Abstract
We have reported that supplemental doses of the α- and γ-tocopherol isoforms of vitamin E decrease and increase, respectively, allergic lung inflammation. We have now assessed whether these effects of tocopherols are reversible. For these studies, mice were treated with antigen and supplemental tocopherols in a first phase of treatment followed by a 4 week clearance phase and then the mice received a second phase of antigen and tocopherol treatments. The pro-inflammatory effects of supplemental levels of γ-tocopherol in phase 1 were only partially reversed by supplemental α-tocopherol in phase 2 but were completely reversed by raising α-tocopherol levels 10-fold in phase 2. When γ-tocopherol levels were increased 10-fold (highly-elevated tocopherol) so that the lung tissue γ-tocopherol levels were equal to the lung tissue levels of supplemental α-tocopherol, γ-tocopherol reduced leukocyte numbers in the lung lavage fluid. In contrast to the lung lavage fluid, highly-elevated levels of γ-tocopherol increased inflammation in the lung tissue. These regulatory effects of highly-elevated tocopherols on tissue inflammation and lung lavage fluid were reversible in a second phase of antigen challenge without tocopherols. In summary, the pro-inflammatory effects of supplemental γ-tocopherol on lung inflammation were partially reversed by supplemental levels of α-tocopherol but were completely reversed by highly-elevated-levels of α-tocopherol. Also, highly-elevated levels of γ-tocopherol were inhibitory and reversible in lung lavage but, importantly, were pro-inflammatory in lung tissue sections. These results have implications for future studies with tocopherols and provide a new context in which to review vitamin E studies in the literature.
Keywords: inflammation, tocopherol, vitamin E, endothelial cell, leukocyte recruitment
Introduction
Vitamin E is an antioxidant lipid that has been used to regulate inflammatory disease. Vitamin E consists of multiple natural isoforms, including the natural α-, β-, γ-, and δ-tocopherols and the unsaturated α-, β-, γ-, and δ-tocotrienols, which differ in the number of methyl groups on the chromanol head (1, 2). The natural isoforms (d-form) of tocopherols are the R,R,R stereoisomers in the 2, 4’ and 8’ positions of the lipid tail, whereas synthetic tocopherols have R or S racemic conformations at these positions. Dietary tocopherols are taken up from the intestine, transported via chylomicrons in the lymph to the blood and then to the liver. Subcutaneously administered tocopherols, which are used in our studies, are also transported through the lymph to the blood and then to the liver. Though γ-tocopherol is the most abundant vitamin E isoform in diet, it exists in tissue at only 10% the concentration of α-tocopherol due to the preferential uptake of α-tocopherol by the hepatic enzyme α-tocopherol transfer protein (αTTP)(3). Nevertheless, αTTP transfers significant quantities of γ-tocopherol to lipid particles which then enter the circulation (3). Cells acquire tocopherols from plasma lipoproteins by plasma phospholipid transfer protein, scavenger receptors, or the lipoprotein lipase pathway (4). After cell uptake, tocopherols are located in cell membranes. At equal molar concentrations in vitro, α-tocopherol, γ-tocopherol, and the tocotrienol isoforms have a similar capacity to scavenge reactive oxygen species (ROS) during lipid oxidation (1, 5, 6). In addition to serving as antioxidants, vitamin E isoforms have been reported to have non-antioxidant functions (1, 7, 8).
It is reported that asthmatics have low levels of the vitamin E isoform α-tocopherol (9–12), however there are seemingly contradictory reports regarding the effect of vitamin E on allergic/asthmatic inflammation (13–15). It is reported that α-tocopherol is beneficial in reducing asthma in some European countries (Finland and Italy) (16, 17). Disappointingly, clinical trials in the United States and the Netherlands using α-tocopherol supplements have failed to show benefit in asthma (17–20). The plasma levels of tocopherols in Americans and Europeans are reported to differ; several reports indicate that plasma levels of γ-tocopherol in Americans and people in the Netherlands are 2–6 times higher than most Europeans, whereas α-tocopherol plasma levels do not differ among these countries (reviewed in (21)). These plasma tocopherol levels reflect differences in diet since γ-tocopherol is the major form of vitamin E in the diet of Americans but is not in abundance in most European diets (21), which use oils with no or very low γ-tocopherol. Moreover, we recently reported that supplemental levels of γ-tocopherol enhance inflammation and supplemental levels of α-tocopherol reduce inflammation in a murine asthma model (8). It is not known whether the pro-inflammatory and anti-inflammatory effects of tocopherol isoforms are reversible. Therefore, we investigated the reversibility of the effects of tocopherols by determining whether the pro-inflammatory effect of supplemental γ-tocopherol during a phase of three OVA-challenges can be reversed with a 4 week clearance of tissue tocopherols/inflammation, followed by administration of α-tocopherol during a second phase with OVA challenge.
Using purified natural isoforms of tocopherols at supplemental concentrations, we report here the novel findings that the differential effects of supplemental tocopherols on allergic inflammation are only partially reversible. In contrast, this inflammation is completely reversed and reduced by highly-elevated amounts of α-tocopherol. In vivo, raising γ-tocopherol to equal molar concentrations as α-tocopherol inhibited lung lavage fluid inflammation. In vitro, these doses of γ-tocopherol partially blocked leukocyte transendothelial migration, by a direct effect on endothelial cells. Unlike the partial reversibility of tocopherols’ effects at supplemental treatment levels, the anti-inflammatory effects of highly-elevated levels of tocopherols were completely reversible for inflammation in lung lavage. However, for highly-elevated levels of γ-tocopherol, there were differential effects on inflammation in lung lavage and tissue sections since there was reduced inflammation in lung lavage but elevated inflammation in lung tissue sections.
Materials & Methods
Animals
BALB/c mice were from Jackson Laboratories, Bar Harbor, Maine. The studies are approved by the Northwestern University institutional review committee for animals.
Cells
The endothelial cell line mHEVa was cultured as previously described (22, 23). Spleen cells were prepared from freshly isolated male BALB/c mouse spleens (22) and spleen red blood cells were lysed by hypotonic shock (22).
Tocopherol reagents
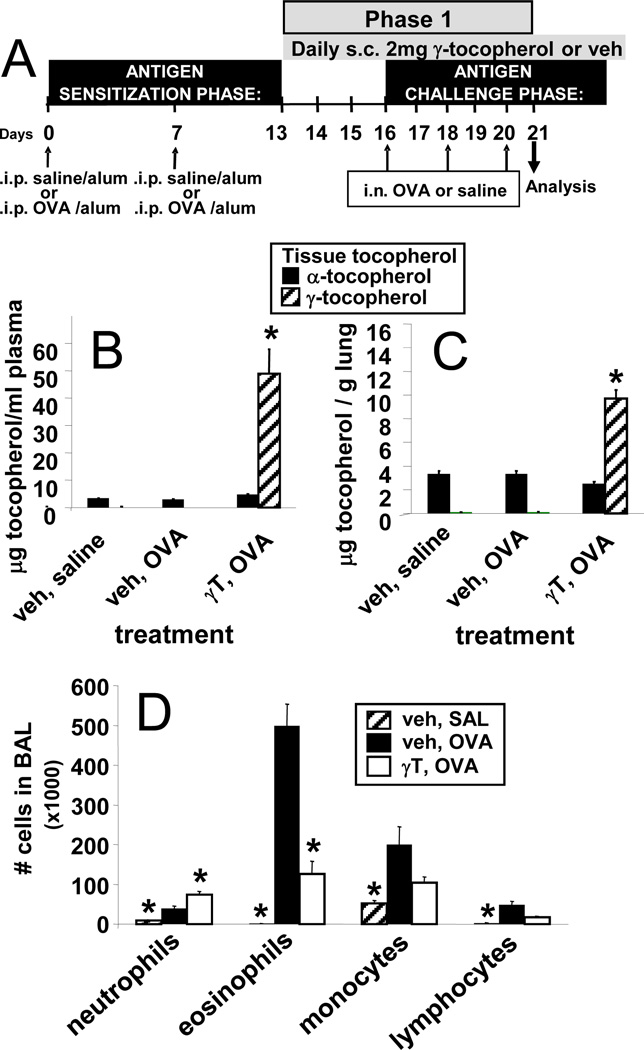
Ethoxylated castor oil was composed of polyethoxylated castor oil (BASF), 20% ethanol, and 1% benzyl alcohol. d-α-tocopherol (MP Biomedicals) was >98% pure. D-γ-tocopherol (Supelco) was 99.9% pure. We confirmed the purity of these tocopherols by HPLC with electrochemical detection as described below. No tocopherols were present in the ethoxylated castor oil, also measured by HPLC. The purified tocopherols were diluted in ethoxylated castor oil for the in vivo studies. It was determined that complete suspension of tocopherols in the ethoxylated castor oil required rolling and inverting mixture at room temperature for 20 minutes (data not shown). This is in contrast to our previous study where the tocopherols were suspended for only a couple of minutes (8). As discussed in the results section (Figure 2), we determined that 0.2 mg subcutaneous tocopherol per day (“supplemental” tocopherol levels) is sufficient to achieve plasma tocopherol levels that were in our previous studies (8) . “Highly elevated” dosing refers to treatment of 2 mg subcutaneous tocopherol per day.
