Abstract
The myristoylated matrix protein (myr-MA) of HIV functions as a regulator of intracellular localization, targeting the Gag precursor polyprotein to lipid rafts in the plasma membrane during virus assembly and dissociating from the membrane during infectivity for nuclear targeting of the preintegration complex. Membrane release is triggered by proteolytic cleavage of Gag, and it has, until now, been believed that proteolysis induces a conformational change in myr-MA that sequesters the myristyl group. NMR studies reported here reveal that myr-MA adopts myr-exposed [myr(e)] and -sequestered [myr(s)] states, as anticipated. Unexpectedly, the tertiary structures of the protein in both states are very similar, with the sequestered myristyl group occupying a cavity that requires only minor conformational adjustments for insertion. In addition, myristate exposure is coupled with trimerization, with the myristyl group sequestered in the monomer and exposed in the trimer (Kassoc = 2.5 ± 0.6 × 108 M–2). The equilibrium constant is shifted ≈20-fold toward the trimeric, myristate-exposed species in a Gag-like construct that includes the capsid domain, indicating that exposure is enhanced by Gag subdomains that promote self-association. Our findings indicate that the HIV-1 myristyl switch is regulated not by mechanically induced conformational changes, as observed for other myristyl switches, but instead by entropic modulation of a preexisting equilibrium.
During the late stage of the HIV replication cycle, several thousand copies of the viral Gag polyprotein colocalize at fatty acid-rich raft-like sites on the plasma membrane, where they assemble and bud to form a new virus particle (1). Targeted assembly at these sites is promoted by an N-terminal myristyl group (myr) and conserved basic patch on the surface of the matrix domain (MA) of Gag, which function synergistically to promote tight membrane binding (2, 3). During or shortly after budding, Gag is cleaved by the viral protease, which liberates the mature MA, capsid (CA), and nucleocapsid (NC) proteins and the unstructured peptides p2, p1, and p6 for viral maturation (4). Upon entering a new cell, a portion of the mature matrix proteins dissociate from the membrane and are incorporated into the preintegration complex (PIC) (5), where they appear to help direct the nuclear localization of the PIC (6–9).
There is considerable evidence that the N-terminal myristyl group of MA plays a role in regulating membrane binding. Mutations that block myristoylation result in aberrant targeting of Gag to intracellular membranes in vivo and an increase in the soluble fraction of Gag in vitro (10–14). In addition, the affinity of MA for membranes is substantially lower than that of the Gag precursor (15), and proteolysis of membrane-bound Gag by the HIV-1 protease leads to the release of the mature MA protein from the membrane (16). These findings indicate that the HIV protease is the trigger for a myristyl switch in which the myristate is exposed in the Gag polyprotein to promote membrane binding but becomes sequestered upon proteolysis to promote cytosolic and nuclear targeting (13, 15–18). Three myristyl switches have been structurally characterized: the calcium-dependent switch of recoverin (19, 20), the hydrolytic switch of the ARF1 GTPase (21), and the autoinhibitory switch of c-Abl tyrosine kinase (22). In all cases, conversion between myristate-exposed and -sequestered states is accompanied by major changes in protein structure, and it has been suggested that the HIV-1 myristyl switch is similarly triggered by a conformational change induced by proteolysis of Gag (13, 15–18).
The unmyristoylated HIV-1 matrix protein [myr(–)-MA] is monomeric at concentrations as high as 3 mM and 35°C (23, 24), but crystallizes as a trimer at reduced temperatures (25). In both the solid- and solution-states, the N- and C-terminal residues of myr(–)-MA are spatially distant, with the distal helix projecting away from the globular portion of the protein and the 12 C-terminal residues being disordered (23–25). These residues are also disordered in a Gag-like construct that includes the independently folded N-terminal domain of capsid [myr(–)-MA–CANTD] (26). Based on these structures, it is difficult to understand how the N-terminal myristyl group would be sensitive to cleavage of the MA–CA junction. To address these issues, we have prepared the myristoylated form of the HIV-1 matrix protein (referred in the remainder of the paper as myr-MA), as well as a myristoylated Gag-like construct that includes the capsid domain (myr-MA–CA; residues Myr-1–Leu-362), for solution-state NMR and equilibrium studies.
Materials and Methods
Sample Preparation. Yeast N-terminal myristyl transferase (yNMT) was amplified from yeast genomic DNA (Promega), and a NdeI restriction site within the yNMT gene was removed by a silent mutation (sequenced at the W. M. Keck Foundation Biotechnology Resource Laboratory, Yale University, New Haven, CT). Genes encoding MA and MA–CA were PCR-amplified from HIV-1 genomic cDNA plasmid pNL4–3 (27), and DNA encoding for a C-terminal His-6-tag was appended to the 3′ primers. The DNA segments for the MA and MA–CA genes were inserted into a coexpression vector (M. Resh, Memorial Sloan–Kettering Cancer Center, New York) and transformed into DH5α-competent cells (Invitrogen), and the plasmids were extracted (Qiagen, Valencia, CA) and sequenced. The MA gene was also subcloned into pET-11a vector (Novagen) for expression of myr(–)-MA. Plasmids were transformed into BL21/DE3 codon-plus RIL-competent cells (Stratagene) for protein expression. Cells were supplemented with myristic acid (10 mg/liter; Sigma) or U-13C-myristic acid (5 mg/liter; Isotec) 1 h before induction with isopropyl β-d-thiogalactoside (1 mM). Cells were lysed (1× Bugbuster, Novagen; followed by French press) and the proteins purified by cobalt affinity chromatography (Clontech). Traces of unmyristoylated species were subsequently removed by butyl-Sepharose hydrophobicity chromatography (Amersham Pharmacia). Samples were stored at room temperature with 1× protease inhibitor mixture Set I (Calbiochem). Molecular weights were confirmed by electrospray ionization mass spectrometry: myr(–)-MA, with C-terminal His-6 tag: Mrcalc = 15,534.4, Mrmeas = 15,534.6 ± 0.9; myr-MA, Mrcalc = 14,921.8, Mrmeas = 14,922.20 ± 0.99; 15N-myr-MA, with C-terminal His-6 tag: Mrcalc = 15,953.6, Mrmeas = 15,953.9 ± 1.1; myr-MA–CA, Mrcalc = 41,329.1, Mrmeas = 41,329.7 ± 11.
