Abstract
Vitamin E isoforms have opposing regulatory effects on leukocyte recruitment during inflammation. Furthermore, in vitro, vitamin E isoforms have opposing effects on leukocyte migration across endothelial cells by regulating vascular cell adhesion molecule (VCAM)-1 activation of endothelial cell protein kinase Cα (PKCα). However, it is not known whether tocopherols directly regulate co-factor-dependent or oxidative activation of PKCα. We report herein that co-factor-dependent activation of recombinant PKCα was increased by γ-tocopherol and was inhibited by α-tocopherol. Oxidative activation of PKCα was inhibited by α-tocopherol at a 10 fold lower concentration than γ-tocopherol. In binding studies, NBD-tagged-α-tocopherol directly bound to full-length PKCα or the PKCα-C1a domain but not PKCζ. NBD-tagged-α-tocopherol binding to PKCα or the PKCα-C1a domain was blocked by diacylglycerol, α-tocopherol, γ-tocopherol, and retinol but not by cholesterol or phosphatidylserine (PS). Tocopherols enhanced PKCα-C2 domain binding to PS-containing lipid vesicles. In contrast, the PKCα-C2 domain did not bind to lipid vesicles containing tocopherol without PS. The PKCα-C1b domain did not bind to vesicles containing tocopherol and PS. In summary, α-tocopherol and γ-tocopherol bind the diacylglycerol binding site on PKCα-C1a and can enhance PKCα-C2 binding to PS-containing vesicles. Thus, the tocopherols can function as agonists or antagonists for differential regulation of PKCα.
Keywords: α-tocopherol, γ-tocopherol, protein kinase Cα, co-factors, oxidation, vitamin E
Introduction
Tocopherols are antioxidant lipids that function by donating a hydrogen from the chromanol head hydroxyl group to lipid radicals produced in lipid peroxidation chain reactions [1, 2]. Tocopherols also have non-antioxidant functions and are reported to modulate disease, protein expression, and cell signaling [3–5]. There are multiple natural isoforms of vitamin E, which differ in number of methyl groups on the chromanol head, including the saturated α-, β-, γ-, and δ-tocopherols and unsaturated α-, β-, γ-, and δ-tocotrienols. The most abundant forms of vitamin E in tissues and in the diet are α-tocopherol and γ-tocopherol. γ-tocopherol has one less methyl group on the chromanol head than α-tocopherol.
We have reported that in vivo α-tocopherol decreases and γ-tocopherol increases leukocyte recruitment during allergic lung inflammation in mice [6–8]. Consistent with this, α-tocopherol decreases and γ-tocopherol increases endothelial cell signaling during leukocyte migration across endothelial cells in vitro [6–8]. During this leukocyte migration, leukocytes bind to the endothelial cell adhesion molecules VCAM-1 and ICAM-1. VCAM-1 and ICAM-1 signal through activation of PKCα in the endothelial cells [9, 10]. We have reported that VCAM-1’s activation of PKCα is regulated by tocopherols [6–8]; α-tocopherol decreases and γ-tocopherol increases activation of endothelial cell PKCα [6–8]. It is also reported that tocopherols regulate activation of PKC in other cell systems [6, 11–16]. However, it is not known whether tocopherols can directly regulate PKCα.
PKCα is a serine/threonine kinase that utilizes the cofactors phosphatidylserine (PS), diacylglycerol (DAG), and calcium for activation [17–19]. PKCα is comprised of the domains C1a, C1b, C2, C3, and C4. C1a and C1b bind DAG and phorbol esters with differential affinities; the C1a domain preferentially binding DAG and the C1b domain preferentially binding phorbol esters. The C2 domain binds calcium and PS. The C3 catalytic domain binds ATP and the C4 catalytic domain binds substrates. During cofactor-dependent (non-oxidative) activation, calcium recruits PKCα to the membrane where PKCα’s C2-domain directly interacts with PS in the membrane [18, 20, 21]. Upon C2-domain association with the membrane, PKCα’s C1-domain and C2-domain fold opens and the C1-domain then interacts with DAG in the membrane [22–24]. In addition to cofactor-dependent activation, we and others have shown that PKCα can be activated via direct oxidation of its regulatory domain [10, 25]. In mild oxidizing conditions, peroxide oxidizes sulfhydryls in the two zinc finger regions within the C1a and C1b-domains of PKCα, thus activating PKCα.
PKCα is transiently oxidized and activated during VCAM-1 signaling in endothelial cells [10]. Briefly, VCAM-1 activates NOX2 that generates reactive oxygen species for the oxidation and activation of PKCα [10]. In addition, during VCAM-1 activation of PKCα, there is an increase in intracellular calcium, a PKCα cofactor [26], but there is no increase in the PKCα cofactor diacylglycerol; however, there is a reduction in endogenous cellular diacylglycerol [10]. Thus, VCAM-1’s transient activation of PKCα is directly regulated by oxidation and the cofactors calcium and diacylglycerol. The total VCAM-1 activation of PKCα in cells is therefore the sum of the oxidative and cofactor-dependent activation.
Previous reports indicate that activation of PKCα in cells can be altered by tocopherol treatment of cells or tissues but it has not been reported whether tocopherols directly bind and regulate PKCα [6, 11–16]. We report here that tocopherols directly bind and regulate PKCα. Alpha-tocopherol decreases and γ-tocopherol enhances PS-dependent activation of recombinant PKCα. Also, α-tocopherol ablates the γ-tocopherol-induced increase in PS-dependent activation of recombinant PKCα. Both α-tocopherol and γ-tocopherol significantly inhibit oxidative-activation of PKCα; however, the α-tocopherol inhibits oxidative-activation at 10 fold lower doses than γ-tocopherol. α-tocopherol and γ-tocopherol enhance PKCα-C2 binding to PS-containing phospholipid layers. Moreover, these tocopherols directly bind to PKCα-C1a at the DAG binding site. Thus, α-tocopherol is an antagonist and γ-tocopherol is an agonist of PS-dependent PKCα activity. It is the sum of tocopherol isoforms’ antioxidant and agonist/antagonist activities at the doses present in cells that yields the total tocopherol regulation of PKCα activity in a cell and tissue.
Experimental Procedures
Co-factor-dependent PKC activity assay
The non-radioactive PKC assay kit (Calbiochem, Cat #539584) was used as described by the manufacturer, except for those reagents indicated below. Recombinant human HIS-tagged rPKCα (Calbiochem, Cat #539650) or rPKCζ (Enzo Life Sciences, Cat #BML-SE413) was used. Phosphatidylserine supplied in chloroform/methanol (3:1) (PS, Sigma-Aldrich, Cat #P6641) and natural R,R,R-α-tocopherol (MP Biomedicals, Cat #02100562) or natural R,R,R-γ-tocopherol (Sigma-Aldrich, Cat #47785) in hexane was dried under nitrogen in an amber glass vial. For the kinase assay, PS was suspended in ddH2O by 3 rounds of sonication in an iced water bath for 1 min followed by vortexing for 30 sec and placed on ice. To suspend the tocopherols, a reaction mixture containing buffer, CaCl2, and PS were added according to kit procedure to generate final assay buffer concentrations of 6 mM MgCl2, 1 mM EDTA, 2 mM EGTA, pH 7.0, 2 mM CaCl2, and PS (at concentration indicated in experiments). The negative control excluded PKCα’s cofactors, CaCl2 and S. The tocopherols were suspended in buffer by 3 rounds of sonication in an iced water bath for 30 sec followed by vortexing for 30 sec. Then, 100 μM adenosine-5′-triphosphate (ATP, Calbiochem) was added and vortexed briefly. PKCζ, kinase activity assays were analyzed in the presence of 30 μg/ml PS and 2 mM CaCl2. Reaction mixtures were brought to room temperature for 10 min. Recombinant human rPKCα (Calbiochem, Cat #539650) or rPKCα (Enzo Life Sciences, Cat #BML-SE413) was added to the tocopherol/reaction mixture, incubated for 5 min at room temperature, cooled on ice for 5 min, and then added to the substrate-coated plate from the Calbiochem PKC kit on ice. To initiate the kinase activity, the plate was placed on a room temperature water bath for 30 min. The reaction was stopped with 0.1 M H3PO4 and the plate was washed. Biotinylated anti-phospho-substrate Ab from the kit was added to all wells and incubated at room temperature for 1 hour. Wells were washed and the kit’s secondary antibody (horseradish peroxidase conjugated to streptavidin) was added to all wells and incubated for 1 hour at room temperature. The wells were washed and then o-phenylenediamine in substrate buffer (50 mM citric acid/sodium phosphate buffer, pH 5.0, plus H2O2) was added to the wells. When color change was sufficient (1–3 minutes), the reaction was stopped by adding 0.1 M H3PO4 to the wells. Absorbance was read on a luminescent plate reader at 492 nm. Data is presented as relative fluorescence from the sample minus the fluorescence signal from the blank.