Figure 2. Opposing functions of supplemental levels of natural d-α-tocopherol and d-γ-tocopherol.
A. Structure of natural d-α-tocopherol (d-α-T) and natural d-γ-tocopherol (d-γ-T), which differ by one methyl group (arrows). B. Tocopherol in plasma and perfused lung tissue after 8 daily subcutaneous (s.c.) doses of 0.04, 0.2 or 0.6 mg αT or γT. Tocopherol was measured by HPLC. n = 4–5 animals per group. C. Allergic lung inflammation protocol with intraperitoneal (i.p.) sensitization with chicken egg white albumin (OVA) fraction V and tocopherol treatment starting after OVA-sensitization. Intranasal (i.n.) OVA challenge was with fraction VI OVA. Inflammation was analyzed 24 hours after the last OVA challenge. D. Bronchoalveolar lavage (BAL) neutrophils, eosinophils, monocytes and lymphocytes were counted with a hemocytometer and cytospun BAL cells were counted by standard morphological criteria. Veh, vehicle.
OVA / tocopherol administration and inflammation
BALB/c female mice, 4–6 weeks of age at start of experiment, were maintained on chow diet. The mice were sensitized by intraperitoneal injection (200 µl) of OVA grade V (10 µg)/alum or saline/alum on days 0 and 7 (24). Then the mice received either one phase or two phases of treatments with tocopherols during OVA challenge (Figure 1) as indicated for each study.
Figure 1. Timeline synopsis for tocopherol and OVA treatments.
A) Tocopherol treatment during a single phase of OVA challenges. B) Tocopherol treatment during two phases of OVA challenges.
Briefly, for studies with only one phase of tocopherol treatments and OVA challenges (Figure 1A), on days 13–20, the mice received daily subcutaneous injections (50µl) of “supplemental” (0.2 mg) or “highly elevated” (2 mg) doses of tocopherols in 50 µl ethoxylated castor oil (25). Vehicle mice were injected with ethoxylated castor oil alone. Mice treated with both isoforms of tocopherols at supplemental levels were injected with 0.2 mg of α-tocopherol and 0.2 mg γ-tocopherol in 50 µl vehicle; mice treated with both isoforms at highly elevated levels were injected with 2 mg of α-tocopherol and 2 mg γ-tocopherol in 50 µL vehicle. The tocopherol doses for each study are as indicated in the figures for the studies. On days 16, 18, and 20, the mice were challenged with 150 µg intranasal OVA fraction VI (Sigma) in saline or saline alone (24). On day 21, mice were weighed and sacrificed for tissue collection.
In studies with two phases of tocopherol treatments and OVA challenges (Figure 1B), after phase 1 OVA challenges, mice entered a clearance phase from days 21–50, during which tocopherol was cleared from tissues and lung inflammation receded. Starting on day 51, mice received daily vehicle or tocopherol treatments. In the studies with supplemental levels of tocopherols, mice were challenged once on day 53 with 150 µg intranasal OVA fraction VI (Sigma) in saline or saline alone to examine the initial response to rechallenge with antigen (Figure 1B). In contrast, in the studies with highly-elevated tocopherols in two phases of treatments, mice received three rechallenges (days 53, 55, 57) with 150 µg intranasal OVA fraction VI (Sigma) in saline or saline alone to examine the ability to regulate the response to repeated challenges with antigen (Figure 1B).
Twenty-four hours after the last OVA challenge (Figure 1), plasma and lung tocopherol levels were measured. Bronchoalveolar lavage (BAL) cells and blood eosinophils were stained and counted as previously described (24). Frozen lung tissue sections were stained with hematoxylin and eosin. OVA-specific IgE was determined by ELISA as previously described (26). Lung lavage and lung tissue were examined for cytokines by ELISA and qRT-PCR, respectively.
Tocopherol measurement
Lungs were perfused, weighed and homogenized in absolute ethanol with 5% ascorbic acid on ice. The internal standard tocol is added to each lung to determine recovery. mHEVa cells, plasma or homogenate were extracted with an equal volume of 0.37 wt% butylated hydroxytoluene in hexane to prevent oxidation and increase recovery of tocopherol. The samples were vortexed and then centrifuged for 5 minutes at 2,000 rpm at room temperature. The hexane layer was removed to a separate vial and the hexane extraction step was repeated two more times for a total of three hexane extractions per sample. The hexane layer was dried under nitrogen and stored at −20°C. The samples were reconstituted in methanol and then tocopherols were separated using a reverse phase C18 HPLC column and HPLC (Waters Company, Milford, MA) with 99% methanol-1% water as a mobile phase with detection with an electrochemical detector (potential 0.7V) (Waters Company).
Cytokines and chemokines measurement
The BAL were tested for levels of cytokines using Invitrogen’s mouse Th1/Th2 multiplexing detection kit plus IL-13 singleplex kit (both from Life Technologies) using the Luminex 200 multiplexing system and xPONENT analysis software (Luminex Corporation, Austin, TX). CCL11 and CCL24 were determined by quantitative RT-PCR from lung tissue. Total RNA was isolated from 10–15 mg lung tissue using the QIAGEN RNeasy Mini Kit. cDNA was prepared using Quanta’s qScript cDNA synthesis kit and analyzed by PCR on an ABI 7300 Thermal Cycler (Applied Biosystems, Carlsbad, CA). Taqman primers/probes and Taqman Universal Master Mix were used as directed (Applied Biosystems).
In vitro cell association and migration assays with laminar flow
For endothelial cell pre-treatment, 50–70% confluent endothelial cells (mHEVa cells) grown in a monolayer were treated with γ-tocopherol in DMSO overnight; migration assay was run the following day when cells reached 100% confluence. For leukocyte pre-treatment, 4–6-week old male BALB/c mice were treated daily for 4 days with subcutaneous injections of highly elevated γ-tocopherol or vehicle, as described above. Spleen cells were freshly prepared on day 5 and migration assay was completed using untreated confluent mHEVa monolayers. A parallel plate flow chamber was used to examine leukocyte migration under conditions of laminar flow of 2 dynes/cm2 as previously described (23, 27). Leukocyte transendothelial migration was examined at 15 minutes by fixing the cells and examination of phase dark cells by phase light microscopy (23, 27). The transendothelial migration of leukocytes in this assay is induced by the endothelial cell-derived chemokine MCP-1 (28).
Statistics
Data were analyzed by a one way ANOVA followed by Tukey’s or Dunn’s multiple comparisons test (SigmaStat, Jandel Scientific, San Ramon, CA). Presented are the means ± the standard errors.
Results
Reversibility of tocopherol regulation of allergic inflammation
We previously reported that α-tocopherol supplementation reduces and γ-tocopherol elevates leukocyte recruitment during allergic lung inflammation by, at least, direct effects of tocopherols on the endothelium (8). Additionally, when supplemental α-tocopherol and γ-tocopherol are administered together, an intermediate phenotype is observed such that inflammation is not significantly different from allergen-challenged vehicle-treated mice (8). Therefore, we determined whether these regulatory effects of purified natural d-α-tocopherol (α-tocopherol) and d-γ-tocopherol (γ-tocopherol) isoforms (Figure 2A) were reversible. As in previous studies (8), ethoxylated castor oil was used as the vehicle for the tocopherols because it does not contain tocopherols or compounds that react with tocopherols and is used for pharmaceutical suspension of viscous lipids. A few days of subcutaneous administration of tocopherols was used in these studies rather than feeding for weeks with tocopherol-containing diets to change tissue tocopherol levels (25, 29) so that tissue tocopherol levels were altered in the few days before antigen challenge (Figure 2C).
Before administering the tocopherols to the mice, purity (>98%) and concentration of tocopherols in the vehicle was determined by HPLC with electrochemical detection (data not shown). In contrast to our previous studies in which 2mg/day doses of tocopherols were suspended for only a couple of minutes (8), we have determined that complete suspension of tocopherols in ethoxylated castor oil requires at least 20 minutes as determined by HPLC (data not shown). Therefore, a dose curve for subcutaneous tocopherol administration was used to determine the quantities of completely suspended tocopherols that will raise plasma concentrations to the 10–12 µg α-tocopherol/ml and 3–5 µg γ-tocopherol/ml as in our previous report (8). Plasma and lung tocopherols for mice treated with 0.2 mg α-tocopherol or γ-tocopherol in 50 µl ethoxylated castor oil per day for 8 days (Figure 2B) is consistent with plasma and lung tocopherol levels in our previous report (8). The fold increase in plasma tocopherols in these studies is similar to the fold increase in human plasma levels from tocopherol supplementation (30, 31). Furthermore, the 0.2 mg dose of tocopherols administered daily during OVA antigen challenge (Figure 2C) demonstrated opposing immune-regulatory functions of α-tocopherol and γ-tocopherol (Figure 2D), as we previously described (8). Tocopherols did not alter basal levels of leukocytes in saline-challenged mice (data not shown)(8). In these and previous reports of tocopherol supplementation (8), the body weights of the mice were unaltered by tocopherol treatments (data not shown). In summary, we define 0.2 mg tocopherol treatment/day as “supplemental tocopherol treatment” and the resulting tissue uptake of tocopherols as “supplemental tissue tocopherol levels”.