NMR Spectroscopy. NMR data were collected with Bruker 600 MHz and 800 MHz instruments using protein samples of 30–200 μM, unless otherwise stated (35°C, 50 mM sodium phosphate, pH 5.5/100 mM NaCl/5 mM DTT/1× protease inhibitor mixture). Backbone assignments for myr-MA were obtained with standard triple resonance experiments (28), and side chain assignments and [1H–1H]NOEs (nuclear Overhauser effects) for structure calculations were obtained from 3D 15N-edited NOE-SY–heteronuclear sequential quantum correlation (HSQC), 3D 13C-edited heteronuclear multiple quantum correlation (HMQC)–NOESY and 4D 13C/15N-edited HMQC–NOESY–HSQC spectra (29). The myristate 1H–13C signals were differentiated from protein signals by comparison of 2D [1H– 13C]HMQC spectra obtained for constructs containing unlabeled and 13C-labeled myristate (Fig. 5, which is published as supporting information on the PNAS web site). The myristate signals were assigned on the basis of 13C chemical shifts and sequential connectivities observed in the NOESY spectra, and are consistent with previous assignments (30). The myr C5–11 methylene signals were degenerate, but the remaining 1H–13C correlation signals were resolved. The terminal myr-C14H3 methyl group exhibited an unusual upfield chemical shift (0.45 ppm) due to its close proximity to Trp-16 (confirmed by myr-C14H3 to Trp-aromatic proton NOEs). NMR data were processed with nmrpipe (31) and analyzed with nmrview (32).
Structure Calculations. Conservative distance bounds for [1H–1H]NOEs and backbone dihedral angle restraints based on 13Cα, 13Cβ, 1Hα, 13C, and 15N chemical shifts (33) were used for structure calculations with cyana (34) as described (26). NOEs involving the degenerate myr-C5–11 methylenes were treated by a 3.5 Å pseudoatom correction to the central C8 atom. Structures were assessed with procheck-nmr (35) and visualized with pymol (36). Statistical information associated with the structure calculations is given in Table 1.
Table 1. Structural statistics.
| NMR-derived restraints* | |
| Interproton restraints | 891 |
| Intraresidue | 43 |
| Sequential | 165 |
| Medium range (1 < j — i < 5) | 572 |
| Long range (|j — i| > 4) | 111 |
| Protein—myristate | 17 |
| Hydrogen bond restraints | 154 |
| Dihedral angle restraints (f, y) | 138 |
| Total restraints | 1,183 |
| Average restraints per residue | 18 |
| Residual restraint violations | |
| CYANA target function, Å2 | 0.10 ± 0.05 |
| Maximum violations | |
| Upper limits, Å2 | 0.12 ± 0.10 |
| Lower limits, Å2 | 0.04 ± 0.04 |
| van der Walls, Å2 | 0.07 ± 0.02 |
| Torsion angles, radian | 0.79 ± 0.39 |
| Structure convergence | |
| Pairwise rms deviations† | |
| bb heavy atoms, Å | 0.45 ± 0.09 |
| all heavy atoms, Å | 1.01 ± 0.08 |
| Pairwise rms deviations (relative to myr (—)-MA) | |
| NMR structures, Ň | 0.48 ± 0.1 |
| X-ray structures, Ň | 0.96 ± 0.07 |
| Ramachandran analyses§ | |
| Most favored regions, % | 88 |
| Additional allowed regions, % | 11 |
| Generously allowed regions, % | 1 |
Gly-123 through the C-terminal Tyr-132 were not restrained.
Relative to mean atom positions for residues Val-7-Ile-104.
PDB accession number 1HIW for the myr(—)-MA X-ray structure.
Gly and Pro residues were not included in the analysis.
Analytical Ultracentrifugation. Sedimentation equilibrium measurements were made with a Beckman XL-I Optima system equipped with a four-hole An-60 rotor (Beckman Coulter). Cells were equipped with double-sectored charcoal filled epon centerpieces of path length 12 or 3 mm and quartz windows. Protein samples were prepared in 50 mM sodium phosphate buffer at pH 5.5 containing 100 mM NaCl and 2 mM Tris (2-carboxy-ethyl)-phosphine-HCl at loading concentrations of 20–200 μM for myr-MA, 2.5–100 μMfor myr-MA–CA, and 100–200 μM for myr(–)-MA. Rotor speeds ranged from 18,000 to 40,000 rpm for myr-MA and from 10,000 to 22,000 rpm for myr-MA–CA, and temperatures ranged from 20 to 35°C. Scans were obtained by using the absorption optics system with 0.002 cm step size and four averages per point, and acquired at wavelengths of 295 nm [myr-MA and myr(–)-MA] or 280 nm (myr-MA–CA). Partial specific volumes (v-bar) and molar extinction coefficients were calculated by using the program sednterp (www.jphilo.mail-way.com) and buffer densities were measured pycnometrically. Data analysis was performed by using nonlin (37). Equilibrium association constants were determined by global analysis of data acquired from samples prepared at a minimum of four loading concentrations and centrifuged at a minimum of four rotor speeds.
