Abstract
Purpose
To examine the mortality risk associated with diabetes in the Mexico City Diabetes Study (MCDS) and the San Antonio Heart Study (SAHS).
Methods
Prospective cohorts conducted 1990-2007 in MCDS and 1979-2000 in SAHS. Mortality risk was examined using Cox proportional hazard models in 1,402 non-Hispanic whites (NHW), 1,907 U.S.-born Mexican Americans (MA), 444 Mexican-born MA, 2,281 Mexico City residents (MCR) between the ages of 35 and 64.
Results
Age- and sex-adjusted mortality HRs comparing U.S.-born MA, Mexican-born MA and MCR to NHW were 1.09 (95% CI: 0.86, 1.37), 1.23 (95% CI: 0.86, 1.76) and 0.97 (95% CI: 0.77, 1.23), respectively, in non-diabetic individuals; in contrast, mortality risk varied in diabetic individuals with respective HRs of 1.77 (95% CI: 1.20, 2.61), 1.08 (95% CI: 0.59, 1.97) and 2.27 (95% CI: 1.53, 3.35) (interaction p-value=0.0003). Excluding Mexican-born MA and non-diabetic individuals, controlling for medication use, insulin use, fasting glucose levels and duration of diabetes explained a significant proportion of the mortality differential (HRs relative to NHW were 1.31 (95% CI: 0.87, 1.98) in U.S.-born MA and 1.38 (95% CI: 0.89, 2.12) in MCR).
Conclusions
This study provides evidence that diabetes is more lethal in U.S.-born MA and MCR than in NHW.
Diabetes, the number one cause of death in Mexico, poses a public health risk particularly for Mexicans and Mexican Americans(1). In the United States the incidence of diabetes is two-to three-fold higher in Mexican Americans (MA) than among non-Hispanic whites (NHW), and evidence from NHANES III and the San Antonio Heart Study (SAHS) suggests that the prevalence of diabetes is rapidly increasing(2-7). Moreover, among individuals with diabetes the mortality risk is higher in MA relative to NHW(8).
Previous comparisons of Mexico City (MC) residents and San Antonio MA indicate that the prevalence of diabetes is lower in MC residents than in San Antonio MA(9) which may be a result of a higher case fatality rate or a lower incidence of diabetes(10). Moreover, examining the mortality risks associated with diabetes helps determine the public health burden of diabetes in these populations. Factors potentially associated with mortality include biologic differences in diabetes severity triggered by genetic or environmental factors as well as differences in health-care access, treatment practices and on-going prevention efforts. Unfortunately, available markers of biologic differences in disease severity including medication use, insulin use, fasting glucose levels and duration of clinically recognized diabetes are intrinsically tied to health-care use/treatment. Increased access to care and disease awareness likely result in a shorter time to recognition and treatment of disease, a higher prevalence of recognized diabetes, a lower prevalence of unrecognized diabetes and improved outcomes. Therefore, in the Mexico City Diabetes Study (MCDS) and the SAHS we examined the mortality risk associated with prevalent diabetes as well as factors associated with mortality in individuals with diabetes. Our hypothesis was that the mortality risk associated with diabetes would be higher in MC residents and in MA than in NHW.
MATERIALS AND METHODS
The Mexico City Diabetes Study (MCDS)
The MCDS is a population-based cohort of 2282 men and women first examined between 1990 and 1992(11). Participants were randomly selected from six low-income census tracts in Mexico City and were 35-64 years of age at entry into the study(9,11,12). A complete enumeration of the colonias was carried out, and 3326 study-eligible individuals were identified. Of these, 2282 participated for a response rate of 68.5%(9,11,12). The Institutional Review Boards of the University of Texas Health Science Center at San Antonio (UTHSCSA) and the Centro de Estudios en Diabetes approved the study. All participants gave informed consent.
The San Antonio Heart Study (SAHS)
The SAHS cohort consists of 5158 participants, recruited in two phases: phase one, 1979 to 1982; and phase two, 1984 to 1988(12-15). Households were randomly sampled in three types of San Antonio neighborhoods: inner city, essentially 100% MA neighborhoods; middle-income neighborhoods; and high-income suburbs. Men and non-pregnant women between the ages of 25 and 64 years residing in the selected households were eligible and invited to participate. The combined response rate for both phases of the study was 65.3%. The Institutional Review Board of the UTHSCSA approved the study. All participants gave informed consent.
Baseline SAHS and MCDS Examinations
The MCDS and SAHS cohort examinations were standardized, followed similar protocols with core components being identical and used the same laboratory for biomarker measurement(14-16). A history of CVD was defined as self-reported physician-diagnosed heart attack or stroke. Hypertension was defined as systolic/diastolic≥140/90 mm Hg or reported current treatment with anti-hypertensive medication. Diabetes was defined as fasting/2-hour postload plasma glucose ≥126/200 mg/dL, or reported physician-diagnosed diabetes and current medication use to treat diabetes(17). Undiagnosed diabetes was diabetes first identified at the clinical examination. Fasting glucose and insulin levels were used to calculate Homeostasis Model Assessment of beta cell function (HOMA-β-cell) and Homeostasis Model of Insulin Resistance (HOMA-IR) as described by Matthews et al(18).
