Abstract
Methylphenidate (MPD) is the most widely used drug in the treatment of attention-deficit hyperactivity disorder (ADHD). ADHD has a high incidence in children and can persist in adolescence and adulthood. The relation between sex and the effects of acute and chronic MPD treatment was examined using adolescent male and female rats from three genetically different strains: spontaneously hyperactive rat (SHR), Wistar-Kyoto (WKY) and Sprague-Dawley (SD). Rats from each strain and sex were randomly divided into a control group that received saline injections and three MPD groups that received either 0.6 or 2.5 or 10 mg/kg MPD injections. All rats received saline on experimental day 1 (ED1). On ED2 to ED7 and ED11, the rats were injected either with saline or MPD and received no treatment on ED8 to ED10. The open field assay was used to assess the dose response of acute and chronic MPD administration. Significant sex differences were found. Female SHR and SD rats were significantly more active after MPD injections than their male counterparts, while the female WKY rats were less active than the male WKY rats. Dose dependent behavioral sensitization or tolerance to MPD treatment was not observed for SHR or SD rats, but tolerance to MPD was found in WKY rats for the 10 mg/kg MPD dose. The use of dose response protocol and evaluating different locomotor indices provides the means to indentify differences between the sexes and the genetic strain in adolescent rats. In addition these differences suggest that the differences to MPD treatment between the sexes are not due to the reproductive hormones.
Keywords: Ritalin, SHR, WKY and SD rats, Open-field activity, Locomotor activity
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most prevalent diagnosed neuropsychiatric disorders in children, conservatively estimated to occur in 3.0 to 7.5% of school-aged children (Goldman et al., 1998; Wilens et al., 2008a). In another report, it was estimated that up to 20% of boys in public schools are treated for ADHD with psychostimulants (Faraone and Wilens, 2007; LeFever et al., 1999). School-aged children suffering of ADHD are inattentive or hyperactive-impulsive (American Psychiatric Association 1994, Swanson et al., 1998), having disorders in motor control and perception (Landgren et al., 2000; Wilens et al., 2008a). The pharmacotherapy treatments of choice are methylphenidate (MPD) and/or amphetamine (Castellanos and Tannock, 2002). Although these drugs are very effective in treating the short-term behavioral symptoms of ADHD, there is growing concern about the potential of MPD to increase the risk for drug dependence (Kollins, et al., 2001; Vendruscolo et al., 2008) i.e. they can produce abuse and dependence in a long-term treatment (Dafny and Yang, 2006; Goldman et al., 1998; Levin and Kleber, 1995). For example, exposure to MPD in adolescent rats has been shown to endure changes in the neurobiology of the adult brain reward systems (Moll et al., 2001), or alter the responsiveness to cocaine in adulthood (Brandon et al., 2001): while others reported that stimulant treatment for ADHD causes a significant protective effect on the development of any substance use disorder (Wilens et al., 2008a; Faraone and Wilens, 2007). Despite their potential long-term adverse effects on the developing brain, psychostimulants for ADHD treatment are increasingly being administered to children as young as two years of age (Zito et al., 2000).
There are significant gender differences in ADHD expression (Cornforth et al., 2010). An early study showed that the disorder is more often diagnosed in boys than in girls (Anderson et al., 1987). Gender differences in ADHD expression were also found in adults. For example, Biederman et al. (1994) stressed the viability and importance of identification of female subjects with ADHD. Although there are reports indicating that the response to psychostimulants is sex-dependent (Berger and Sagvolden, 1998; Camp and Robinson, 1988;; Cornforth et al., 2010; Glick et al., 1984; Robinson, 1984), the vast majority of experimental studies related to ADHD in rats utilized male rats (Askenasy et al., 2007). MPD bind to dopamine (DA) transporter (DAT) that results in an increase DA concentration in the synaptic cleft (Volkow et al., 2005) therefore MPD is consider as an indirect dopamine (DA) agonist (Challman and Lipsky, 2000; Gatley et al., 1999; Solanto, 1998), DA functioning varies by sex and age which may result in the effect of MPD being different in male and female of different ages (Cornforth et al., 2010). Prenatal cocaine dampened behavioral responses to MPD in male and female adolescent rats exhibits sex differences in their response to the drug (Torres-Reveron and Dow-Edwards, 2006), as well as using incentive processing (Brenhouse et al., 2009), anxiety-related behavior (Vendruscolo et al., 2008), juvenile toxicity assessment (Beckman et al., 2008; Teo et al., 2002), impairment of attention (Rezvani et al., 2009), behavioral performance (Wagner et al., 2007), and circadian activity pattern (Algahim et al., 2009; Lee et al., 2009) procedures. However, controversial observations following MPD administration among the sexes were reported (Cornforth et al., 2010; Dafny and Yang, 2006). Each of the previous studies used only one strain and reported sex differences. The objective of this study is to clarify this controversy using several different rat strains in the same dose response protocol of MPD on different sexes of adolescent rats.
Since MPD is used in ADHD therapy we selected to investigate the dose response property of MPD in an animal model for ADHD-the spontaneous hyperactive rat (SHR) (McCarty and Kopin, 1979; Pires et al., 2009; Roessner et al., 2010; Sagvolden, 2000; Thanos et al., 2010). The SHR strain was bred from progenitor Wistar-Kyoto (WKY) rats. Therefore, the WKY rat strain was selected as control to the SHR strain group (Roessner et al,2010;Simchon et al., 2010). In addition, we selected the Sprague-Dawley (SD) rat strain since the SD strain rat is used most frequently in psychostimulant studies as “normal” rat (Askenasy et al, 2007). Each strain of rats comprised a different gene pool that resulted in different susceptibility to psychostimulants and its chronic effects like sensitization (Dafny and Yang, 2006).
Several different factors can alter the rate of drug effect. The most important factors are sex and genetically determined polymorphins in drug excitation and conjugation (Benet et al., 1996; Cornforth et al., 2010). Therefore, the objective of this study is to investigate whether differences in strain and sex influence MPD response. For this purpose, an MPD dose-response study was carried out in three different rat strains as well as in male and female adolescent rats using an open-field assay.
Our hypothesis is that MPD will show different effects in the adolescent male compared to the adolescent female and that the different strains of adolescent rats will respond to MPD differently.
2. Methods
2.1. Subjects
Male (N=112) and female (N=104) SHR, WKY, and SD rats, 34 days old, were purchased from Harlan Laboratories (Indianapolis, IN, USA). For adaptation, animals were housed in the experimental room in groups of four per cage. The ambient temperature of the room was 21±2°C, with relative humidity of 37 to 42%. Animals were maintained on a 12:12h light/dark cycle (05:30–17:30h light on) with food and water given ad libitum. The initial 5–6 days prior to the recording sessions were used for acclimation. One day prior to the first recording day, the rats of the same sex from each strain were randomly divided into four groups (each group having N=8 animals, unless indicated otherwise – see Table 1), and each animal was placed in a separate testing cage that became the home cage for the eleven experimental days (Table 1). At a particular time, only one sex and one rat strain was used. The experiment was done in the home cage to eliminate the novelty of the test cage as an additional stimulus to our treatment. Many investigators have used rats of different ages and correlated the rat’s ages to human ages, such as juvenile, periadolescent, adolescent, young adult and adult (Bolonos et al., 1998; Brandon et al., 2001; Campbell et al., 2000; Collins et al., 2004; Laviola et al., 1995; McDougall et al., 1999; Rezvoui and Levin, 2004; Roffman and Raskin, 1997). Based on these reports, the following can be derived: from postnatal day 21 (P-21 to P-30; P-31 to P-39; P-40 to P-50; P-60 to P-75 and P-76) and above the rats are considered as juveniles, periadolescent, adolescents, young adults and adult rats, respectively. Therefore, all experiments start on P-40. The experiment was carried out in accordance with the guidelines of the NIH and the declaration of Helsinki and was approved by our local Animal Welfare Committee.
Table 1. Treatment protocol.