Results
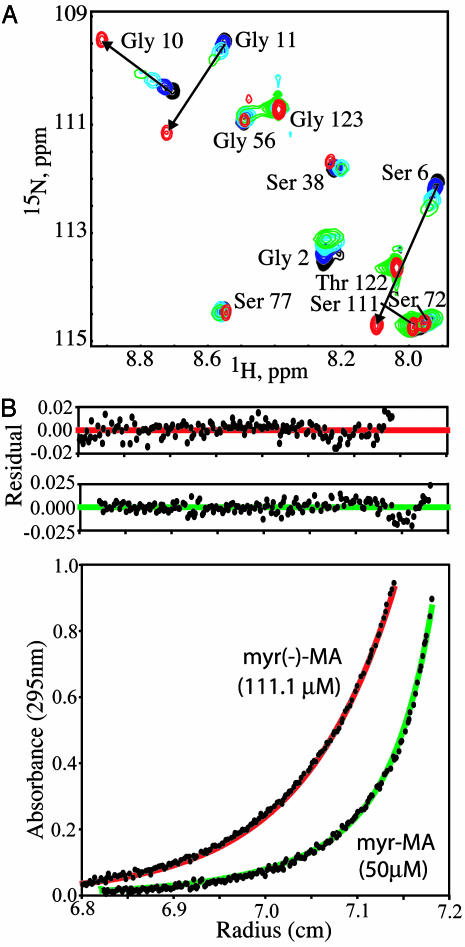
Myr-MA Exists as an Equilibrium Mixture of Monomeric (Myr-Sequestered) and Trimeric (Myr-Exposed) Species. High-quality 2D [1H–15N]HSQC spectra were obtained for myr-MA at concentrations of 200 μM or less (Fig. 5). Although most signals (>80%) were insensitive to concentration under the conditions used, concentration-dependent deviations were observed for two subsets of signals. Chemical shifts for one subset deviate significantly from those of the unmyristoylated protein (myr(–)-MA) at concentrations <100 μM, but progressively shift toward the frequencies of myr(–)-MA as the concentration is increased (Fig. 1A; Δδ 1H >0.2 ppm or Δδ 15N > 1.0 ppm; residues Ala-5–Gly-11, Arg-39, and Gly-49). A second subset exhibits chemical shifts that match those of myr(–)-MA at concentrations <100 μM but progressively broaden and/or deviate (by a smaller magnitude) with increasing concentration (residues Leu-41–Asn-47 and Gly-71–Leu-75).
Fig. 1.
(A) Overlay of expanded portions of the 2D [1H–15N]HSQC spectra obtained for myr-MA [25 μM (black), 100 μM (blue), 400 μM (cyan), and 800 μM (green)] and myr(–)-MA (500 μM, red). A subset of myr-MA signals differ significantly from those of myr(–)-MA at low concentration due to sequestration of the myristyl group but shift progressively toward the frequencies of myr(–)-MA as the concentration is increased (denoted by arrows), consistent with an equilibrium shift toward a myristate-exposed species at higher concentrations. (B) Representative sedimentation equilibrium data obtained for myr-MA and myr(–)-MA at 20°C. Data for myr-MA fit to a momomer–trimer equilibrium with association constant (Kassoc) of 2.5 ± 0.6 × 108 M–2 (theoretical curve shown in green), whereas the myr(–)-MA data fit best to a homogeneous, monomeric species (red). Higher-order myr-MA oligomers are formed at concentrations >100 μMat20°C. At 35°C, where optimal NMR data were obtained, Kassoc for myr-MA shifts to 4.0 ± 0.8 × 107 M–2.
[1H–1H]NOE data obtained for myr-MA indicate that the chemical shift deviations observed at low concentrations (150–200 μM, 35°C) are caused by interactions between the myristyl group and the protein (see below). The progressive shift of these signals toward values of the unmyristoylated protein as the concentration is increased is indicative of an equilibrium shift toward a multimeric, myristate-exposed species. Sedimentation equilibrium (SE) measurements confirm that myr-MA self-associates, and quantitative analyses indicate that the protein trimerizes with association constants (Kassoc) of 2.5 ± 0.6 × 108 M–2 and 4.0 ± 0.8 × 107 M–2 at 20°C and 35°C, respectively (Fig. 1B). Thus, the combined NMR and SE data indicate that myr-MA exists as a momomer–trimer equilibrium, with the myristate sequestered [myr(s)] in the monomer and exposed [myr(e)] in the trimer.
