Abstract
The development of topical microbicides for intravaginal use to prevent HIV infection requires that the drugs and formulated products be nontoxic to the endogenous vaginal Lactobacillus. In 30 min exposure tests we found dapivirine, tenofovir and UC781 (reverse transcriptase inhibitor anti-HIV drugs) as pure drugs or formulated as film or gel products were not deleterious to Lactobacillus species; however, PSC-RANTES (a synthetic CCR5 antagonist) killed 2 strains of Lactobacillus jensenii. To demonstrate the toxicity of formulated products a new assay was developed for use with viscous and non-viscous samples that we have termed the Lactobacillus toxicity test. We found that the vortex mixing of vaginal Lactobacillus species can lead to reductions in bacterial viability. Lactobacillus can survive brief, about 2 sec, but viability declines with increased vortex mixing. The addition of heat inactivated serum or bovine serum albumin, but not glycerol, prevented the decrease in bacterial viability. Bacillus atrophaeus spores also demonstrated loss of viability upon extended mixing. We observed that many of the excipients used in film formulation and the films themselves also afford protection from the killing during vortex mixing. This method is of relevance for toxicity for cidal activities of viscous products.
Keywords: HIV, Microbicide, Lactobacillus, Vortex mixing, bacterial quantitation
1. Introduction
Different solid or viscous formulations are under development for microbicide delivery including vaginal rings, gels and films. These products should optimally prevent infection by HIV without disturbing the normal vaginal microflora since this flora is a key component of the innate immune environment which can reduce the HIV risk(Martin et al., 1999). We have focused on films and gel formulations; both of which rely on similar or the same viscosity enhancing agents such as species of polyvinyl alcohols and methylcelluloses.
The minimum cidal concentration (MCC) assay detects a decrease in microbial viability after a 30 min exposure of 104 colony forming units (CFUs)/mL or greater (Moncla and Hillier, 2005, Moncla et al., 2008). The test was useful in our search for materials that would kill HIV and bacterial sexually transmitted pathogens but lacked the sensitivity to determine Lactobacillus killing of less than 4 logs. Minimum inhibition concentration tests (MIC) with microbicides do not provide relevant information because they only determine inhibition. The MCC assay may suggest that inhibition is occurring but more complex methods are required to differentiate killing from inhibition (Moncla and Hillier, 2005, Moncla et al., 2008). However, the MCC assay is difficult to use with drugs formulated for vaginal use because they contain viscous excipients. In order to test these products for cidal activity against Lactobacillus, the products must be diluted before testing, resulting in drug and excipient concentrations that are far below that expected during product use. Therefore, the assay may underestimate deleterious effects, undermine toxicity testing against Lactobacillus or misrepresent the efficacy of the drug. We therefore developed a new assay to assess the effects of drugs, excipients and formulated products that closely mimic concentrations anticipated during use.
In the studies reported here two different classes of anti-HIV drugs (reverse transcriptase inhibitors, RTIs and antimicrobial peptides) were screened for toxicity (non-deleterious effects) against Lactobacillus species. A new assay for toxicity against Lactobacillus was developed and we found vortex mixing killed Lactobacillus species; however, most of the films, gels, excipients and formulations were not detrimental to Lactobacillus species.
2. Materials and methods
2.1. Bacterial strains and growth conditions
Reference strains of Lactobacillus were obtained from the American Type Culture Collection, ATCC (Manassas, VA 20109, USA). Field isolates were obtained from human vaginal samples. Lactobacillus species were identified using colony morphology, Gram-stain reaction and catalase production and were confirmed using DNA-DNA hybridization to DNA from reference strains (Antonio et al., 1999). Organisms were stored frozen at −70° C in litmus milk until needed. Stock cultures were revived by plating onto blood agar plates (Columbia blood agar base, PML Microbiologicals, Wilsonville, OR 97070-9234, USA). Cultures were incubated at 35° C in air enriched to 6 % CO2 overnight or until good growth was observed. Bacillus atrophaeus spore suspensions at a concentration of 2.6 × 107/mL were obtained from STERIS Corporation (Mentor, OH 44060, USA).
2.2. Lactobacillus toxicity testing (LTT) and MCC testing
Saline and ACES buffer [(N-2-acetamido)-2- aminoethanesulfonic acid] (pH 7.0) (Sigma-Aldrich, St. Louis, MO 63178, USA) were prepared in house. The isotonic strength of each lot of buffer was determined using a Vapro Pressure Osmometer 5520 (Westcor Inc., Logan UT, 84321 USA) and adjusted to 200–300 mosm/kg with sodium chloride prior to use. Heat inactivated fetal bovine serum (FBS) was obtained from Cellgro, (Mediatech Herndon, VA 20109, USA) bovine serum albumin (BSA) was obtained from Sigma-Aldrich (St. Louis, MO 63178, USA). Serum, BSA and glycerol were added to buffer at a final concentration of 7.5 %, 3.75% and 3.75% (vol/vol) respectively and filter sterilized through a 0.45 μ filter and stored at 4° C until used; buffers were prepared fresh weekly.