The table described the treatment on each experimental day of the WKY, SHR and SD male and female rat groups. Treatment groups I, II, III, and IV were injected with saline, 0.6, 2.5 or 10.0 mg/kg ip methylphenidate (MPD), respectively.
| Treatment group | Strain | N males |
N females |
Experimental day | |||
|---|---|---|---|---|---|---|---|
| 1 | 2–7 | 8–10 | 11 | ||||
| I. Saline | WKY | 8 | 8 | Saline | Saline | Washout | Saline |
| SHR | 8 | 8 | Saline | Saline | Washout | Saline | |
| SD | 8 | 8 | Saline | Saline | Washout | Saline | |
| II. 0.6 mg/kg MPD | WKY | 8 | 8 | Saline | 0.6 mg/kg | Washout | 0.6 mg/kg |
| SHR | 8 | 8 | Saline | 0.6 mg/kg | Washout | 0.6 mg/kg | |
| SD | 8 | 8 | Saline | 0.6 mg/kg | Washout | 0.6 mg/kg | |
| III. 2.5 mg/kg MPD | WKY | 13 | 12 | Saline | 2.5 mg/kg | Washout | 2.5 mg/kg |
| SHR | 12 | 8 | Saline | 2.5 mg/kg | Washout | 2.5 mg/kg | |
| SD | 11 | 12 | Saline | 2.5 mg/kg | Washout | 2.5 mg/kg | |
| IV. 10 mg/kg MPD | WKY | 12 | 8 | Saline | 10 mg/kg | Washout | 10 mg/kg |
| SHR | 8 | 8 | Saline | 10 mg/kg | Washout | 10 mg/kg | |
| SD | 8 | 8 | Saline | 10 mg/kg | Washout | 10 mg/kg | |
2.2. Drugs
Methylphenidate hydrochloride (MPD) was procured from Mallinckrodt (Hazelwood, MO). MPD was dissolved in a 0.9% isotonic NaCl solution (saline) and dosages were calculated as free base. Injections were given intra-peritoneally (i.p.) and equalized to a volume of 0.8cc with 0.9% saline, so that the volume of each injection was the same for all animals. The MPD doses of 0.6, 2.5, and 10 mg/kg used in this study corresponded to low, medium, and high doses respectively (Dafny and Yang, 2006; Gaytan et al., 1996). Saline injections consisted of 0.8cc isotonic saline solution (0.9% NaCl) administered i.p.
2.3. Saline and MPD treatment
All animals were treated with saline on experimental day 1 (ED1). Then, during ED2 to ED7 the animals in the control group received saline, while the animals in the other groups were treated with 0.6 or 2.5 or 10 mg/kg MPD, i.p. All animals had a washout phase for three days at ED8 to ED10 in which they did not receive any treatment. Finally, on the last experimental day, ED11, the animals received the same treatment as in ED2 to ED7 (Table 1). All animals were injected between 6:30–7:00 a.m. because in a previous dose-response study, it was found that MPD injection in the morning exerted the most significant effects (Gaytan et al., 1996, 1997, 2000).
2.4. Apparatus and open field activity recording
Open-field testing introduced over 70 years ago resembles a natural behavioral pattern and is one of the most widely used methods in animal behavioral research (Askenasy et al., 2007). Its popularity stems from the simplicity of the apparatus and of clearly defined behavior. Open-field locomotor behaviors represent the interaction of the whole animal with the experimental situations. The augmentation or attenuation of locomotor activity following repeated psychostimulant administration has been referred to as behavioral sensitization or tolerance respectively (Algahim et al., 2009; Barron et al., 2009; Gaytan et al., 1997; Lee et al., 2008, 2009; Robinson, 1984; Yang et al., 2001, 2006, 2008, 2010).
Sixteen transparent (acrylic) boxes (40.5cm× 40.5cm× 31.5cm), each containing two arrays of 16 infrared motion sensors, were used to record the locomotor activity after treatment. The arrays with motion sensors were located at 6 and 12.5cm above the box floor. The activity monitoring system checked each of the sensor beams every 10 msec to determine whether beams were interrupted. Interruption of any beam was counted as an activity. Cumulative counts were compiled by Accuscan analyzer (Columbus, OH, USA) and downloaded every 10 mins to a computer, equipped with OASIS data collection software, which computed different locomotor activity indices (Gaytan et al., 1996, 1997, 2000; Yang et al., 2001, 2003, 2006, 2010). Four locomotor indices were studied: horizontal activity (HA), vertical activity (VA), the total distance traveled (TDT), and the number of stereotypic movements (NOSM). HA and VA were computed as the total number of beam interruptions that occurred in the horizontal and vertical plane, respectively; the TDT recorded the forward ambulation in centimeters; and the NOSM was computed as the number of repetitive, purposeless movements having at least 1 sec interval between them (Gaytan et al., 1997, 2000; Yang et al.,2000,2003,2006,2010). The locomotor activities were recorded on ED1–ED7 and ED11 for 2h, after saline or MPD injection. On ED8–ED10 (washout period) the activity was recorded for 2h, at the same time as in the previous experimental days, without injecting with saline or MPD (see Table 1).
2.5. Data analysis
For each analyzed activity the data had two formats: 1) sum of activity for the initial 2h after saline/MPD injection; 2) temporal variation of activity computed over consecutive intervals of 10 mins each, for the initial 2h after saline/MPD injection. The acute effect of MPD was evaluated by comparing the activities on ED2 and ED1, while the chronic effect of MPD was evaluated by comparing the activities on ED11 i.e. the last MPD administration to activities on ED1 and/or ED2. All comparisons were made with multifactor ANOVA and Post-hoc analysis with Fischer’s LSD test. Significance for all of the comparisons was set at the level of p<0.05 along with their corresponding F-values. The power of ANOVA for this study is estimated to be from 0.83 to 0.94 with a sample size of 8 rats/group for each motor intex.
3. RESULTS
3.1 Saline control
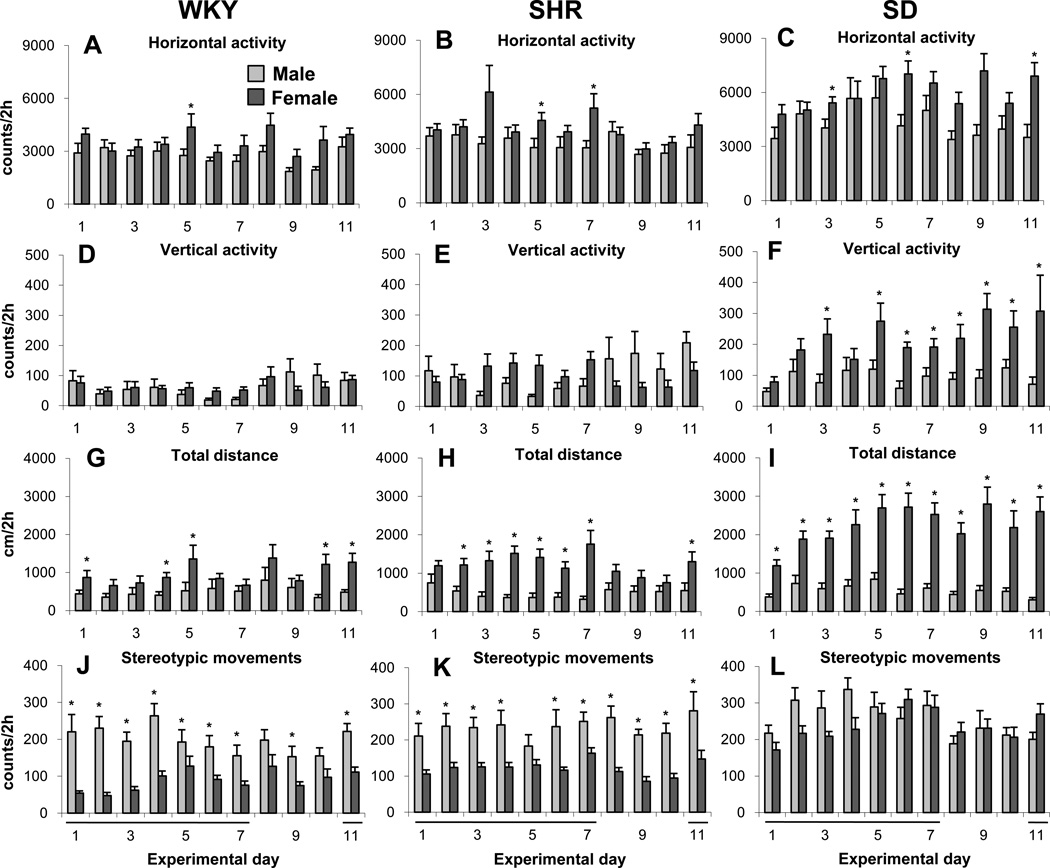
Saline was injected on seven consecutive days to female and male adolescent WKY, SHR, and SD rats followed by three days without treatment (washout period) and an additional day of saline injection on ED11 (Table 1). Four locomotor indices were studied: horizontal activity (HA), vertical activity (VA), total distance travels (TDT), and number of steroptipic movement (NOSM). Figure 1 summarizes the four locomotor indices of male and female adolescent WKY, SHR, and SD groups and shows that for the same sex and group, the rats exhibited similar activity, with no significant fluctuation, during all 11 experimental days. Significant (F2, 32 = 5.96; p<0.05) differences between male and female rats were observed in the VA of the SD rat groups (Fig. 1F), the TDT of the WKY, SHR, and SD rat groups (Fig. 1 G, H, and I), and in the NOSM of the WKY and SHR groups (Fig. 1J and K). In general, strain and sex differences (F6, 123 = 28.15; p<0.05) were found when females and males of all three strains were compared.