Oxidative activation of PKCα activity
Methods are as above in the Co-factor-dependent Protein Kinase activity assay except for the following: 1) Glycerol from the commercial rPKCα was removed by dialysis since glycerol is an antioxidant, and 2) No PS or CaCl2 was used in these assays since oxidative activation of PKCα is cofactor-independent. To remove glycerol, rPKCα was dialyzed using 0.025 um pore (Millipore, Cat #VSWP02500) against a reaction buffer with iron (6 mM MgCl2, 50 mM Tris-HCl, 45 μM FeCl2, 1 mM EDTA, 2 mM EGTA, pH 7.0) for 30 min on ice. Following addition of tocopherol and reaction buffer with 45 μM FeCl2, oxidative activation of 15 ng rPKCα was initiated by addition of 1 or 10 mM H2O2 as previously described [25]. After 2 min, the reaction was stopped with 9 mM DTT as previously described [25]. To examine PKC activity, the samples were then added to the PKC kit substrate plate and examined for generation of fluorescence as described above in the Co-factor-dependent Protein Kinase activity assay.
Cloning, protein expression and purification of PKCα domains C1a, C1b, and C2
GST-PKCα-C1a fusion protein on a pGEX vector (kind gift from Dr. Alexandra Newton, UCSD) was expressed in BL-21 cells (GE Healthcare) and purified using glutathione-Sepharose beads 4B (GE Healthcare) according to standard methods with the following conditions: induction was 18 hours at 25°C in the presence of 0.01 mM ZnSO4 and isopropyl β-D-1-thiogalactopyranoside (IPTG); 50 μM ZnSO4 was added to all buffers following induction to allow proper folding of the GST-PKCα-C1a domain. Expression of GST-PKCα-C1a was determined by Coomassie stain and a western blot using an anti-GST Ab (Cell Signaling, Cat #2622) showed only two bands at 25 kDa (GST) and 31 kDa (GST-PKCα-C1a) (data not shown). GST-PKCα-C1a was stored in 25 mM Tris-HCl, 75 mM NaCl, 50 μM ZnSO4 containing 50% glycerol.
PKCα-C1a, PKCα-C1b and PKCα-C2 domains were cloned into a pET21a vector with 6xHis tag as previously described [27]. To improve the expression and stability of PKCα-C1a and PKCα-C1b in E. coli BL21 RIL codon plus (Stratagene) cells, enhanced-GFP was inserted at the C-terminal of the C1-domain to produce eGFP-fused C1a, C1b and C2-domains. E. coli were grown in LB media containing 100 μg/ml of ampicillin at 37 °C until the OD600 reached 0.8. Then, overexpression was induced by addition of 0.5 mM IPTG for 6 to 10 hours at 25 °C. Cells were centrifuged, resuspended in 25 mM Tris-HCl buffer (pH 7.4) containing 160 mM KCl, 1 mM phenylmethanesulphonylfluoride, and 5 mM of dithiothreitol, and lysed by sonication. The lysate was centrifuged at 4 °C. Ni-NTA (Qiagen) was added into the cell lysate and shaken for 30 minutes at 4 °C. The mixture was applied to an anti-His column and the column was washed with 25 mM Tris-HCl buffer (pH 7.4)/160 mM KCl/25mM imidazole. Proteins were eluted from the column by a gradient increase of imidazole in the buffer and then applied to an ion exchange column for further purification. Purity and concentration of recombinant proteins were determined by SDS-PAGE and a bicinchoninic acid assay, respectively.
Surface Plasmon Resonance (SPR) for PKCα-C2 binding to large unilamellar vesicles (LUVs)
1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine (POPS), and the diacylglycerol derivative of 1-steroyl-2-arachidonyl-sn-glycerol (SAG) were from Avanti Polar Lipids, Inc. (Alabaster, AL). All SPR measurements were at 24 °C using a lipid-coated L1 chip (GE Healthcare) in the BIACORE X system as described previously [28] Large unilamellar vesicles (LUVs) were extruded using a 100 nm-pore membrane as previously described [28]. The SPR active surface and control surface were coated with POPC/POPE/POPS (70:20:10 mole %) and POPC vesicles, respectively, as previously described [28]. Alternatively, the control surface was coated with POPC/POPE/POPS/tocopherols in (70-x:20:10:x, x = 0–10 mole %). After washing the sensor chip surface with running buffer (25mM Tris-HCl, pH 7.4, containing 0.16 M KCl), the active surface and control sensor chip surface were coated with the indicated lipid composition to give the same resonance unit (RU) values. The level of lipid coating for both surfaces was kept at the minimum necessary for preventing non-specific adsorption to the sensor chips. This low surface coverage minimized the mass transport effect and kept the total protein concentration above the total concentration of protein binding sites on vesicles. For kinetic SPR measurements, the flow rate was 30μl/min for association and dissociation phases. The protein association with the lipid layer is presented as the difference between the active chips’ signals and the background signals from the control chip.
Binding of eGFP-PKCα-C1b to giant unilamellar vesicles (GUVs)
Giant unilamellar vesicles (GUVs) were prepared by electroformation as described previously [29]. GUVs were grown in a sucrose solution (350 mM) while an electric field (3V, 20Hz frequency) was applied for 5 hours at room temperature. GUVs were comprised of PC/PE/PS (65:20:10 mole%) with 5 mole% of SAG (positive control), α-tocopherol or γ-tocopherol. The 1–2 μL of sucrose-loaded GUV solution was added into an 8-well chamber containing 200 μL of 25 mM Tris-HCl buffer, pH 7.4, with 0.16 M KCl solution. GUVs, which were 5–30 μm diameter, were mixed with 100nM of eGFP-PKCα-C1b and fluorescence intensity was examined at room temperature using a custom-built multi-photon, multi-channel microscope with SimFCS software as described previously [30]. eGFP-PKCα-C1b was two-photon excited at 900 nm by a tunable Tsunami laser (Spectra Physics) and a 525 ± 25 band pass filter was used for emission. The images (256 × 256 pixels) were collected with the pixel dwell time of 32 millisecond using Peltier-cooled 1477P style Hamamatsu photomultiplier tubes. For determination of eGFP-PKCα-C1b binding, 5 GUVs were selected and for each GUV, an averaged image of a total of 10 frames was collected for further analysis by MATLAB. The total photon counts of the image were read into a 256 × 256 matrix to recreate the averaged image. Then a binary image mask was created using this image matrix by analyzing the photon count histogram of the image. The image matrix and its binary mask were multiplied to extract the photon counts only from GUV. The total photon counts of GUV were divided by the total area of the pixels that constitute each GUV to yield the photon counts per pixel. Data are presented as mean ± standard deviation of [average photon count per pixel of the GUV]/[average photon count per pixel outside the GUV].
Fluorescent tocopherol ELISA for binding to PKCα
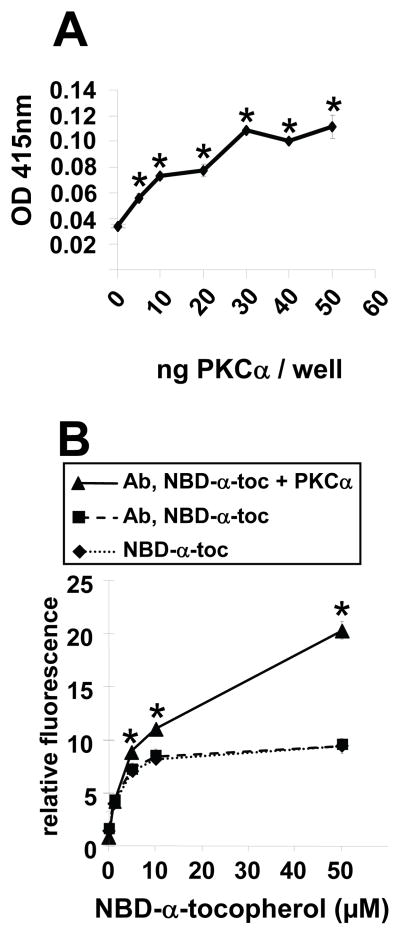
A half-area 96-well plate (Costar, Cat #3690) was coated overnight with 2 μg/ml anti-6X His tag Ab (Abcam, Cat #ab9108) in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3, pH 9.0) and then washed with PBS/0.05% Tween and blocked with PBS/3% BSA for 2 hours. Saturation of 6XHis-tagged-rPKCα binding to the anti-6X His tag Ab on the plate was determined by labeling with anti-PKCα Ab (Abcam, Cat #ab4124), which had been biotinylated using the EZ-Link Biotinylation Kit (Pierce, Cat #21343), and then addition of streptavidin-horseradish peroxidase/o-phenylenediamine. We found that 30 ng rPKCα per well was the lowest concentration to saturate the plate and thus 30 ng was used in the fluorescent lipid ELISA.
