Abstract
Information on dietary supplements, medications, and other xenobiotics in epidemiologic surveys is usually obtained from questionnaires and is subject to recall and reporting biases. The authors used metabolite data obtained from hydrogen-1 (or proton) nuclear magnetic resonance (1H NMR) analysis of human urine specimens from the International Study of Macro-/Micro-Nutrients and Blood Pressure (INTERMAP Study) to validate self-reported analgesic use. Metabolic profiling of two 24-hour urine specimens per individual was carried out for 4,630 participants aged 40–59 years from 17 population samples in Japan, China, the United Kingdom, and the United States (data collection, 1996–1999). 1H NMR-detected acetaminophen and ibuprofen use was low (∼4%) among East Asian population samples and higher (>16%) in Western population samples. In a comparison of self-reported acetaminophen and ibuprofen use with 1H NMR-detected acetaminophen and ibuprofen metabolites among 496 participants from Chicago, Illinois, and Belfast, Northern Ireland, the overall rate of concordance was 81%–84%; the rate of underreporting was 15%–17%; and the rate of underdetection was approximately 1%. Comparison of self-reported unspecified analgesic use with 1H NMR-detected acetaminophen and ibuprofen metabolites among 2,660 Western INTERMAP participants revealed similar levels of concordance and underreporting. Screening for urinary metabolites of acetaminophen and ibuprofen improved the accuracy of exposure information. This approach has the potential to reduce recall bias and other biases in epidemiologic studies for a range of substances, including pharmaceuticals, dietary supplements, and foods.
Keywords: analgesics, non-narcotic; anti-inflammatory agents, non-steroidal; epidemiologic studies; metabolomics; pharmacoepidemiology; questionnaires; reproducibility of results
In the United States, acetaminophen is one of the most commonly used drugs for treating pain, with 29 billion units of acetaminophen-containing products having been sold to retail and nonretail pharmacies in 2005 (1). Of the total US sales, approximately 60% of these products were sold as over-the-counter medicines (1). Similarly, in the United Kingdom, use of acetaminophen is high, with annual sales of approximately 166 tons in 2000 (2). Other analgesics such as ibuprofen are used commonly in Western countries; for example, in the United Kingdom, total ibuprofen sales of 46 tons were reported in 2000 (2).
Despite endorsement by regulatory bodies of over-the-counter analgesics as safe and effective for use without a physician’s prescription, approximately 40% of reported cases of liver injury in the United States in 2005 were due to over-the-counter acetaminophen (1). Likewise, recent United Kingdom surveys indicate that a high percentage of the population may be unaware of the risks associated with analgesic use (3, 4). In one study, 16% of university students surveyed admitted that they had knowingly exceeded the maximum daily dose of analgesics (4); the authors of another survey concluded that patient knowledge is insufficient to ensure safe usage of acetaminophen (3). Acetaminophen use has been associated with nephropathy, asthma, and other lung conditions (5–7), while ibuprofen use has been linked to increased risk of stroke and cardiovascular events (8, 9). However, some clinical and observational studies have yielded conflicting results (10–12), which could be due in part to difficulties in obtaining accurate information on use of acetaminophen and/or ibuprofen.
Typically in epidemiologic surveys information on dietary supplements, medications, and other xenobiotics is obtained from questionnaires, with resultant data being subject to possible recall and reporting biases. Reported medication use is sometimes compared with a secondary measure, such as general practitioner (13) or pharmacy (14, 15) databases, to validate the information. However, these secondary measures are themselves subject to incompleteness and coding errors and often may not include data on use of over-the-counter medications. Therefore, objective methods are needed to reduce bias and misclassification.
High-throughput metabonomic screening methods for biofluids, such as hydrogen-1 (or proton) nuclear magnetic resonance (1H NMR) spectroscopy and mass spectrometry, are now being applied in epidemiologic studies (16–18). These methods can simultaneously detect hundreds of metabolites related to diet, the gut microbiome, and the xenometabolome, including low-molecular-weight drug metabolites. Hence, they offer greater utility and economy than single-substance assay approaches—for example, approaches for detecting drug metabolites (19–21) and for assessing adherence to medication regimens (22).