Study Population, Follow-up and Events
The current analyses included MCDS (n=2282) and SAHS (n=3769) participants between the ages of 35-64 years at study enrollment. In the MCDS cohort 1 individual was excluded due to missing information and 93 people had incomplete ascertainment through January 1st 2007 (ascertainment rate=95.9%). In the SAHS cohort 16 individuals were excluded due to missing information and 22 people had incomplete ascertainment through January 1st 2000 (ascertainment rate=99.4%).
Statistical Analyses
Prospective analyses were carried out using country, ethnicity and birthplace as a person’s exposure status and all-cause mortality as the outcome. For initial analyses individuals were stratified into four country/ethnicity categories; NHW from San Antonio (NHW), U.S.-born MA from San Antonio, foreign-born MA from San Antonio (i.e., largely Mexican born; Mexican-born MA) and MC residents. Foreign-born MA from San Antonio were excluded due to their small sample size from analyses limited to individuals with diabetes.
Age-, and sex-adjusted means and proportions were determined for participant baseline characteristics stratified by country/ethnic group. Cox proportional hazard models were used to calculate age- and sex-adjusted hazard ratios (HRs) for all-cause mortality in relation to country/ethnic group. The association between country/ethnic group and all-cause mortality was then assessed after stratifying by diabetes status using appropriate interaction terms. Additional covariates adjusted for in subsequent models included hypertension, smoking status, CVD history, total cholesterol and HDL-cholesterol.
Country/ethnic stratum-specific age- and sex- adjusted mortality HRs obtained from Cox proportional hazard models were determined for each cardiovascular risk factor using the appropriate interaction terms to determine whether each risk factor conveyed the same risk across the three populations. Additional covariates adjusted for in subsequent models included hypertension, smoking status, CVD history, total cholesterol and HDL-cholesterol. Finally, after excluding foreign-born MAs from San Antonio due to their small sample size, we limited the population to those with diabetes and calculated HRs for all-cause mortality among individuals with diabetes to examine what covariates associated with diabetes severity and treatment may account for the different mortality risk associated with diabetes across the three populations.
Throughout all analyses, a p-value of 0.05 for pair-wise comparisons between country/ethnic strata was used as a nominal value for statistically significant interactions. Moreover, the assumption of proportional hazards was evaluated for country/ethnic group as well as diabetes status.
RESULTS
During an average follow-up of 15.1 years MCDS participants experienced a total of 270 deaths prior to January 1st 2007. During an average follow-up of 15.4 years SAHS participants experienced a total of 514 deaths prior to January 1st 2000.
At baseline, after adjusting for age and sex the prevalence of diabetes was lower in NHW [5.7% (95% CI: 4.7, 6.9)] than in U.S.-born MA [15.0% (95% CI: 13.4, 16.7)], Mexican-born MA [14.4% (95% CI: 11.4, 18.0)] or MC residents [12.8% (95% CI: 11.4, 14.3)] (Table 1). The prevalence of undiagnosed diabetes was similar in NHW [3.8% (95% CI: 3.0, 4.9)] and MC residents [4.8% (95% CI: 4.0, 5.8)], but higher in U.S.-born MA [7.0% (95% CI: 6.0, 8.3)] and Mexican-born MA [8.3% (95% CI: 6.1, 11.2)]. Prevalence of smoking was higher in MC residents than in NHW or U.S.-born MA. Prevalence of hypertension was higher in U.S.-born MA than in NHW, but similar in MC residents and NHW. Use of blood pressure lowering medication was lower in MC residents than in NHW or U.S.-born MA. Additionally, HDL levels were much lower and triglycerides much higher in MC residents than in any of the three San Antonio populations. Use of cholesterol lowering medication was very low across all four populations (i.e., less than 1.0%).
Table 1.