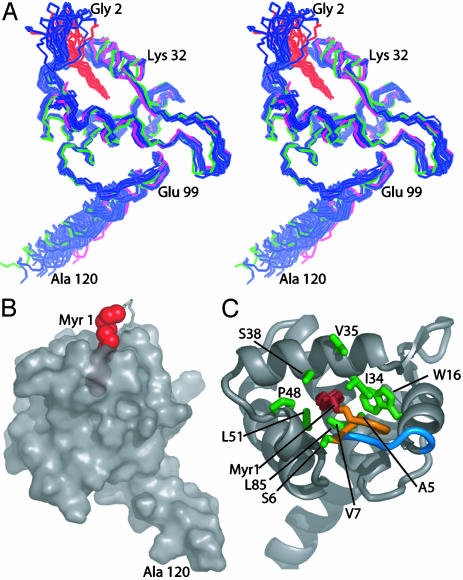
Structure of Myr-MA in the Monomeric, Myr(s) State. A superposition of 20 myr(s)-MA structures generated from NMR data collected at concentrations of 150–200 μM and 35°C is shown in Fig. 2A. For comparison, representative x-ray and NMR structures of the unmyristoylated protein are also shown. The backbone atoms of Val-7–Ile-104 are well defined (0.45 ± 0.09-Å rms deviation relative to mean atom positions) and are in close agreement with coordinates of the unmyristoylated protein [0.48 ± 0.10-Å and 0.96 ± 0.07-Å rms deviation relative to the NMR and x-ray structures of myr(–)-MA, respectively]. The myristyl group adopts an extended conformation and penetrates ≈10 Å beneath the surface of the protein (Fig. 2B). The terminal methyl group (C14H3) packs in close proximity to side chains of core residues Trp-16, Ile-34, and Leu-85, and other myristate methylene groups interact with the side chains of Ala-5, Ser-6, Val-7, Ile-34, Ser-38, Pro-48, Val-35, Leu-51, and Glu-52 (Fig. 2C). Significantly, ≈40% of the myristyl group remains exposed to the solvent in the sequestered form of the protein (Fig. 2B), which may explain the concentration-dependent coupling of protein oligomerization to myristate exposure. This contrasts with the sequestered form of recoverin, in which the myristyl group is fully buried beneath the surface of the protein and exposure is accompanied by major internal conformational changes triggered by calcium binding (20).
Fig. 2.
(A) Stereoview of 20 superposed NMR structures determined for myr(s)-MA (backbone atoms of Gly-2–Ala-120 in blue and carbon atoms of myr 1 in red). For comparison, representative NMR (magenta) and x-ray (green) structures of myr(–)-MA are also shown. (B) Semitransparent surface representation of myr(s)-MA showing the partial penetration of the myristyl group (red spheres). (C) Ribbon drawing of myr(s)-MA showing side chains (green) that exhibit [1H–1H]NOEs with the myristyl group (red). The orange segment is fully disordered in myr(–)-MA, and the blue segment undergoes a minor conformational adjustment (reflected by chemical shift changes) upon insertion of the myristyl group.
NOE data obtained under equilibrium conditions can be complicated by contributions from the minor species. In all spectra obtained, NOEs to the myristate were observed, but no new intraprotein NOEs were detected that would be indicative of an altered protein conformation. In addition, the relative NOE intensities and cross-peak patterns that were assigned in the low-concentration 2D NOESY data (30 μM) matched the data obtained at higher concentrations (100–200 μM). Thus, if the trimer was contributing to the NOEs assigned to the monomer, its structure must not be significantly different from that of the monomer. The only spectral differences that could be unambiguously attributed to a structural perturbation in the backbone of the protein involved the [1H–15N]NMR chemical shifts of Ser-9–Gly-11. These residues are removed from the myristate-binding site, yet are sensitive to the position of the myr(s)/myr(e) equilibrium (Fig. 1 A). [1H–15N]NMR relaxation data obtained for myr(s)-MA (not shown) indicate that Val-7, Ser-9, and Asp-14 are involved in chemical exchange. In addition, the dihedral angles of Gly-10 and Gly-11 exhibit substantial variation among the six models of the myr(–)-MA x-ray structure, and Val-7–Lys-15 exhibit relatively high thermal parameters (25). Thus, structural changes implicated by the chemical shift data involve only a few residues, located near the N terminus of the protein, that have higher than average intrinsic conformational mobility. Interestingly, these residues appear to be critical determinants of the myr(s)/myr(e) equilibrium, as mutation of these or nearby residues can dramatically inhibit membrane binding, presumably due to enhanced sequestration of the myristyl group (17, 38).
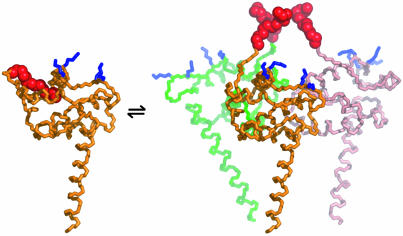
Model for the Trimeric [Myr(e)-MA]3 Species. The structure of the trimeric form of myr-MA could not be determined to atomic resolution because of the tendency of the protein to precipitate in a matter of hours at concentrations >400 μM. However, the concentration-dependent chemical shift changes are consistent with a trimer interface similar to those observed in the crystal structures of both the HIV-1 and simian immunodeficiency virus (SIV) myr(–)-MA proteins (25, 39). In particular, the subset of myr-MA signals that match those of myr(–)-MA at low concentrations, but deviate with increasing concentration, correspond to residues at or near the trimer interface of the myr(–)-MA crystal structure. The fact that myr(–)-MA does not readily form trimers in solution at 20–35°C (Fig. 1B and refs. 23 and 24) suggests that assembly is promoted mainly by intermolecular myr–myr interactions. Modeling studies indicate that such interactions can be readily accommodated in trimers without distorting the tertiary structure or disrupting intermolecular contacts observed in the myr(–)-MA crystal structure (Fig. 3).
Fig. 3.