For the fluorescent lipid ELISA, 7-nitrobenz-2-oxa-1,3-diazole(NBD)-α-tocopherol (gift of Jeffrey Atkinson) [31] was diluted in ethanol and briefly vortexed. NBD-α-tocopherol is non-fluorescent in hydrophilic environments but fluoresces in hydrophobic environments as previously described for NBD-α-tocopherol binding to α-tocopherol transfer protein (αTTP) [31]. In this PKCα binding assay, NBD fluorescence is increased when NBD-α-tocopherol binds to hydrophobic environments within the lipid binding domains of PKCα. NBD-α-tocopherol in ethanol or ethanol control was added to His-tagged-rPKCα (30 ng per well) (Calbiochem) in the reaction buffer from the PKC activity kit (Calbiochem, Cat #539584), generating a final concentration of 1% ethanol in reaction buffer (6 mM MgCl2, 1 mM EDTA, 2 mM EGTA, pH 7.0, 2 mM CaCl2). The NBD-α-tocopherol/rPKCα samples were protected from light for 5 minutes at room temperature and then applied to an anti-HIS Ab-coated ELISA plate. The plate was rotated at room temperature for 10 minutes and then washed 10 times with PBS/0.05% Tween to remove unbound NBD-α-tocopherol. Reaction buffer was added to the plate and relative fluorescence units were measured on a fluorescence reader at 469 nm excitation, 535 nm emission.
Suspension assay for tocopherol binding to rPKCα, rPKCζ and GST-rPKCα-C1a
This binding assay functions similar to previous studies on NBD-α-tocopherol binding to α-tocopherol transfer protein (αTTP) in which NBD becomes fluorescent when in hydrophobic environments within αTTP [31]. NBD-α-tocopherol was not prepared in lipid vesicles since the hydrophobic environment of vesicles induces fluorescence. Specificity of binding is examined by competition with nonlabeled tocopherols, known ligands of PKCα, and control lipids such as cholesterol. Briefly, NBD-α-tocopherol (gift of Jeff Atkinson) and/or the competitors α-tocopherol (MP Biomedicals), γ-tocopherol (Sigma-Aldrich), α-tocotrienol (Cayman Chemical, Cat #10008377), γ-tocotrienol (Cayman Chemical, Cat #10008494), retinol (Sigma, Cat #R732), 1,2-dioctanoyl-sn-glycerol (DOG, Avanti), or cholesterol (Sigma, Cat #C8667) were prepared in ethanol at 200x so that the final ethanol concentration in the assay was 1%. PS was prepared at 20x by adding reaction buffer to dried-down PS and alternating sonication and vortexing 30 seconds each, 3 times. To prepare the GST-rPKCα-C1a for the assay, it was dialyzed (to remove storage glycerol) for 1 hour at 4°C against the reaction buffer from the PKC activity kit (Calbiochem, Cat #539584). Full length commercial rPKCα and rPKCζ were not dialyzed. For the assay, the rPKCα (0.1μM), rPKCζ (0.1μM) or GST-PKCα-C1a (0.2 μM) were added to the reaction buffer followed by addition of NBD-α-tocopherol (gift of Jeff Atkinson). The samples were briefly vortexed three times for 1 second. In assays with competitors, NBD-α-tocopherol was added to reaction buffer containing the indicated PKC enzyme, samples were vortexed (3x 1s), competitor was added, and samples were vortexed again (3x 1s). Samples were rotated for 30 minutes at room temperature while protected from light. Samples were plated on a half-area 96-well plate (Costar, Cat#3690) and relative fluorescence units were measured on a fluorescence reader at 469 nm excitation, 535 nm emission.
Statistics
Data were analyzed by a one way ANOVA followed by Tukey’s multiple comparisons test (SigmaStat, Jandel Scientific, San Ramon, CA). Presented are the means ± the standard errors.
Results
α-tocopherol and γ-tocopherol differentially modulate cofactor-dependent rPKCα activity
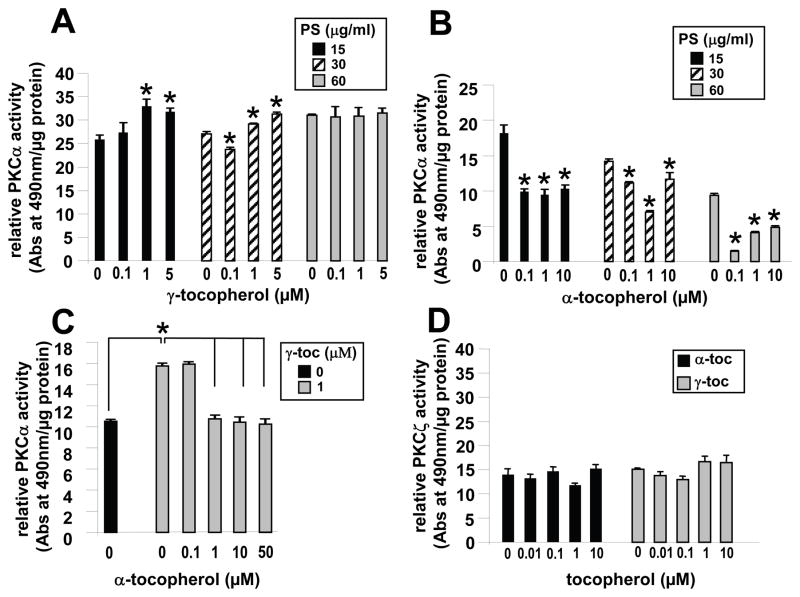
We determined whether γ-tocopherol or α-tocopherol directly regulate PS cofactor-dependent activation of PKCα or oxidative activation of PKCα. In the presence of the cofactor calcium (2 mM CaCl2), γ-tocopherol consistently induced a significant, albeit small, increase in recombinant PKCα (rPKCα) activity in the presence of 15 μg/ml and 30 μg/ml PS (Figure 1A). This small increase is consistent with reports that γ-tocopherol induces a small significant increase in VCAM-1-activated PKCα in endothelial cells that then can result in large increases in leukocyte recruitment during inflammation in vivo [6, 8]. At high PS (60 μg/ml) concentrations, rPKCα activity is elevated to the level of activity observed with 1 μM γ-tocopherol plus 15–30 μg/ml PS. In the absence of PS, γ-tocopherol did not increase the low rPKCα activity (data not shown). Alpha-tocopherol at 0.1 to 10 μM inhibited rPKCα activity in the presence of 15–60 μg/ml PS (Figure 1B). Furthermore, α-tocopherol at 1–50 μM ablated the γ-tocopherol (1 μM)-induced increase in rPKCα activity (Figure 1C). To determine if the effects of γ-tocopherol and α-tocopherol on PKC activity are limited to DAG cofactor-dependent PKCs, we determined whether tocopherols modulate PKCζ which is active independent of the cofactor DAG [32]. Neither γ-tocopherol nor α-tocopherol significantly modulated PKCζ activity (Figure 1D).
Figure 1. Gamma-tocopherol enhances and α-tocopherol inhibits non-oxidative cofactor-dependent rPKCα activity.
A,B) In the presence of 2 mM CaCl2 and PS (15 μg/ml, 30 μg/ml, or 60 μg/ml), 15 ng rPKCα was incubated with γ-tocopherol or α-tocopherol for 5 min at room temperature before addition to the PKC substrate plate. Then, the plate was incubated for 30 min at room temperature. Without PS or H2O2, 1 μM γ-tocopherol did not enhance PKCα activity (data not shown). C) α-tocopherol’s ability to inhibit the enhancing effect of γ-tocopherol (γ-toc) on PKCα was measured using calcium and 30 μg/mL PS. For A-C: a background value of 2.6 relative units, which is rPKCα activity in the absence of PS and calcium, was subtracted from each sample. D) In the presence of 30 μg/mL PS, 15 ng rPKCζ was incubated with γ-tocopherol (γ-toc) or α-tocopherol (α-toc) for 5 min at room temperature before addition to the substrate plate. Then, the plate was incubated for 30 min at room temperature. For D: a background value of 7.2 relative units, which is rPKCζ activity in the absence of PS and calcium, was subtracted from each sample. Data are the mean ± SEM of triplicates from a representative experiment of three experiments. A–B) *, p<0.05 compared to the no tocopherol group. C) *, p<0.05 compared to the indicated groups.