Bacterial suspensions were prepared in saline or ACES buffer with or without additions as indicated in the figures to a density of 2 McFarland units, approximate concentration of 2.0 × 108 bacteria/mL. Sample materials were weighed into the bottom of a 50 mL conical centrifuge tube (about 0.25 to 0.5 g) and equivalent volume of buffer added and mixed. Then an equal volume of bacterial suspensions were added, mixed for 2 sec, and sampled for colony forming units (CFUs). The samples were then mixed on a vortex mixer set to speed of 10 (the fastest available speed) for 30 sec, unless otherwise indicated. Alternatively, the mixtures were mixed by hand using a gentle swirling motion. The starting concentrations of bacteria in the inoculum were determined by plate count of the initial suspension of bacteria at a density of 2 McFarland units. Experiments where the CFUs, as determined from the starting solution and the CFUs from the 2 sec sample did not agree were rejected. The number of CFUs for each datum was taken from the dilution plate that contained between 50 and 300 colonies. All reported values represent the average of triplicate samples. If the number of colonies on the individual sample plates did not agree within 20% they were rejected. MCCs and MICs (agar dilution) assays were performed exactly as previously described (Moncla and Hillier, 2005, Moncla et al., 2008). When drug availability limited the number of tests we could perform the drug was tested at the concentration anticipated during use or the highest obtainable concentration. Octylglycerol (OG), a well characterized microbicide was used as a control. The OG was kindly provided by Charles Isaacs (New York State Institute of Basic Research in Developmental Disabilities, Staten Island, NY 10314 USA). Unless stated otherwise each experiment was repeated on at least 2 separate occasions.
2.3. Excipients, Drugs, and Substances tested
The viscosity increasing excipients evaluated in these studies were methylcellulose (4000 centipoise) and polyvinyl alcohol (PVA) [30 to 70 kilo-daltons (kDA) and 9 to 10 kDa], Methy-β-cyclodextrin MβCD (approximate formula weight 1320) and hydroxypropyl- β-cyclodextrin, HPβCD (approximate FW 1380) were from Sigma Aldrich (St. Louis, MO 63178, USA). Carbopol 974P was obtained from Noveon, Inc., (Cleveland, OH 44141, USA), and hydroxyethycellulose (HEC) 250HX was from Hercules Incorporated (Wilmington, DE 19801). PSC-RANTES (a synthetic peptide CCR5 antagonist) was synthesized by the Peptide Synthesis Facility at the University of Pittsburgh (Fontenot et al., 1991), and the molecular mass confirmed by mass spectrometry. The non-nucleoside reverse transcriptase inhibitors (NNRTIs) UC781 drug and tenofovir drug and gel product (1% drug) were provided by CONRAD (the Contraceptive Research and Development a Division of the Department of Obstetrics and Gynecology of Eastern Virginia Medical School, 1911 North Fort Myer Drive, Suite 900, Arlington, Virginia 22209) (Schwartz et al., 2006). A gel containing 0.01% UC781 was prepared in a carbomer/methylcellulose base formulated as previously described in animal studies (Patton et al., 2006, Patton et al., 2007). The hydroxyethyl cellulose (HEC) universal placebo was prepared as previously described (Tien et al., 2005). A proprietary formulation of dapivirine (TMC120) gel was provided by the International Partnership for Microbicides (Silver Spring, MD 20910, USA). Octylglycerol, dapivirine and UC781 containing films were formulated using a polyvinyl alcohol (PVA) polymer base.
3. Theory/Calculations
Vaginal products and their components should optimally have no cidal activity against Lactobacillus species, a key component of the vaginal ecosystem. L. crispatus and L. jensenii are used as representative species since they are the most common vaginal Lactobacillus (Antonio et al., 1999, Gustafsson et al., 2011). If formulated products or their components cause a reduction in the number of viable bacteria, equal to or greater than 1 Log 10, of two different strains of any of our representative species, the substance being tested is considered to potentially have a negative effect on the Lactobacillus if used clinically. Many of the materials are difficult to test because they are so viscous they must be diluted to low concentration. We reasoned that if we could mix high concentrations of bacteria with the tested material and allow them to interact for 30 minutes prior to diluting; we would be exposing the organisms to the materials under conditions similar to their in vivo use.