Representation of the myr(s)-MA [myr(e)-MA]3 equilibrium showing the experimentally determined NMR structure of myr(s)-MA and a proposed model for the [myr(e)-MA]3 trimer. The model was generated by superpositioning three identical copies of a representative NMR structure of myr-MA [generated by using only myr(–)-MA NMR restraints] onto the coordinates of the trimeric myr(–)-MA x-ray structure. The model is consistent with the NMR chemical shift changes observed for myr-MA at protein concentrations that favor self-association. Lysine residues that are important for efficient virus replication (67) and have been proposed to interact with the membrane surface (25) are shown in blue. The intermolecular myr–myr interactions may be disrupted upon binding to membranes.
[myr(e)-MA]3 equilibrium showing the experimentally determined NMR structure of myr(s)-MA and a proposed model for the [myr(e)-MA]3 trimer. The model was generated by superpositioning three identical copies of a representative NMR structure of myr-MA [generated by using only myr(–)-MA NMR restraints] onto the coordinates of the trimeric myr(–)-MA x-ray structure. The model is consistent with the NMR chemical shift changes observed for myr-MA at protein concentrations that favor self-association. Lysine residues that are important for efficient virus replication (67) and have been proposed to interact with the membrane surface (25) are shown in blue. The intermolecular myr–myr interactions may be disrupted upon binding to membranes.
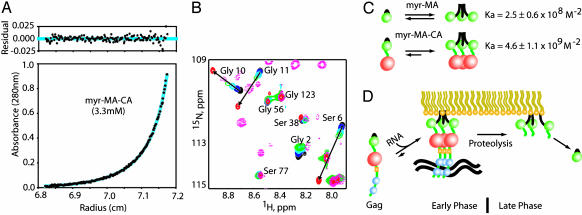
The Capsid Domain Enhances Myristate Exposure by Promoting Self-Assembly. To gain insight into the mechanism by which proteolysis of Gag modulates the myristyl switch, we prepared a Gag-like construct that contains both the myr-MA and CA domains (myr-MA–CA, residues myr-1–Leu-362). The CA domain is located immediately C-terminal to the myr-MA domain of the Gag precursor and contains a C-terminal subdomain that is required for capsid and Gag oligomerization (40–44) and for virion assembly (45–49). As observed for myr-MA, sedimentation equilibrium data obtained for myr-MA–CA are consistent with a monomer/trimer equilibrium (2.5–10 μM, 20°C) (Fig. 4A). Significantly, the association equilibrium constant measured for myr-MA–CA (4.6 ± 1.1 × 109 M–2) is nearly 20-fold greater than that determined for myr-MA. Thus, the concentrations at which 50% of the protein is monomeric are 73 μM for myr-MA and 14 μM for myr-MA–CA at 20°C.
Fig. 4.
(A) Sedimentation equilibrium data obtained for myr-MA–CA (3.3 μM, 20°C) and best-fit theoretical curve (cyan) for a monomer–trimer equilibrium (Kassoc = 4.6 ± 1.1 × 109 M–2). (B) Portions of 2D [1H–15N]HSQC spectra obtained for myr-MA–CA (50 μM; pink dashed lines), myr(–)-MA (500 μM, red), and concentration-dependent spectra obtained for myr-MA (see Fig. 1 caption for color definitions). The data obtained for myr-MA–CA follow trends observed for myr-MA. (C) Schematic representation of the monomer–trimer and myr(s)–myr(e) equilibrium (myr, MA, and CA are shown as black lines and green and red spheres, respectively). The CA domain promotes self-association and thereby shifts the equilibrium toward the myr(e) state. (D) Other Gag domains (yellow spheres, p2; blue spheres, NC) that promote assembly and membrane binding are expected to also shift the equilibrium toward the myr(e) state. Proteolytic cleavage of the MA–CA junction during the early phase of replication eliminates these effects, allowing the equilibrium to shift toward the myr(s) state.
NMR signals observed for the myr-MA domain of myr-MA–CA follow the trends observed for the isolated myr-MA protein, and indicate greater exposure of the myr group at lower protein concentrations compared to myr-MA (Fig. 4B). In addition, 1H and 15N chemical shifts and signal intensities for the flexible residues that connect the globular portions of the myr-MA and CA domains of myr-MA–CA match those observed previously for an unmyristoylated MA–CA C-terminal domain construct (26). Thus, the CA-induced equilibrium shift toward the trimer is not due to changes in intra- or intermolecular MA–CA interactions, but instead results from a synergistic effect associated with the covalent linking of the two moderately self-associating domains (Fig. 4C). This is in some ways analogous to the synergistic effect of the basic patch and myr group of MA (and other myristoylated proteins) on membrane binding (3). Neither element binds membranes with sufficient affinity to serve as a functional membrane anchor, but substantial binding occurs when both groups are presented (3).
Discussion
Previous mutagenesis studies have identified a link between membrane binding and Gag self-association (50). Mutations in both the CA C-terminal domain and p2 domains that inhibit Gag assembly disrupt membrane binding (51–53), and sequentially incremented C-terminal truncations of Gag result in progressive decreases in Gag multimerization and membrane affinity (54). These findings can now be explained in terms of the observed coupling of Gag assembly with myristate exposure. Thus, subdomain interactions that promote Gag assembly entropically shift the myr(s)/myr(e) equilibrium toward the myr(e) state. Recent mutgenesis studies indicate that the NC domain also plays a role in promoting the binding of Gag to membranes (55–57). NC is a basic domain that does not independently self-associate, but promotes Gag assembly by binding cooperatively to nucleic acids templates (58–61). RNA-promoted Gag assembly is therefore also likely to shift the myr(s)/myr(e) equilibrium toward the myr-exposed state, thereby enhancing the affinity of Gag for membranes.