α-tocopherol and γ-tocopherol inhibit oxidative activation of PKCα
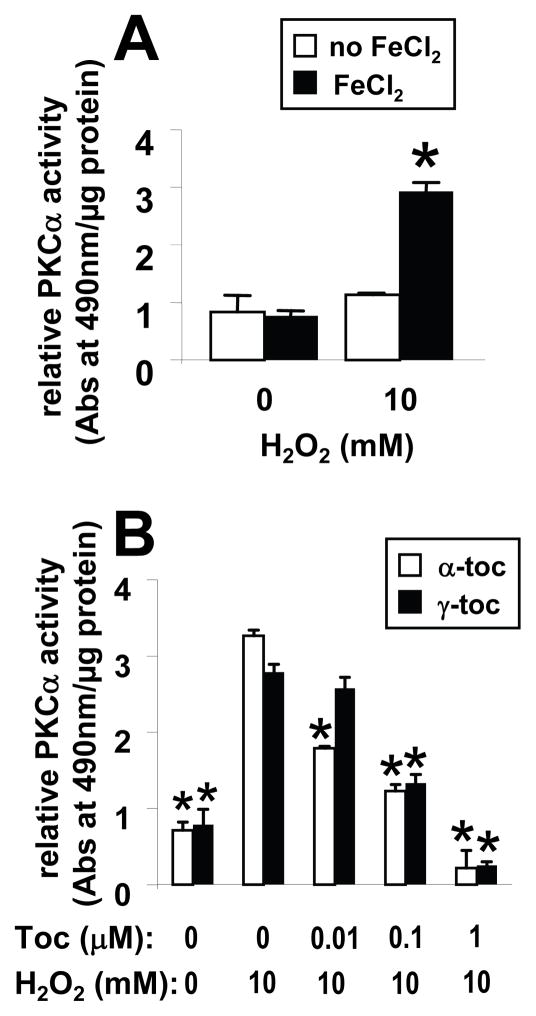
VCAM-1-induced ROS oxidizes and directly activates PKCα [10]. It is reported that the oxidative activation of PKCα, by peroxide, is accomplished by the Fenton reaction which requires catalysis by iron [25]. Therefore, iron was added to assay buffers to determine whether tocopherol regulates H2O2-induced activation of rPKCα. rPKCα was activated by H2O2 in the presence of FeCl2 (Figure 2A). Both γ-tocopherol and α-tocopherol inhibited H2O2-induced oxidative activation of rPKCα (Figure 2B). However, α-tocopherol (0.01 μM) was able to significantly inhibit rPKCα activity at lower doses that γ-tocopherol (0.1 μM) (Figure 2B).
Figure 2. Alpha-tocopherol and γ-tocopherol inhibit peroxide-induced oxidative activation of rPKCα.
A) In a cofactor-independent assay (no PS or calcium), rPKCα was activated with 10 mM H2O2 and 45 μM FeCl2 for 2 min before the reaction was quenched with 9 mM DTT. The oxidatively activated rPKCα was then applied to the substrate plate. B) rPKCα was incubated with α-tocopherol (α-toc) or γ-tocopherol (γ-toc) for 5 min at room temperature prior to H2O2/FeCl2 activation and then examined for PKC activity as in A. Data are the mean ± SEM of triplicates from a representative experiment of three experiments. *, p<0.05 as compared to the no tocopherol/10mM H2O2-treated group.
α-tocopherol and γ-tocopherol enhance rPKCα-C2 binding to lipid layers containing PS
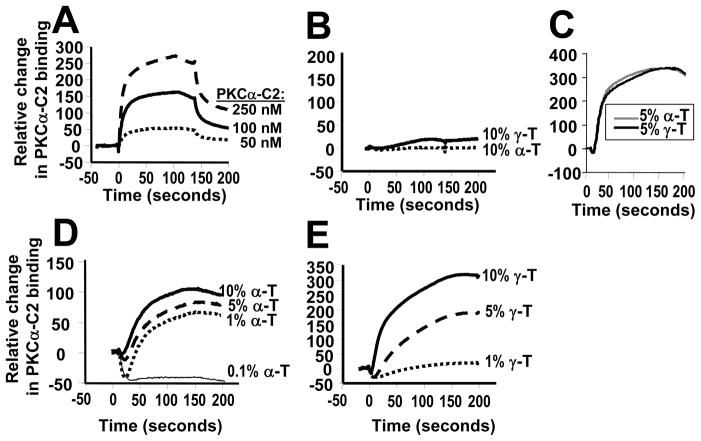
When PKCα is activated, it translocates to the plasma membrane and interacts with lipid cofactors including PS. These membranes also contain tocopherols, but it is not known whether tocopherols in membranes regulate recruitment of PKCα. Therefore, using surface plasmon resonance (SPR), it was determined whether PKCα-C2 domain binding to lipid-coated surfaces is regulated by tocopherols. With SPR analysis, the relative change in binding differs among experiments; therefore comparisons among groups analyzed by SPR are made within each experiment and these are presented as separate panels in Figure 3. In Figure 3A, there was a dose-dependent PKCα-C2 domain binding to PS-containing lipid surfaces (POPC/POPE/POPS [70:20:10 mol%]). The PKCα-C2 domain did not bind in the absence of PS (data not shown). The PKCα-C2 domain did not bind to 90% POPC surfaces with 10 mol% α-tocopherol or 10 mol% γ-tocopherol (Figure 3B), indicating that α-tocopherol or γ-tocopherol alone was not sufficient for PKCα-C2 binding to a lipid surface without PS. Interestingly, addition of 5 mol% α-tocopherol or γ-tocopherol to a PS-containing lipid surface equally enhanced binding of PKCα-C2 as compared to the PS-containing lipid surface without tocopherols as shown by the change in binding in Figure 3C. However, just 1 mol% α-tocopherol enhanced association of PKCα-C2 with the lipid layer (Figure 3D), whereas 5 mol% γ-tocopherol was required for enhanced association of PKCα-C2 (Figure 3E).
Figure 3. Alpha-tocopherol and γ-tocopherol enhance PKCα-C2 association to PS-containing vesicles.
A) Dose-dependent PKCα-C2 binding to the PS-containing lipid layer (POPC/POPE/POPS, 70:20:10 mole%). FC1 control channel (100 mole% POPC) has been subtracted from FC2 test channel (POPC/POPE/POPS, 70:20:10 mole%). B) Tocopherol was not sufficient for PKCα-C2 (250 nM) binding to lipid layers without POPS (POPC/tocopherol, 90:10 mole%). FC1 control channel (100 mole% POPC) has been subtracted from FC2 test channel (POPC/tocopherol, 90:10 mole%). C) α-tocopherol (α-T) and γ-tocopherol (γ-T) enhanced binding of PKCα-C2 (250 nM) to the lipid layers with PS (POPC/POPE/POPS/tocopherol, 65:20:10:5 mole %). FC1 control channel (POPC/POPE/POPS, 70:20:10 mole%) has been subtracted from FC2 test channel (POPC/POPE/POPS/tocopherol, 65:20:10:5 mole %. D) ≥1% α-tocopherol enhanced binding of PKCα-C2 (250 nM) to the lipid layers with PS (POPC/POPE/POPS/tocopherol, 70-x:20:10:x mole %, x=1–10). FC1 control channel (POPC/POPE/POPS, 70:20:10 mole%) has been subtracted from FC2 test channel (POPC/POPE/POPS/tocopherol, 70-x:20:10:x mole %, x=1–10). E) ≥5% γ-tocopherol enhanced binding of PKCα-C2 (250 nM) to the lipid layers with PS (POPC/POPE/PS/tocopherol, 70-x:20:10:x mole %, x=1–10). FC1 control channel (POPC/POPE/POPS, 70:20:10 mole%) has been subtracted from FC2 test (POPC/POPE/PS/tocopherol, 70-x:20:10:x mole %, x=1–10). Data can only be compared within an experiment because the Y-axis values vary among experiments. Data in each panel are from a representative experiment of two experiments.