Acetaminophen, ibuprofen, and their related metabolites are readily identifiable in 1H NMR urine spectra, and their spectral signatures are distinctive (see Web Figure 1, which is posted on the Journal’s Web site (http://aje.oxfordjournals.org/)) (23–26). Utilizing these features, we recently developed and validated a method for detecting the presence/absence of acetaminophen and ibuprofen in human urine spectra with sensitivity greater than 98% (27). This was achieved by performing targeted xenometabolome analyses for acetaminophen and ibuprofen metabolites from untargeted 1H NMR urinary spectra previously acquired for participants in the International Study of Macro-/Micro-Nutrients and Blood Pressure (INTERMAP Study). Here we compare acetaminophen and ibuprofen use detected by means of 1H NMR spectroscopy of urine specimens with self-reported analgesic use among participants in the INTERMAP Study.
MATERIALS AND METHODS
Participants and INTERMAP study design
The INTERMAP Study surveyed 4,680 women and men aged 40–59 years from Japan (4 samples), the People’s Republic of China (3 samples), the United Kingdom (2 samples), and the United States (8 samples) (28). Participants were randomly recruited from general and occupational populations in 1996–1999. Each participant made 4 clinic visits, the first 2 on consecutive days and the second 2 on consecutive days 3 weeks later, on average. Dietary data were collected at each visit by a trained interviewer using the in-depth multipass 24-hour recall method. All foods and drinks consumed in the previous 24 hours, including dietary supplements, were recorded.
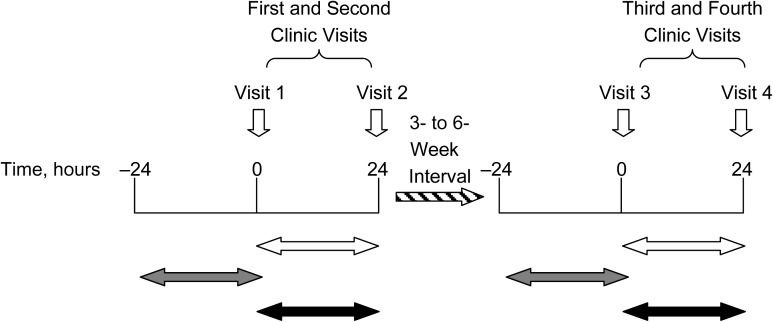
Prior to the first clinic visit, participants were asked to bring all of their medications with them, and on both the first and the third clinic visits, participants were asked to name the medications they were currently taking for high blood pressure and any other medications they were taking. Each participant provided 2 timed 24-hour urine collections, with both the start and the end of each 24-hour urine collection being done at the research center, between the first and second clinic visits and the third and fourth clinic visits (Figure 1). Urine volume was measured, and aliquots were obtained and stored at −20°C and then air-freighted on dry ice to the INTERMAP Central Laboratory (Leuven, Belgium) for urinary biochemistry (sodium, potassium, calcium, magnesium, and creatinine) and amino acid analysis by means of ion-exchange chromatography. Frozen aliquots were also sent from Leuven to Imperial College London for 1H NMR spectroscopy. The participants’ responses to questions on medication use, obtained during the first and third clinic visits, were subsequently coded by trained staff into 55 categories according to standard procedure—for example, yes/no for current use of oral antidiabetic, analgesic, or lipid-lowering agents. Names of medications were not entered into the INTERMAP database.
Figure 1.
Twenty-four-hour urine collection periods and corresponding self-reported data on analgesic use, International Study of Macro-/Micro-Nutrients and Blood Pressure, 1996–1999. The white horizontal arrows indicate 24-hour urine collection periods; the gray arrows indicate corresponding information on self-reported food and medication intake based on visits 1 and 3; and the black arrows indicate corresponding information on self-reported food intake based on visits 2 and 4 only and no available information on medication intake.