To examine the reversibility of supplemental tocopherol treatment in the OVA-asthma model (timeline in Figure 3A), mice were sensitized with OVA followed by subcutaneous treatment with daily supplemental α-tocopherol or γ-tocopherol and challenged three times with OVA intranasally in phase 1; next, these mice entered a 4 week clearance phase without tocopherol or antigen administration; then, the mice entered phase 2 in which they received daily subcutaneously tocopherol treatment and were re-challenged once with OVA to examine the effects of tocopherols at the initiation of antigen re-challenge (Figure 3A). In phase 2, the mice received the same tocopherol isoform or the other tocopherol isoform; the tocopherol treatment groups are shown in Figure 3A. In addition to treatment groups that were given supplemental tocopherol treatments (0.2 mg/day) during phase 2, two treatment groups were administered α-tocopherol at 5 times (1 mg/day) and 10 times (2 mg/day) the supplemental tocopherol levels (Figure 3A). Twenty-four hours following the end of phase 1 or phase 2 (Day 21 and day 54) (Figure 3A), there was the expected elevation in plasma tocopherols after tocopherol treatment (Figure 3B,D). A baseline of α-tocopherol but not γ-tocopherol is observed in groups that were not treated with tocopherols because α-tocopherol is present in standard rodent chow, whereas γ-tocopherol is low to not detected in rodent chow. The four week clearance phase was sufficient time to clear tocopherol from plasma (Figure 3C) and resolve inflammation from the lung (32, 33).
Figure 3. Timeline and plasma tocopherol levels during analysis of the reversibility of the regulatory effects of supplemental tocopherol on allergic lung inflammation.
A. Mice were sensitized with OVA and then administered subcutaneous 0.2 mg tocopherol daily during OVA challenge in phase 1 of treatments. Following phase 1, mice entered a 4-week clearance phase during which they were not treated with tocopherols or challenged with OVA. At the end of the clearance phase, mice were treated subcutaneously with 0.2 mg tocopherols and rechallenged with OVA in phase 2 of treatments. In phase 2, mice were again treated with daily doses of supplemental tocopherol (same isoform as phase 1, different isoform than phase 1, or vehicle) and re-challenged one time with OVA. In addition, two treatment groups were daily administered 5× and 10× supplemental αT in phase 2. Inflammation was analyzed 24 hours following this re-challenge. n, number of animals per group. B. Tocopherol in plasma on day 21 as measured by HPLC. C. Tocopherol in plasma on day 49 as measured by HPLC. D. Tocopherol in plasma on day 54 as measured by HPLC. Note that on day 21, day 49 or day 54, plasma tocopherol in saline-treated groups receiving tocopherols were the same as those treated with OVA and tocopherols (data not shown). i.p., intraperitoneal; i.n., intranasal; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as in panel A.
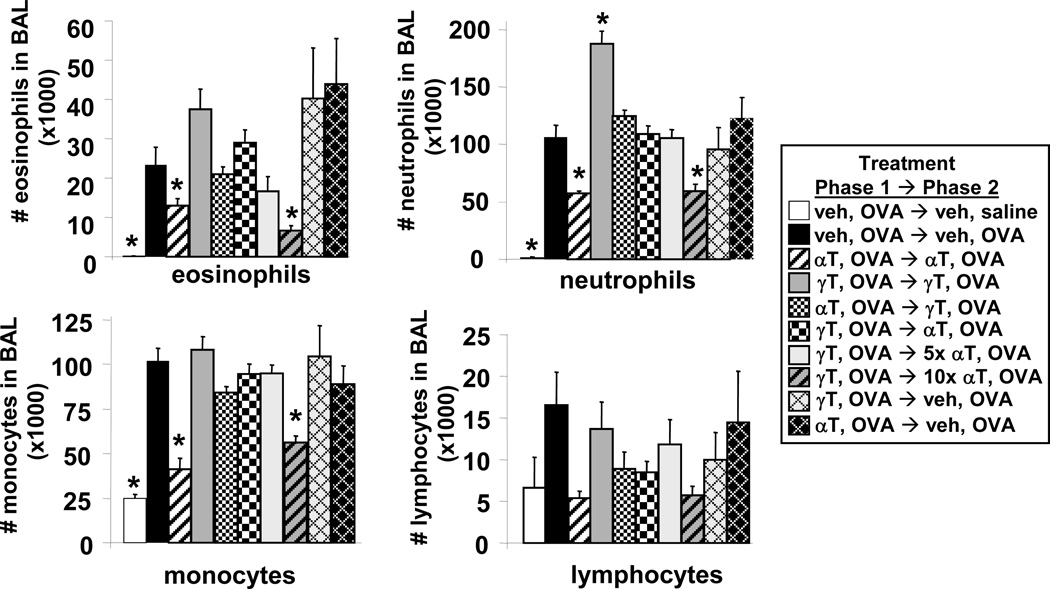
As expected, the single OVA re-challenge, that was used to examine the initial response to antigen rechallenge, induced an increase in leukocytes in the bronchoalveolar lavage (BAL) including eosinophils, neutrophils, monocytes and lymphocytes as compared to saline controls (Figure 4). It is reported that at 24 hours after a single OVA challenge, there is recruitment of several leukocyte cell types including eosinophils, neutrophils, monocytes and lymphocytes, whereas during allergic inflammation, the peak accumulation in eosinophils occurs after several OVA challenges (24, 34). Supplemental α-tocopherol and γ-tocopherol treatments did not alter basal levels of the lung leukocytes in saline-treated mice (data not shown). Treatment with supplemental α-tocopherol in both phase 1 and 2 significantly reduced OVA-challenged recruitment of lung lavage eosinophils, neutrophils, and monocytes as compared to vehicle-treated OVA-challenged mice (Figure 4). In contrast, treatment with supplemental γ-tocopherol in both phases raised OVA-induced lung lavage eosinophils and significantly increased OVA-induced neutrophils (Figure 4). Groups that received phase 1→phase 2 treatments of α-tocopherol,OVA→γ-tocopherol,OVA or received γ-tocopherol,OVA→α-tocopherol,OVA had an intermediate phenotype such that the number of lung lavage leukocytes was similar to that for vehicle-treated OVA-challenged mice (Figure 4). Thus, switching tocopherol isoform in phase 2 did not completely reduce lung lavage inflammation. In addition, the lung leukocytes in the tocopherol,OVA →vehicle,OVA groups were not different than the vehicle,OVA→vehicle,OVA group (Figure 4). However, raising α-tocopherol in phase 2 (the γ-tocopherol,OVA→10X α-tocopherol,OVA group) completely reduced the inflammation to the level in the α-tocopherol,OVA→α-tocopherol,OVA group (Figure 4). We define these 10-fold higher levels of tocopherols (10 × 0.2mg/day = 2mg tocopherol/day) in phase 2 as “highly-elevated” doses of tocopherols (Figure 3).
Figure 4. Reversibility of the regulatory effects of supplemental tocopherol levels on allergic bronchoalveolar lavage inflammation.
Mice were treated with tocopherols and OVA as in Figure 3. On day 54, bronchoalveolar lavage (BAL) neutrophils, eosinophils, monocytes and lymphocytes were counted with a hemocytometer and cytospun BAL cells were counted by standard morphological criteria. veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 3A; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA→veh OVA group.
The tocopherols also regulated the lung tissue inflammation. Alpha-tocopherol was anti-inflammatory (Figure 5C) and γ-tocopherol was pro-inflammatory (Figure 5D). Switching the tocopherol isoform in phase 2 (Figure 5E,F) or omitting tocopherol during phase 2 with OVA restimulation (Figure 5G,H) resulted in an intermediate phenotype where inflammation was similar to the levels in the vehicle-treated OVA-restimulated group (Figure 5A). However, the γ-tocopherol,OVA→10X α-tocopherol,OVA group had reduced inflammation in the lung tissue (Figure 5I). Tocopherols did not alter blood eosinophils (Figure 5J). Consistent with OVA-specific IgE antibody production during the sensitization phase, the tocopherols that were administered after sensitization did not affect OVA-specific IgE (Figure 6A).
Figure 5. Reversibility of the regulatory effects of supplemental tocopherol on allergic lung tissue inflammation.
Mice were treated with tocopherols as in Figure 3A. A–I. Representative micrographs (40× objective) of perivascular regions in the lung tissue from day 54 as in Figure 3A. Lung tissues were stained with hematoxylin and eosin. J. number of blood eosinophils on day 54 of treatments as in Figure 3A. white arrow, vessel lumen; black arrow, bronchial airway; veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 3A; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol.