4. Results
4.1. Effects of drugs on Bacteria
The Minimum Cidal Concentration test (MCC) format was used to expose the organisms to drug and formulations (Table 1). The reverse transcriptase inhibitors (RTIs) tenofovir, dapivirine (TMC120), and UC781 in both the formulated (film or gel) or unformulated states; were not toxic to Lactobacillus. Tenofovir at a concentration of 1 mg/mL and the gel, diluted 1:10 for use, were not toxic. However, these concentrations did not represent the concentration anticipated during use. PSC-RANTES killed 20% of the L. jensenii isolates tested and inhibition of an unknown quantum was observed in 4 strains. However, these data, in the MCC format, did not address the question as to whether or not inhibition of these strains reaches the requisite 1 log decrease.
TABLE 1.
Materials tested using MCC assaysa
| Organism | Tenofovir 1mg/ml | Tenofovir 1% gel | UC-781 39 ng/mL | PSC- RANTES 300 μ/mL |
|---|---|---|---|---|
| No. killed/No/tested | No. killed/No/tested | No. killed/No/tested | No. killed/No/tested | |
| Lactobacillus crispatus | 0/8 | 0/8 | 0/6 | 0/9 |
| L. jensenii | 0/11 | 0/11 | 0/3 | 2/10 (4)b |
| L. vaginalis | 0/10 | 0/10 | NTc | 0/10 |
| L. gasseri | 0/10 | NT | 0/9 | NT |
| Gardnerella vaginalis | 0/14 | 0/14 | 0/14 | NT |
| Neisseria gonorrhoeae | 0/8 | NT | 0/10 | 0/7 (7) |
All strains were tested at pH 4 and pH 7 and no differences were observed. The Minimum Cidal Concentration test (MCC) is an exposure assay that is a pass/fail tests that detects a 4 log10 kill in 30 min.
Numbers in parenthesis are the number of strains tested demonstrating a reduction in viability without reaching a 4 log10 kill in 30 min.
NT not tested.
4.2. Effects of Excipients on bacterial viability
To define the LTT and make it objective we averaged all the data in Table 2 and set the cutoff as 2 standard deviations of the mean. Therefore, all results ≥ 1 Log 10 decrease in CFUs is a failed test; if the material has a failed test with two different isolates it fails the LTT test and is not used in our development scheme. Toxicity testing of different grades of PVAs as candidates for film preparation was concurrent with film development; therefore, testing was halted on any excipient that was rejected during formulation. The results of studies of effects on the viability of Lactobacillus species for the most promising varieties of PVAs, films and gels are presented in Table 2. The PVAs dissolved in buffer resulted in slight increases in the number of CFUs observed that ranged from 0.004 to 0.702 log 10. However, the placebo and active films were observed to reduce the CFUs for some species and these reductions appear to be strain specific. Using saline in place of ACES buffer did not alter the results (data not shown and Fig. 1). Values less than one log10 could represent pipetting or indeterminate errors. Table 2 demonstrates the difference in killing observed between the strains of different species, the L. jensenii are more sensitive compared to L. crispatus. The L. jensenii, 3 of 5 strains, had viability losses when exposed to the gel formulations that were greater than 1 log10 and therefore failed the LTT. The 30 day tenofovir films also demonstrated viability losses with L. jensenii > 1 Log and also failed the LTT. However, these decreases in viability are much less than would be observed with a truly toxic compound such as OG, see figure 1, where killing is 7 log10 in 30 min.
TABLE 2.
Log decrease or increase in CFUs when assayed in the presence of PVAs and films.
| Material testeda | L. crispatusb | L. jensenii |
|---|---|---|
| PVA powder-9–10 kDa (Sigma) | Passc (0.010–0.370) | Pass (0.015–0.733) |
| PVA powder30–70 kDa (Sigma) | Pass (0.006–0.410) | Pass (0.004–0.702) |
| Methyl –cellulose Powder 4000 cps (Spectrum) | Pass (0.072–0.420) | Pass (0.291–0.794) |
| Tenofovir film T0d | Pass (-0.048–−0. 414) | Pass (− 0.141–0.323) |
| Tenofovir film T30 | Pass (0.069–0.139) | Fail (−0.471–−2.120) |
| UC781 film | Pass (0.010–0.270) | Pass (−0.371–0.197) |
| UC781 gel | Pass (0.005–0.280) | Fail (0.209–1.659) |
| Placebo Film | Pass (0.0024–−0.159) | Pass (−0.171–0.398) |
| Dapivirine Film | Pass (0.042–0.130) | Pass (−0.171–0.607) |
| Dapivirine gel | Pass (0.025–0.280) | Fail (−0.873–1.486) |
Active, placebo and UC781 films use PVA (PVA 9–10 kDa). UC781 gel was formulated in a methylcellulose base.
L. crispatus ATCC 20225, ATCC 33197 and 3 field isolates were used. L. jensenii ATCC 25258 and four field isolates were tested with each of the material listed. All strains were tested using the LTT with vortex mixing.