Baseline Characteristics (means or proportions and 95% confidence intervals) of the Study Population Stratified by Country, Birthplace and Ethnicity
| NHW (n=1,402) |
U.S.-born MA (n=1,907) |
Mexican-born MA (n=444) |
Mexico City (n=2,281) |
|
|---|---|---|---|---|
| Unadjusted | ||||
|
| ||||
| Age (years) | 49.4 | 48.3 | 47.5 | 47.3 |
| Male [% (n)] | 44.3 (621) | 43.1 (821) | 38.1 (169) | 41.2 (940) |
| Deceased [% (n)] | 12.6 (176) | 14.8 (283) | 12.4 (55) | 11.8 (270) |
|
| ||||
| Adjusted for Age and Sex | ||||
|
| ||||
| Diabetes (%) | 5.7 (4.7, 6.9) | 15.0 (13.4, 16.7) | 14.4 (11.4, 18.0) | 12.8 (11.4, 14.3) |
| Undiagnosed (%) | 3.8 (3.0, 4,9) | 7.0 (6.0, 8.3) | 8.3 (6.1, 11.2) | 4.8 (4.0, 5.8) |
| Using any medication (%) | 1.3 (0.9, 1.9) | 5.2 (4.3, 6.2) | 4.0 (2.6, 6.1) | 6.0 (5.1, 7.1) |
| Insulin Use (%) | 0.7 (0.4, 1.3) | 2.1 (1.6, 2.9) | 0.6 (0.2, 1.8) | 0.5 (0.3, 0.9) |
| Hypertension (%) | 16.2 (14.3, 18.2) | 19.1 (17.3, 21.0) | 15.9 (12.8, 19.7) | 16.5 (15.0, 18.2) |
| Blood Pressure Medication (%) | 11.4 (9.8, 13.2) | 9.8 (8.6, 11.2) | 8.3 (6.1, 11.1) | 5.8 (4.9, 6.9) |
| Current smoker (%) | 26.9 (24.6, 29.3) | 27.1 (25.1, 29.2) | 27.3 (23.3, 31.7) | 31.0 (29.1, 33.0) |
| Overweight: BMI 25-30 kg/m2 (%) | 35.0 (32.5, 37.5) | 41.1 (38.9, 43.4) | 43.3 (38.7, 48.0) | 48.8 (46.8, 50.9) |
| Obese: BMI ≥ 30 kg/m2 (%) | 18.3 (16.3, 20.4) | 32.7 (30.6, 34.8) | 32.3 (28.1, 36.8) | 28.6 (26.8, 30.5) |
| Lipid lowering medication (%) | 0.8 (0.5, 1.4) | 0.8 (0.5, 1.3) | 0.6 (0.2, 1.7) | 0.2 (0.1, 0.4) |
| CVD history (%) | 3.9 (3.1, 5.0) | 4.2 (3.4, 5.2) | 2.6 (1.5, 4.4) | 2.1 (1.6, 2.8) |
| Education (years) | 14.1 (13.9, 14.3) | 10.5 (10.4, 10.7) | 8.2 (7.8, 8.5) | 5.2 (5.1, 5.4) |
| BMI (kg/m2) | 26.3 (26.0, 26.5) | 28.7 (28.5, 28.9) | 28.6 (28.2, 29.1) | 28.1 (27.9, 28.3) |
| Fasting glucose (mmol/L) | 5.10 (4.98, 5.22) | 5.74 (5.64, 5.84) | 5.56 (5.35, 5.77) | 5.50 (5.41, 5.59) |
| 2-hour glucose (mmol/L) | 6.26 (6.05, 6.50) | 8.14 (7.96, 8.32) | 7.77 (7.40, 8.15) | 6.64 (6.48, 6.81) |
| Systolic BP (mm Hg) | 116 (115, 116) | 120 (119, 121) | 119 (118, 121) | 119 (118, 119) |
| Diastolic BP (mm Hg) | 71 (71, 72) | 74 (73, 74) | 73 (72, 74) | 73 (73, 74) |
| Total cholesterol (mmol/L) | 5.41 (5.35, 5.47) | 5.39 (5.34, 5.44) | 5.38 (5.28, 5.48) | 4.98 (4.94, 5.03) |
| HDL cholesterol (mmol/L) | 1.42 (1.40, 1.43) | 1.29 (1.28, 1.31) | 1.30 (1.27, 1.33) | 0.85 (0.83, 0.86) |
| Triglyceridesa (mmol/L) | 1.32 (1.29, 1.36) | 1.57 (1.54, 1.61) | 1.50 (1.43, 1.58) | 2.07 (2.02, 2.12) |
CI, confidence interval; MA, Mexican American; NHW, non-Hispanic white; BMI, body mass index; HDL, high-density lipoprotein; CVD, cardiovascular disease; BP, blood pressure;
Due to non-normal distributions geometric means are presented.
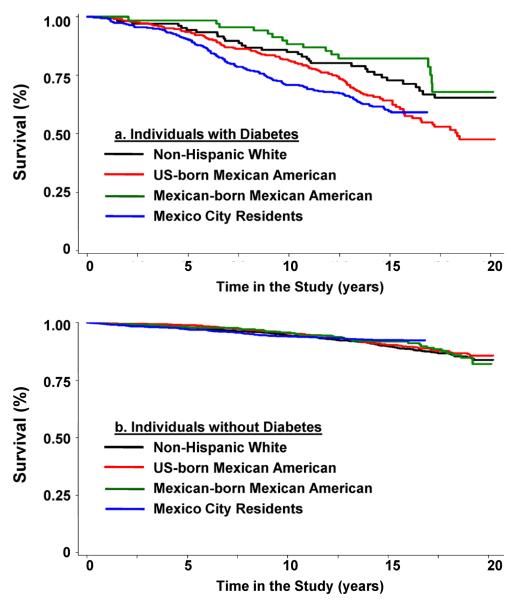
The age- and sex-adjusted HRs for all-cause mortality comparing U.S.-born MA, Mexican-born MA and MC residents to NHW were 1.45 (95% CI: 1.20, 1.75), 1.32 (95% CI: 0.98, 1.79) and 1.41 (95% CI: 1.16, 1.71), respectively (Table 2). Among individuals without diabetes the age- and sex-adjusted HRs for all-cause mortality comparing U.S.-born MA, Mexican-born MA and MC residents to NHW were 1.09 (95% CI: 0.86, 1.37), 1.23 (95% CI: 0.86, 1.76) and 0.97 (95% CI: 0.77, 1.23), respectively (Table 2). In contrast, mortality risk varied across the four populations among individuals with diabetes with HRs of 1.77 (95% CI: 1.20, 2.61), 1.08 (95% CI: 0.59, 1.97) and 2.27 (95% CI: 1.53, 3.35) for U.S.-born MAs, Mexican-born MAs and MC residents compared to NHW, respectively (Table 2). Kaplan-Meier survival curves reflected this pattern (available online in “Figure 1”).