α-tocopherol and γ-tocopherol enhance rPKCα-C1a but not rPKCα-C1b binding to lipid GUVs
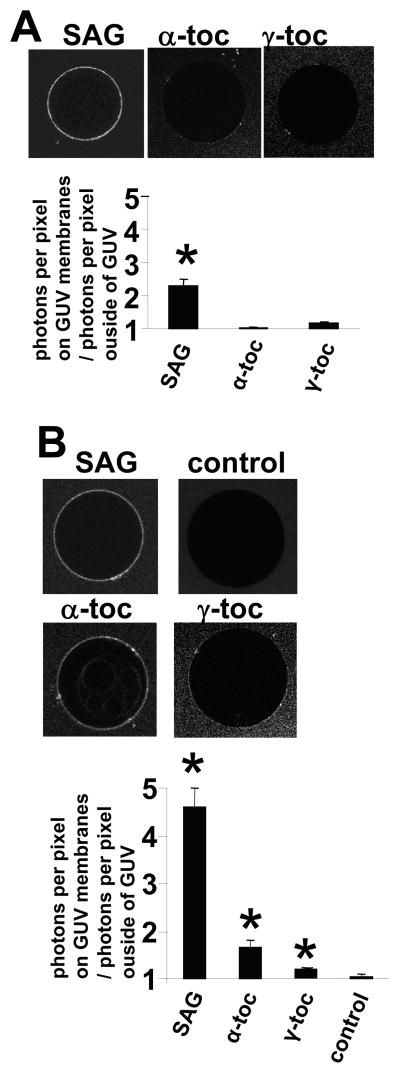
Since membrane binding of PKCα-C1a and PKCα-C1b domain is difficult to monitor by SPR analysis, we measured its binding to membranes containing tocopherols by fluorescence microscopy using eGFP-PKCα-C1a, eGFP-PKCα-C1b and giant unilamellar vesicles (GUVs) comprised of POPC/POPE/POPS (65:20:10 mol%) or POPC/POPE/POPS (65:20:10 mol%) with 5 mol% α-tocopherol, γ-tocopherol, or the positive control 1-steroyl-2-arachidonyl-sn-glycerol (SAG). Fluorescence microscopy of the vesicles and fluorescence intensity analysis of the GUV surfaces showed that eGFP-PKCα-C1a and eGFP-PKCα-C1b bound the GUVs containing the positive control SAG (Figure 4). The eGFP-PKCα-C1a bound the GUVs containing α-tocopherol or γ-tocopherol (Figure 4). In contrast, eGFP-PKCα-C1b did not bind to the GUVs containing α-tocopherol or γ-tocopherol (Figure 4). This indicates that α-tocopherol or γ-tocopherol induce the membrane binding of PKCα-C1a domain but not the PKCα-C1b domain.
Figure 4. Alpha-tocopherol and γ-tocopherol bind PKCα-C1a but do not bind PKC.
α-C1b.
A) eGFP-PKCα-C1b and B) eGFP-PKCα-C1a were added to control giant unilamellar vesicles (GUVs) comprised of PC/PE/PS (65:20:10 mole%) or GUVs comprised of PC/PE/PS (65:20:10 mole%) with 5 mole% 1-stearoyl,2-arachidonoyl-snglycerol (SAG), α-tocopherol (α-toc) or γ-tocopherol (γ-toc). Micrographs show fluorescence of representative GUVs. In the graph, the data is presented as the ratio of average photon counts per pixel within the GUV membrane to average photon counts outside of the GUV membrane ([average photon count per pixel within GUV]/[average photon count per pixel outside GUV]) as determined by fluorescence microscopy. A) The SAG positive control induced binding of eGFP-PKCα-C1b, but neither α-tocopherol or γ-tocopherol enhanced its binding in the presence of PS. B) SAG (the positive control), α-tocopherol or γ-tocopherol in the GUVs induced binding of eGFP-PKCα-C1a as compared to the control GUVs comprised of PC/PE/PS. Data are from a representative experiment of two experiments. *, p<0.05 as compared to the control group.
NBD-α-tocopherol directly binds full length rPKCα
Although in Figures 1–2 α-tocopherol or γ-tocopherol regulated activation of PKCα and in Figure 3 these tocopherols regulated recruitment of PKCα to PS-containing membranes, it is not known whether tocopherols directly interact with PKCα. Therefore, it was determined whether tocopherols bind to full length His-tagged rPKCα using anti-His tag coated/BSA blocked ELISA plates and NBD-tagged α-tocopherol. In this assay, NBD-α-tocopherol becomes fluorescent when inserted into a hydrophobic pocket in PKCα as has been described for NBD-α-tocopherol binding to α-tocopherol transfer protein [31]. We did not use NBD-γ-tocopherol because it is difficult to synthesize and is not available. We determined that, for this assay, the maximum HIS-tagged rPKCα binding to the anti-His-tag-coated ELISA plates was 30 ng/well as measured by labeling with an anti-PKC antibody (Figure 5A). To examine NBD-α-tocopherol binding, NBD-α-tocopherol was added to rPKCα; this was then applied to an anti-HIS Ab-coated ELISA plate; the plate was washed and then fluorescence was determined in a fluorescence plate reader. NBD-α-tocopherol directly bound to rPKCα with a significant increase in fluorescence signal at 5 μM NBD-α-tocopherol (Figure 5B). Though this lipid ELISA assay demonstrates that NBD-α-tocopherol directly binds to rPKCα, there was high background from lipid binding to BSA used for blocking the plate (data not shown).
Figure 5. NBD-α-tocopherol binds full length rPKCα.
A) Doses for rPKCα loading of anti-His Ab-coated ELISA plates were examined with biotinylated anti-PKCα as described in Methods. B) NBD-α-tocopherol (NBD-α-toc) background binding (to the plate or to the plate with anti-His Ab alone) and NBD-α-tocopherol binding to the anti-His Ab/30ng rPKCα was measured by examining NBD fluorescence. The NBD moiety fluorescence (excitation/emission = 469/535 nm) is induced by incorporation into a hydrophobic pocket. Data are the mean ± SEM of triplicates from a representative experiment of three experiments. Symbols with no errors bars have error bars than the symbol. *, p<0.05 as compared to the group(s) without PKC.
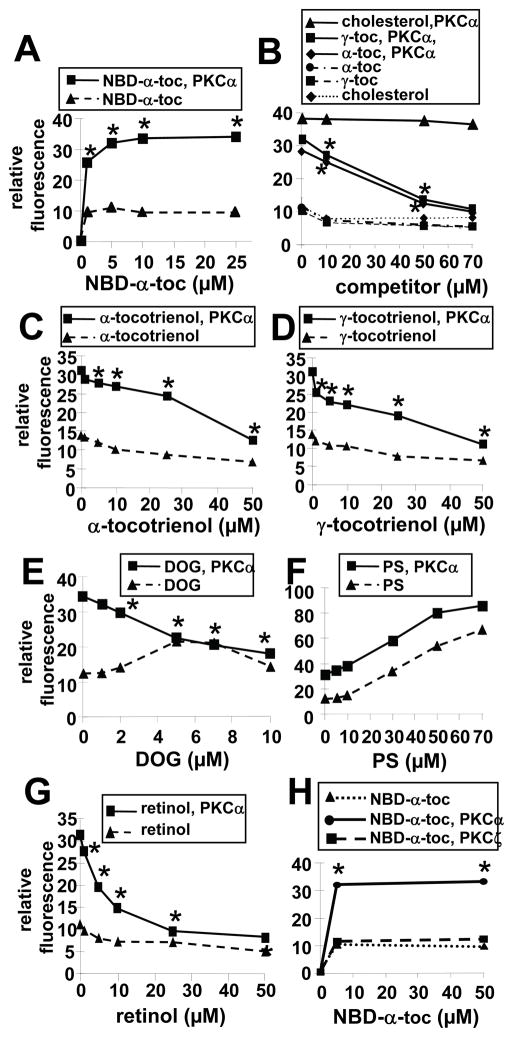
For greater assay sensitivity, NBD-α-tocopherol was added to rPKCα in solution or control buffer and change in fluorescence was determined as NBD-α-tocopherol fluoresces when it binds in a hydrophobic environment. NBD-α-tocopherol bound to rPKCα at 1 μM NBD-α-tocopherol (Figure 6A). To examine specificity of tocopherol binding to rPKCα, 5 μM NBD-α-tocopherol was added to rPKCα in the presence of increasing doses of the unlabeled amphipathic lipids α-tocopherol, γ-tocopherol, α-tocotrienol, γ-tocotrienol, or cholesterol. Alpha-tocopherol, γ-tocopherol, α-tocotrienol, and γ-tocotrienol competed with NBD-α-tocopherol binding to rPKCα (Figure 6B–D). In contrast, the negative control cholesterol did not compete with NBD-α-tocopherol binding to rPKCα (Figure 6B).