For this report, the specific names of analgesics used were obtained from the paper records stored for the Belfast, Northern Ireland (216 of 220 participants) and Chicago, Illinois (280 of 315 participants) samples. Therefore, for persons from these 2 Western population samples, it was possible to directly compare self-reported acetaminophen and ibuprofen use with urinary 1H NMR-detected acetaminophen and ibuprofen use. In subsequent analyses, self-reported analgesic use (yes/no) from the INTERMAP database was compared with the presence/absence of urinary 1H NMR-detected acetaminophen and ibuprofen for participants from all 17 population samples.
1H NMR spectroscopic analysis of urine specimens
Preparation and 1H NMR spectroscopic analysis of INTERMAP urine specimens have been described in detail (16). Briefly, 500 μL of thawed urine specimen was mixed with 250 μL of phosphate buffer (0.2 M disodium hydrogen phosphate/0.2 M sodium dihydrogen phosphate, pH 7.4 (range, ±0.5)) for stabilization of urine, and 75 μL of sodium 3-trimethylsilyl-(2,2,3,3-2H4)-1-propionate in deuterium monoxide solution (final concentration 0.1 mg/mL) was added for internal calibration at δ 0.0. Each specimen was placed into a 96-well plate and left to stand for 10 minutes before centrifugation at 1,500 g for 10 minutes to remove precipitate. Urine specimens were automatically delivered using the Bruker Efficient Sample Transfer System (Bruker BioSpin, Karlsruhe, Germany), and conventional 1H NMR spectra were acquired using a Bruker Avance 600 spectrometer (Bruker BioSpin, Rheinstetten, Germany) operating at 600.29 MHz in flow-injection mode. A standard 1-dimensional pulse sequence was used with presaturation of water resonance (recycle delay − 90° − t1 − 90° − tm − 90° acquisition; XWIN-NMR 3.5; Bruker BioSpin, Karlsruhe, Germany), with t1 of 2 seconds and tm of 100 ms. For each specimen, 64 free induction decays were collected into 32,000 data points using a spectral width of 20 ppm and a total repetition time of 4.8 seconds. The free induction decays were multiplied by an exponential weighting function corresponding to a line broadening of 0.3 Hz, and data were zero-filled to 64,000 data points prior to Fourier transformation.
All 1H NMR spectra were baseline-corrected and phased using an in-house routine written in MATLAB 7.0.1 (MathWorks, Natick, Massachusetts). All spectra were referenced to sodium 3-trimethylsilyl-(2,2,3,3-2H4)-1-propionate automatically, and each spectrum was digitized into 0.01-ppm spectral regions. The region of the spectra containing the water and urea resonances (δ 4.5–6.4) was removed. Of the 4,680 INTERMAP participants, valid 1H NMR spectra were not acquired for one of the 2 urine collections for 50 participants, leaving 4,630 persons (9,260 urine spectra) for the present report.
Multivariate analysis: acetaminophen and ibuprofen assessment models
Details on the development and validation of the assessment models, based on orthogonal projection to latent structure discriminant analysis with a Monte Carlo resampling procedure, have been published (27). Briefly, a subsample of INTERMAP urine specimens was visually inspected for the presence or absence of acetaminophen and ibuprofen metabolites; these spectra were then used to validate statistical models for detection of each analgesic. Optimal models were constructed on the basis of detailed spectral regions containing acetaminophen (δ 1.84–1.88; δ 2.13–2.25; and δ 7.13–7.49) and ibuprofen (δ 0.87–0.9; δ 1.05–1.09; δ 1.21–1.23; δ 1.38–1.48; and δ 1.51–1.55) metabolites, each with a bin size width of 0.01 ppm. For both acetaminophen and ibuprofen models, spectra were initially normalized to the spectral regions containing the drug metabolites. Spectra were then mean-centered prior to further analysis. Based on the optimal parameters, models for acetaminophen and ibuprofen showed sensitivity greater than 98% in identifying urine specimens containing acetaminophen and ibuprofen metabolites (27). The acetaminophen model was subsequently applied to 8,436 urine spectra and the ibuprofen model to 8,604 urine spectra, after excluding persons whose urine specimens were used to validate the prediction models. Here, we apply the model to the whole INTERMAP 1H NMR data set (9,260 urine spectra) to objectively assess reported analgesic use based on the presence or absence of urinary metabolites of acetaminophen and ibuprofen.