Table 2.
Age- and sex- adjusted hazard rate ratios (and 95% confidence intervals) from multivariate Cox models for a given difference in risk factor level for all-cause mortality in the total population and stratified by diabetes status.
| Total Population | Baseline Diabetes Status | ||||||
|---|---|---|---|---|---|---|---|
| N=6,034a | No (n=5,130a) | Yes (n=815a) | |||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | Pb | |
| City/Ethnicity | |||||||
| U.S.-born MA versus NHW | 1.45 | (1.20, 1.75) | 1.09 | (0.86, 1.37) | 1.77 | (1.20, 2.61) | 0.03 |
| Mexican-born MA versus NHW | 1.32 | (0.98, 1.79) | 1.23 | (0.86, 1.76) | 1.08 | (0.59, 1.97) | 0.72 |
| Mexican versus NHW | 1.41 | (1.16, 1.71) | 0.97 | (0.77, 1.23) | 2.27 | (1.53, 3.35) | <0.001 |
The discrepancy between the n in the total population and those with and without diabetes is due to 89 individuals with missing information on diabetes status.
Two-sided p-value for interaction between diabetes and mortality risk for each city/ethnic group relative to NHW. In addition the global two-sided p-value for interaction between diabetes and mortality risk across the four city/ethnic groups is 0.0003.
Figure 1a. and 1.b.
Unadjusted Kaplan-Meier all-cause survival estimates for non-Hispanic White, U.S.-born Mexican American, Mexican-born Mexican American and Mexico City residents (a) with and (b) without diabetes.
Controlling for cardiovascular risk factors including age, sex, hypertension, smoking status, CVD history, total cholesterol and HDL-cholesterol, diabetes remained a strong modifier of the mortality risk across the four populations (global p-value <0.0001 for interaction). After adjusting for cardiovascular risk factors, among individuals without diabetes HRs adjusted for cardiovascular risk factors for all-cause mortality comparing U.S.-born MA, Mexican-born MA and MC residents to NHW were 1.11 (95% CI: 0.88, 1.40), 1.20 (95% CI: 0.83, 1.75) and 0.98 (95% CI: 0.74, 1.28), respectively. In contrast, in individuals with diabetes mortality risk was higher in U.S.-born MA [HR=1.83 (95% CI: 1.20, 2.78)] and MC residents [HR=2.62 (95% CI: 1.70, 4.06)] than in NHW.
The age- and sex- adjusted mortality risk associated with diabetes was over 4-fold in MC residents [HR=4.38 (95% CI: 3.43, 5.59)] and significantly higher than in NHW [HR=1.88 (95% CI: 1.28, 2.77)] (Table 3). The mortality risk associated with diabetes was also high in U.S.-born MA [HR=3.06 (95% CI: 2.40, 3.89)]. Another risk factor with a statistically significant interaction across the three populations was current smoking status which was significantly associated with higher mortality risk in NHW and US-born MA but not in Mexico-City residents. The mortality risk associated with smoking was low in MC residents [HR=1.19 (95% CI: 0.92, 1.54)], 50% in U.S.-born MA [HR=1.50 (95% CI: 1.17, 1.91)] and over 2-fold in NHW [HR=2.25 (95% CI: 1.67, 3.03)]. After additionally controlling for hypertension, current smoking status, a history of CVD, total cholesterol and HDL cholesterol, the mortality risks associated with diabetes were 1.69 (95% CI: 1.11, 2.56), 2.76 (95% CI: 2.17, 3.56), 1.70 (95% CI: 0.90, 3.23) and 4.54 (95% CI: 3.54, 5.81) in NHW, U.S.-born MA, Mexican-born MA and MC residents, respectively.
Table 3.
Age- and sex- adjusted hazard rate ratios (and 95% confidence intervals) from separate Cox models for a given difference in risk factor level for all-cause mortality stratified by country, birthplace and ethnicity.
| NHW | U.S.-born MA | Mexican-born MA | Mexico City | Pa | |
|---|---|---|---|---|---|
| Age (1 year increase) | 1.12 (1.10, 1.15) | 1.12 (1.10, 1.13) | 1.12 (1.08, 1.17) | 1.10 (1.08, 1.11) | 0.19 |
| Gender (men versus women) | 1.54 (1.15, 2.07) | 1.88 (1.49, 2.38) | 1.72 (1.01, 2.92) | 1.49 (1.17, 1.89) | 0.54 |
| Diabetes Status (yes versus no) | 1.88 (1.28, 2.76) | 3.06 (2.40, 3.89) | 1.65 (0.92, 2.96) | 4.38 (3.43, 5.59) | <0.001 |
| Hypertension Status (yes versus no) | 1.21 (0.88, 1.67) | 1.35 (1.06, 1.73) | 1.87 (1.08, 3.22) | 1.26 (0.97, 1.64) | 0.57 |
| Current smoker versus others | 2.25 (1.67, 3.03) | 1.50 (1.17, 1.91) | 1.97 (1.16, 3.34) | 1.19 (0.92, 1.54) | 0.01 |
| History of CVD (yes versus no) | 2.08 (1.39, 3.12) | 1.97 (1.42, 2.75) | 3.43 (1.54, 7.63) | 1.93 (1.15, 3.25) | 0.64 |
| Total cholesterol (1 mmol/L increase) | 1.01 (0.87, 1.17) | 0.96 (0.87, 1.07) | 1.15 (0.97, 1.36) | 0.92 (0.83, 1.03) | 0.19 |
| HDL-cholesterol (1 mmol/L increase) | 0.73 (0.50, 1.06) | 0.86 (0.62, 1.20) | 1.69 (0.86, 3.23) | 0.95 (0.56, 1.60) | 0.20 |
CI, confidence interval; MA, Mexican American; NHW, non-Hispanic white; CVD, cardiovascular disease; HDL, high-density lipoprotein;
Two-sided p-value is for the global interaction test addressing whether the hazard ratios differ across the three strata.