The coupling of MA self-association with myristate exposure allows at least three biologically relevant mechanisms for modulating the HIV-1 myristyl switch: (i) changes in local protein concentration (for example, the concentration of myr-MA is high in virions, ≈14 mM, but becomes diluted upon viral entry, ref. 62), (ii) binding of factors to Gag domains that promote Gag–Gag interactions (e.g., RNA binding to the NC domain), and (iii) proteolytic removal of Gag domains that either directly (CA, p2) or indirectly (NC binding to RNA) promote Gag–Gag interactions. The present findings suggest that membrane release during viral infectivity is regulated by the latter mechanism, in which synergistic intermolecular interactions between Gag subdomains are eliminated upon proteolysis. Although the high concentration of myr-MA in mature virions (≈14 mM) (62) is expected to favor protein self-association and membrane binding, the equilibrium would shift toward the monomeric, myr(s)-MA species in the diluting environment of the cell being infected, allowing myr-MA to dissociate from the membrane. During the late phase of the replication cycle, the binding of the NC domain to the viral genome is expected to promote Gag assembly and myristate exposure, facilitating assembly of nucleic acid-containing virus particles (Fig. 4D).
The myr-MA domain of Gag also contains a CRM1-dependent nuclear export signal (NES), and there is evidence that Gag transiently accesses the nucleus during the late phase of the replication cycle (9). The function of the NES could be to simply counteract the nuclear localization signal (NLS), because nuclear accumulation of Gag would be detrimental to viral assembly (9, 63). However, Gag may also play a direct role in the export of the viral genome (63, 64). Mutations in the myr-MA domain of HIV-1 Gag that block the NES result in the aberrant accumulation of both Gag and the viral genome in the nucleus and in the production of RNA-deficient virions (9). Similar results were obtained recently for the Rous sarcoma virus (RSV) (63, 65). In addition, mutations in RSV MA that block nuclear localization of Gag lead to the production of particles deficient in genomic RNA (63, 65). The murine leukemia virus Gag proteins also transiently access the nucleus and may function in nuclear export of the viral genome (64). A mechanism consistent with these observations has been proposed in which the NLS of MA initially targets Gag to the nucleus for genome binding, and the NES subsequently targets the Gag–RNA complex to the cytosol where additional Gag molecules assemble on the RNA and target the complex to the plasma membrane (63). Although this hypothesis remains controversial (65), our findings are compatible. Thus, subsequent to translation, an equilibrium distribution of monomeric and multimeric Gag species is likely to coexist in the cytosol. The population of monomeric, myr-sequestered Gag probably displays the NLS, directing its transport to the nucleus. Cooperative assembly of Gag on the viral RNA could then promote exposure of the myristate, targeting the myristoylated HIV-1 Gag polyprotein (myr-Gag)–RNA complex out of the nucleus and to the membrane for further assembly. In this regard, mutations in HIV-1 myr-MA that lead to aberrant accumulation of myr-Gag in the nucleus are at sites that could easily affect the myr(s)/myr(e) equilibrium. Indeed, mutations at nearby sites have been shown to reduce the affinity of myr-Gag for membranes, and this has been attributed to enhanced sequestration of the myristate group (17, 38).
In summary, we have shown that the HIV-1 myristyl switch is coupled to protein assembly, and is regulated not by major conformational changes, but instead by entropic modulation of a preexisting equilibrium. Regulated membrane binding is now explained by synergistic intermolecular interactions between Gag subdomains that cooperatively promote assembly, thereby shifting the equilibrium toward the myr-exposed state. Subsequent proteolysis of Gag eliminates these cooperative effects, allowing the mature myr-MA domain to dissociate and sequester the myr group. Our findings explain the sensitivity of membrane binding to mutations in the CA, p2, and NC domains of Gag, provide a mechanistic basis for genome binding as a trigger for myristate exposure and membrane localization, and suggest a potential role for the myristyl switch in modulating NES/NLS activity. The structural information should facilitate efforts to develop therapeutic strategies that target the HIV-1 myristyl signal (66).
Supplementary Material
Acknowledgments
We thank M. Resh (Memorial Sloan–Kettering Cancer Center, New York) for the coexpression vector and D. King (University of California, Berkeley) for mass spectrometry measurements. Support from the National Institutes of Health (Grant AI30917 to M.F.S.) is gratefully acknowledged. Funds for the purchase of the ultracentrifuge were provided by National Institutes of Health Shared Instrumentation Grant S10-RR15899 (to D.B.). E.L., P.L., and I.K. are University of Maryland Baltimore County Presidential, Meyerhoff, and Howard Hughes Medical Institute/Minority Access to Research Careers/Meyerhoff undergraduate scholars, respectively.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CA, capsid protein; HSQC, heteronuclear single quantum coherence; MA, matrix protein; myr-MA, myristoylated HIV-1 MA; myr(–), unmyristoylated; myr(s), myristate sequestered state; myr(e), myristate exposed state; NC, nucleocapsid protein; NOE, nuclear Overhauser effect; NES, nuclear export signal; NLS, nuclear localization signal.
Data deposition: The NMR chemical shift assignments for myr-MA have been deposited at the BioMagResBank, http://bmrb.wisc.edu (accession no. 5960). Coordinates for 20 conformers of the myristoylated matrix protein have been deposited in the Protein Data Bank, www.rcsb.org (PDB ID code 1UPH).
See Commentary on page 417.