Figure 1.
Summarizes the control groups and show the mean locomotor activity of the initial 2 hour post-saline injection of the male and female WKY, SHR, and SD rat groups during the 11 consecutive experimental days. Saline injection was give on ED1 to ED7 and ED11 (see Table 1). Label * indicates significant (p<0.05) sex differences between the daily and locomotor activities using the Fisher LSD test. Error bars are SEM.
3.2 Acute dose response characteristics of MPD in adolescent males and females of three rat strains
3.2.1. Comparing the acute MPD effect of each group i.e., 0.6mg/kg MPD effect on on male WKY rats only
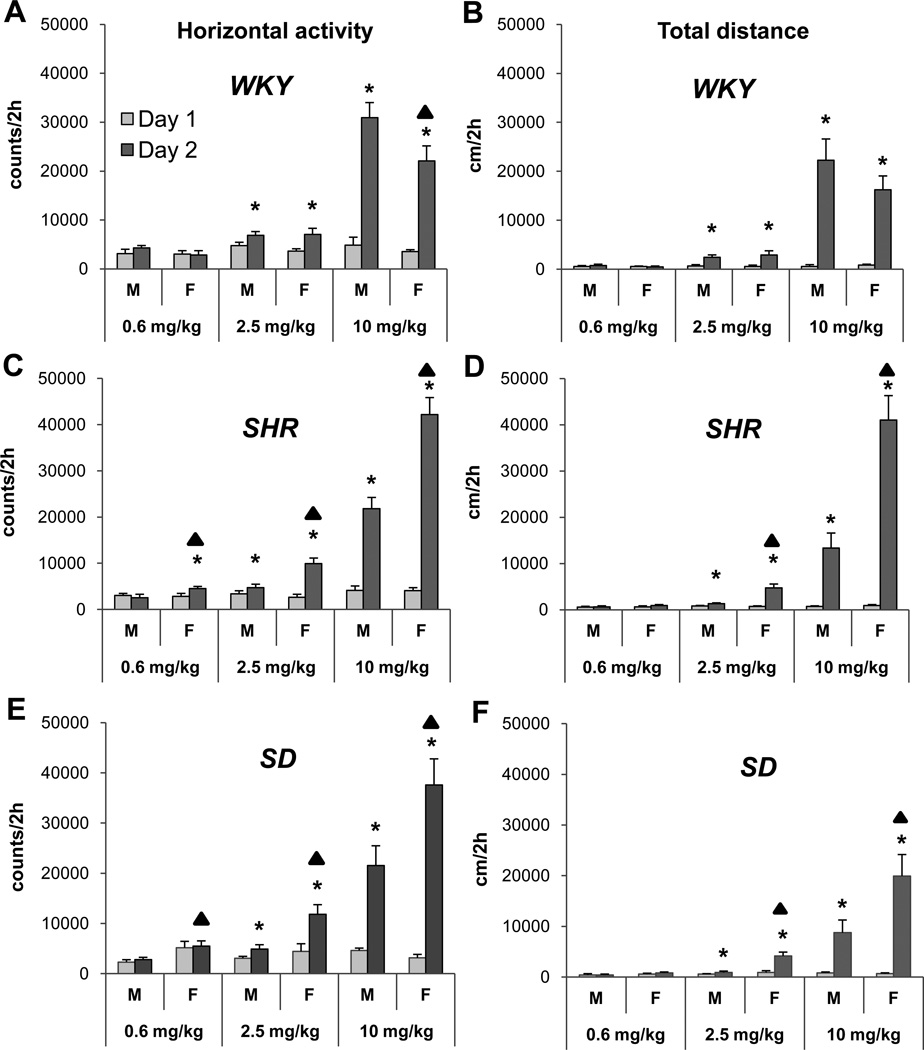
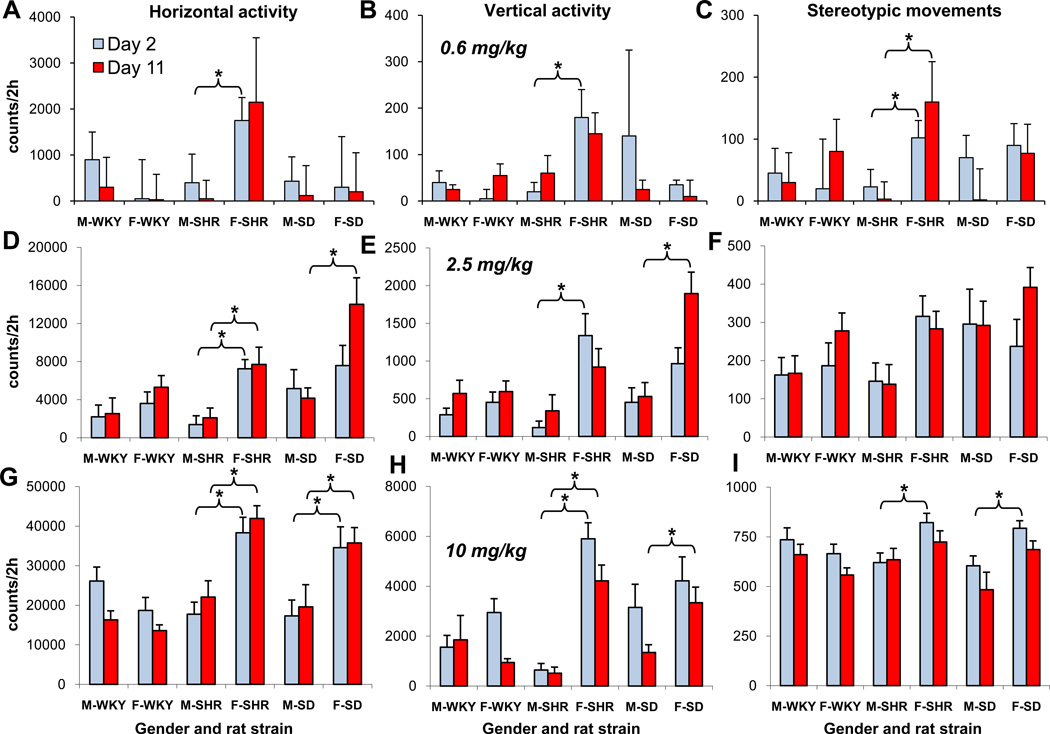
Figure 2 summarizes the HA and TDT as a function of acute MPD dose administered to adolescent male and female WKY, SHR, and SD rats. The 0.6 mg/kg MPD dose significantly (F1, 23 = 4.37; *p<0.05) increased only the HA (Fig. 2C) and VA (data not shown) of female SHR when activity on ED2 was compared to that of ED1. The 2.5 mg/kg MPD dose significantly (F1, 15 = 7.06; *p<0.05) increased the HA and TDT (Fig. 2), as well as the VA and NOSM (data not shown), in both female and male WKY, SHR, and SD rats when their response on ED2 was compared to that on ED1. The 10 mg/kg MPD dose resulted in a robust significant (F1, 23 = 4.11; p<0.05) increase of HA and TDT on ED2 versus ED1 (Fig. 2), as well as the VA and NOSM (data not shown)using the Fisher LSD test.
Figure 2.
Acute effect of methylphenidate (MPD) of the horizontal activity and total distance traveling. Label * indicates significant (p<0.05) acute MPD effect by comparing the activity after the first MPD treatment (ED2) to that obtained after saline injection on ED1 of each group, using the Fisher LSD test i.e. male WKY ED1 was compared to male WKY ED2. Label ▲ indicates significant (p<0.05) sex difference in response to acute MPD administration. For easy comparison all the scales for all of the MPD doses kept the same.