Limiting the population to those with diabetes, population specific age- and sex-adjusted means and proportions were determined for factors likely to reflect diabetes severity stratifying across the three populations of interest (Table 4). Among participants with diabetes, MC residents [35.1% (95% CI: 30.0, 40.6)] were less likely to have undiagnosed diabetes than NHW [59.9% (95% CI: 50.3, 68.9)] or U.S.-born MA [44.6% (95% CI: 39.2, 50.1)]. Moreover, among participants with undiagnosed diabetes, MC residents had higher fasting and two-hour glucose levels, higher triglycerides, higher insulin resistance as assessed by HOMA-IR and lower HDL-cholesterol levels than NHW. Among participants with diabetes, MC residents [53.7% (95% CI: 48.0, 59.3)] were more likely to use medication to treat their diabetes than NHW [24.6% (95% CI: 17.4, 33.6)] or U.S.-born MA [39.8% (95% CI: 34.6, 45.3)]. Among individuals using medication to treat their diabetes, MC residents were less likely to be using insulin, their duration of diabetes was shorter and their HDL-cholesterol levels were lower than NHW or U.S.-born MAs. In contrast, fasting glucose was similar in MC residents and U.S.-born MA, but elevated relative to NHW.
Table 4.
Age- and sex- adjusted characteristics (means or proportions and 95% confidence intervals) of individuals with diabetes stratified by country, birthplace, ethnicity and medication use.
| NHW | U.S.-born MA | Mexico City | |
|---|---|---|---|
| Undiagnosed (n) | 62 | 144 | 114 |
| Agea (years) | 55.0 (53.1, 56.9) | 52.1 (50.9, 53.4) | 49.9 (48.5, 51.3) |
| Malea (%) | 50.0 | 44.4 | 29.0 |
| Deceaseda [% (n)] | 25.8 (16) | 28.5 (41) | 22.8 (26) |
| Fasting glucose (mmol/L) | 7.38 (6.39, 8.36) | 8.17 (7.53, 8.82) | 8.62 (7.89, 9.36) |
| Two-hour glucose (mmol/L) | 13.0 (11.7, 14.3) | 15.6 (14.8, 16.5) | 16.0 (15.0, 17.0) |
| Duration of diabetes (years) | 0 | 0 | 0 |
| HDL (mmol/L) | 1.33 (1.25, 1.42) | 1.18 (1.13, 1.24) | 0.84 (0.78, 0.90) |
| Triglyceridesb (mmol/L) | 1.98 (1.72, 2.27) | 2.08 (1.90, 2.28) | 2.84 (2.56, 3.16) |
| Hypertension (%) | 44.0 (32.1, 56.7) | 39.4 (31.6, 47.7) | 30.9 (22.7, 40.5) |
| Blood pressure Medication (%) | 31.7 (21.1, 44.5) | 18.5 (12.8, 25.8) | 9.8 (5.5, 16.9) |
| Fasting insulin (IU/ml)b | 17.9 (14.7, 21.8) | 20.6 (18.1, 23.4) | 20.3 (17.6, 23.4) |
| HOMA-IRb | 5.6 (4.5, 6.9) | 6.9 (6.0, 8.0) | 7.4 (6.3, 8.6) |
| HOMA-βb,c | 110 (85, 142) | 112 (95, 132) | 97 (80, 117) |
| Diagnosed no medication (n) | 17 | 49 | 39 |
| Agea (years) | 55.6 (52.0, 59.3) | 52.6 (50.4, 54.7) | 52.4 (50.0, 54.8) |
| Malea (%) | 41.2 | 36.7 | 51.3 |
| Deceaseda [% (n)] | 41.2 (7) | 32.7 (16) | 28.2 (11) |
| Fasting glucose (mmol/L) | 8.43 (6.56, 10.3) | 10.3 (9.22, 11.4) | 12.6 (11.4, 13.9) |
| Two-hour glucose (mmol/L) | 16.0 (13.5, 18.4) | 19.2 (17.8, 20.7) | 18.7 (16.3, 21.1) |
| Duration of diabetes (years) | 6.4 (2.6, 10.1) | 7.7 (5.5, 10.0) | 6.2 (3.7, 8.8) |
| HDL (mmol/L) | 1.31 (1.15, 1.47) | 1.17 (1.08, 1.26) | 0.86 (0.81, 0.91) |
| Triglyceridesb (mmol/L) | 1.89 (1.45, 2.47) | 2.03 (1.74, 2.37) | 2.60 (2.18, 3.09) |
| Hypertension (%) | 20.8 (7.8, 45.0) | 34.9 (22.8, 49.3) | 20.2 (10.3, 35.7) |
| Blood pressure Medication (%) | 16.9 (5.4, 42.0) | 11.9 (5.4, 24.2) | 5.2 (1.3, 18.6) |
| Fasting insulin (IU/ml)b | 21.6 (14.3, 32.6) | 17.8 (14.3, 22.1) | 14.4 (11.3, 18.3) |
| HOMA-IRb | 8.2 (5.2, 12.8) | 7.9 (6.2, 10.0) | 7.2 (5.5, 9.4) |
| HOMA-βb,a | 89 (52, 152) | 58 (43, 77) | 40 (29, 54) |
| Using medication (n) | 28 | 131 | 162 |
| Agea (years) | 53.7 (50.8, 56.6) | 54.9 (53.5, 56.2) | 53.9 (52.7, 55.1) |
| Malea (%) | 42.9 | 36.6 | 43.2 |
| Deceaseda [% (n)] | 32.1 (9) | 51.2 (67) | 53.7 (87) |