References
- 1.Nguyen, D. H. & Hildreth, J. E. (2000) J. Virol. 74, 3264–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan, X., Yu, X., Lee, T.-H. & Essex, M. (1993) J. Virol. 67, 6387–6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou, W., Parent, L. J., Wills, J. W. & Resh, M. D. (1994) J. Virol. 68, 2556–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogt, V. M. (1997) in Retroviruses, eds. Coffin, J. M., Hughes, S. H. & Varmus, H. E. (Cold Spring Harbor Lab. Press, Plainview, N.Y.), Vol. 1, pp. 27–69. [Google Scholar]
- 5.Miller, M. D., Farnet, C. M. & Bushman, F. D. (1997) J. Virol. 71, 5382–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukrinsky, M. I., Sharova, N., Dempsey, M. P., Stanwick, T. L., Bukrinskaya, A. G., Haggerty, S. & Stevenson, M. (1992) Proc. Natl. Acad. Sci. USA 89, 6580–6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bukrinsky, M. I., Haggerty, S., Dempsey, M. P., Sharova, N., Adzhubei, A., Spitz, L., Lewis, P., Goldfarb, D., Emerman, M. & Stevenson, M. (1993) Nature 365, 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukrinsky, M. I., Sharova, N., McDonald, T. L., Pushkarskaya, T., Tarpley, W. G. & Stevenson, M. (1993) Proc. Natl. Acad. Sci. USA 90, 6125–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dupont, S., Sharova, N., DeHoratius, C., Virbasius, C.-M. A., Zhu, X., Bukrinskaya, A. G., Stevenson, M. & Green, M. R. (1999) Nature 402, 681–685. [DOI] [PubMed] [Google Scholar]
- 10.Bryant, M. & Ratner, L. (1990) Proc. Natl. Acad. Sci. USA 87, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Copeland, N. G., Jenkins, N. A., Nexo, B., Schultz, A. M., Rein, A., Mikkelsen, T. & Jorgensen, P. (1988) J. Virol. 62, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Göttlinger, H. G., Sodroski, J. G. & Haseltine, W. A. (1989) Proc. Natl. Acad. Sci. USA 86, 5781–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spearman, P., Horton, R., Ratner, L. & Kuli-Zade, I. (1997) J. Virol. 71, 6582–6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spearman, P., Wang, J.-J., Vander Heyden, N. & Ratner, L. (1994) J. Virol. 68, 3232–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou, W. & Resh, M. D. (1996) J. Virol. 70, 8540–8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hermida-Matsumoto, L. & Resh, M. D. (1999) J. Virol. 73, 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paillart, J.-C. & Gottlinger, H. G. (1999) J. Virol. 73, 2604–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouamr, F., Scarlata, S. & Carter, C. A. (2003) Biochemistry 42, 6408–6417. [DOI] [PubMed] [Google Scholar]
- 19.Ames, J. B., Ishima, R., Tanaka, T., Gordon, J. I., Stryer, L. & Ikura, M. (1997) Nature 389, 198–202. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka, T., Ames, J. B., Harvey, T. S., Stryer, L. & Ikura, M. (1995) Nature 367, 444–447. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg, J. (1998) Cell 95, 237–248. [DOI] [PubMed] [Google Scholar]
- 22.Nagar, B., Hantschel, O., Young, M. A., Scheffzek, K., Veach, D., Bornmann, W., Clarkson, B., Superti-Furga, G. & Kuriyan, J. (2003) Cell 112, 859–871. [DOI] [PubMed] [Google Scholar]
- 23.Massiah, M. A., Starich, M. R., Paschall, C., Summers, M. F., Christensen, A. M. & Sundquist, W. I. (1994) J. Mol. Biol. 244, 198–223. [DOI] [PubMed] [Google Scholar]
- 24.Matthews, S., Barlow, P., Boyd, J., Barton, G., Russell, R., Mills, H., Cunningham, M., Meyers, N., Burns, N., Clark, N., et al. (1994) Nature 370, 666–668. [DOI] [PubMed] [Google Scholar]
- 25.Hill, C. P., Worthylake, D., Bancroft, D. P., Christensen, A. M. & Sundquist, W. I. (1996) Proc. Natl. Acad. Sci. USA 93, 3099–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang, C., Ndassa, Y. & Summers, M. F. (2002) Nat. Struct. Biol. 9, 537–543. [DOI] [PubMed] [Google Scholar]
- 27.Adachi, A., Gendelman, H. E., Koenig, S., Folks, T., Willey, R., Rabson, A. & Martin, M. A. (1986) J. Virol. 59, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kay, L. E., Ikura, M., Tschudin, R. & Bax, A. (1990) J. Magn. Reson. 89, 496–514. [DOI] [PubMed] [Google Scholar]
- 29.Clore, G. M., Kay, L. E., Bax, A. & Gronenborn, A. M. (1991) Biochemistry 30, 12–18. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka, T., Ames, J. B., Kainosho, M., Stryer, L. & Ikura, M. (1998) J. Biomol. NMR 11, 135–152. [DOI] [PubMed] [Google Scholar]
- 31.Delaglio, F., Grzesiek, S., Vuister, G. W., Zhu, G., Pfeifer, J. & Bax, A. (1995) J. Biomol. NMR 6, 277–293. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, B. A. & Blevins, R. A. (1994) J. Biomol. NMR 4, 603–614. [DOI] [PubMed] [Google Scholar]
- 33.Cornilescu, G., Delaglio, F. & Bax, A. (1999) J. Biomol. NMR. 13, 289–302. [DOI] [PubMed] [Google Scholar]
- 34.Güntert, P., Mumenthaler, C. & Wüthrich, K. (1997) J. Mol. Biol. 273, 283–298. [DOI] [PubMed] [Google Scholar]
- 35.Laskowski, R. A., Rullmann, J. A. C., MacArthur, M. W., Kaptein, R. & Thornton, J. M. (1996) J. Biomol. NMR 8, 477–486. [DOI] [PubMed] [Google Scholar]
- 36.DeLano, W. L. (2002) The PyMOL User's Manual, http://www.pymol.org (DeLano Scientific, San Carlos, CA).