Figure 6. Tocopherol binds to rPKCα but not rPKCζ.
A) rPKCα (0 or 0.1 μM) was incubated with NBD-α-tocopherol (NBD-α-toc) for 30 min at room temperature. B) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of α-tocopherol (α-toc), γ-tocopherol (γ-toc) or cholesterol for 30 min at room temperature. C) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of α-tocotrienol for 30 min at room temperature. D) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of γ-tocotrienol for 30 min at room temperature. E) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of DOG for 30 min at room temperature. F) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of PS for 30 min at room temperature. G) 5 μM NBD-α-tocopherol was incubated with rPKCα (0 or 0.1 μM) in the presence of the indicated doses of retinol for 30 min at room temperature. H) rPKCα or rPKCζ was incubated with NBD-α-tocopherol (NBD-α-toc) for 30 mins at room temperature.
After the 30 minutes incubation with NBD-α-tocopherol, NBD fluorescence, which is increased in a hydrophobic environment, was examined in a fluorescence plate reader with a 469 nm excitation and a 535 nm emission. Data are the mean ± SEM of triplicates from a representative experiment of three experiments. Symbols with no errors bars have error bars smaller than the symbol. A, H) *, p<0.05 compared to the no NBD-α-tocopherol group. B–G) *, p<0.05 compared to the group without competitor to determine whether there was inhibition of NBD-α-tocopherol binding to rPKCα.
It was determined whether diacylglycerol competes with NBD-α-tocopherol binding since diacylglycerol contains structural similarities to tocopherols in that they each have a hydroxyl group and lipid tail. At just 5 μM DOG, there was complete ablation of 5 μM NBD-α-tocopherol binding to rPKCα as compared to NBD-α-tocopherol background fluorescence in the presence of DOG without rPKCα (Figure 6E). Furthermore, retinol, which is reported to bind to the diacylglycerol-site of C1a [33, 34], competed for NBD-α-tocopherol binding to rPKCα (Figure 6G). In contrast to competitors of the C1a domain, PS, which binds the PKCα-C2 domain, did not compete for NBD-α-tocopherol binding to rPKCα (Figure 6F). The dose-dependent increase in fluorescence in the presence of PS without rPKCα (Figure 6F) likely occurred as a result of NBD-α-tocopherol association with hydrophobic environments within PS complexes. It was also determined whether NBD-α-tocopherol binds to PKCζ because PKCζ is activated independent of diacylglycerol [32]. NBD-α-tocopherol did not bind PKCζ at the 5 μM dose used in rPKCα binding studies and still did not bind at 50 μM NBD-α-tocopherol (Figure 6H). Thus, tocopherols directly bind to rPKCα but not PKCζ. Moreover, tocopherols, tocotrienols, and PKCαC1a-binding lipid cofactors compete with NBD-α-tocopherol’s binding to rPKCα.
NBD-α-tocopherol directly and specifically binds to the PKCα-C1a domain
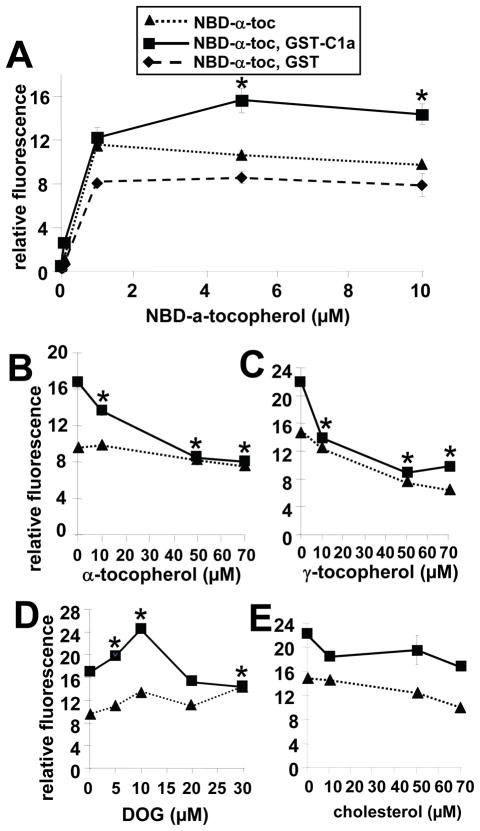
It was determined whether NBD-α-tocopherol directly binds to PKCα-C1a domain, using GST-PKCα-C1a. GST was not removed from the GST-PKCα-C1a because C1a is relatively hydrophobic and the GST tag stabilizes the PKCα-C1a domain in solution. NBD-α-tocopherol bound to GST-PKCα-C1a as compared to GST alone at a 5 μM tocopherol dose, which is the same dose that optimally bound full length rPKCα (Figure 6A and 7A). This NBD-α-tocopherol binding to GST-PKCα-C1a was competed with unlabeled α-tocopherol (Figure 7B), γ-tocopherol (Figure 7C) and DOG, the PKCα-C1a cofactor (Figure 7D) but not the control amphipathic lipid cholesterol (Figure 7E). DOG (5–10μM) enhancement of NBD-α-tocopherol binding to PKCα-C1a (Figure 7D) suggests that perhaps DOG binding to the PKCα-C1a domain exchanges with NBD-α-tocopherol facilitating tocopherol binding. In summary, NBD-α-tocopherol directly binds full-length rPKCα and the PKCα-C1a domain, resulting in regulation of PKCα activity.
Figure 7. Alpha-tocopherol, γ-tocopherol and DOG compete with NBD-α-tocopherol for binding to PKCα-C1a.
A) 0.2 μM GST-PKCα-C1a or 0.2 μM GST was incubated with the indicated doses of NBD-α-tocopherol (NBD-α-toc) for 30 min at room temperature. B–E) 5 μM NBD-α-tocopherol was incubated with GST-PKCα-C1a (0 or 0.2 μM) in the presence of the indicated doses of α-tocopherol (B), γ-tocopherol (C), DOG (D) or cholesterol (E) for 30 min at room temperature. After the 30 minutes incubation with NBD-α-tocopherol, NBD fluorescence, which is increased in a hydrophobic environment, was examined in a fluorescence plate reader with a 469 nm excitation and a 535 nm emission. Data are the mean ± SEM of triplicates from a representative experiment of three experiments. Symbols with no errors bars have error bars smaller than the symbol. A) *, p<0.05 compared to the NBD-α-tocopherol group. B–D) *, p<0.05 compared to the group with no competitor to determine whether there was inhibition of NBD-α-tocopherol binding to rPKCα.
Discussion
In these studies, we demonstrate that α-tocopherol and γ-tocopherol directly modulate cofactor-dependent activation of rPKCα and oxidative-activation of rPKCα. This regulation occurs through direct binding of tocopherol to PKCα’s C1a domain and through enhancement of PKCα binding to PS in tocopherol-containing lipid layers. These innovative studies are the first to demonstrate that tocopherols directly bind and modulate PKCα activity.
The PKCα-C1a regulatory domain contains a high-affinity binding site for DAG and retinol [33, 34]. Cofactor binding to PKCα-C1a is influenced by cofactor fatty acid chain length and saturation and by the hydroxyl group donation of a hydrogen to a recipient atom in the PKCα-C1a domain [23, 35–37]. We found that tocopherols, which have a reactive hydroxyl group on the chromanol head and an unsaturated lipid tail, compete with DAG binding to the PKCα-C1a domain. We also report that the binding of tocopherols regulate PKCα activity. γ-tocopherol elevates cofactor-dependent PKCα activity and α-tocopherol inhibits cofactor-dependent PKCα activity. Furthermore, 1 μM α-tocopherol blocks this enhancing effect of 1 μM γ-tocopherol. Therefore, γ-tocopherol serves as an agonist and α-tocopherol serves as an antagonist of cofactor-dependent PKCα activity. Without PS, γ-tocopherol does not increase the low PKCα activity (data not shown), suggesting that PS is necessary for γ-tocopherol enhancement of cofactor-dependent PKCα activity. At high PS concentrations (60 μg/ml) without tocopherol, PKCα activity is elevated to the level of PKCα activity with tocopherol and 30 μg/ml PS, suggesting that high PS induces maximal activation of PKCα activity without further agonist regulation through the PKCα-C1a domain.