| Fasting glucose (mmol/L) | 8.90 (7.41, 10.4) | 10.8 (10.1, 11.5) | 10.9 (10.3, 11.5) |
| Duration of diabetes (yrs) | 11.6 (8.52, 14.7) | 10.8 (9.41, 12.1) | 8.53 (7.32, 9.75) |
| Insulin Use (%) | 50.4 (32.5, 68.2) | 39.3 (31.1, 48.1) | 8.7 (5.2, 14.1) |
| HDL (mmol/L) | 1.32 (1.20, 1.44) | 1.15 (1.09, 1.21) | 0.86 (0.81, 0.91) |
| Triglyceridesb (mmol/L) | 1.92 (1.55, 2.36) | 2.17 (1.97, 2.39) | 2.30 (2.11, 2.51) |
| Hypertension (%) | 38.0 (22.1, 57.0) | 36.0 (28.1, 44.6) | 32.2 (25.4, 39.8) |
| Blood pressure Medication (%) | 40.1 (22.9, 60.2) | 26.2 (19.3, 34.4) | 17.2 (12.6, 24.3) |
Unadjusted;
Due to non-normal distributions geometric means are presented.
Adjusted for age, sex and HOMA-IR.
Among those with diabetes the age- and sex-adjusted HRs for all-cause mortality comparing U.S.-born MA, and MC residents to NHW were 1.67 (95% CI: 1.13, 2.47) and 2.05 (95% CI: 1.38, 3.05), respectively. Markers of diabetes severity including medication use, insulin use and fasting glucose levels were independent predictors of all-cause mortality. Controlling for these covariates as well as duration of clinically recognized diabetes (which grouped those with undiagnosed diabetes at zero) moderately attenuated the HRs for all-cause mortality; HRs comparing U.S.-born MA and MC residents to NHW were 1.31 (95% CI: 0.87, 1.98) and 1.38 (95% CI: 0.89, 2.12), respectively. Additionally, controlling for hypertension status, blood pressure medication, smoking status, a history of cardiovascular disease, total cholesterol and HDL-cholesterol levels did not further attenuate the HRs; HRs comparing U.S.-born MA and MC residents to NHW were 1.33 (95% CI: 0.86, 2.06) and 1.61 (95% CI: 0.98, 2.66), respectively.
After stratifying by diabetes status, there was no evidence that the assumption of proportional hazards was violated in individuals with diabetes. However, in participants without diabetes in MC we observed a higher mortality hazard relative to NHW in the first 10 years of the study, but a lower mortality hazard relative to NHW during the remainder of the study.
DISCUSSION
Previous studies report up to a 3-fold increase in mortality risk associated with diabetes (10,19-23). The age- and sex- adjusted HR for diabetes in NHW in the SAHS is 1.88 (95% CI: 1.28, 2.76). In contrast, in U.S.-born MA there is a 3-fold increased mortality risk and in MC residents over a 4-fold increased mortality risk associated with diabetes. Unexpectedly, adjusting for cardiovascular risk factors including age, sex, hypertension, smoking status, CVD history, total cholesterol and HDL-cholesterol altered associations only slightly with the point estimate for diabetes associated mortality increasing slightly in MC residents. This result indicates that the increased mortality risk associated with diabetes in U.S.-born MA and MC residents is independent of cardiovascular risk factors at least to the extent that we were able to adjust for them.
International studies of migrant populations can be used to assess environmental impact on disease rates while controlling for genetic effects. Previously we found Native American genetic admixture to be similar in MC residents and MA living in San Antonio(9); hence, genetic factors presumably would convey similar mortality risk associated with diabetes in these populations. The “healthy migrant effect” is one possible explanation for differences between U.S.-born MA and MA who immigrated to the US(24, 25). A second explanation is chance given the low number of Mexican-born MAs (n = 444) and the resulting wide confidence intervals which led us to exclude these individuals from some analyses.