3.2.2. Comparing the acute MPD effect of the same dose between the sexes
In general, differences between the sexes of all three rat strains were observed following the three MPD doses. The 0.6 mg/kg dose elicited a significant (F2, 49 = 3.74; F2, 49 = 4.02; ▲p<0.05) increase in activity between the sexes of SHR and SD rats, respectively i.e., female SHR and SD rats expressed higher HA compared to the male rats (Fig. 2C and E). Following the 2.5 mg/kg MPD dose, female SHR and SD rats expressed a significant (F2, 49 = 4.01; F2, 49 = 4.35i ▲p<0.05) increase in HA respectively (Fig. 2C and E), TDT (F2, 49 = 3.98; F2, 49 = 4.31; p<0.05) respectively (Fig. 2D and F), and NOSM (data not shown). Similar sex differences in response to MPD were observed following the 10 mg/kg dose (F2, 49 = 4.12; F2, 49 = 4.33; p<0.05) respectively, (Fig. 2). At this dose, the male WKY rats expressed significantly (F2, 49 = 3.96; p<0.05) higher HA than their female counterparts (Fig. 2A); while opposite differences were found between the sexes of SHR and SD rats (Fig. 2C, D, E, and F; F2, 49 = 3.82; F2, 49 = 3.91; F2, 49 = 4.04; F2, 49 = 4.07; p<0.05, respectively). Both female and male WKY, SHR, and SD rats exhibited different responses to the three MPD doses. Female SHR and SD rats exhibited a more robust increase in locomotion compared to male rats (F10, 49 = 4.12; F10, 49 = 4.03; F10, 49 = 4.02; F10, 49 = 3.87; p<0.05, Fig. 2C, D, E, and F, respectively), except for the male WKY, which showed a more robust (F10, 49 = 3.92; ▲p<0.05) increase in HA and TDT compared to female WKY rats (Fig. 2A).
3.3. Chronic MPD dose response characteristics in adolescent male and female WKY, SHR, and SD rats
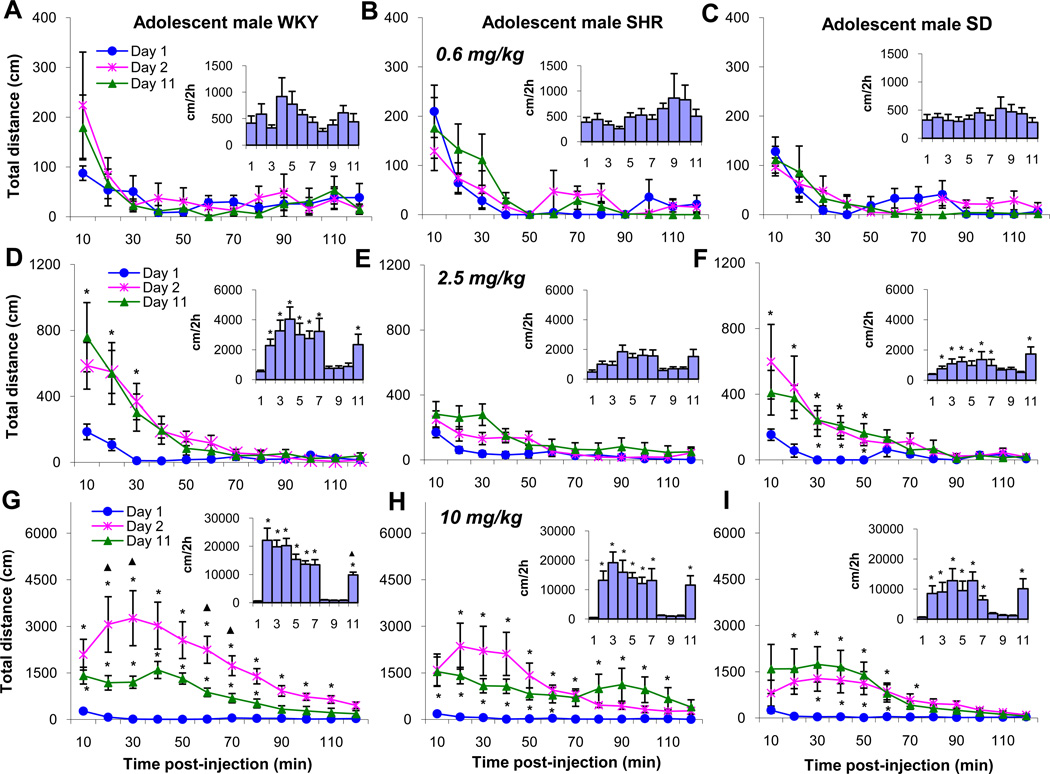
The chronic dose response characteristics for TDT in adolescent male WKY, SHR, and SD rats was examined next. Fig. 3 summarizes the sequential 10 mins temporal effects for the initial 2h post-injection of MPD on ED1, ED2, and ED11 and the TDT under the temporal curve of 2h post drug injection for all experimental days (Fig. 3 inserts). Chronic treatment with the lowest MPD dose (0.6 mg/kg) had no effect on total distance on all experimental days in all the three strains (Fig. 3A, B, and C). When the animals were treated with 2.5mg/kg MPD, the temporal graph shows that MPD (2.5 mg/kg) caused the WKY rats to significantly (F1, 23 = 4.96; p<0.05) increase their TDT during the initial 30 min post-injection period (Fig. 3D). The SHR group was not affected by this MPD dose (Fig. 3E), while the SD rats experienced a significant (F1, 23 = 4.82; p<0.05) increase in TDT for 50 mins post-injection (Fig. 3F). However, when the TDT for ED2 to ED7 and ED11 was summarized and compared to baseline activity on ED1, a significant (F10, 129 = 32.87; F10, 129 = 34.03; *p<0.05) increase in locomotor activity in the WKY and SD rats was observed respectively (Fig. 3D and F).
Figure 3.
Sequential, 10 minute distance traveled for 2 hours of ED1, ED2, and ED11 (see Table 1), to show the acute and chronic dose response effect of the 3 MPD doses as well as the total activity (the insert) under the temporal graph for each of the experimental days for the male WKY, SHR and SD rat groups. Label * indicates significant (p<0.05) difference compared to ED1: ▲ indicates significant (p<0.05) difference compared to ED2. The scale for each dose was different to show the dose response.
The 10 mg/kg MPD dose exerted a robust significant (F10, 129 = 32.66; p<0.05) increase in TDT in adolescent male rats for each of the three rat strains (Fig. 3G, H, and I). For the WKY rats, the peak effect occurred 30 mins. post-injection and the drug effects remained for 110 mins post-injection (Fig. 3G). The same dose on ED11 elicited a significant increase in TDT compared to saline (ED1). The degree of increased activity on ED11 was significantly (F1, 23 = 4.93; p<0.05) less than that of ED2 and the duration of the MPD effect was shorter at 20 min. i.e. for 80 min. (Fig. 3G), the 10 mg/kg MPD dose given to adolescent male WKY (Fig. 3G) rats produced tolerance (F1, 23 = 4.87; ▲p<0.05) to its effects as measured by TDT. Similar observations were obtained for the other locomotor indices (HA, VA, and NOSM) recorded from the WKY strain (data not shown). The SHR group exhibited a different response to the 10 mg/kg MPD dose. The response exhibited double peaking at 20 mins and 90 mins post-injection on ED2 to ED7. This increase lasted for about 110 mins. (F1, 23 = 4.69; p<0.05; Fig. 3H). On ED11, the 10 mg/kg MPD dose produced an effect that lasted for 60 mins, compared to the 110 min duration observed on previous experimental days. The locomotor activity of the SHR group remained about the same during all the injection days (Fig. 3H). SD rats exhibited a different response to the same MPD dose compared to the WKY and SHR groups. The peak effect was reached within the initial 10 mins post-injection. This effect remained for 70 mins on ED2 and 60 mins on ED11 (Fig. 3I). The total activity under the curve was similar for all the post-injection days (F1, 23 = 5.02; p<0.05).