Pass = the test material pasted the Lactobacillus Toxicity Test defined as no reduction in viability. The numbers in parenthesis are the ranges of values observed in the LTT. Data are presented as log10 change in CFUs, compared to the controls incubated in buffer only. The Students’t-test was used for statistical analysis (P< 0.05). A material demonstrating a reduction in viability ≥ 1 Log 10 for two or more of 10 isolates of Lactobacillus tested fails the test. All but three of the materials listed passed the LTT.
Tenofovir films were tested after preparation and again after one month. T0 samples of the films examined within 3 days of preparation, and T30 are the results of films stored for 30 days at 50° C. Note the films degraded by thirty days and became toxic to two strains L. jensenii (log reductions on the order of 1.0 to 2.1 were observed).
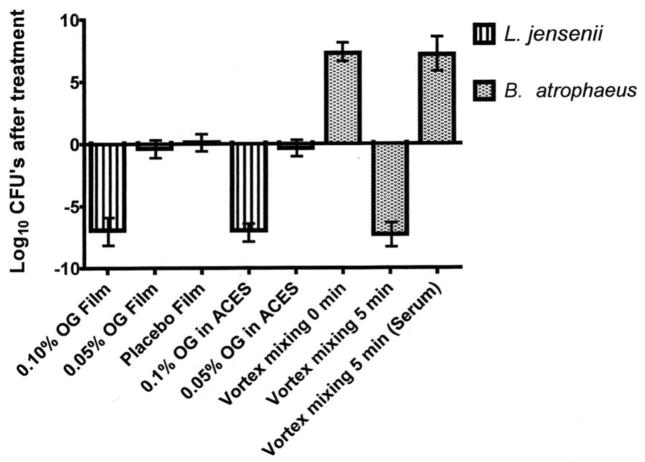
Fig. 1. Comparison of MCC method with new Lactobacillus Safety Test: viability of Lactobacillus and the effects of vortex mixing on viability.
Octylglycerol was used to demonstrate that comparable results are observed using either the Lactobacillus Toxicity Test (bars 1, 2, and 3) or the Minimum Cidal Concentration test, (bars 4 and 5). Films were prepared so they would give a final concentration of 0.10%, 0.05% or 0.00% when dissolved in ACEs buffer for the LST, bars 1, 2 and 3 respectively. Bacteria were added to give a final concentration of approximately 2–20 × 107/mL. After 30 min at 37°C, samples were taken and the CFUs determined. Bacteria were also diluted and used in the MCC test at final OG concentrations of 0.1%, or 0.05%, bars 4 and 5 respectively. Both tests were conducted to detect as little as 50 CFUs/mL in the test solutions. In both tests Lactobacillus were killed (6 logs) at 0.1% but not 0.05% OG in either film formulation or a solution demonstrating the LTT test gives comparable results to the standard test (MCC). Bars 6, 7 and 8 are the results of experiments using B. atrophaeus spores to show the effects of vortex mixing. Spore suspensions at a concentration of 2.61 × 107/ml were prepared in ACEs buffer and mixed on a vortex mixer for 2 sec, bar 6 or 5 min bar 7. The addition of serum to the spore suspensions protected them from killing during mixing for 5 min on a vortex mixer, bar 8.
4.3 Formulated products
From an extensive screening process several viscosity increasing excipients were selected for use in preparing film and gel based preparation of the NNRTIs, Tables 2 and 3. The effects on viability were very much dependent on the species and strains used for the test. L. jensenii consistently demonstrated reductions in viability approaching 1 log 10 Table 2. The calculated mean of all the data presented in Table 2 is 0.050 and the standard deviation (SD) is 0.477. Values greater than a −2 times the SD indicated killing has occurred; therefore, anything more than a one log reduction is a failure. Both of the gels and the thirty day tenofovir film failed the LTT because there were two isolates that failed for each test material (Table 2). Table 3 demonstrated the sensitivity of L jensenii LPB28Ab to the testing; this strain approaches Log 10 values of −1 for four different formulations. It also demonstrates that the gels and films may be reformulated to prevent the killing of specific strains of Lactobacillus.
TABLE 3.