Statistical analysis
Initial analyses were performed on data from the Belfast and Chicago samples. Self-reported current acetaminophen or ibuprofen use was compared with the presence/absence of 1H NMR-detected urinary acetaminophen and ibuprofen metabolites obtained from the prediction models. Rates of concordance and 95% confidence intervals were calculated, along with rates of underreporting/underdetection of these 2 common analgesics, assuming that 1) the presence of acetaminophen and ibuprofen urinary metabolites reflected previous-day intake and 2) where participants reported having used acetaminophen or ibuprofen when metabolites were not detected in the urine, these self-reports were accurate and indicated underdetection of acetaminophen and ibuprofen by 1H NMR. Then self-reported questionnaire data on current analgesic use (yes/no) for all 17 INTERMAP Western population samples were compared with the presence/absence of 1H NMR-detected urinary acetaminophen and ibuprofen metabolites obtained from the prediction models, by country, gender, and age group. We used χ2 tests to assess intersubgroup differences in the prevalence of analgesic use.
RESULTS
Acetaminophen and ibuprofen use among INTERMAP participants from Belfast and Chicago
Among persons who reported analgesic use, the proportion using acetaminophen and/or ibuprofen was higher in Belfast than in Chicago for both visits (for the first clinic visit, 46.7% and 20.7%, respectively; for the third clinic visit, 27.3% and 16.3%). Use of other analgesics was similar for Belfast and Chicago (for the first clinic visit, 40.0% and 34.5%, respectively; for the third clinic visit, 36.4% and 32.7%), with aspirin being the most commonly reported alternative analgesic. The proportion of reported analgesic users for whom the name of the analgesic was not recorded was higher for Belfast than for Chicago (for the first clinic visit, 13.3% and 44.8%, respectively; for the third clinic visit, 36.4% and 51.0%). Reported analgesic users for whom the name of the analgesic was not recorded were excluded from comparisons with 1H NMR-detected urinary acetaminophen and ibuprofen metabolites; therefore, 216 (98.2%) of 220 participants from Belfast and 280 (88.9%) of 315 participants from Chicago were available for these analyses.
In comparison of self-reported acetaminophen and/or ibuprofen data with 1H NMR-detected urinary acetaminophen and/or ibuprofen metabolites (Table 1), overall rates of concordance were high for both populations: 83.8% (95% confidence interval (CI): 78.9, 88.7) in Belfast and 81.1% (95% CI: 76.5, 85.7) in Chicago. Concordance rates were comparable across gender and age groups. Overall rates of underdetection of acetaminophen and ibuprofen were low (∼1%) and were comparable for both population samples. Rates of underreporting for both visits were higher in Chicago than in Belfast (6.1% (95% CI: 3.3, 8.9) and 1.4% (95% CI: 0, 2.9); P = 0.01). Rates of underreporting at 1 visit only were comparable for the 2 populations: 10.7% (95% CI: 7.1, 14.3) for Chicago and 13.9% (95% CI: 9.3, 18.5) for Belfast (P = 0.28).
Table 1.