Environmental determinants are also likely responsible for the increased mortality associated with diabetes in both U.S.-born MA and MC residents relative to NHW. Unfortunately, available markers of biologic differences in disease severity are intrinsically tied to health-care use/treatment. Focusing only on individuals with diabetes and controlling for markers of diabetes severity as well as differences in health-care use/treatment attenuated differences in mortality risk across the three populations in individuals with diabetes. This indicates that diabetes severity and differences in health-care use/treatment may contribute to differences in the mortality risk associated with diabetes.
Duration of diagnosed and duration of undiagnosed diabetes (i.e., time to recognition and treatment) may also be differentially impacting severity and outcomes across populations. Among individuals with diabetes the prevalence of undiagnosed diabetes was highest in NHW at 59.9%, decreased slightly in U.S.-born MA at 44.6% and was lowest in MC residents at 35.1%. In individuals with diabetes, fasting and two-hour glucose levels were higher in MC residents than in NHW. Hence, while undiagnosed diabetes accounted for a smaller proportion of individuals with diabetes among MC residents their undiagnosed diabetes appeared to be more severe than undiagnosed diabetes in NHW.
The lower proportion of undiagnosed diabetes among those with diabetes in MC residents and the higher prevalence of medication use to treat diabetes in MC residents indicate that in some respects access to and quality of medical care is higher in MC residents recruited between 1990-1992 than in NHW or U.S.-born MA recruited between 1979 and 1988. In contrast, among individuals with diabetes lower use of insulin and blood pressure medications in MC residents relative to NHW or U.S.-born MA suggest access to and quality of care may be lower in MC residents relative to SAHS participants. However, the lower use of insulin in MC residents may not necessarily suggest lower access/quality care, but may be related to the shorter diabetes duration, particularly among those using medication: 8.5 yrs in Mexico City vs. 10.8 yrs for US-born MA and 11.6 years in NHW. Recent clinical trials including ACCORD and VADT which each compared two different levels of intensity for glycemic control, with ACCORD also including comparisons for blood pressure as well as lipid levels, indicate that beyond a certain level access to intensive care and tight glucose control is not associated with a decrement in mortality(26,27). It should be noted that mortality for the non diabetic population is similar in both cities and for all ethnic groups: this finding provides evidence in favor of similar quality of care.
The current study combines the MCDS, the first population-based cohort study to follow Mexican individuals over an extended time period for mortality, with the SAHS, one of the first population-based cohort studies to follow MA individuals over a similar time period. The MCDS was specifically designed to ensure comparability across the two studies and methodologies and questionnaires developed for the SAHS were used at the MCDS. The high vital status ascertainment rates make it unlikely that differential bias with respect to mortality could explain our finding.
A limitation is that the recruitment time frame varied across the two studies. Hence, secular trends in diabetes awareness and treatment may contribute to mortality differences and bias the study findings; SAHS participants were recruited between 1979 and 1988 while MC residents were recruited between 1990 and 1992. For instance, the proportion of undiagnosed diabetes among individuals with diabetes is lowest in MC residents and the prevalence of medication use to treat diabetes was also highest in MC residents with diabetes who were recruited in the early 1990’s when diabetes awareness was increasing globally. However, because mortality rates among individuals with diabetes were higher in MC residents who were recruited later than SAHS participants when treatment and outcomes would hopefully have improved, it is unlikely that secular trends explain the higher mortality risk in MC residents with diabetes relative to NHW with diabetes. A second limitation of the study is that the generalizability of our results may be limited given that participant recruitment occurred in only two cities.
Our study provides evidence that diabetes is more lethal in U.S.-born Mexican Americans and Mexico City residents than in non-Hispanic whites. These findings indicate that diabetes may in part be responsible for national as well as international health disparities between non-Hispanic whites, Mexican Americans and Mexicans. Given that diabetes is the number one cause of death in Mexico and its incidence continues to increase in the U.S. and internationally, these disparities warrant further examination.
ACKNOWLEDGEMENTS
1 This work was supported by grants from the Consejo Nacional de Ciencia y Tecnologia Apoyo (14502); National Heart Lung and Blood Institute (R01-HL24799 and R01-HL36820); National Institute of Diabetes and Digestive and Kidney Diseases (K01-DK064867); and National Center on Minority Health and Health Disparities (R01-MD004251); ); and the Office of Research and Development, Department of Veterans Affairs, and the Ralph H. Johnson VAMC. Further support was provided through VA HSR&D REAP Award (grant #IIR-06-219). The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
2. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs.
Abbreviations Used
- HOMA-β-cell
Homeostasis Model Assessment of beta cell function
- HOMA-IR
Homeostasis Model of Insulin Resistance
- HR
hazard ratio
- MA
Mexican American
- MC
Mexico City
- MCDS
Mexico City Diabetes study
- NHW
non-Hispanic white
- SAHS
San Antonio Heart Study
- TX
Texas
- US
United States
- UTHSCSA
University of Texas Health Science Center at San Antonio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Instituto Nacional de Estadística y Geografía Mujeres y Hombres en México. Decimotercera Edición. 2009. ISBN:978-970-13-5169-7.