Gel versus Films: Effects on viability of Lactobacillus crispatus and L. jensenii
| Test product | Log 10 change in CFUsa |
||
|---|---|---|---|
| L. crispatus ATCC 33197 | L. jensenii LBP28Aa | L. jensenii ATCC 25258 | |
| PVA Film Placebo | −0.338 | +0.008 | −0.1686 |
| HEC Gel Placebo | −0.062 | −0.272 | +0.034 |
| MC Gel Placebo | −0.049 | −0.189 | +0.166 |
| PVA Film 0.01% UC781 | −0.450 | −0.343 | −0.095 |
| HEC Gel 0.01% UC781 | +0.119 | −0.880 | −0.285 |
| MC Gel 0.01% UC781 | +0.132 | −1.137 | +0.132 |
| PVA Film 0.01% UC781 HPC | +0.088 | −0.454 | −0.105 |
| HEC Gel 0.01% UC781 HPC | −0.105 | −0.660 | −0.053 |
| MC Gel 0.01% UC781 HPC | −0.098 | −0.982 | +0.062 |
| PVA Film 0.01% UC781 MC | −0.055 | −0.486 | −0.042 |
| HEC Gel 0.01% UC781 MC | −0.115 | −0.916 | −0.215 |
| MC Gel 0.01% UC781 MC | +0.068 | −0.958 | −0.402 |
Data are present as log10 change in CFUs, compared to the controls incubated in buffer only. (+) Values indicate the number of CFUs increase over controls while (−) values indicate there was decrease in the CFUs, zero would represent no change. Data presented are averaged from two or in some cases three separate experiments.
To differentiate the underlying cause, the effects of vortex mixing were studied using L. crispatus and two strains of L. jensenii. Fig. 2 demonstrates the killing occurs over a brief time, 30 sec. The ATCC type strain of L. crispatus (ATCC 33197) demonstrated only a slight decrease in the colony forming units after 30 seconds of vortex mixing while L. jensenii strains were more sensitive to killing (Fig. 2). Killing was found to be time dependent. The addition of 7.5% heat inactivated horse serum (Fig. 1), 3.75% BSA but not 3.75% glycerol prevented L. jensenii killing during vortex mixing (data not shown). Bacillus atrophaeus was also tested by mixing for up to 5 minutes. This resulted in a reduction in viability > 6 logs, the limit of detection in our system. B. atrophaeus was not destroyed when 7.5% serum was present during the mixing (Fig. 1). Loss of viability over that observed in controls was never observed when bacterial or spore suspensions were mixed by hand or when vortex mixing was performed in a round bottom 50 mL centrifuge tube (data not shown). Using phase contrast microscopy we were unable to discern any differences in the chain lengths or clumping of organisms after mixing by either method.
FIG. 2.
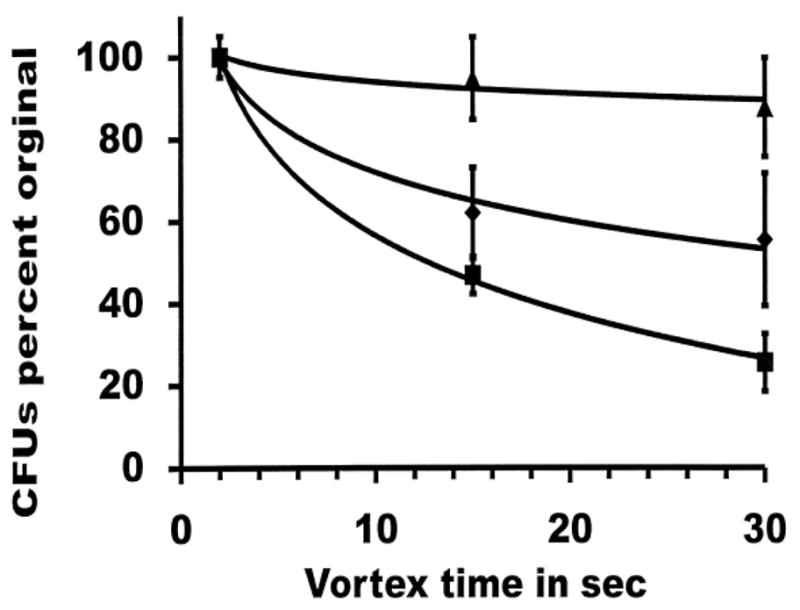
Effects of vortexing on CFUs for different Lactobacillus species. Strains were vortexed in ACES buffer as described in the text for the indicated seconds. For baselines (100%) samples were vortexed for 2 seconds and sampled for colony forming units. L. crispatus ATCC 33197 [▲], L. jensenii ATCC 25258 [■], L. jensenii LBP 90Aa [◆].
4.4. Underlying mechanism
The reductions in CFUs were only observed in the films and not with the unformulated PVA used suggesting: the polymers were protective; manipulation of the bacteria was causing the decrease in viability observed or the films were toxic.
5. Discussion
The reverse transcriptase inhibitors (RTIs) used in this study did not demonstrate toxicity against any of bacteria used in our study. This was an expected result since reverse transcriptases have not been reported in these organisms. The killing and inhibition of Lactobacillus by peptide anti-retroviral PSC-RANTES was surprising. Lactobacillus lack any known targets for these compounds. Killing was inconsistent among species and strains. For example, two isolates of L. jensenii were killed by PSC-RANTES while four isolates were inhibited. The L. vaginalis and L. gasseri strains were not affected. Intra-species variations in S-layer proteins could explain these differences (Antikainen et al., 2002, Claus et al., 2005).