Comparison of Self-Reported Acetaminophen and Ibuprofen Use With Detection of Acetaminophen and/or Ibuprofen Metabolites in Urine Specimens of Belfast and Chicago Participants, by Gender and Age, International Study of Macro-/Micro-Nutrients and Blood Pressure, 1996–1999
| Concordance of Urinary and Self-Report Data | Location, Gender, and Age Group, years |
|||||||||||||||||||
| Belfast, Northern Ireland (n = 216) |
Chicago, Illinois (n = 280) |
|||||||||||||||||||
| Men (n = 125) |
Women (n = 91) |
Subtotal (n = 216) |
Men (n = 138) |
Women (n = 142) |
Subtotal (n = 280) |
|||||||||||||||
| 40–49 (n = 59) |
50–59 (n = 66) |
40–49 (n = 59) |
50–59 (n = 32) |
40–49 (n = 59) |
50–59 (n = 66) |
50–59 (n = 32) |
50–59 (n = 32) |
|||||||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Concordancea at both visits | 89.8 | 82.1, 97.5 | 92.4 | 86.0, 98.8 | 74.6 | 63.4, 85.6 | 71.9 | 56.2, 87.4 | 83.8 | 78.9, 88.7 | 84.7 | 76.4, 93.0 | 80.3 | 70.7, 89.8 | 80.0 | 70.6, 89.3 | 79.2 | 69.7, 88.5 | 81.1 | 76.5, 85.7 |
| Underreportingb | ||||||||||||||||||||
| At 1 visit onlyc | 10.2 | 2.5, 17.8 | 7.6 | 1.2, 13.9 | 23.7 | 12.8, 34.5 | 15.6 | 3.0, 28.2 | 13.9 | 9.3, 18.5 | 6.9 | 1.1, 12.8 | 10.6 | 3.2, 18.0 | 11.4 | 4.0, 18.8 | 13.9 | 5.9, 21.8 | 10.7 | 7.1, 14.3 |
| At both visits | 0 | 0, 0 | 0 | 0, 0 | 1.7 | 0, 5.0 | 6.3 | 0, 14.6 | 1.4 | 0, 2.9 | 4.2 | 0, 8.8 | 6.1 | 0.3, 11.8 | 8.6 | 2.0, 15.1 | 5.6 | 0.3, 10.8 | 6.1 | 3.3, 8.9 |
| Subtotal | 10.2 | 2.5, 17.8 | 7.6 | 1.2, 13.9 | 25.4 | 14.3, 36.5 | 21.9 | 7.6, 36.1 | 15.3 | 10.5, 20.1 | 11.1 | 3.9, 18.3 | 16.7 | 7.7, 25.6 | 20.0 | 10.6, 29.3 | 19.4 | 10.3, 28.5 | 16.8 | 12.4, 21.2 |
| Underdetectiond | ||||||||||||||||||||
| At 1 visit onlyc | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 6.3 | 0, 14.6 | 0.9 | 0, 2.2 | 4.2 | 0, 8.8 | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 1.1 | 0, 2.3 |
| At both visits | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 0 | 0 | 0 | 0, 0 | 1.5 | 0, 4.5 | 0 | 0, 0 | 0 | 0, 0 | 0.4 | 0, 1.1 |
| Subtotal | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 6.3 | 0, 14.6 | 0.9 | 0, 2.2 | 4.2 | 0, 8.8 | 1.5 | 0, 4.5 | 0 | 0, 0 | 0 | 0, 0 | 1.4 | 0, 2.8 |
| Mixed findingse | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 0 | 0, 0 | 0 | 0 | 0 | 0, 0 | 1.5 | 0, 4.5 | 0 | 0, 0 | 1.4 | 0, 4.1 | 0.7 | 0, 1.7 |
Abbreviation: CI, confidence interval.
Detection of urinary acetaminophen and/or ibuprofen metabolites matched with self-reported use of acetaminophen and/or ibuprofen.
Detection of urinary acetaminophen and/or ibuprofen metabolites but no reported use of acetaminophen and/or ibuprofen.
Indicates concordance for the other visit.
Reported use of acetaminophen and/or ibuprofen but no detection of urinary acetaminophen and/or ibuprofen metabolites.
Underdetection of urinary acetaminophen and/or ibuprofen metabolites at one visit and underreporting of acetaminophen and/or ibuprofen use at the other visit.