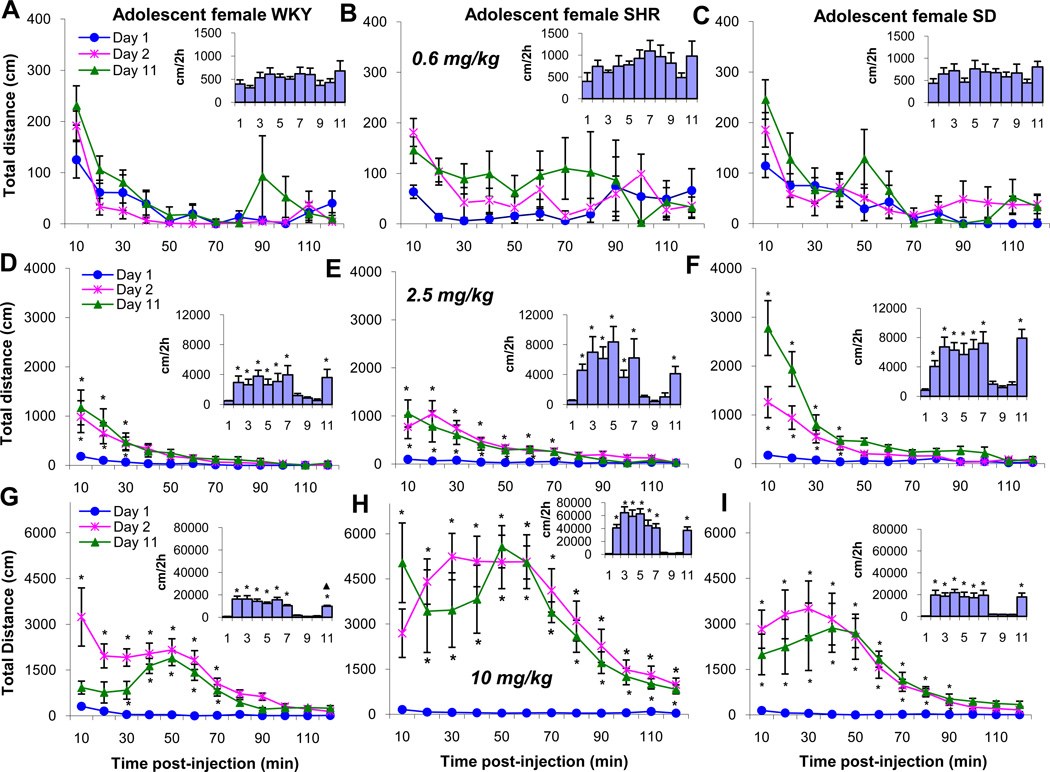
Figure 4 summarizes the same experimental protocol as figure 3, except adolescent female WKY, SHR, and SD rats were used. In the WKY female rats, a 0.6 mg/kg MPD dose did not modulate the TDT (Fig. 4A). The 2.5 mg/kg MPD dose significantly (F1, 23 = 3.98; F1, 23 = 4.13; F1, 23 =3.88; p<0.05) elevated the TDT on ED2 to ED7 and on ED11 for about 30 mins post injection (Fig. 4D) compared to saline baseline (ED1). Administration of 10 mg/kg MPD to female WKY rats on ED2 increased significantly (F1, 23 = 3.84; p<0.05) the TDT immediately post-injection for about 70 mins compared to ED1, while the same dose given on ED11 exhibited a delayed effect. The significant (F1,23 =5.13; p<0.05) increase in TDT started 30 mins post-injection and the increase in TDT lasted for only 50 mins post-injection (Fig. 4G). The amount of TDT was significantly (F1,23 =3.74;▲p<0.05) less than that observed on ED2, i.e., tolerance was obtained for this dose.
Figure 4.
Summary of the same experimental protocol as Figure 3 show for the female WKY, SHR, and SD rat groups.
The 0.6 mg/kg MPD dose given to adolescent female SHR group did not modulate the TDT (Fig. 4B) and exhibited a similar pattern with female WKY rats. However, the 2.5 and 10 mg/kg doses produced significantly (F10, 129 = 33.17; F10, 129 = 31.25; p<0.05) different effects on the TDT when WKY and SD rats were compared respectively (Fig. 4F and I). The duration of the 2.5 mg/kg MPD effect on TDT was longer i.e. 70 mins. compared to 30 to 40 mins. in the WKY and SD rats, respectively. In the adolescent female SHR group, the 10 mg/kg MPD dose significantly (F1, 23 = 3.95; F1, 23 = 4.18; p<0.05) increased the TDT for 120 mins post-injection on ED2 and ED11 (Fig. 4I).
The 0.6 mg/kg MPD dose given to adolescent female SD rats did not exert any effect on locomotion (Fig. 4C), while the 2.5 mg/kg MPD dose significantly (F1, 23 = 3.84; p<0.05) increased the TDT for about 40 mins. (Fig 4F). Similar increases in locomotion were observed on all the experimental days. The 10 mg/kg MPD dose elicited significantly (F1,23 = 4.16; p<0.05) robust increases in activity for about 90 mins. on all the experimental days (Fig. 4I). Comparing the duration effects of 10mg/kg MPD between the three rat strain show that the female SHR group responded with the longest distance traveled followed by SD and WKY rats, respectively (Fig. 4H).
3.4. Comparison within and between groups
Figure 5 compares the HA, VA, and NOSM obtained on ED2 to ED11 in adolescent male and female WKY, SHR, and SD rats. This comparison tested whether there were differences in the locomotor responses between the female and male rat groups following acute and chronic 0.6, 2.5, or 10 mg/kg MPD doses. This figure shows that adolescent female and male rats responded to MPD with significantly (F10, 23 = 33.33; F10, 23 = 29.92; p<0.05) different intensities of increased locomotion. When the HA for 2h post-injection on ED2 was compared to ED11, male and female WKY and SD rats showed similar levels of HA, while the female SHR group showed significantly (F10, 23 = 31.08; p<0.05) higher activity after the 0.6 mg/kg MPD dose compared to male SHR group, as well as other rat groups (Fig. 5A). Following 2.5 mg/kg MPD administration, the HA of male and female WKY rats exhibited similar activity while the female SHR and SD rats exhibited significantly (F10, 23 =31.17; F10, 23 = 33.47; p<0.05) higher activity than the male rats (Fig. 5D). In addition, the female SD rats exhibited the most significantly (F10, 23 = 34.05; p<0.05) robust increase in HA following a dose of 2.5 mg/kg MPD compared with the other groups, with the exception that the NOSM of all groups exhibited a similar intensity (Fig. 5D, E, and F). After 10 mg/kg MPD administration, the male WKY, SHR, and SD rats and female WKY rats exhibited a similar robust increase in locomotor activity on both ED2 and ED11. However, female SHR and SD rats exhibited significantly (F10, 23 = 33.02; F10, 23 = 35.03; p<0.05) higher HA when compared to the WKY group (Fig. 5G). Similar observations were obtained for the VA (Fig. 5H). The NOSM was similar for both sexes among WKY, SHR, and SD rats on ED2 and ED11 (Fig. 5I).
Figure 5.
Horizontal activity, vertical activity, and stereotypic movement of male and female WKY, SHR, and SD rat groups in ED 11 (the last day of MPD administration) compared to that obtained in ED 2 (the first MPD administration). Label * indicates significant (p<0.05) difference between the compared groups using the post-hoc analysis with Fisher’s LSD test.
4. Discussion
It is suggested that individuals both with and without ADHD misuse stimulant medication (Wilens et al., 2008a). A meta analysis of 21 studies representing 113,401 subjects showed that 5 to 9% of grade school and high school age children and 5 to 35% of college age individuals used stimulants (Wilens et al., 2003). Moreover, today on school campuses around the world, “students are striking deals to buy and sell prescription drugs such as Adderall and Ritalin (MPD) to improve their academic performance (Greely et al., 2008)”. An article in our local newspaper (Houston Chronicle, October, 2009) read, “Let healthy people take Ritalin to boost their brains with pills, like those prescribed for ADHD kids”. A Nature Commentary (Greely et al., 2008) suggested that “we should welcome new methods of improving our brain function and doing it with pills is no more morally objectionable than eating right or getting a good night sleep”. Safe and effective cognitive enhancers will benefit both the individual and society. But it would also be foolish to ignore problems that such use of cognitive enhance drug could create or exacerbate (Greely et al., 2008). The objective of this study is to investigate the effects of chronic daily MPD treatment in male and female adolescent rats of three different genetic strains.