Women in different parts of the world appear to have the same dominant species of vaginal Lactobacillus species: L. crispatus, L. jensenii and L. gasseri (Antonio et al., 1999, Anukam et al., 2006, Gustafsson et al., 2011, Pavlova et al., 2002, Vasquez et al., 2002). Therefore, our findings are relevant to locations outside of North America.
Most other studies have used gel formulated microbicides (Patton et al., 2006, Patton et al., 2007, Schwartz et al., 2006) and we encountered sampling difficulties with these formulations. In our studies we observed killing by gel formulations. As we explored other delivery vehicles such as films it became obvious the current methods (MCC) require sample dilution to concentrations far less than encounter during use, for example we diluted tenofovir gels 1:10 before assaying. Thus an alternative method for toxicity testing was needed that would approximate in vivo concentrations of drugs or excipients.
Killing during vortex mixing was observed for 3 of 4 strains of L. crispatus and all 4 strains of L. jensenii examined; suggesting the effect may be widespread among the Lactobacillus. Importantly, the organisms can be protected by the addition of BSA or serum to the diluent. B. atrophaeus spores were studied because we reasoned they are more resistant to killing by mechanical forces, and would not show an increase in CFUs due to chain cleavage as could be with Lactobacillus. As with the Lactobacillus, B. atrophaeus spores were killed by vortex mixing that did not occur in the presence of added protein or in a round bottom tubes. This suggests that conical tubes disrupt the smooth mixing process and introduce deleterious mechanical stresses that lead to cellular damage that is not limited to species of Lactobacillus.
The use of the deleterious effects of vortex mixing have been noted in microbiology (Gerhardt, 1981, Holmquist and Kjelleberg, 1993, Ranhand, 1974, Sadhu et al., 1989, Seung Won et al., 2008). Sadhu et al., 1989 used extensive vortex mixing to examine vaginal morphotypes but did not report the effects on flora viability. Vortexing Lactobacillus with glass beads disrupts Lactobacillus chain lengths to varying degrees depending on the species (Ranhand, 1974). Strains of S. pyogenes exhibited 100% increase in CFUs after 30 sec but with increased mixing viability decreased (Ranhand, 1974). This demonstrates that mechanical action was disrupting groups of streptococci resulting in higher colony counts. We did not observe this to any significant degree.
Seung et al. noted a lower recovery of Bacillus from the ventilation filters if the samples were mixed using a vortex mixer than if they were mixed by hand. Our data suggests they were observing loss of viability rather than a decrease in recovery of organisms from the filter materials(Seung Won Kim, 2008).
Most of the microbicides developed and tested have been gel based. In our studies only the films tested proved safe. The gel formulations always yielded greater losses in viability than film preparation, Tables 2 and 3. Setting the cutoff for passing the LTT to > two standard deviations gave us a clear and reasonable point for passing the LTT.
A cautionary note should be taken concerning the presentation of bacterial killing; for example, Simpson et al. and Baranger et al. both used percent reduction in bacterial viability in their reports of “antimicrobial” agents elafin and pre-elafin (Baranger et al., 2008, Simpson et al., 1999). Such reports tend to over emphasis small reductions in bacterial viability. In Figure 2 where the data are presented as percent of original viable bacteria the differences appear to be quite large. However, the differences are less than 1 Log 10 and are a reminder that in reporting large numbers, such as the number of bacteria in a culture the difference in the viability or killing should use Log 10.
Taken together the data suggest that the use of a vortex mixer be limited in duration and for mixing only. Dispersal, or recovery of organisms from the environment or other materials should use other methods or include protective agents such as BSA, heat inactivated serum or gelatin (Gerhardt, 1981). These results demonstrate that round bottom tubes should always be used and that there is a need for careful selection and testing of diluents when sampling and dispersing Lactobacillus species.
Lactobacillus toxicity testing has become an integral part of microbicide development and we have developed accurate and sensitive tools and have monitored the effects by some candidates on Lactobacillus species viability. Currently, we feel loss of viability of < 1 log to be unimportant; however, greater loss of viability or the observation of inhibition indicates the flora should be carefully monitored during trials. Other compounds such as nonoxynol-9 that demonstrate extensive and high rates of killing should probably not enter clinical trials. The significance of the test should become known as the data from current drug trials are revealed.