Tocopherols also function as antioxidants. We report that both α-tocopherol (0.01 μM) and γ-tocopherol (0.1 μM) inhibited oxidative activation of rPKCα suggesting an antioxidant function for tocopherols. However, α-tocopherol significantly decreased peroxide activation of PKCα at a 10-fold lower dose compared to γ-tocopherol, even though α-tocopherol and γ-tocopherol have roughly equal anti-oxidant ability towards lipids in solution [38–40] and equal ability to bind PKCα (Figure 6B). Therefore, tocopherol isoforms differ in their antioxidant capacity towards PKCα.
PKC activity is also reported to positively correlate with membrane bilayer curvature, nonbilayer phases, and dehydration of the membrane by DAGs in the presence of calcium [41, 42]. Using cell-free systems, it has been reported that the DAG polar head group spacing and degree of acylated chain saturation contribute to PKC activation [37, 43]. In our studies, α-tocopherol, at 5 fold lower concentrations than γ-tocopherol enhanced PKCα-C2 domain interaction with PS-containing lipid surfaces without direct tocopherol interaction with the PKCα-C2 domain. This may be consistent with α-tocopherol’s significantly greater partitioning than γ-tocopherol into polyunsaturated lipid-rich domains for differential regulation of membrane structure [44]. Since it is reported that the PKCα-C2 domain binds to membranes and then, subsequently, the PKCα-C1a domain associates with DAG [27], it suggests that tocopherols influence the association of the PKCα-C2 domain with PS in the membrane and then tocopherols in the membrane compete with membrane DAG for binding to the PKCα-C1a domain. Thus, α-tocopherol and γ-tocopherol directly bind the PKCα-C1a domain, regulate cofactor-dependent activation of PKCα, regulate oxidative activation of PKCα, and regulate recruitment of PKCα to lipid membranes This suggests that antioxidant and non-antioxidant effects of these tocopherols contribute to the overall regulation of PKCα activity in cells and tissues.
Tocopherols have been reported to modulate PKCα activation in cells [16]. In cells, PKCα is recruited to the cell membrane where it interacts with PS and DAG [18, 22, 45]. PKCα is also transiently activated by oxidation [10]. We have previously reported that α-tocopherol pre-treatment of endothelial cells inhibits VCAM-1-induced oxidative activation of PKCα [6]. However, during VCAM-1 activation of PKCα, in addition to oxidative activation of PKCα, there is also generation of calcium [26] and consumption of the PKCα cofactor diacylglycerol [10], suggesting a contribution of both oxidative activation of PKCα and cofactor-dependent activation of PKCα during VCAM-1 signaling in endothelial cells. Therefore, α-tocopherol may inhibit VCAM-1 signaling by functioning both as an antioxidant and as an antagonist of PKCα. In contrast, γ-tocopherol, which is at 1/10 the tissue concentration of α-tocopherol, elevates VCAM-1 activation of PKCα and ablates the inhibitory effects of α-tocopherol on VCAM-1 activation of PKCα in endothelial cells [6]. Since in tissues, γ-tocopherol is at 1/10 the concentration of α-tocopherol [6], but we report here that 10 times more γ-tocopherol than α-tocopherol was required to have equal antioxidant capacity towards PKCα, it suggest that, in cells γ-tocopherol has much lower total antioxidant capacity towards PKCα than α-tocopherol. Therefore, nonantioxidant functions for γ-tocopherol are consistent with the potent γ-tocopherol enhancement of VCAM-1 activation of PKCα in cells. This enhancing effect of γ-tocopherol in cells may occur through γ-tocopherol’s direct co-factor-dependent agonist activation PKCα and/or γ-tocopherol’s enhancement of PKCα recruitment to PS-containing membranes as observed in our studies in this report. An enhancing effect of γ-tocopherol on cofactor-dependent PKCα activity is consistent with a contribution of cofactor-dependent (calcium and DAG) activation of PKCα during VCAM-1 signaling [10, 26]. In vivo, the anti-inflammatory effect of α-tocopherol and pro-inflammatory effect of γ-tocopherol on leukocyte recruitment [6, 8, 46] is the sum of tocopherol isoform antioxidant and agonist/antagonist functions.
In summary, α-tocopherol inhibits and γ-tocopherol elevates PKCα activity in the presence of PS. In contrast, both α-tocopherol and γ-tocopherol inhibit PS-independent oxidative activation of PKCα, although α-tocopherol significantly inhibits this oxidative PKCα activation at one-tenth the concentration required for γ-tocopherol inhibition. Alpha-tocopherol and γ-tocopherol modulate PKCα activity by enhancing association of the PKCα-C2 domain to PS-containing lipid layers and the tocopherols directly bind to the PKCα-C1a domain. Thus, tocopherols can function as antioxidants and function as PKCα agonists or antagonists for the regulation of PKCα activity in cells.
Acknowledgments
We thank Drs. Sean Davidson, Cara Gottardi, Phil Howles, and Alexandra Newton for their helpful advice and suggestions regarding protein-lipid interactions. We thank David Escobar and Dr. Rigen Mo for helpful advice on the expression of the GST-PKCα-C1a domain.
Funding.
These studies were supported by National Institutes of Health Grants R01 AT004837 (J.M.C-M) and GM68849 (W.C.).
References
- 1.Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids. 1996;31:671–701. doi: 10.1007/BF02522884. [DOI] [PubMed] [Google Scholar]
- 2.Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- 3.Azzi A, Gysin R, Kempna P, Munteanu A, Negis Y, Villacorta L, Visarius T, Zingg JM. Vitamin E mediates cell signaling and regulation of gene expression. Ann N Y Acad Sci. 2004;1031:86–95. doi: 10.1196/annals.1331.009. [DOI] [PubMed] [Google Scholar]
- 4.Azzi A, Breyer I, Feher M, Pastori M, Ricciarelli R, Spycher S, Staffieri M, Stocker A, Zimmer S, Zingg JM. Specific cellular responses to alpha-tocopherol. J Nutr. 2000;130:1649–1652. doi: 10.1093/jn/130.7.1649. [DOI] [PubMed] [Google Scholar]
- 5.Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39:231–255. doi: 10.1016/s0163-7827(00)00006-0. [DOI] [PubMed] [Google Scholar]
- 6.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of Vitamin E have Opposing Immunoregulatory Funcitons during Inflammation by Regulating Leukocyte Recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook-Mills JM, Marchese M, Abdala-Valencia H. VCAM-1 Expression and Signaling during Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid Redox Signal. 2011 doi: 10.1089/ars.2010.3522. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCary CA, Abdala-Valencia H, Berdnikovs S, Cook-Mills JM. Supplemental and Highly Elevated Tocopherol Doses Differentially Regulate Allergic Inflammation: Reversibility of α-Tocopherol and γ-Tocopherol’s Effects. J Immunol. 2011;186:3674–3685. doi: 10.4049/jimmunol.1003037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumagin R, Lomakina E, Sarelius IH. Leukocyte-endothelial cell interactions are linked to vascular permeability via ICAM-1-mediated signaling. Am J Physiol Heart Circ Physiol. 2008;295:H969–H977. doi: 10.1152/ajpheart.00400.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdala-Valencia H, Cook-Mills JM. VCAM-1 Signals Activate Endothelial Cell Protein Kinase Cα Via Oxidation. J Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boscoboinik D, Szewczyk A, Hensey C, Azzi A. Inhibition of cell proliferation by alpha-tocopherol. Role of protein kinase C. J Biol Chem. 1991;266:6188–6194. [PubMed] [Google Scholar]
- 12.Tasinato A, Boscoboinik D, Bartoli GM, Maroni P, Azzi A. d-alpha-tocopherol inhibition of vascular smooth muscle cell proliferation occurs at physiological concentrations, correlates with protein kinase C inhibition, and is independent of its antioxidant properties. Proc Natl Acad Sci U S A. 1995;92:12190–12194. doi: 10.1073/pnas.92.26.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahoney CW, Azzi A. Vitamin E inhibits protein kinase C activity. Biochem Biophys Res Commun. 1988;154:694–697. doi: 10.1016/0006-291x(88)90195-7. [DOI] [PubMed] [Google Scholar]