Unspecified analgesic use among INTERMAP participants from Japan, China, the United Kingdom, and the United States
Self-reported analgesic use was similar for men and women (for the first clinic visit, 7.2% and 7.5%, respectively (P = 0.73); for the third clinic visit, 7.1% and 7.5%, respectively (P = 0.69)) (Tables 2 and 3). The prediction models indicated that 644 (7.0%) of 9,260 urine spectra contained acetaminophen metabolites and 382 (4.1%) contained ibuprofen metabolites. The prevalence of detected acetaminophen and ibuprofen metabolites was significantly higher in urine specimens obtained from Western participants than from urine specimens obtained from East Asian participants (P < 0.0001), indicating wider acetaminophen and ibuprofen use among participants from the United Kingdom and the United States (overall rate of 16.4% for the first urine collection and 17.5% for the second urine collection; 9.8%–16.0% for men, 15.6%–24.3% for women) than among participants from China and Japan (overall rate of 4.8% for the first urine collection and 4.2% for the second urine collection; 0.4%–3.8% for men, 1.4%–4.9% for women). Based on detection of acetaminophen and ibuprofen metabolites in urinary specimens, the numbers of acetaminophen and ibuprofen users were mostly higher than the numbers of participants who reported current use of any analgesic medication, indicating systematic underreporting of analgesic use. This discrepancy was more prominent among women than among men.
Table 2.
Prevalence of Analgesic Use According to Detection of Urinary Acetaminophen and Ibuprofen Metabolites and According to Self-Reports Among Male Participants, by Country and Age Group, International Study of Macro-/Micro-Nutrients and Blood Pressure, 1996–1999
| Source of Data on Analgesic Use | Country and Age Group, years |
|||||||||||||||||
| Japan (n = 570) |
China (n = 416) |
United Kingdom (n = 265) |
United States (n = 1,085) |
Total (All Ages) (n = 2,336) |
||||||||||||||
| 40–49 (n = 287) |
50–59 (n = 283) |
40–49 (n = 210) |
50–59 (n = 206) |
40–49 (n = 133) |
50–59 (n = 132) |
40–49 (n = 535) |
50–59 (n = 550) |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Acetaminophen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 3 | 1.0 | 2 | 0.7 | 7 | 3.3 | 4 | 1.9 | 11 | 8.3 | 13 | 9.8 | 37 | 6.9 | 48 | 8.7 | 125 | 5.4 |
| Second urine specimen | 2 | 0.7 | 0 | 0 | 5 | 2.4 | 4 | 1.9 | 10 | 7.5 | 14 | 10.6 | 42 | 7.9 | 51 | 9.3 | 128 | 5.5 |
| First or second urine specimen | 5 | 1.7 | 2 | 0.7 | 11 | 5.2 | 7 | 3.4 | 16 | 12.0 | 23 | 17.4 | 64 | 12.0 | 78 | 14.2 | 206 | 8.8 |
| First and second urine specimens | 0 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0.5 | 5 | 3.8 | 4 | 3.0 | 15 | 2.8 | 21 | 3.8 | 47 | 2.0 |
| Ibuprofen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 0 | 0 | 1 | 0.4 | 1 | 0.5 | 0 | 0 | 2 | 1.5 | 1 | 0.8 | 25 | 4.7 | 39 | 7.1 | 69 | 3.0 |