In summary the non-nucleoside reverse transcriptase inhibitors studied did not exhibit toxic or inhibitory activities towards our test panel of Lactobacillus species while the protein based anti-HIV drug PSC-RANTES did. Analysis of formulated products requires a significant dilution before the products could be manipulated for testing. In many cases, the resulting drug concentrations after dilution are far below the concentration anticipated in use making it difficult to determine whether the data are meaningful. By starting at very high bacterial concentrations we could circumvent these problems; but introduced an artifact of vortex mixing. However, we have always allowed for a drop in viability less than 1 Log10 before we were concerned about damage to the Lactobacillus in the microflora. We observed protection from killing by some of the polymer excipients, films and in some cases the gel. Comparing L. jensenii to L. crispatus, L. jensenii was more sensitive to killing by vortex mixing and most sensitive to killing by gel formulations. This would suggest closer monitoring of the species is needed in future clinical trials. Selecting the proper test for Lactobacillus toxicity depends on the drug, its formulation and whether or not the drug inhibits or kills Lactobacillus. Our algorithm is to test pure materials with the MCC assay at the intended in vivo concentration. If killing is observed the MCC is defined and if inhibition is observed the MIC is determined. Formulated materials are tested using the Lactobacillus toxicity test and are considered safe if the loss of viability is less than one Log.
6. Conclusions
Round bottom tubes should be used when bacteria are mixed with a vortex mixer. The reverse transcriptase inhibitors did not demonstrate toxicity against any of our study bacteria while some peptide antimicrobials were active against some of our test species. Methylcellulose and the various molecular species polyvinyl alcohol all passed the LTT. The Lactobacillus toxicity test performs better than the test currently used in the field of microbicide development.
Research highlights.
The standard Lactobacillus toxicity test for anti-HIV microbicides is presented.
Vortex mixers may kill large numbers of organisms and result in false values.
Non-nucleoside reverse transcriptase inhibitors should continue development.
Polyvinyl alcohol based films are safe and not deleterious to Lactobacillus.
Film based anti-HIV microbicides should continue development.
Acknowledgments
This work was supported by grants from the International Partnership for Microbicides, CONRAD, Pendleton Charitable Trust Fund, NIH NIAID Grant U01 AI068633 and NIAID Grant AI39061.
Abbreviations
- PSC-RANTES
synthetic peptide CCR5 antagonist
- MCC
minimum cidal concentration
- CFUs
colony forming units
- MIC agar dilution
the minimum inhibitory concentration test
- NNRTIs
non-nucleoside reverse transcriptase inhibitors
- LL-37 and LSA-5
cathelicidin derived antimicrobial peptides
- RC 101
an anti-HIV peptide microbicides
- ATCC
American Type Culture Collection
- STDs
sexually transmitted diseases
- UC781
a thiocarboxanilide nonnucleoside reverse transcriptase inhibitor of human immunodeficiency virus (HIV-1)
- TMC120
TMC120-R147681 (dapivirine) is a reverse transcriptase inhibitor
- CONRAD
the Contraceptive Research and Development a Division of the Department of Obstetrics and Gynecology of Eastern Virginia Medical School, 1911 North Fort Myer Drive, Suite 900, Arlington, Virginia 22209
- ACES
buffer [(N-2-acetamido)-2- aminoethanesulfonic acid]
Footnotes
Parts of this work were presented at the Microbicides 2010 Conference in Pittsburgh, PA. USA. Parts of this work are contained in the Ph.D. Dissertation of H. Yang.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antikainen J, Anton L, Sillanpaa J, Korhonen TK. Domains in the S-layer protein CbsA of Lactobacillus crispatus involved in adherence to collagens, laminin and lipoteichoic acids and in self-assembly. Molecular microbiology. 2002;46:381–394. doi: 10.1046/j.1365-2958.2002.03180.x. [DOI] [PubMed] [Google Scholar]
- Antonio MA, Hawes SE, Hillier SL. The identification of vaginal Lactobacillus species and the demographic and microbiologic characteristics of women colonized by these species. J Infect Dis. 1999;180:1950–1956. doi: 10.1086/315109. [DOI] [PubMed] [Google Scholar]
- Anukam KC, Osazuwa EO, Ahonkhai I, Reid G. Lactobacillus Vaginal Microbiota of Women Attending a Reproductive Health Care Service in Benin City, Nigeria. Sexually Transmitted Diseases. 2006;33:59–62. doi: 10.1097/01.olq.0000175367.15559.c4. [DOI] [PubMed] [Google Scholar]