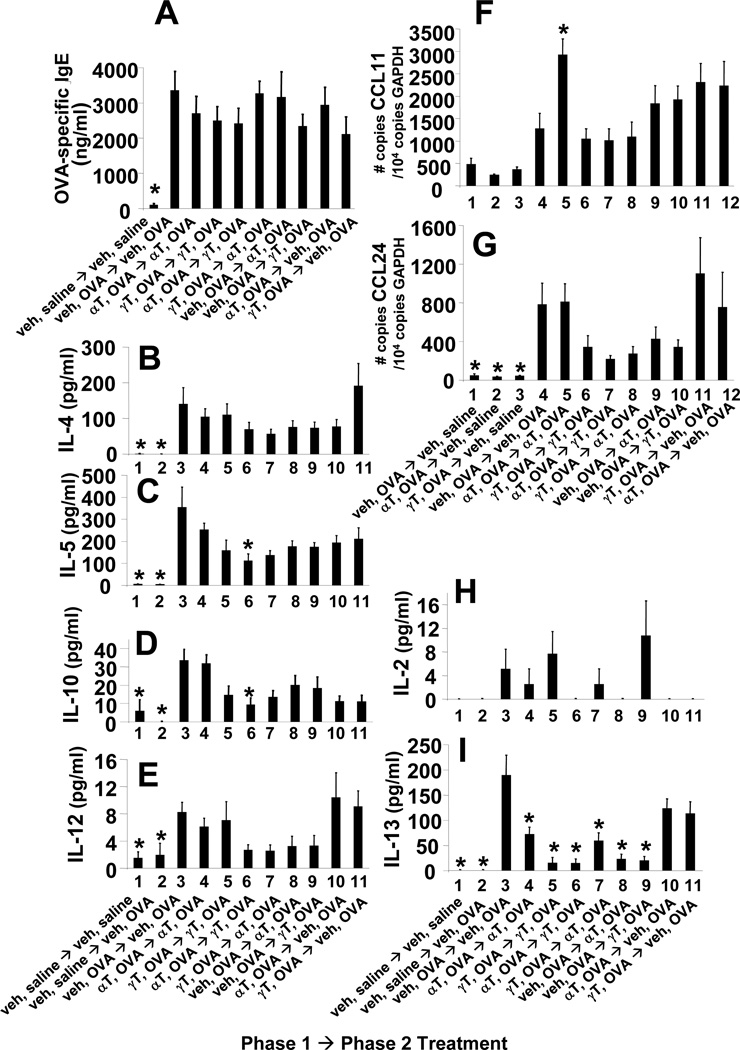
Figure 6. Switching tocopherol isoforms at supplemental levels in phase 2 did not alter OVA-specific IgE, lung cytokines or lung chemokines.
Mice were treated with supplemental levels of tocopherols as in Figure 3A. A. serum OVA-specific IgE as measured by ELISA. B-F,I,J. BAL supernatants were examined for cytokines using the Th1/Th2 mouse cytokine multiplexing kit with IL-13 (Life Technologies). G,H. lung tissue was placed in RNAlater then examined for eotaxin 1 (CCL11) and eotaxin 2 (CCL24) expression by real-time PCR. veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 3A; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA→veh OVA group.
Supplemental levels of tocopherols do not alter OVA-induced Th1/Th2 cytokines
Since tocopherols regulated inflammation in Figures 4–5, we determined whether tocopherols affected regulatory cytokines and chemokines. The Th1 cytokines IFNγ and IL-2 were not affected by treatment with supplemental tocopherols (Figure 6I,J). The OVA-induced increases in IL-4, IL-5, CCL11 and CCL24 were not affected by the supplemental tocopherol treatments or the 10X α-tocopherol treatment (Figure 6B,C,G,H). OVA-induced IL-13 was not altered by tocopherols (Figure 6F). This is consistent with reports that leukocyte infiltration can be regulated in Th2-driven models with no change in cytokine production (8, #2953, #2621, 35, 36). IL-12 and IL-10 were altered in two treatment groups. IL-12 expression was increased in the γ-tocopherol,OVA→γ-tocopherol,OVA group compared to the vehicle,OVA→vehicle,OVA group (Figure 6E). IL-10 in the γ-tocopherol,OVA→10× α-tocopherol,OVA group was reduced compared to the vehicle,OVA→vehicle,OVA group (Figure 6D). Overall, supplemental levels of tocopherols did not greatly impact expression of cytokines or chemokines.
Highly-elevated γ-tocopherol reduces lung lavage inflammation
When equal amounts of α-tocopherol or γ-tocopherol (0.2mg tocopherol/day) are administered to mice (Figures 2 and 3), 10 times more α-tocopherol than γ-tocopherol is acquired by tissues due to the preferential transfer of α-tocopherol to lipid particles in the liver by α-tocopherol transfer protein (α-TTP) (reviewed in (3, 37)). To examine the functional effects of equal molar tissue levels of γ-tocopherol versus α-tocopherol, it was determined that 10 times more γ-tocopherol (2 mg γ-tocopherol/day) must be subcutaneously administered to achieve approximately equal molar lung tissue levels of γ-tocopherol as that for lung tissue levels of supplemental α-tocopherol (Figure 7C versus Figure 2B). We have defined 10 times the supplemental treatment levels as “highly-elevated tocopherol treatment” and the resulting tissue levels from this highly-elevated treatment as “highly-elevated tissue tocopherol levels”. For these studies, mice were treated with highly-elevated γ-tocopherol and three OVA re-challenges as in the timeline in Figure 7A. After the three OVA re-challenges, the highly-elevated γ-tocopherol treatments inhibited lung lavage eosinophilia as compared to vehicle controls (Figure 7D). In addition, highly-elevated γ-tocopherol treatment significantly reduced IL-5, IL-10, MIP-1α and MCP-1 but not IFNγ, IL-2, CCL11 or CCL24 (Figure 8). Thus, at equal molar lung tissue levels of α-tocopherol and γ-tocopherol, α-tocopherol (Figure 2D) and γ-tocopherol (Figure 7D) both function to inhibit lung lavage inflammation.
Figure 7. Highly-elevated γ-tocopherol decreases lung bronchoalveolar lavage inflammation.
A. Timeline for treatments with OVA and highly-elevated γ-tocopherol (γT) during allergic lung inflammation (only 1 phase of OVA treatments). On day 21 (panel A), plasma was collected and lungs were perfused free of blood. Tocopherol was measured by HPLC. Lung lavage leukocytes were examined on day 21. B. Plasma tocopherol. C. Lung tocopherols. D. BAL neutrophils, eosinophils, monocytes and lymphocytes were counted with a hemocytometer and cytospun BAL cells were counted by standard morphological criteria. γT, d-gamma-tocopherol; veh, vehicle; *, p<0.05 as compared to veh, OVA group. n = 4 mice per group for the saline group and 8 mice per group for the OVA-stimulated groups.
Figure 8. Highly-elevated γ-tocopherol treatment decreases Th2 cytokines and chemokines in the lung.
Mice were treated with highly-elevated γ-tocopherol as in Figure 7A. BAL supernatants were examined for cytokines (IL-4, IL-5, IL-10, IFNγ, IL-2, MIP-1α, MCP-1) using a mouse cytokine multiplexing assay (Millipore). Lung tissue was placed in RNAlater and then examined for eotaxin 1 (CCL11) and eotaxin 2 (CCL24) expression by quantitative real-time PCR. γT, d-gamma-tocopherol; veh, vehicle; *, p<0.05 as compared to veh, OVA group.
Highly-elevated γ-tocopherol in endothelial cells partially inhibits leukocyte transendothelial migration in vitro
We have previously reported that supplemental levels of γ-tocopherol enhance leukocyte transendothelial migration in vitro by a direct effect on endothelial cells (8). Since the highly-elevated γ-tocopherol reduced lung lavage inflammation (Figure 7D), we determined whether highly-elevated levels of γ-tocopherol modulated endothelial cell function during MCP-1-induced leukocyte transendothelial migration in vitro. Exogenous γ-tocopherol was loaded into endothelial cells overnight to generate tocopherol levels equivalent to highly-elevated lung tissue γ-tocopherol (Figures 7C,9A); this required addition of exogenous 20 µM γ-tocopherol overnight followed by 3 washes (Figure 9A). Highly-elevated levels of γ-tocopherol in endothelial cells in vitro inhibited leukocyte transendothelial migration by 40% under physiological laminar flow (Figure 9B). To examine effects of highly-elevated γ-tocopherol on leukocyte function during transmigration, mice were treated with highly-elevated levels of γ-tocopherol for 8 days, spleen leukocytes were isolated and examined in vitro for transendothelial migration. Pre-loading leukocytes in vivo for 4 days with highly-elevated γ-tocopherol had no effect on leukocyte transendothelial migration in vitro (Figure 9C). Thus, highly-elevated levels of γ-tocopherol partially inhibited endothelial cell function but not leukocyte function during leukocyte transendothelial migration.