It was reported (Lee et al., 2009; Yang et al., 2007) that six single daily MPD injections can be considered as a chronic treatment for rats. Life expectancy of the average Western population is about 78 years and the life expectancy of a rat is about two years. Six days in a rat’s life accounts for about 0.86% of a rat’s life expectancy, which is equal to about 33.4 weeks in a human’s life, i.e. more than 33 weeks of drug treatment can be considered as chronic treatment (Lee et al., 2009; Yang et al., 2007)
In the present study, three doses of MPD was used. Reviewing the available publications reveals that there is no universal recognized MPD dosage guidelines for clinical treatment or what is the required blood level to achieve to obtain optimum treatment for ADHD patients (Eichlseder, 1985; Gadow, 1997, Pelham et al., 2001, White and Yadao, 2000). In a study of 289 patients treated with MPD White and Yadao, (2000) reported that the range of MPD doses ingested by these patients was from 0.006 to 29.3 mg/kg while other studies reported that the majority of patients were treated with 1.0 to 3.0 mg/kg MPD (Eichlseder, 1985; El-Zeim et al., 2005; Kollins et al., 2001; Pelham et al., 2001; Solanto, 1988; Wilens, 2003). The dose below 5.0 mg/kg i.p. in rodents is considered as a low dose and is comparable to doses in clinical use (Rush and Baker, 2001). MPD doses between 0.5 to 3.5 mg/kg i.p. were reported to promote peak plasma concentration within the typical clinical range (Rush and Baker, 2001) and considered as low MPD dose. The range of 5.0 to 10.0 mg/kg i.p. MPD considered moderate to high dose, respectively (Rush and Baker, 2001; Kollins et al., 2001; Stewart and Badiani, 1993; Solanto, 1998; Santosh and Taylor, 2000; Yang et al., 2007,2010). It is known that drug effects in rodents require higher doses on a milligram per kilogram body weight compared to humans since rodent exhibit a more rapid metabolism (Gately et al., 1999). It is believed that the rats require higher doses of MPD than humans to reach therapeutic levels because of the differences in pharmacokinetics, drug metabolism, excretion, and gastric absorption between humans and rodents. Therefore, the doses of 0.6 and 2.5mg/kg MPD in this study can be considered clinically relevant.
The main findings of this study are 1) each control group (WKY, SHR, and SD) of male adolescent rats and each control group of female adolescent rats exhibited similar activity patterns and levels throughout all 11 experimental days following saline administration; 2) the females of the SHR and SD groups traveled a significantly higher distance than their male counterparts; 3) the female SD rats exhibited a higher VA than the male SD rats; 4) the male WKY and SHR rats exhibited a higher NOSM than their female counterparts. The observed sex differences in the activity of control animals were dependent on both the strain and locomotor indices, but none of the factors alone could explain the observed significant sex differences. For example, in the case of SHR group, HA and VA were not significantly different for males and females, while TDT and NOSM were significantly different. For WKY rats, TDT and NOSM were significantly different, while for the SD rats, VA and TDT were significantly different. This observation suggests that each locomotor activity index is regulated by different neuronal circuits and indicates that MPD affects each circuit differently thus several locomotor indices need to be studied simultaneously.
Both sexes exhibit dose response effects of MPD treatment, however each strain and sex exhibit different levels of change. SHR and SD females exhibited significantly higher activity than their male counterparts for MPD doses of 2.5 and 10 mg/kg, while the WKY females exhibited significantly lower activity than the WKY males for the dose of 10 mg/kg. The MPD dose of 0.6 mg/kg did not produce a significant increase in activity (excepting for the SHR group, Fig. 2C), while higher MPD doses of 2.5 and 10 mg/kg produced a robust activity increase for all animals. In studies examining the expression of genes involved in dopamine (DA) signaling and metabolism and the quantitative real time RT-PCR procedure using SHR and WKY rats–following MPD administration, significant differences were reported between these two animal strains in the striatal DA transporter (DAT) density strains (Roessner et al., 2010). Similar observations studying the density of DAT was reported by others (Simchon et al., 2010) which may explain the differences in the MPD effects on their locomotor behavior observed in this study.
The SHR and SD strain rats exhibited no significant differences in response to MPD injection on ED11 compared to MPD injection on ED2, indicating that no behavioral sensitization or tolerance was induced by chronic MPD treatment, independent of MPD dose and sex similar observation was reported by Valvassori et al., (2007) using Wistar rats. A similar experimental protocol, designed to study the effects of the chronic MPD treatment in adult rats, indicated that behavioral sensitization was induced by MPD doses of 2.5 mg/kg and 10 mg/kg (Gaytan et al., 1997, 2000; Yang et al., 2003, 2006). This suggests that, in addition to MPD dose, age also influences whether behavioral sensitization or tolerance after chronic administration of MPD will be expressed (Dafny and Yang, 2006). The higher dose of 10 mg/kg MPD produced tolerance in both adolescent male and female WKY rats. It is possible that this dose caused stereotypic behavior and, thus, interpreted as tolerance for the HA and TDT.; but our stereotypic activity did not increase which suggests that this attenuation of activity can express tolerance. The amount of TDT was significantly smaller on ED11 than that obtained on ED2. For adolescent male WKY rats, an MPD dose of 10 mg/kg also produced a significant decrease in sequential TDT on ED11 in comparison with sequential TDT on ED2. This effect was not observed for adolescent female WKY rats.
Sex differences in the locomotor response to MPD treatment were not found in two other recent experiments using adolescent rats. Those experiments utilized lower MPD doses or different rat strains (Ferguson and Cada, 2003; Wooters et al., 2006). Using male and female SHR, WKY, and SD adolescent rats, the acute response to MPD was studied by recording open field activity 1 hr before (baseline) and 1 hr after an i.p. injection of 2 mg/kg MPD, given on postnatal days P45 and P88 (Ferguson and Cada, 2003). Significant differences were found only in the post drug activity between the same-sex strains, but not between the males and females of the same strain. In another experiment (Wooters et al., 2006), the locomotor activity of periadolescent (P25) and adult (P60) male and female SD rats, first classified as high responders (HR) or low responders (LR) based on the level of their activity in an inescapable novel environment, was subsequently assessed after ten daily injections of MPD (3 or 10 mg/kg, s.c.) or saline. After fifteen days, the rats were challenged with saline or MPD (10 mg/kg) over two days. The adult females showed greater MPD-induced hyperactivity than adult males during the repeated MPD treatment phase, as we have observed previously (Gaytan et al., 1997, 2000; Yang et al., 2003, 2006). No significant difference in MPD-induced hyperactivity was found between HR and LR rats of either age and sex.
The vast majority of experimental studies in rats related to ADHD were carried out using male rats (Askenasy et al., 2007), despite the remarkable gender difference in ADHD expression in humans. For example, a study based on positron emission tomography found that the global Cerebral glucose metabolism (CMRglu) in ADHD girls (N=5) was 15% lower than in normal girls (N=6) while the CMRglu in ADHD boys did not differ from that of normal boys (Ernst et al., 1994). ADHD is more often diagnosed in males than in females (Anderson et al., 1987), but the females exhibit more severe symptoms than males (Biederman et al., 1994), perhaps due to their “higher genetic loading for the ADHD” (Pauls, 1991). In one of the few animal studies on sex differences in an ADHD model (Anderson et al., 2000), it was suggested that these differences might be explained by differences in dopamine receptor density. Between 24–40 days (the onset of puberty for rats) the striatal male D2 receptor density increases 144±26%, while female D2 receptor increases only 31±7%.
The SHR rat is considered one of the best rodent models for studying the ADHD disorder (Barron et al., 2009; McCarty and Kopin, 1979; Pires et al., 2009; Roessner et al., 2010; Sagvolden, 2000; Thanos et al., 2010). WKY rats, from which the SHR rats were bred, were also used in this study. Usually in ADHD studies, the WKY rats are used as controls for the SHR rats (Roessner et al., 2010; Sagvolden et al., 1993; Sagvolden and Sergeant, 1998; Simchon et al., 2010; Thanos et al., 2010), therefore, this rat strain was used in this study. The SD strain was also included in this study because this strain was often used in psychostimulant studies (Askenasy et al, 2007; Dafny and Yang, 2006), and because in our previous experiments, we studied the effects of multiple MPD doses on locomotor activity of adolescent and adult SD rats (Algahim et al., 2009; Gaytan et al., 1996, 1997, 2000; Lee et al., 2008, 2009; Sripada et al., 1998; Yang et al., 2001, 2003, 2006, 2007, 2010).