- 37.Johnson, M. L. & Faunt, L. M. (1992) Methods Enzymol. 210, 1–37. [DOI] [PubMed] [Google Scholar]
- 38.Ono, A. & Freed, E. O. (1999) J. Virol. 73, 4136–4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao, Z., Belyaev, A. S., Fry, E., Roy, P., Jones, I. M. & Stuart, D. I. (1995) Nature 378, 743–747. [DOI] [PubMed] [Google Scholar]
- 40.Rosé, S., Hensley, P., O'Shannessy, D. J., Culp, J., Debouck, C. & Chaiken, I. (1992) Proteins Struct. Funct. Genet. 13, 112–119. [DOI] [PubMed] [Google Scholar]
- 41.Gitti, R. K., Lee, B. M., Walker, J., Summers, M. F., Yoo, S. & Sundquist, W. I. (1996) Science 273, 231–235. [DOI] [PubMed] [Google Scholar]
- 42.Yoo, S., Myszka, D. G., Yeh, C., McMurray, M., Hill, C. P. & Sundquist, W. I. (1997) J. Mol. Biol. 269, 780–795. [DOI] [PubMed] [Google Scholar]
- 43.Gamble, T. R., Yoo, S., Vajdos, F. F., von Schwedler, U. K., Korthylake, D. K., Wang, H., McCutcheon, J. P., Sundquist, W. I. & Hill, C. P. (1997) Science 278, 849–853. [DOI] [PubMed] [Google Scholar]
- 44.Franke, E. K., Yuan, H. E. H., Bossolt, K. L., Goff, S. P. & Luban, J. (1994) J. Virol. 68, 5300–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jowett, J., Hockley, D., Nermut, M. V. & Jones, I. M. (1992) J. Gen. Virol. 73, 3079–3086. [DOI] [PubMed] [Google Scholar]
- 46.Von Poblotzki, A., Wagner, R., Niedrig, M., Wanner, G., Wolf, H. & Modrow, S. (1993) Virology 193, 981–985. [DOI] [PubMed] [Google Scholar]
- 47.Dorfman, A. T., Bukovsky, A., Ohagen, A. S., Hoglund, H. & Gottlinger, G. (1994) J. Virol. 68, 8180–8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang, W.-H., Hockley, D. J., Nermut, M. V., Morikawa, Y. & Jones, I. M. (1996) J. Gen. Virol. 77, 743–751. [DOI] [PubMed] [Google Scholar]
- 49.Reicin, A. S., Ohagen, A., Yin, L., Hoglund, S. & Goff, S. P. (1996) J. Virol. 70, 8645–8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindwasser, O. W. & Resh, M. D. (2001) J. Virol. 75, 7913–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ebbets-Reed, D., Scarlata, S. & Carter, C. A. (1996) Biochemistry 35, 14268–14275. [DOI] [PubMed] [Google Scholar]
- 52.Liang, C., Hu, J., Russell, R. S., Roldan, A., Kleiman, L. & Wainberg, M. A. (2002) J. Virol. 76, 11729–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Accola, M. A., Hoglund, S. & Gottlinger, H. G. (1998) J. Virol. 72, 2072–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ono, A., Demirov, D. & Freed, E. O. (2000) J. Virol. 74, 5142–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Platt, E. J. & Haffar, O. K. (1994) Proc. Natl. Acad. Sci. USA 91, 4594–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sandefur, S., Varthakavi, V. & Spearman, P. (1998) J. Virol. 72, 2723–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sandefur, S., Smith, R. M., Varthakavi, V. & Spearman, P. (2000) J. Virol. 74, 7238–7249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feng, Y.-X., Li, T., Campbell, S. & Rein, A. (2002) J. Virol. 76, 11757–11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campbell, S. & Vogt, V. M. (1995) J. Virol. 69, 6487–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muriaux, D., Mirro, J., Harvin, D. & Rein, A. (2001) Proc. Natl. Acad. Sci. USA 98, 5246–5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu, F., Joshi, S. M., Ma, Y. M., Kingston, R. L., Simon, M. N. & Vogt, V. M. (2001) J. Virol. 75, 2753–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang, C., Loeliger, E., Kinde, I., Kyere, S., Mayo, K., Barklis, E., Sun, Y., Huang, M. & Summers, M. F. (2003) J. Mol. Biol. 327, 1013–1020. [DOI] [PubMed] [Google Scholar]
- 63.Scheifele, L. Z., Garbitt, R. A., Rhoads, J. D. & Parent, L. J. (2002) Proc. Natl. Acad. Sci. USA 99, 3944–3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nash, M. A., Meyer, M. K., Decker, G. L. & Arlinghaus, R. B. (1993) J. Virol. 67, 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Callahan, E. M. & Wills, J. W. (2003) Virology 77, 9388–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindwasser, O. W. & Resh, M. D. (2002) Proc. Natl. Acad. Sci. USA 99, 13037–13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Freed, E. O., Englund, G. & Martin, A. M. (1995) J. Virol. 69, 3949–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.