Figure 9. Highly-elevated γ-tocopherol reduces leukocyte transendothelial migration through direct regulation of endothelial cells.
A. 90% confluent endothelial cell monolayers were treated overnight with a dose curve of γ-tocopherol. After washing, cells were harvested and tocopherol was measured by HPLC. B. 90% confluent endothelial cell monolayers were treated overnight with 20 µM γ-tocopherol. Cells were washed 5 times before the start of the leukocyte migration assay with physiological laminar flow as detailed in Methods. C. Mice were treated with highly-elevated (2 mg/day) γ-tocopherol or vehicle (ethoxylated castor oil) for 4 days as we previously reported for in vivo loading of tocopherols in leukocytes (8). Spleens were collected and red blood cells were lysed by hypotonic lysis. Spleen leukocytes from vehicle mice or spleen leukocytes from mice treated with highly-elevated γ-tocopherol were added to untreated endothelial cell monolayers and examined for transendothelial migration under physiological laminar flow conditions as detailed in methods. Leukocytes in each treatment group were not pooled; leukocytes from each mouse were processed individually. γT, d-gamma-tocopherol; veh, vehicle; *, p<0.05 as compared to vehicle-treated group. n = 3 migration assays per treatment.
Regulation of lung inflammation by highly-elevated tocopherols is reversible
It was determined whether the regulatory effects of highly-elevated levels of tocopherols on lung lavage inflammation are reversible when tocopherols are withdrawn before antigen re-challenge. Highly-elevated levels of tocopherols (2 mg/day) were administered and the mice were treated with three OVA re-challenges as in the timeline in Figure 10A). At the end of phase 1 (day 21), significantly more tocopherol was present in the plasma of mice that received highly-elevated levels of tocopherols (Figure 10B) as compared to the supplemental (0.2 mg/day) tocopherol treated mice (Figure 2B). At the completion of the clearance phase, the tocopherols were cleared from the plasma (Figure 10C). At the end of phase 2 (day 58), there were significant increases in tocopherols in plasma and lung tissue as compared to vehicle controls (Figures 10D,E).
Figure 10. Timeline and plasma tocopherol levels during analysis of the reversibility of the regulatory effects of highly-elevated tocopherol levels on allergic lung inflammation.
A. Mice were sensitized with OVA and then administered subcutaneous highly-elevated (2 mg) tocopherol daily during OVA challenge in phase 1 of treatments. Following phase 1, mice entered a 4-week clearance phase during which they were not treated with tocopherols or challenged with OVA. At the end of the clearance phase, mice were treated subcutaneously with highly-elevated (2 mg) tocopherols and rechallenged with OVA in phase 2 of treatments. In phase 2, mice were again treated with daily doses of supplemental tocopherol (same isoform as phase 1, different isoform than phase 1, or vehicle) and re-challenged three times with OVA. All treatment groups are listed. B. Plasma tocopherol on day 21. C. Plasma tocopherol on day 49. D. Plasma tocopherol on day 58. E. Tocopherol in perfused lung on day 58. veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 10A; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA on days 21 or 49 or as compared to veh OVA→veh OVA group on day 58 where indicated. n, number of mice per group.
The vehicle,OVA→vehicle,saline group had the same baseline numbers of lung lavage cells as the vehicle,saline→vehicle,saline group (Figure 11), indicating that OVA treatment from phase 1 did not alter background lung lavage leukocytes during phase 2. Regardless of whether the OVA-challenged mice were treated in phase 1 with tocopherol or vehicle, in phase 2 the highly-elevated α-tocopherol or γ-tocopherol reduced lung lavage eosinophils (Figure 11) as compared to mice that received OVA,vehicle in both phase 1 and 2. Interestingly, the effects of highly-elevated tocopherols in phase 1 are reversible when tocopherol is withdrawn in phase 2 because the lung lavage eosinophil numbers in the α-tocopherol,OVA→vehicle,OVA group and the γ-tocopherol,OVA→vehicle,OVA group were not different than the lung lavage eosinophil numbers in the vehicle,OVA→vehicle,OVA group (Figure 11). Blood eosinophils were not significantly altered by the highly-elevated tocopherols, except for the γ-tocopherol,OVA→γ-tocopherol,OVA group (Figure 12F).
Figure 11. Reversibility of the regulatory effects of highly-elevated tocopherol levels on allergic bronchoalveolar lavage inflammation.
Mice were treated with highly-elevated tocopherols as in Figure 10A. On day 58, bronchoalveolar lavage (BAL) neutrophils, eosinophils, monocytes and lymphocytes were counted with a hemocytometer. Cytospun BAL cells were counted by standard morphological criteria. veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA→veh OVA group on day 58.
Not shown are two saline groups: leukocytes in the BAL of the αT,OVA→veh,saline and γT,OVA→veh,saline groups had BAL cell infiltrate not significantly different than veh,saline→veh,saline group.
Figure 12. Reversibility of the regulatory effects of highly-elevated tocopherol on allergic lung tissue inflammation.
Mice were treated with highly-elevated tocopherols as in Figure 10A. A–E. Representative micrographs (20× objective) of perivascular regions in the lung tissue from day 58 as in Figure 10A. Lung tissue was stained with hematoxylin and eosin. F. number of blood eosinophils on day 58 of treatments as in Figure 10A. white arrow, vessel lumen; black arrow, bronchial airway; veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 10A; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA→veh OVA group.
Surprisingly, although highly-elevated γ-tocopherol reduced leukocyte numbers in the lung lavage (Figure 11), the γ-tocopherol,OVA→γ-tocopherol,OVA treatment elevated lung tissue leukocytes and induced lung tissue remodeling (Figure 12C) as compared to the vehicle,OVA→vehicle,OVA treatment (Figure 12A). In contrast, α-tocopherol,OVA→α-tocopherol,OVA treatment reduced lung tissue inflammation (Figure 12B). These regulatory effects of highly-elevated levels of tocopherols in lung tissue were reversible because the γ-tocopherol,OVA→vehicle,OVA group and the α-tocopherol,OVA→vehicle group (Figure 12D and E) were not different than the vehicle,OVA→vehicle,OVA group (Figure 12A). In summary, in the lung lavage and lung tissue, highly-elevated α-tocopherol significantly reduces OVA-induced inflammation. However, highly-elevated γ-tocopherol elevates lung tissue inflammation but reduces lung lavage inflammation. These effects of highly-elevated tocopherols were reversible.
Highly-elevated tocopherols reduce expression of IL-13
Since lung tissue and lung lavage inflammation were significantly affected by the highly-elevated tocopherol treatments, regulatory Th1/Th2 cytokines, chemokines and OVA-specific IgE were examined. The OVA-specific IgE antibodies at the end of phase 2 were not affected by the tocopherol treatments (Figure 13A). As expected, Th1 cytokines IFNγ (not detected) and the low levels of IL-2 (Figure 13H) were not affected by tocopherol treatments. Highly-elevated levels of α-tocopherol or γ-tocopherol treatments in phase 1 and/or 2 did alter affect OVA-restimulated IL-4, IL-5, IL-10 or IL-12 (Figure 13B,C,D,E), except one group (α-tocopherol,OVA→γ-tocopherol,OVA) which had reduced IL-5 and IL-10 production. (Figure 13C,D). The OVA-induced lung tissue chemokine CCL11 was not reduced by the tocopherols (Figure 13F) and there was a trend in reduced CCL24 with phase 2 tocopherol treatments that did not reach significance (Figure 13G). In contrast, OVA-induced IL-13 production was reduced by highly-elevated tocopherols in phase 2 and this was reversed with antigen re-challenge in the absence of tocopherols in phase 2 (ie, γ-tocopherol,OVA→vehicle,OVA and α-tocopherol,OVA→vehicle,OVA groups) (Figure 13I). In summary, highly-elevated tocopherols reduced reduced lung lavage inflammation and reduced lung lavage IL-13 but highly-elevated γ-tocopherol increased lung tissue inflammation.
Figure 13. Switching tocopherol isoforms at highly-elevated levels in phase 2 decreased IL-13 but not other lung cytokines, lung chemokines or OVA-specific IgE.
Mice were treated with highly-elevated levels of tocopherols as in Figure 10A. A. serum OVA-specific IgE was measured by ELISA. B–E, H–I. BAL supernatants were examined for cytokines using the Th1/Th2 mouse cytokine multiplexing kit plus IL-13 singleplex supplement (Life Technologies). F,G. Lung tissue was placed in RNAlater then examined for eotaxin 1 (CCL11) and eotaxin 2 (CCL24) expression by quantitative real-time PCR. veh OVA→veh OVA group, indicates phase 1 treatments followed by phase 2 treatments as described in Figure 10A; veh, vehicle; αT, d-alpha-tocopherol; γT, d-gamma-tocopherol; *, p<0.05 as compared to veh OVA→veh OVA group.