Sex differences in the acute and chronic effects of MPD treatment were found mostly in SHR, then in SD rats, and none in WKY adolescent rats. On the other hand, WKY adolescent rats were the only ones that exhibited tolerance to MPD chronic administration. The results obtained in our open-field assay experiment are at odds with results obtained in a study of temporal processing (Ferguson et al., 2007) in which the same rat strains were used. Ferguson et al., (2007) observed that the baseline behavior differed among rat strains while the sensitivity to administered stimulant drugs (MPD and d-amphetamine) did not differ among strains. They concluded that the SHR rat was not a suitable model for ADHD. Strain differences in addition to sex differences were observed in this study for MPD doses in the same range, which suggests that movements in open field assay experiments are more sensitive than temporal processing in studying the MPD effects among rat strains.
In conclusion, the above described experiments demonstrate that MPD treatment of WKY, SHR, and SD adolescent male and female rats elicits significantly different locomotor effects as measured by the open field assay. These differences were revealed by a dose response protocol, recording of different locomotor indices such as HA, VA, TDT, and NOSM, statistical analysis of 10 min sequential (temporal) activity for 2 hours showing the latency, duration and the MPD peak effect as well as the sum of activity under a 2 hour of the temporal graph. Sex differences in response to MPD were observed in adolescent rats prior to puberty; therefore, it is possible to assume that these differences to MPD treatment are not due to the reproductive hormones. Since MPD treatment in adolescence critically influences the effects of MPD treatment in adulthood (Barron et al., 2009; Yang et al., 2010), further studies are necessary in order to evaluate the influence of MPD on adult rat strains of both sexes, treated previously with MPD in their adolescence.
Research Highlights.
Sex plays major role in animal’s response to methylphenidate
Genotype plays major role in animal’s response to methylphenidate
Each sex exhibits different dose response characteristics
Each genotype exhibits different dose response characteristics
The highest dose of MPD elicits similar effects in all the groups
Acknowledgments
The authors wish to thank Mallinckrodt Inc. for their gift of MPD and to Drs. A.C. Swann, J. E. Lever and A. Levine and to Ms. R. Cornell and D. Wood for manuscript preparation. This research was supported in part by the National Research Service Award from the National Institute of Health #F31-DA14441 and NIH R01 DA 027222.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algahim MF, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Prolonged methylphenidate treatment alters the behavioral diurnal activity pattern of adult male Sprague-Dawley rats. Pharmacol Biochem Behav. 2009;92:93–99. doi: 10.1016/j.pbb.2008.10.021. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr. Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev. 2000;24:137–141. doi: 10.1016/s0149-7634(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Williams S, McGee R, Silva PA. DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psychiatry. 1987;44:69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- Askenasy EP, Taber KH, Yang PB, Dafny N. Methylphenidate (Ritalin): behavioral studies in the rat. Int J Neurosci. 2007;117:757–794. doi: 10.1080/00207450600910176. [DOI] [PubMed] [Google Scholar]
- Barron E, Yang PB, Swan AC, Dafny N. Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin) Inter. J. of Neurosc. 2009;119:40–58. doi: 10.1080/00207450802330546. [DOI] [PubMed] [Google Scholar]
- Beckman DA, Schneider M, Youreneff M, Tse FL. Juvenile toxicity assessment of d,l-methylphenidate in rats. Birth Defects Res B Dev Reprod Toxicol. 2008;83:48–67. doi: 10.1002/bdrb.20143. [DOI] [PubMed] [Google Scholar]
- Benet LZ, Kroetz DL, Sheiner LB. Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, and Elimination. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. the Pharmacological Basis of Therapeutics. 9th ed. N.Y.: McGraw Hill; 1996. p. 11. [Google Scholar]
- Berger DF, Sagvolden T. Sex differences in operant discrimination behavior in an animal model of attention-deficit hyperactivity disorder. Behav Brain Res. 1998;94:73–82. doi: 10.1016/s0166-4328(97)00171-x. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Spencer T, Wilens T, Mick E, Lapey KA. Gender differences in a sample of adults with attention deficit hyperactivity disorder. Psychiatry Res. 1994;53:13–29. doi: 10.1016/0165-1781(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Glatt SJ, Jackson D. Subsensitivity to dopaminergic drugs in periadolescent rats: a behavioral and neurochemical analysis. Brain Res Dev Brain Res. 1998;111:25–33. doi: 10.1016/s0165-3806(98)00116-3. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulnerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–661. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Napierata L, Kussmaul L, Leussis M, Andersen SL. Juvenile methylphenidate exposure and factors that influence incentive processing. Dev Neurosci. 2009;31(1–2):95–106. doi: 10.1159/000207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- Campbell JO, Wood RD, Spear LP. Cocaine and morphine-induced place conditioning in adolescent and adult rats. Physiol Behav. 2000;68:487–493. doi: 10.1016/s0031-9384(99)00225-5. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clin Proc. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S. Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev Brain Res. 2004;153:175–187. doi: 10.1016/j.devbrainres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Cornforth C, Sonuga-Barke E, Coghill D. Stimulant drug effects on attention deficit/hyperactivity disorder: a review of the effects of age and sex of patients. Curr Pharm Des. 2010;16:2424–2433. doi: 10.2174/138161210791959827. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: a review of its locomotor effects. Brain Res Bull. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Eichlseder W. Ten years of experience with 1,000 hyperactive children in a private practice. Pediatrics. 1985;76:176–184. [PubMed] [Google Scholar]
- El-Zein RA, Abdel-Rahman SZ, Hay MJ, Lopez MS, Bondy ML, Morris DL, Legator MS. Cytogenetic effects in children treated with methylphenidate. Cancer Lett. 2005;230:284–291. doi: 10.1016/j.canlet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ. Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Wilens TE. Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry. 2007;11:15–22. [PubMed] [Google Scholar]
- Ferguson SA, Cada AM. A longitudinal study of short- and long-term activity levels in male and female spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rats. Behav Neurosci. 2003;117:271–282. doi: 10.1037/0735-7044.117.2.271. [DOI] [PubMed] [Google Scholar]
- Ferguson SA, Paule MG, Cada A, Fogle CM, Gray EP, Berry KJ. Baseline behavior, but not sensitivity to stimulant drugs, differs among spontaneously hypertensive, Wistar-Kyoto, and Sprague-Dawley rat strains. Neurotoxicol Teratol. 2007;29:547–561. doi: 10.1016/j.ntt.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Gadow KD. An overview of three decades of research in pediatric psychopharmacoepidemiology. J Child Adolesc Psychopharmacol. 1997;7:219–236. doi: 10.1089/cap.1997.7.219. Review. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Volkow ND, Gifford AN, Fowler JS, Dewey SL, Ding YS, Logan J. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl) 1999;146:93–100. doi: 10.1007/s002130051093. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Yang P, Swann A, Dafny N. Diurnal differences in sensitization to methylphenidate. Brain Res. 2000;864:24–39. doi: 10.1016/s0006-8993(00)02117-x. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur J Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- Goldman LS, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Council on Scientific Affairs, American Medical Association. JAMA. 1998;279:1100–1107. doi: 10.1001/jama.279.14.1100. [DOI] [PubMed] [Google Scholar]
- Greely H, Sahakian B, Harris J, Kessler RC, Gazzaniga M, Campbell P, Farah MJ. Towards responsible use of cognitive-enhancing drugs by the healthy. Nature. 2008;456:702–705. doi: 10.1038/456702a. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. Review. [DOI] [PubMed] [Google Scholar]
- Landgren M, Kjellman B, Gillberg C. Deficits in attention, motor control and perception (DAMP): a simplified school entry examination. Acta Paediatr. 2000;89:302–309. [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Lee MJ, Swann AC, Dafny N. Methylphenidate sensitization is prevented by prefrontal cortex lesion. Brain Res. Bull. 2008;76:131–140. doi: 10.1016/j.brainresbull.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Yang PB, Wilcox VT, Burau KD, Swann AC, Dafny N. Does repetitive Ritalin injection produce long-term effects on SD female adolescent rats? Neuropharmacology. 2009;57:201–207. doi: 10.1016/j.neuropharm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- LeFever GB, Dawson KV, Morrow AL. The extent of drug therapy for attention deficit-hyperactivity disorder among children in public schools. Am J Public Health. 1999;89:1359–1364. doi: 10.2105/ajph.89.9.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Kleber DH. Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harv Rev Psychiatry. 1995;2:246–258. doi: 10.3109/10673229509017144. [DOI] [PubMed] [Google Scholar]