- Baranger K, Zani ML, Chandenier J, Dallet-Choisy S, Moreau T. The antibacterial and antifungal properties of trappin-2 (pre-elafin) do not depend on its protease inhibitory function. The FEBS journal. 2008;275:2008–2020. doi: 10.1111/j.1742-4658.2008.06355.x. [DOI] [PubMed] [Google Scholar]
- Claus H, Akca E, Debaerdemaeker T, Evrard C, Declercq JP, Harris JR, Schlott B, Konig H. Molecular organization of selected prokaryotic S-layer proteins. Canadian journal of microbiology. 2005;51:731–743. doi: 10.1139/w05-093. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Ball JM, Miller MA, David CM, Montelaro RC. A survey of potential problems and quality control in peptide synthesis by the fluorenylmethoxycarbonyl procedure. Pept Res. 1991;4:19–25. [PubMed] [Google Scholar]
- Gerhardt P. Diluents and biomass measurement. In: Gerhardt P, editor. Manual of methods for general bacteriology. American Society for Microbiology; Washington DC: 1981. pp. 504–507. [Google Scholar]
- Gustafsson R, Ahrne S, Jeppsson B, Benoni C, Olsson C, Stjernquist M, Ohlsson B. The Lactobacillus flora in vagina and rectum of fertile and postmenopausal healthy Swedish women. BMC Women’s Health. 2011;11:17. doi: 10.1186/1472-6874-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist L, Kjelleberg S. Changes in viability, respiratory activity and morphology of the marine Vibrio sp. strain S14 during starvation of individual nutrients and subsequent recovery. FEMS Microbiology Ecology. 1993;12:215–223. [Google Scholar]
- Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis. 1999;180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- Moncla BJ, Hillier SL. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex Transm Dis. 2005;32:491–494. doi: 10.1097/01.olq.0000170444.13666.e9. [DOI] [PubMed] [Google Scholar]
- Moncla BJ, Rohan LC, Pryke K, Graebing PW. Degradation of Naturally occurring and engineered antimicrobial peptides by preoteases. Abstr. 174; International Microbicide Conference; Pittsburgh, PA USA. 2010. p. 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncla BJ, Pryke K, Isaacs CE. Killing of Neisseria gonorrhoeae, Streptococcus agalactiae (group B streptococcus), Haemophilus ducreyi, and vaginal Lactobacillus by 3-O-octyl-sn-glycerol. Antimicrob Agents Chemother. 2008;52:1577–1579. doi: 10.1128/AAC.01023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50:1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Sweeney YT, Balkus JE, Rohan LC, Moncla BJ, Parniak MA, Hillier SL. Preclinical safety assessments of UC781 anti-human immunodeficiency virus topical microbicide formulations. Antimicrob Agents Chemother. 2007;51:1608–1615. doi: 10.1128/AAC.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova SI, Kilic AO, Kilic SS, So JS, Nader-Macias ME, Simoes JA, Tao L. Genetic diversity of vaginal lactobacilli from women in different countries based on 16S rRNA gene sequences. Journal of Applied Microbiology. 2002;92:451–459. doi: 10.1046/j.1365-2672.2002.01547.x. [DOI] [PubMed] [Google Scholar]
- Ranhand JM. Simple, Inexpensive Procedure for the Disruption of Bacteria. Appl Environ Microbiol. 1974;28:66–69. doi: 10.1128/am.28.1.66-69.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadhu K, Domingue PAG, Chow AW, Nelligan J, Bartlett K, Costerton JW. A Morphological Study of the in situ Tissue-Associated Autochthonous Microflora of the Human Vagina. Microbial Ecology in Health and Disease. 1989;2:99–106. [Google Scholar]
- Schwartz JL, Mauck C, Lai JJ, Creinin MD, Brache V, Ballagh SA, Weiner DH, Hillier SL, Fichorova RN, Callahan M. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind Phase I safety study. Contraception. 2006;74:133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Seung Won K, Peter CR, Thomas HK, Sagar MG, Ramakrishnan MA, Senthilvelan A, James EF. Optimizing the Recovery of Surrogates for Bacterial Bioterrorism Agents from Ventilation Filters. CLEAN - Soil, Air, Water. 2008;36:601–608. [Google Scholar]
- Simpson AJ, Maxwell AI, Govan JR, Haslett C, Sallenave JM. Elafin (elastase-specific inhibitor) has anti-microbial activity against gram-positive and gram-negative respiratory pathogens. FEBS letters. 1999;452:309–313. doi: 10.1016/s0014-5793(99)00670-5. [DOI] [PubMed] [Google Scholar]
- Tien D, Schnaare RL, Kang F, Cohl G, McCormick TJ, Moench TR, Doncel G, Watson K, Buckheit RW, Lewis MG, Schwartz J, Douville K, Romano JW. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS research and human retroviruses. 2005;21:845–853. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- Vasquez A, Jakobsson T, Ahrne S, Forsum U, Molin G. Vaginal Lactobacillus Flora of Healthy Swedish Women. J Clin Microbiol. 2002;40:2746–2749. doi: 10.1128/JCM.40.8.2746-2749.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]