Discussion
The opposing effects of supplemental α- and γ-tocopherol are partially reversible
We previously reported that supplemental levels of α-tocopherol and γ-tocopherol have anti-inflammatory and pro-inflammatory effects, respectively, during experimental asthma in mice (8). In addition, tocopherols regulate infiltration of all the leukocyte cell types in response to antigen challenge by a direct effect on the endothelium (8). In the studies herein, the regulatory effects of tocopherols on inflammation were partially reversed when tocopherol-treated mice were cleared of plasma tocopherol for 1 month and then supplemented with the opposing tocopherol in a second phase of tocopherol and antigen treatments. There was only partial reversal despite the mild inflammation upon a single re-challenge with antigen. In contrast, the pro-inflammatory effects of supplemental levels of γ-tocopherol in phase 1 were completely reversed by treatment with highly-elevated α-tocopherol (10 times supplemental amounts) in the second phase with a single antigen-rechallenge. At 24 hours after the single antigen re-challenge, there was the expected early neutrophil and monocyte infiltrate (34) as well as the beginning of eosinophil accumulation in the lung in the OVA-challenged vehicle-treated control group, since eosinophil infiltration peaks after several antigen challenges (24). The tocopherol effects were not accompanied by differences in expression of cytokines or chemokines. This is consistent with reports by us and others demonstrating that leukocyte recruitment to the lung, in response to antigen challenge, can be regulated in the absence of changes in cytokine or chemokine expression when there is a functional effect on the endothelium (8, 24, 35).
Tocopherol isoforms and doses differentially regulate lung lavage inflammation, leukocyte transendothelial migration, and lung tissue inflammation
Interestingly, the opposing regulatory effects of supplemental α-tocopherol and γ-tocopherol on lung inflammation occur when γ-tocopherol is present in tissues at just 10% the concentration of α-tocopherol. Physiological levels of γ-tocopherol are lower than α-tocopherol in vivo because of the selective transfer of α-tocopherol to lipid particles by the hepatic enzyme α-tocopherol transfer protein (αTTP) (3). However, when we increased tissue γ-tocopherol levels to that of supplemental α-tocopherol tissue levels (10 µg/g lung) by administration of 10 fold higher levels of γ-tocopherol, the lung lavage inflammation was decreased after three antigen challenges despite the very high eosinophil levels that normally occur after three antigen challenges in phase 2. Importantly, highly-elevated γ-tocopherol elevated the lung tissue inflammation and airway remodeling after three antigen challenges.
The different regulatory effects of supplemental versus highly-elevated γ-tocopherol are consistent with tocopherols having both anti-oxidant and non-antioxidant properties (38). Moreover, the non-antioxidant properties of tocopherols can be specific to the tocopherol isoform (39). In our previous report (8), supplemental levels of γ-tocopherol exerted non-antioxidant effects by enhancing VCAM-1 activation of endothelial cell protein kinase Cα, resulting in increased VCAM-1-dependent leukocyte recruitment. In contrast, α-tocopherol at supplemental levels blocked VCAM-1-induced oxidative activation of protein kinase Cα and thus likely functioned as an antioxidant (8). Administration of supplemental levels of α-tocopherol and γ-tocopherol results in a 10 fold higher level of α-tocopherol than γ-tocopherol in the tissues as expected (38). Therefore, since α-tocopherol and γ-tocopherol possess approximately equal anti-oxidant activity, the higher concentration of α-tocopherol in vivo results in a greater total anti-oxidant capacity by α-tocopherol than γ-tocopherol. In our studies herein, when γ-tocopherol was highly-elevated in the tissues such that γ-tocopherol tissue concentrations were equivalent to the tissue concentrations of supplemental α-tocopherol, the highly-elevated γ-tocopherol would have approximately equal total anti-oxidant capacity to that for α-tocopherol. Nevertheless, in vivo, highly-elevated levels of γ-tocopherol increased accumulation of leukocytes in the tissue but decreased lung lavage inflammation at 24 hours after the third antigen challenge. Thus, even though in vitro transendothelial migration was partially inhibited at 15 minutes by highly-elevated levels of γ-tocopherol, the in vivo data suggests a continued recruitment of leukocytes in lung tissue. This regulatory effect of highly-elevated γ-tocopherol is likely a result of both its anti-oxidant functions and its non-antioxidant enhancement of PKCα activity in lung tissue cells.
Since the highly-elevated γ-tocopherol reduced leukocytes in the lung lavage despite the high numbers of leukocytes in the lung tissue, it suggests that highly-elevated γ-tocopherol reduces recruitment of the leukocytes across the epithelium. It is reported that CCL11 has a role in transendothelial recruitment of eosinophils whereas CCL24 has a greater role in the transepithelial recruitment of eosinophils (40). Therefore, a reduction in leukocyte migration across the epithelium is consistent with the trend in reduction in CCL24 but not CCL11 in the OVA-rechallenged high γ-tocopherol group as compared to OVA-rechallenged vehicle treatment group. In addition, in the lung tissues, there was airway remodeling with epithelial hyperplasia and narrowing of the airways. This may also contribute to reduced leukocyte migration through the epithelium.
Although highly-elevated tissue levels of γ-tocopherol were achieved in our studies by subcutaneous tocopherol treatments, lower levels of tissue γ-tocopherol are reported to be achieved by dietary means. In a report where mice were fed a diet containing 1150 mg α-tocopheryl acetate per kg diet for 15 days, they achieved approximately 13 µg/ml α-tocopherol in plasma (41) if dietary γ-tocopherol had been administered at this dose, the plasma level of γ-tocopherol would be lower than 13 µg/ml. In our studies with subcutaneous administration of tocopherols, the excretion is likely lower than that for dietary tocopherols and thus equal molar lung tissue levels of α-tocopherol and γ-tocopherol (10 µg tocopherol/g lung) were achieved by subcutaneous administration of supplemental levels of α-tocopherol (0.2mg/day for 8 days) and highly-elevated levels of γ-tocopherol (2mg/day for 8 days). The supplemental α-tocopherol elevated plasma α-tocopherol to 12 µg/ml and the highly-elevated γ-tocopherol elevated plasma γ-tocopherol to 25 µg/ml. In addition, subcutaneous administration of highly-elevated α-tocopherol treatment (2 mg/day for 8 days) results in 75 µg/ml α-tocopherol in plasma. This very high α-tocopherol plasma level that we observed through subcutaneous tocopherol administration has not been reported for animal or human studies and may not be achievable through dietary means. Nevertheless, our studies indicate that the pro-inflammatory physiological tissue levels of γ-tocopherols function differently than the anti-inflammatory physiological levels of α-tocopherol; however, at equal-molar tissue levels of these tocopherols, the isoforms can have a similar function for inhibition of lung lavage inflammation but high levels of γ-tocopherol elevate tissue inflammation. Although high doses of α-tocopherol reversed the pro-inflammatory effects of supplemental γ-tocopherol in our studies, reports indicating that high doses of tocopherol can significantly increase the incidence of hemorrhagic stroke, elevate blood pressure, and increase all-cause mortality (42–45) suggest that administration of high-dose α-tocopherol may be a potentially risky approach for reversing the pro-inflammatory effects of supplemental levels of γ-tocopherol. Nevertheless, in our studies, there was an isoform-specific and dose-dependent regulation of inflammation by tocopherols.
Anti-inflammatory effects of highly-elevated α-tocopherol and γ-tocopherol in the bronchoalveolar lavage are reversible
In contrast to the partial reversibility of the regulatory effects of supplemental levels of tocopherols, the anti-inflammatory effects of highly-elevated α-tocopherol and γ-tocopherol in the bronchoalveolar lavage were reversible to the levels of the antigen-challenged vehicle controls when tocopherols were cleared from plasma during a 4-week phase and mice were re-challenged three times with antigen in the absence of tocopherol. In contrast to the anti-inflammatory effects of highly-elevated γ-tocopherol in the lung lavage, inflammation in the lung tissue was increased. This increase in tissue inflammation by highly-elevated γ-tocopherol is consistent with reports of some adverse cardiovascular effects that can occur when elevating α-tocopherol or γ-tocopherol (15, 42–46). The increase in tissue inflammation by highly-elevated γ-tocopherol, in our studies, was reversed in a second phase with three antigen challenges without tocopherol administration.
Highly-elevated levels of γ-tocopherol reduced the antigen-induced pro-inflammatory mediators IL-5, IL-13, MIP-1α and MCP-1 in the lung lavage, whereas supplemental levels of tocopherols did not alter cytokine or chemokine expression. The Th1 cytokines IFNγ and IL-2 were not induced by highly-elevated tocopherol treatments during OVA-challenges. There were some alterations in IL-10 in the studies with supplemental or highly-elevated tocopherols. IL-10 is a cytokine with broad anti-inflammatory properties (47–49). However, IL-10 can also be elevated in the presence of increased inflammation (50). Enhanced IL-10 with concomitant increases in inflammation is not limited to allergic Th2-type disease as it also occurs in Th1/IFNγ-driven Celiac Disease (51). Therefore, IL-10 can be elevated to limit further inflammation. In figures 6, 8 and 13, there is a trend of elevated IL-10 during elevated lung lavage inflammation and reduced IL-10 during reduced lung lavage inflammation, although some groups do not reach significance. In addition to IL-10, inflammation is controlled by a combination of multiple regulators in the microenvironment.