- 2.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354:617–621. [PubMed] [Google Scholar]
- 3.Burke JP, Williams K, Gaskill SP, Hazuda HP, Haffner SM, Stern MP. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med. 1999;159:1450–1456. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- 4.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in U.S. population aged 20-74 yr. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 5.Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–524. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- 6.Park MK, Menard SW, Schoolfield J. Prevalence of overweight in a triethnic pediatric population of San Antonio, Texas. Int J Obes Relat Metab Disord. 2001;25:409–416. doi: 10.1038/sj.ijo.0801550. [DOI] [PubMed] [Google Scholar]
- 7.Stern MP, Mitchell BD. Diabetes in Hispanic Americans. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. 2nd Edition National Institutes of Health, National Institutes of Diabetes and Digestive Kidney Diseases; Bethesda: 1995. [Google Scholar]
- 8.Hunt KJ, Williams K, Resendez RG, Hazuda HP, Haffner SM, Stern MP. All-Cause and Cardiovascular Mortality Among Diabetic Participants in the San Antonio Heart Study: Evidence against the “Hispanic Paradox”. Diabetes Care. 2002;25:1557–1563. doi: 10.2337/diacare.25.9.1557. [DOI] [PubMed] [Google Scholar]
- 9.Stern MP, Gonzalez-Villalpando C, Mitchell BD, Villalpando E, Haffner SM, Hazuda HP. Genetic and environmental determinants of type II diabetes in Mexico City and San Antonio. Diabetes. 1992;41:484–492. doi: 10.2337/diab.41.4.484. [DOI] [PubMed] [Google Scholar]
- 10.Burke JP, Williams K, Haffner SM, Gonzalez-Villalpando C, Stern MP. Elevated incidence of type 2 diabetes in San Antonio, Texas, compared with that of Mexico City, Mexico. Diabetes Care. 2001;24:1573–1578. doi: 10.2337/diacare.24.9.1573. [DOI] [PubMed] [Google Scholar]
- 11.Haffner SM, Gonzalez-Villalpando C, Mykkanen L, Stern M. Total immunoreactive proinsulin, immunoreactive insulin and specific insulin in relation to conversion to NIDDM: the Mexico City Diabetes Study. Diabetologia. 1997;40:830–837. doi: 10.1007/s001250050756. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell BD, Gonzalez-Villalpando C, Arredondo PB, Garcia MS, Valdez R, Stern MP. Myocardial infarction and cardiovascular risk factors in Mexico City and San Antonio, Texas. Arterioscler Thromb Vasc Biol. 1995;15:721–725. doi: 10.1161/01.atv.15.6.721. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell BD, Hazuda HP, Haffner SM, Patterson JK, Stern MP. Myocardial infarction in Mexican-Americans and non-Hispanic whites. The San Antonio Heart Study. Circulation. 1991;83:45–51. doi: 10.1161/01.cir.83.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Hazuda HP, Comeaux PJ, Stern MP, Haffner SM, Eifler CW, Rosenthal M. A comparison of three indicators for identifying Mexican Americans in epidemiologic research. Methodological findings from the San Antonio Heart Study. Am J Epidemiol. 1986;123:96–112. doi: 10.1093/oxfordjournals.aje.a114228. [DOI] [PubMed] [Google Scholar]
- 15.Stern MP, Rosenthal M, Haffner SM, Hazuda HP, Franco LJ. Sex difference in the effects of sociocultural status on diabetes and cardiovascular risk factors in Mexican Americans. The San Antonio Heart Study. Am J Epidemiol. 1984;120:834–851. doi: 10.1093/oxfordjournals.aje.a113956. [DOI] [PubMed] [Google Scholar]
- 16.Haffner SM, Stern MP, Hazuda HP, Rosenthal M, Knapp JA. The role of behavioral variables and fat patterning in explaining ethnic differences in serum lipids and lipoproteins. Am J Epidemiol. 1986;123:830–839. doi: 10.1093/oxfordjournals.aje.a114312. [DOI] [PubMed] [Google Scholar]
- 17.Alberti KGMM, Aschner P, Assal JP, Bennett PH, Groop L, Jervell J, Kanazawa Y, Keen H, Klein R, Mbanya JC, McCarty D, Motala A, Pan XR, Ramachandran A, Samad N, Unwin N, Vardi P, Zimmet PZ. Part 1: Diagnosis and classification of diabetes mellitus. World Health Organization; Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr., Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281:1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D’Agostino RB, Sr., Wilson PW, Savage PJ. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 22.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147:149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 23.Uusitupa M, Peltonen M, Lindstrom J, Aunola S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Valle TT, Eriksson JG, Tuomilehto J. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS One. 2009;4:e5656. doi: 10.1371/journal.pone.0005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Razum O, Zeeb H, Akgun HS, Yilmaz S. Low overall mortality of Turkish residents in Germany persists and extends into a second generation: merely a healthy migrant effect? Trop Med Int Health. 1998;3:297–303. doi: 10.1046/j.1365-3156.1998.00233.x. [DOI] [PubMed] [Google Scholar]
- 25.Razum O, Zeeb H, Rohrmann S. The ‘healthy migrant effect’--not merely a fallacy of inaccurate denominator figures. Int J Epidemiol. 2000;29:191–192. doi: 10.1093/ije/29.1.191. [DOI] [PubMed] [Google Scholar]
- 26.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang DG. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 27.Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr., Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]