| Second urine specimen | 2 | 0.7 | 1 | 0.4 | 1 | 0.5 | 2 | 1.0 | 4 | 3.0 | 4 | 3.0 | 28 | 5.2 | 41 | 7.5 | 83 | 3.6 |
| First or second urine specimen | 2 | 0.7 | 1 | 0.4 | 2 | 1.0 | 2 | 1.0 | 5 | 3.8 | 4 | 3.0 | 44 | 8.2 | 62 | 11.3 | 122 | 5.2 |
| First and second urine specimens | 0 | 0 | 1 | 0.4 | 0 | 0 | 0 | 0 | 1 | 0.8 | 1 | 0.8 | 9 | 1.7 | 18 | 3.3 | 30 | 1.3 |
| Acetaminophen and/or ibuprofen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 3 | 1.0 | 3 | 1.1 | 8 | 3.8 | 4 | 1.9 | 13 | 9.8 | 14 | 10.6 | 60 | 11.2 | 84 | 15.3 | 189 | 8.1 |
| Second urine specimen | 4 | 1.4 | 1 | 0.4 | 6 | 2.9 | 6 | 2.9 | 13 | 9.8 | 18 | 13.6 | 67 | 12.5 | 88 | 16.0 | 203 | 8.7 |
| First or second urine specimen | 7 | 2.4 | 3 | 1.1 | 13 | 6.2 | 9 | 4.4 | 20 | 15.0 | 27 | 20.5 | 99 | 18.5 | 131 | 23.8 | 309 | 13.2 |
| First and second urine specimens | 0 | 0 | 1 | 0.4 | 1 | 0.5 | 1 | 0.5 | 6 | 4.5 | 5 | 3.8 | 28 | 5.2 | 41 | 7.5 | 83 | 3.6 |
| Self-reported analgesic use | ||||||||||||||||||
| First clinic visit | 2 | 0.7 | 4 | 1.4 | 3 | 1.4 | 5 | 2.4 | 10 | 7.5 | 15 | 11.4 | 42 | 7.9 | 87 | 15.8 | 168 | 7.2 |
| Third clinic visit | 2 | 0.7 | 4 | 1.4 | 4 | 1.9 | 6 | 2.9 | 7 | 5.3 | 10 | 7.6 | 43 | 8.0 | 91 | 16.5 | 167 | 7.1 |
| First or third clinic visit | 4 | 1.4 | 6 | 2.1 | 7 | 3.3 | 11 | 5.3 | 13 | 9.8 | 15 | 11.4 | 58 | 10.8 | 114 | 20.7 | 228 | 9.8 |
| First and third clinic visits | 0 | 0 | 2 | 0.7 | 0 | 0 | 0 | 0 | 4 | 3.0 | 10 | 7.6 | 27 | 5.0 | 64 | 11.6 | 107 | 4.6 |
Abbreviation: 1H NMR, hydrogen-1 nuclear magnetic resonance.
Table 3.
Prevalence of Analgesic Use According to Detection of Urinary Acetaminophen and Ibuprofen Metabolites and According to Self-Reports Among Female Participants, by Country and Age Group, International Study of Macro-/Micro-Nutrients and Blood Pressure, 1996–1999
| Source of Data on Analgesic Use | Country and Age Group, years |
|||||||||||||||||
| Japan (n = 568) |
China (n = 416) |
United Kingdom (n = 231) |
United States (n = 1,079) |
Total (All Ages) (n = 2,294) |
||||||||||||||
| 40–49 (n = 291) |
50–59 (n = 277) |
40–49 (n = 212) |
50–59 (n = 204) |
40–49 (n = 128) |
50–59 (n = 103) |
40–49 (n = 541) |
50–59 (n = 538) |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Acetaminophen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 7 | 2.4 | 8 | 2.9 | 5 | 2.4 | 7 | 3.4 | 19 | 14.8 | 18 | 17.5 | 62 | 11.5 | 73 | 13.6 | 199 | 8.7 |
| Second urine specimen | 8 | 2.7 | 4 | 1.4 | 6 | 2.8 | 3 | 1.5 | 17 | 13.3 | 22 | 21.4 | 54 | 10.0 | 78 | 14.5 | 192 | 8.4 |
| First or second urine specimen | 13 | 4.5 | 11 | 4.0 | 10 | 4.7 | 10 | 4.9 | 32 | 25.0 | 30 | 29.1 | 90 | 16.6 | 118 | 21.9 | 314 | 13.7 |
| First and second urine specimens | 2 | 0.7 | 1 | 0.4 | 1 | 0.5 | 0 | 0 | 4 | 3.1 | 10 | 9.7 | 26 | 4.8 | 33 | 6.1 | 77 | 3.4 |
| Ibuprofen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1.5 | 3 | 2.3 | 4 | 3.9 | 43 | 7.9 | 54 | 10.0 | 107 | 4.7 |