- 14.Ricciarelli R, Tasinato A, Clement S, Ozer NK, Boscoboinik D, Azzi A. alpha-Tocopherol specifically inactivates cellular protein kinase C alpha by changing its phosphorylation state. Biochem J. 1998;334:243–249. doi: 10.1042/bj3340243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger T, Hammer A, Wintersperger A, Goti D, Malle E, Sattler W. Modulation of microglial superoxide production by alpha-tocopherol in vitro: attenuation of p67(phox) translocation by a protein phosphatase-dependent pathway. J Neurochem. 2001;79:1169–1182. doi: 10.1046/j.1471-4159.2001.00641.x. [DOI] [PubMed] [Google Scholar]
- 16.Venugopal SK, Devaraj S, Yang T, Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes. 2002;51:3049–3054. doi: 10.2337/diabetes.51.10.3049. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Alfaro JA, Gomez-Fernandez JC, Corbalan-Garcia S. Role of the lysine-rich cluster of the C2 domain in the phosphatidylserine-dependent activation of PKCalpha. J Mol Biol. 2004;335:1117–1129. doi: 10.1016/j.jmb.2003.10.080. [DOI] [PubMed] [Google Scholar]
- 18.Bolsover SR, Gomez-Fernandez JC, Corbalan-Garcia S. Role of the Ca2+/phosphatidylserine binding region of the C2 domain in the translocation of protein kinase Calpha to the plasma membrane. J Biol Chem. 2003;278:10282–10290. doi: 10.1074/jbc.M212145200. [DOI] [PubMed] [Google Scholar]
- 19.Gould CM, Newton AC. The life and death of protein kinase C. Curr Drug Targets. 2008;9:614–625. doi: 10.2174/138945008785132411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conesa-Zamora P, Lopez-Andreo MJ, Gomez-Fernandez JC, Corbalan-Garcia S. Identification of the phosphatidylserine binding site in the C2 domain that is important for PKC alpha activation and in vivo cell localization. Biochem. 2001;40:13898–13905. doi: 10.1021/bi011303o. [DOI] [PubMed] [Google Scholar]
- 21.Verdaguer N, Corbalan-Garcia S, Ochoa WF, Fita I, Gomez-Fernandez JC. Ca(2+) bridges the C2 membrane-binding domain of protein kinase Calpha directly to phosphatidylserine. EMBO J. 1999;18:6329–6338. doi: 10.1093/emboj/18.22.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bittova L, Stahelin RV, Cho W. Roles of ionic residues of the C1 domain in protein kinase C-alpha activation and the origin of phosphatidylserine specificity. J Biol Chem. 2001;276:4218–4226. doi: 10.1074/jbc.M008491200. [DOI] [PubMed] [Google Scholar]
- 23.Ganong BR, Loomis CR, Hannun YA, Bell RM. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986;83:1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ananthanarayanan B, Stahelin RV, Digman MA, Cho W. Activation mechanisms of conventional protein kinase C isoforms are determined by the ligand affinity and conformational flexibility of their C1 domains. J Biol Chem. 2003;278:46886–46894. doi: 10.1074/jbc.M307853200. [DOI] [PubMed] [Google Scholar]
- 25.Gopalakrishna R, Anderson WB. Ca2+- and phospholipid-independent activation of protein kinase C by selective oxidative modification of the regulatory domain. Proc Natl Acad Sci U S A. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook-Mills JM, Johnson JD, Deem TL, Ochi A, Wang L, Zheng Y. Calcium mobilization and Rac1 activation are required for VCAM-1 (vascular cell adhesion molecule-1) stimulation of NADPH oxidase activity. Biochem J. 2004;378:539–547. doi: 10.1042/BJ20030794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medkova M, Cho W. Interplay of C1 and C2 domains of protein kinase C-alpha in its membrane binding and activation. J Biol Chem. 1999;274:19852–19861. doi: 10.1074/jbc.274.28.19852. [DOI] [PubMed] [Google Scholar]
- 28.Stahelin RV, Cho W. Differential roles of ionic, aliphatic, and aromatic residues in membrane-protein interactions: a surface plasmon resonance study on phospholipases A2. Biochem. 2001;40:4672–4678. doi: 10.1021/bi0020325. [DOI] [PubMed] [Google Scholar]
- 29.Yoon Y, Tong J, Lee PJ, Albanese A, Bhardwaj N, Kallberg M, Digman MA, Lu H, Gratton E, Shin YK, Cho W. Molecular basis of the potent membrane-remodeling activity of the epsin 1 N-terminal homology domain. J Biol Chem. 2010;285:531–540. doi: 10.1074/jbc.M109.068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stahelin RV, Digman MA, Medkova M, Ananthanarayanan B, Rafter JD, Melowic HR, Cho W. Mechanism of diacylglycerol-induced membrane targeting and activation of protein kinase Cdelta. J Biol Chem. 2004;279:29501–29512. doi: 10.1074/jbc.M403191200. [DOI] [PubMed] [Google Scholar]
- 31.Nava P, Cecchini M, Chirico S, Gordon H, Morley S, Manor D, Atkinson J. Preparation of fluorescent tocopherols for use in protein binding and localization with the alpha-tocopherol transfer protein. Bioorg Med Chem. 2006;14:3721–3736. doi: 10.1016/j.bmc.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 32.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyos B, Imam A, Chua R, Swenson C, Tong GX, Levi E, Noy N, Hammerling U. The cysteine-rich regions of the regulatory domains of Raf and protein kinase C as retinoid receptors. J Exp Med. 2000;192:835–845. doi: 10.1084/jem.192.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyos B, Jiang S, Hammerling U. Location and functional significance of retinol-binding sites on the serine/threonine kinase, c-Raf. J Biol Chem. 2005;280:6872–6878. doi: 10.1074/jbc.M412695200. [DOI] [PubMed] [Google Scholar]
- 35.Madani S, Hichami A, Legrand A, Belleville J, Khan NA. Implication of acyl chain of diacylglycerols in activation of different isoforms of protein kinase C. FASEB J. 2001;15:2595–2601. doi: 10.1096/fj.01-0753int. [DOI] [PubMed] [Google Scholar]
- 36.Lapetina EG, Reep B, Ganong BR, Bell RM. Exogenous sn-1,2-diacylglycerols containing saturated fatty acids function as bioregulators of protein kinase C in human platelets. J Biol Chem. 1985;260:1358–1361. [PubMed] [Google Scholar]
- 37.Slater SJ, Kelly MB, Taddeo FJ, Ho C, Rubin E, Stubbs CD. The modulation of protein kinase C activity by membrane lipid bilayer structure. J Biol Chem. 1994;269:4866–4871. [PubMed] [Google Scholar]
- 38.Yoshida Y, Saito Y, Jones LS, Shigeri Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: physiological significance and prospects as antioxidants. J Biosci Bioeng. 2007;104:439–445. doi: 10.1263/jbb.104.439. [DOI] [PubMed] [Google Scholar]
- 39.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem. 2004;11:1113–1133. doi: 10.2174/0929867043365332. [DOI] [PubMed] [Google Scholar]
- 41.Micol V, Sanchez-Pinera P, Villalain J, de Godos A, Gomez-Fernandez JC. Correlation between protein kinase C alpha activity and membrane phase behavior. Biophys J. 1999;76:916–927. doi: 10.1016/S0006-3495(99)77255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Garcia F, Micol V, Villalain J, Gomez-Fernandez JC. Infrared spectroscopic study of the interaction of diacylglycerol with phosphatidylserine in the presence of calcium. Biochim Biophys Acta. 1993;1169:264–272. doi: 10.1016/0005-2760(93)90250-d. [DOI] [PubMed] [Google Scholar]
- 43.Bolen EJ, Sando JJ. Effect of phospholipid unsaturation on protein kinase C activation. Biochem. 1992;31:5945–5951. doi: 10.1021/bi00140a034. [DOI] [PubMed] [Google Scholar]
- 44.Urano S, Yano K, Matsuo M. Membrane-stabilizing effect of vitamin E: effect of alpha-tocopherol and its model compounds on fluidity of lecithin liposomes. Biochem Biophys Res Commun. 1988;150:469–475. doi: 10.1016/0006-291x(88)90544-x. [DOI] [PubMed] [Google Scholar]
- 45.Takai Y, Kishimoto A, Iwasa Y, Kawahara Y, Mori T, Nishizuka Y. Calcium-dependent activation of a multifunctional protein kinase by membrane phospholipids. J Biol Chem. 1979;254:3692–3695. [PubMed] [Google Scholar]
- 46.Cook-Mills JM, McCary CA. Isoforms of vitamin E differentially regulate inflammation. Endocr Metab Immune Disord Drug Targets. 2010;10:348–366. doi: 10.2174/1871530311006040348. [DOI] [PMC free article] [PubMed] [Google Scholar]