Alternative interpretations for previous studies of tocopherol regulation of inflammation
Interpretations of animal studies with conflicting effects of tocopherols on allergic disease are influenced by our studies demonstrating opposing functions of supplemental levels of tocopherols and partial reversibility of tocopherol immunoregulation. In a report by Suchankova et al (52), antigen-sensitized rats were treated by oral gavage with α -tocopherol in soy oil for 10 days and then challenged with antigen. These α -tocopherol-treated rats did not exhibit changes in bronchoconstriction or lung inflammation when compared to controls. Since the soy oil vehicle in these studies would contain significant amounts of γ-tocopherol, the results are consistent with the interpretation that the γ-tocopherol in the vehicle ablated the anti-inflammatory benefit of α -tocopherol. In a report by Wagner et al (53), inhibition of lung lavage inflammation was observed when OVA-sensitized rats were treated daily by oral gavage with 100 mg γ-tocopherol/kg body weight and administered two OVA challenges. In their report, perivascular areas of the lung were not shown and therefore lung tissue inflammation is not known. In contrast, in our current and previous report (8), supplemental and highly-elevated γ-tocopherol levels increased lung tissue inflammation. Consistent with our current study in which highly-elevated levels of γ-tocopherol inhibited lung lavage inflammation after three OVA challenges, the report by Wagner et al (53) showed reduced lung lavage inflammation after γ-tocopherol treatment. However, in their studies, the lung lavage inflammation was predominantly neutrophils rather than the expected predominance of eosinophils at 48 hours post-2nd challenge with OVA (53).
The outcomes of clinical studies that focused on α-tocopherol were likely influenced by tissue γ-tocopherols. It is reported that the average baseline human plasma concentration of α-tocopherol (10 µg/ml or 23 µM α-tocopherol) is the same among various countries (21, 54). In contrast, the average human plasma γ-tocopherol level is 2–5 times higher in the United States (2.4 µg/ml or 5.8 µM γ-tocopherol) and the Netherlands (1 µg/ml or 2.3 µM γ-tocopherol) than in European and Asian countries (0.6 µg/ml or 1.6 µM γ-tocopherol) including Italy (0.5 µg/ml or 1.2 µM γ-tocopherol) (21, 54). These differences in human plasma tocopherol are consistent with dietary consumption of tocopherols (21, 38, 54, 55). Although it is acknowledged that species differ in basal levels of tocopherols and metabolism, it is interesting that the outcomes and the fold increase in human plasma γ-tocopherol in the United States compared to other countries is similar to that in our animal studies on the reversibility of the pro-inflammatory effects of supplemental γ-tocopherol. The clinical studies indicate that α-tocopherol supplementation of asthmatic patients is beneficial in Italy and Finland but α-tocopherol is not beneficial for asthmatic patients in studies in the United States or the Netherlands (16–20). Since we report that the pro-inflammatory effects of γ-tocopherol are only partially reversible, elevated human plasma γ-tocopherol in the United States may have influenced the outcomes of α-tocopherol on allergic inflammation in the clinical studies. In addition, in one study, asthmatics were given an α-tocopherol dietary supplement (or soy oil placebo, which is rich in γ-tocopherol) for 6 weeks and there was no beneficial effect of α-tocopherol on lung function (56). In their study, the γ-tocopherol in the vehicle may have ablated the benefit of α-tocopherol supplementation. It is acknowledged that although there are many other differences regarding the environment and genetics of the people in the clinical studies, the data are, at least, consistent with our animal studies.
Summary
In summary, the regulatory effect of tocopherols on leukocyte recruitment to the lung tissue or lung lavage in antigen-sensitized mice depends on 1) the isoform of the tocopherol used to treat the mice (natural α-tocopherol versus natural γ-tocopherol), 2) the concentration of tocopherol in tissues (supplemental vs. highly-elevated), and 3) the previous levels of tocopherol isoforms in tissues. Alpha-tocopherol at highly-elevated but not supplemental levels, during a second phase of antigen challenges, overcomes the pro-inflammatory effects of supplemental γ-tocopherol in the lung lavage and lung tissue. Changes in inflammation due to supplemental levels of tocopherol treatment are not accompanied by changes in inflammatory modulators. In contrast, highly-elevated γ-tocopherol reduces lung lavage leukocytes but elevates lung tissue inflammation; these effects of γ-tocopherol are accompanied by modest changes in cytokines and chemokines. During a second phase of antigen challenge without highly-elevated tocopherol treatment, inflammation returns back to the levels of inflammation in the vehicle-treated antigen-challenged control. These results have important implications for interpretations of previous studies using supplemental or highly-elevated levels of tocopherol isoforms. Although we have demonstrated the importance of tocopherol dose and isoform in the context of allergic inflammation, tocopherol doses and isoforms may also play a significant role in other inflammatory diseases (38, 54).
Acknowledgments
These studies were supported by National Institutes of Health Grant R01 AT004837 (J.M.C-M) and by American Heart Association 0855583G.
References
- 1.Zingg JM, Azzi A. Non-antioxidant activities of vitamin E. Curr Med Chem. 2004;11:1113–1133. doi: 10.2174/0929867043365332. [DOI] [PubMed] [Google Scholar]
- 2.Brigelius-Flohe R, Traber MG. Vitamin E: function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 3.Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr Rev. 2006;64:295–299. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]
- 4.Desrumaux C, Deckert V, Athias A, Masson D, Lizard G, Palleau V, Gambert P, Lagrost L. Plasma phospholipid transfer protein prevents vascular endothelium dysfunction by delivering alpha-tocopherol to endothelial cells. FASEB J. 1999;13:883–892. doi: 10.1096/fasebj.13.8.883. [DOI] [PubMed] [Google Scholar]
- 5.Atkinson J, Epand RF, Epand RM. Tocopherols and tocotrienols in membranes: a critical review. Free Radic Biol Med. 2008;44:739–764. doi: 10.1016/j.freeradbiomed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida Y, Saito Y, Jones LS, Shigeri Y. Chemical reactivities and physical effects in comparison between tocopherols and tocotrienols: physiological significance and prospects as antioxidants. J Biosci Bioeng. 2007;104:439–445. doi: 10.1263/jbb.104.439. [DOI] [PubMed] [Google Scholar]
- 7.Azzi A, Stocker A. Vitamin E: non-antioxidant roles. Prog Lipid Res. 2000;39:231–255. doi: 10.1016/s0163-7827(00)00006-0. [DOI] [PubMed] [Google Scholar]
- 8.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of Vitamin E have Opposing Immunoregulatory Funcitons during Inflammation by Regulating Leukocyte Recruitment. J Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalayci O, Besler T, Kilinc K, Sekerel BE, Saraclar Y. Serum levels of antioxidant vitamins (alpha tocopherol, beta carotene, and ascorbic acid) in children with bronchial asthma. Turk J Peds. 2000;42:17–21. [PubMed] [Google Scholar]
- 10.Kelly FJ, Mudway I, Blomberg A, Frew A, Sandstrom T. Altered lung antioxidant status in patients with mild asthma [letter] Lancet. 1999;354:482–483. doi: 10.1016/S0140-6736(99)01812-7. [DOI] [PubMed] [Google Scholar]
- 11.Shvedova AA, Kisin ER, Kagan VE, Karol MH. Increased lipid peroxidation and decreased antioxidants in lungs of guinea pigs following an allergic pulmonary response. Tox Appl Pharm. 1995;132:72–81. doi: 10.1006/taap.1995.1088. [DOI] [PubMed] [Google Scholar]
- 12.Schunemann HJ, Grant BJ, Freudenheim JL, Muti P, Browne RW, Drake JA, Klocke RA, Trevisan M. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163:1246–1255. doi: 10.1164/ajrccm.163.5.2007135. [DOI] [PubMed] [Google Scholar]
- 13.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. quiz 1118. [DOI] [PubMed] [Google Scholar]
- 15.Devaraj S, Tang R, Adams-Huet B, Harris A, Seenivasan T, de Lemos JA, Jialal I. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am J Clin Nutr. 2007;86:1392–1398. doi: 10.1093/ajcn/86.5.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Heederik D, Kromhout D. Dietary factors and pulmonary function: a cross sectional study in middle aged men from three European countries. Thorax. 1999;54:1021–1026. doi: 10.1136/thx.54.11.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smit HA, Grievink L, Tabak C. Dietary influences on chronic obstructive lung disease and asthma: a review of the epidemiological evidence. Proc Nutr Soc. 1999;58:309–319. doi: 10.1017/s0029665199000427. [DOI] [PubMed] [Google Scholar]