- McCarty R, Kopin IJ. Patterns of behavioral development in spontaneously hypertensive rats and Wistar-Kyoto normotensive controls. Dev Psychobiol. 1979;12:239–243. doi: 10.1002/dev.420120307. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Collins RL, Karper PE, Watson JB, Crawford CA. Effects of repeated methylphenidate treatment in the young rat: sensitization of both locomotor activity and stereotyped sniffing. Exp Clin Psychopharmacol. 1999;7:208–218. doi: 10.1037//1064-1297.7.3.208. [DOI] [PubMed] [Google Scholar]
- Moll GH, Hause S, Rüther E, Rothenberger A, Huether G. Early methylphenidate administration to young rats causes a persistent reduction in the density of striatal dopamine transporters. J Child Adolesc Psychopharmacol. 2001;11:15–24. doi: 10.1089/104454601750143366. [DOI] [PubMed] [Google Scholar]
- Pauls DL. Genetic factors in the expression of attention-deficit hyperactivity disorder. J. Child Adolesc Psychopharmacol. 1991;1:353–360. doi: 10.1089/cap.1991.1.353. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Burrows-Maclean L, Williams A, Fabiano GA, Morrisey SM, Chronis AM, Forehand GL, Nguyen CA, Hoffman MT, Lock TM, Fielbelkorn K, Coles EK, Panahon CJ, Steiner RL, Meichenbaum DL, Onyango AN, Morse GD. Once-a-day Concerta methylphenidate versus three-times-daily methylphenidate in laboratory and natural settings. Pediatrics. 2001;107:1–15. doi: 10.1542/peds.107.6.e105. [DOI] [PubMed] [Google Scholar]
- Pires VA, Pamplona FA, Pandolfo P, Fernandes D, Prediger RD, Takahashi RN. Adenosine receptor antagonists improve short-term object-recognition ability of spontaneously hypertensive rats: a rodent model of attention-deficit hyperactivity disorder. Behav Pharmacol. 2009;20:134–145. doi: 10.1097/FBP.0b013e32832a80bf. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Adolescent and adult rats respond differently to nicotine and alcohol: motor activity and body temperature. Int J Dev Neurosci. 2004;22:349–354. doi: 10.1016/j.ijdevneu.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl.) 1984;84:466–475. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- Roessner V, Sagvolden T, Dasbanerjee T, Middleton FA, Faraone SV, Walaas SI, Becker A, Rothenberger A, Bock N. Methylphenidate normalizes elevated dopamine transporter densities in an animal model of the attention-deficit/hyperactivity disorder combined type, but not to the same extent in one of the attention-deficit/hyperactivity disorder inattentive type. Neuroscience. 2010;167:1183–1191. doi: 10.1016/j.neuroscience.2010.02.073. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Raskin LA. Stereotyped behavior: effects of d-amphetamine and methylphenidate in the young rat. Pharmacol Biochem Behav. 1997;58:1095–1102. doi: 10.1016/s0091-3057(97)00321-3. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Exp Clin Psychopharmacol. 2001;9:59–73. doi: 10.1037/1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Pettersen MB. Spontaneously hypertensive rat (SHR) as a putative animal model of childhood hyperkinesis: SHR behavior compared to four other rat strains. Physiol Behav. 1993;54:1047–1055. doi: 10.1016/0031-9384(93)90323-8. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Sergeant JA. Attention deficit/hyperactivity disorder--from brain dysfunctions to behaviour. Behav Brain Res. 1998;94:1–10. [PubMed] [Google Scholar]
- Sagvolden T. Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD) Neurosci Biobehav Rev. 2000;24:31–39. doi: 10.1016/s0149-7634(99)00058-5. [DOI] [PubMed] [Google Scholar]
- Santosh PJ, Taylor E. Stimulant drugs. Eur Child Adolesc Psychiatry. 2000;9 Suppl 1:I27–143. doi: 10.1007/s007870070017. Review. [DOI] [PubMed] [Google Scholar]
- Simchon Y, Weizman A, Rehavi M. The effect of chronic methylphenidate administration on presynaptic dopaminergic parameters in a rat model for ADHD. Eur Neuropsychopharmacol. 2010 May 19; doi: 10.1016/j.euroneuro.2010.04.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Sripada S, Gaytan O, Al-rahim S, Swann A, Dafny N. Dose-related effects of MK-801 on acute and chronic methylphenidate administration. Brain Res. 1998;814:78–85. doi: 10.1016/s0006-8993(98)01035-x. [DOI] [PubMed] [Google Scholar]
- Stewart J, Badiani A. Tolerance and sensitization to the behavioral effects of drugs. Behav Pharmacol. 1993;4:289–312. [PubMed] [Google Scholar]
- Swanson JM, Sergeant JA, Taylor E, Sonuga-Barke EJ, Jensen PS, Cantwell DP. Attention-deficit hyperactivity disorder and hyperkinetic disorder. Lancet. 1998;351:429–433. [PubMed] [Google Scholar]
- Teo S, Stirling D, Thomas S, Hoberman A, Kiorpes A, Khetani V. A 90-day oral gavage toxicity study of D-methylphenidate and D,L-methylphenidate in Sprague-Dawley rats. Toxicology. 2002;179:183–196. doi: 10.1016/s0300-483x(02)00338-4. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Ivanov I, Robinson JK, Michaelides M, Wang GJ, Swanson JM, Newcorn JH, Volkow ND. Dissociation between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats in baseline performance and methylphenidate response on measures of attention, impulsivity and hyperactivity in a Visual Stimulus Position Discrimination Task. Pharmacol Biochem Behav. 2010;94:374–379. doi: 10.1016/j.pbb.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Reveron A, Dow-Edwards DL. Prenatal cocaine dampened behavioral responses to methylphenidate in male and female adolescent rats. Neurotoxicol Teratol. 2006;28:165–172. doi: 10.1016/j.ntt.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Valvassori SS, Frey BN, Martins MR, Réus GZ, Schimidtz F, Inácio CG, Kapczinski F, Quevedo J. Sensitization and cross-sensitization after chronic treatment with methylphenidate in adolescent Wistar rats. Behav Pharmacol. 2007;18:205–212. doi: 10.1097/FBP.0b013e328153daf5. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Izídio GS, Takahashi RN, Ramos A. Chronic methylphenidate treatment during adolescence increases anxiety-related behaviors and ethanol drinking in adult spontaneously hypertensive rats. Behav Pharmacol. 2008;19:21–27. doi: 10.1097/FBP.0b013e3282f3cfbe. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Ding YS. Imaging the effects of methylphenidate on brain dopamine: new model on its therapeutic actions for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1410–1415. doi: 10.1016/j.biopsych.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wagner AK, Kline AE, Ren D, Willard LA, Wenger MK, Zafonte RD, Dixon CE. Gender associations with chronic methylphenidate treatment and behavioral performance following experimental traumatic brain injury. Behav Brain Res. 2007;181:200–209. doi: 10.1016/j.bbr.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR, Yadao CM. Characterization of methylphenidate exposures reported to a regional poison control center. Arch Pediatr Adolesc Med. 2000;154:1199–1203. doi: 10.1001/archpedi.154.12.1199. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Faraone SV, Biederman J, Gunawardene S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics. 2003;111:179–185. doi: 10.1542/peds.111.1.179. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008a;47:21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adamson J, Monuteaux MC, Faraone SV, Schillinger M, Westerberg D, Biederman J. Effect of prior stimulant treatment for attention-deficit/hyperactivity disorder on subsequent risk for cigarette smoking and alcohol and drug use disorders in adolescents. Arch Pediatr Adolesc Med. 2008b;162:916–921. doi: 10.1001/archpedi.162.10.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl.) 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- Yang P, Singhal N, Modi G, Swann A, Dafny N. Effects of lithium chloride on induction and expression of methylphenidate sensitization. Eur. J. Pharmacol. 2001;426:65–72. doi: 10.1016/s0014-2999(01)01213-4. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Acute and chronic methylphenidate dose-response assessment on three adolescent male rat strains. Brain Res Bull. 2006;71:301–310. doi: 10.1016/j.brainresbull.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Methylphenidate treated at the test cage--dose-dependent sensitization or tolerance depend on the behavioral assay used. Crit Rev Neurobiol. 2007;19:59–77. doi: 10.1615/critrevneurobiol.v19.i1.20. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Psychostimulants given in adolescents modulate their effects in adulthood using the open field and the wheel-running assays. Brain Res. Bull. 2010;82:208–217. doi: 10.1016/j.brainresbull.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, dosReis S, Gardner JF, Boles M, Lynch F. Trends in the prescribing of psychotropic medications to preschoolers. JAMA. 2000;283:1025–1030. doi: 10.1001/jama.283.8.1025. [DOI] [PubMed] [Google Scholar]