol. 1999;162:4842–4848. [PubMed] [Google Scholar]
- 37.Keidar S, Brook GJ, Rosenblat M, Fuhrman B, Dankner G, Aviram M. Involvement of the macrophage low density lipoprotein receptor-binding domains in the uptake of oxidized low density lipoprotein. Arterioscler Thromb. 1992;12:484–493. doi: 10.1161/01.atv.12.4.484. [DOI] [PubMed] [Google Scholar]
- 38.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 39.Roger PM, Breittmayer JP, Arlotto C, Pugliese P, Pradier C, Bernard-Pomier G, Dellamonica P, Bernard A. Highly active anti-retroviral therapy (HAART) is associated with a lower level of CD4+ T cell apoptosis in HIV-infected patients. Clin Exp Immunol. 1999;118:412–416. doi: 10.1046/j.1365-2249.1999.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung CG, Kamiyama T, Agui T. Elevated apoptosis of peripheral T lymphocytes in diabetic BB rats. Immunology. 1999;98:590–594. doi: 10.1046/j.1365-2567.1999.00907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Courtney PA, Crockard AD, Williamson K, McConnell J, Kennedy RJ, Bell AL. Lymphocyte apoptosis in systemic lupus erythematosus: relationships with Fas expression, serum soluble Fas and disease activity. Lupus. 1999;8:508–513. doi: 10.1191/096120399678840765. [DOI] [PubMed] [Google Scholar]
- 42.Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- 43.Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fiani ML, Beitz J, Turvy D, Blum JS, Stahl PD. Regulation of mannose receptor synthesis and turnover in mouse J774 macrophages. J Leukoc Biol. 1998;64:85–91. doi: 10.1002/jlb.64.1.85. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Pomares L, Crocker PR, Da Silva R, Holmes N, Colominas C, Rudd P, Dwek R, Gordon S. Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J Biol Chem. 1999;274:35211–35218. doi: 10.1074/jbc.274.49.35211. [DOI] [PubMed] [Google Scholar]
- 46.Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–12460. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 48.Sambrano GR, Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc Natl Acad Sci USA. 1995;92:1396–1400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peterson PK, Wilkinson BJ, Kim Y, Schmeling D, Quie PG. Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect Immun. 1978;19:943–949. doi: 10.1128/iai.19.3.943-949.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart SP, Dougherty GJ, Haslett C, Dransfield I. CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J Immunol. 1997;159:919–925. [PubMed] [Google Scholar]