- 18.Weiss ST. Diet as a risk factor for asthma. Ciba Fndn Symp. 1997;206:244–257. [PubMed] [Google Scholar]
- 19.Troisi RJ, Willett WC, Weiss ST, Trichopoulos D, Rosner B, Speizer FE. A prospective study of diet and adult-onset asthma. Am J Respir Crit Care Med. 1995;151:1401–1408. doi: 10.1164/ajrccm.151.5.7735592. [DOI] [PubMed] [Google Scholar]
- 20.Dow L, Tracey M, Villar A, Coggon D, Margetts BM, Campbell MJ, Holgate ST. Does dietary intake of vitamins C and E influence lung function in older people? Am J Respir Crit Care Med. 1996;154:1401–1404. doi: 10.1164/ajrccm.154.5.8912755. [DOI] [PubMed] [Google Scholar]
- 21.Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- 22.Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte Migration through Monolayers of Endothelial Cell Lines involves VCAM-1 Signaling via Endothelial Cell NADPH Oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- 23.Abdala-Valencia H, Cook-Mills JM. VCAM-1 Signals Activate Endothelial Cell Protein Kinase Cα Via Oxidation. J Immunol. 2006;177:6379–6387. doi: 10.4049/jimmunol.177.9.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdala-Valencia H, Earwood J, Bansal S, Jansen M, Babcock G, Garvy B, Wills-Karp M, Cook-Mills JM. Nonhematopoietic NADPH oxidase regulation of lung eosinophilia and airway hyperresponsiveness in experimentally induced asthma. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1111–L1125. doi: 10.1152/ajplung.00208.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mustacich DJ, Leonard SW, Devereaux MW, Sokol RJ, Traber MG. Alpha-tocopherol regulation of hepatic cytochrome P450s and ABC transporters in rats. Free Radic Biol Med. 2006;41:1069–1078. doi: 10.1016/j.freeradbiomed.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 26.Bryce PJ, Geha R, Oettgen HC. Desloratadine inhibits allergen-induced airway inflammation and bronchial hyperresponsiveness and alters T-cell responses in murine models of asthma. J Allergy Clin Immunol. 2003;112:149–158. doi: 10.1067/mai.2003.1616. [DOI] [PubMed] [Google Scholar]
- 27.Deem TL, Abdala-Valencia H, Cook-Mills JM. VCAM-1 Activation of PTP1B in Endothelial Cells. J Immunol. 2007;178:3865–3873. doi: 10.4049/jimmunol.178.6.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qureshi MH, Cook-Mills J, Doherty DE, Garvy BA. TNF-alpha-Dependent ICAM-1- and VCAM-1-Mediated Inflammatory Responses Are Delayed in Neonatal Mice Infected with {I}Pneumocystis carinii. J Immunol. 2003;171:4700–4707. doi: 10.4049/jimmunol.171.9.4700. [DOI] [PubMed] [Google Scholar]
- 29.Meydani SN, Shapiro AC, Meydani M, Macauley JB, Blumberg JB. Effect of age and dietary fat (fish, corn and coconut oils) on tocopherol status of C57BL/6Nia mice. Lipids. 1987;22:345–350. doi: 10.1007/BF02534004. [DOI] [PubMed] [Google Scholar]
- 30.Goti D, Balazs Z, Panzenboeck U, Hrzenjak A, Reicher H, Wagner E, Zechner R, Malle E, Sattler W. Effects of lipoprotein lipase on uptake and transcytosis of low density lipoprotein (LDL) and LDL-associated alpha-tocopherol in a porcine in vitro blood-brain barrier model. J Biol Chem. 2002;277:28537–28544. doi: 10.1074/jbc.M203989200. [DOI] [PubMed] [Google Scholar]
- 31.Vatassery GT, Fahn S, Kuskowski MA. Alpha tocopherol in CSF of subjects taking high-dose vitamin E in the DATATOP study. Parkinson Study Group. Neurology. 1998;50:1900–1902. doi: 10.1212/wnl.50.6.1900. [DOI] [PubMed] [Google Scholar]
- 32.Jungsuwadee P, Dekan G, Stingl G, Epstein MM. Recurrent aerosol antigen exposure induces distinct patterns of experimental allergic asthma in mice. Clin Immunol. 2002;102:145–153. doi: 10.1006/clim.2001.5157. [DOI] [PubMed] [Google Scholar]
- 33.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 34.Tomkinson A, Cieslewicz G, Duez C, Larson KA, Lee JJ, Gelfand EW. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med. 2001;163:721–730. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 35.Pero RS, Borchers MT, Spicher K, Ochkur SI, Sikora L, Rao SP, Abdala-Valencia H, O'Neill KR, Shen H, McGarry MP, Lee NA, Cook-Mills JM, Sriramarao P, Simon MI, Birnbaumer L, Lee JJ. Galphai2-mediated signaling events in the endothelium are involved in controlling leukocyte extravasation. Proc Natl Acad Sci U S A. 2007;104:4371–4376. doi: 10.1073/pnas.0700185104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keshavan P, Deem TL, Schwemberger SJ, Babcock GF, Cook-Mills JM, Zucker SD. Unconjugated bilirubin inhibits VCAM-1-mediated transendothelial leukocyte migration. J Immunol. 2005;174:3709–3718. doi: 10.4049/jimmunol.174.6.3709. [DOI] [PubMed] [Google Scholar]
- 37.Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- 38.Cook-Mills JM, McCary CA. Isoforms of Vitamin E Differentially Regulate Inflammation. Endocr Metab Immune Disord Drug Targets. 2010 doi: 10.2174/1871530311006040348. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider C. Chemistry and biology of vitamin E. Mol Nutr Food Res. 2005;49:7–30. doi: 10.1002/mnfr.200400049. [DOI] [PubMed] [Google Scholar]
- 40.Pope SM, Fulkerson PC, Blanchard C, Akei HS, Nikolaidis NM, Zimmermann N, Molkentin JD, Rothenberg ME. Identification of a cooperative mechanism involving interleukin-13 and eotaxin-2 in experimental allergic lung inflammation. J Biol Chem. 2005;280:13952–13961. doi: 10.1074/jbc.M406037200. [DOI] [PubMed] [Google Scholar]
- 41.Bella DL, Schock BC, Lim Y, Leonard SW, Berry C, Cross CE, Traber MG. Regulation of the alpha-tocopherol transfer protein in mice: lack of response to dietary vitamin E or oxidative stress. Lipids. 2006;41:105–112. doi: 10.1007/s11745-006-5077-7. [DOI] [PubMed] [Google Scholar]
- 42.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 43.Tornwall ME, Virtamo J, Korhonen PA, Virtanen MJ, Albanes D, Huttunen JK. Postintervention effect of alpha tocopherol and beta carotene on different strokes: a 6-year follow-up of the Alpha Tocopherol, Beta Carotene Cancer Prevention Study. Stroke. 2004;35:1908–1913. doi: 10.1161/01.STR.0000131750.60270.42. [DOI] [PubMed] [Google Scholar]
- 44.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 45.Leppala JM, Virtamo J, Fogelholm R, Huttunen JK, Albanes D, Taylor PR, Heinonen OP. Controlled trial of alpha-tocopherol and beta-carotene supplements on stroke incidence and mortality in male smokers. Arterioscler Thromb Vasc Biol. 2000;20:230–235. doi: 10.1161/01.atv.20.1.230. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi K, Komaru T, Takeda S, Takeda M, Koshida R, Nakayama M, Kokusho Y, Kawakami Y, Yamaguchi N, Miyazawa T, Shimokawa H, Shirato K. gamma-tocopherol, but not alpha-tocopherol, potently inhibits neointimal formation induced by vascular injury in insulin resistant rats. J Mol Cell Cardiol. 2006;41:544–554. doi: 10.1016/j.yjmcc.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 47.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. J Immunol. 1993;150:353–360. [PubMed] [Google Scholar]
- 48.Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39:1314–1323. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson DS, Tsicopoulos A, Meng Q, Durham S, Kay AB, Hamid Q. Increased interleukin-10 messenger RNA expression in atopic allergy and asthma. Am J Respir Cell Mol Biol. 1996;14:113–117. doi: 10.1165/ajrcmb.14.2.8630259. [DOI] [PubMed] [Google Scholar]
- 51.Forsberg G, Hernell O, Hammarstrom S, Hammarstrom ML. Concomitant increase of IL-10 and pro-inflammatory cytokines in intraepithelial lymphocyte subsets in celiac disease. Int Immunol. 2007;19:993–1001. doi: 10.1093/intimm/dxm077. [DOI] [PubMed] [Google Scholar]
- 52.Suchankova J, Voprsalova M, Kottova M, Semecky V, Visnovsky P. Effects of oral alpha-tocopherol on lung response in rat model of allergic asthma. Respirology. 2006;11:414–421. doi: 10.1111/j.1440-1843.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 53.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. Gamma-tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin Exp Allergy. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 54.Cook-Mills JM, Marchese M, Abdala-Valencia H. VCAM-1 Expression and Signaling during Disease: Regulation by Reactive Oxygen Species and Antioxidants. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3522. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 56.Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax. 2004;59:652–656. doi: 10.1136/thx.2004.022616. [DOI] [PMC free article] [PubMed] [Google Scholar]