| Second urine specimen | 1 | 0.3 | 0 | 0 | 2 | 0.9 | 1 | 0.5 | 3 | 2.3 | 4 | 3.9 | 55 | 10.2 | 57 | 10.6 | 123 | 5.4 |
| First or second urine specimen | 1 | 0.3 | 0 | 0 | 2 | 0.9 | 3 | 1.5 | 5 | 3.9 | 6 | 5.8 | 75 | 13.9 | 83 | 15.4 | 175 | 7.6 |
| First and second urine specimens | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.5 | 1 | 0.8 | 2 | 1.9 | 23 | 4.3 | 28 | 5.2 | 55 | 2.4 |
| Acetaminophen and/or ibuprofen signals in 1H NMR urine spectra | ||||||||||||||||||
| First urine specimen | 7 | 2.4 | 8 | 2.9 | 5 | 2.4 | 10 | 4.9 | 22 | 17.2 | 21 | 20.4 | 100 | 18.5 | 123 | 22.9 | 296 | 12.9 |
| Second urine specimen | 9 | 3.1 | 4 | 1.4 | 8 | 3.8 | 4 | 2.0 | 20 | 15.6 | 25 | 24.3 | 107 | 19.8 | 127 | 23.6 | 304 | 13.3 |
| First or second urine specimen | 14 | 4.8 | 11 | 4.0 | 12 | 5.7 | 13 | 6.4 | 36 | 28.1 | 35 | 34.0 | 153 | 28.3 | 186 | 34.6 | 460 | 20.1 |
| First and second urine specimens | 2 | 0.7 | 1 | 0.4 | 1 | 0.5 | 1 | 0.5 | 6 | 4.7 | 11 | 10.7 | 54 | 10.0 | 64 | 11.9 | 140 | 6.1 |
| Self-reported analgesic use | ||||||||||||||||||
| First clinic visit | 5 | 1.7 | 4 | 1.4 | 5 | 2.4 | 6 | 2.9 | 6 | 4.7 | 8 | 7.8 | 50 | 9.2 | 87 | 16.2 | 171 | 7.5 |
| Third clinic visit | 2 | 0.7 | 2 | 0.7 | 6 | 2.8 | 14 | 6.9 | 7 | 5.5 | 11 | 10.7 | 51 | 9.4 | 79 | 14.7 | 172 | 7.5 |
| First or third clinic visit | 5 | 1.7 | 5 | 1.8 | 10 | 4.7 | 18 | 8.8 | 10 | 7.8 | 15 | 14.6 | 71 | 13.1 | 106 | 19.7 | 240 | 10.5 |
| First and third clinic visits | 2 | 0.7 | 1 | 0.4 | 1 | 0.5 | 2 | 1.0 | 3 | 2.3 | 4 | 3.9 | 30 | 5.5 | 60 | 11.2 | 103 | 4.5 |
Abbreviation: 1H NMR, hydrogen-1 nuclear magnetic resonance.
The prevalence of acetaminophen and ibuprofen use varied among the Western population samples: Honolulu, Hawaii (all participants of Japanese ancestry) had the lowest prevalence, with 7.8% of urine specimens containing acetaminophen and/or ibuprofen metabolites for the first urine collection and 9.3% for the second urine collection (Web Table 1). Pittsburgh, Pennsylvania, and Corpus Christi, Texas (non-Hispanic participants) showed the highest prevalence of analgesic use, with acetaminophen and/or ibuprofen metabolites being detected in more than 20% of the first and second urine collections. Among Western participants, those aged 50–59 years had higher acetaminophen and/or ibuprofen use than those aged 40–49 years (for the first and second urine specimens, P = 0.01 and P = 0.006, respectively), and more women used acetaminophen and/or ibuprofen than men (P < 0.0001 for both urine specimens).
Self-reported data on analgesic use (Web Table 2) were similar to urinary data, with Honolulu showing the lowest proportion of reported analgesic users (3.5% for both the first and third visits). Prevalences of self-reported analgesic use at the first and third visits were greater than 15% for the Chicago, Minneapolis (Minnesota), Pittsburgh, and Corpus Christi (non-Hispanic) samples (Web Table 2). High-level concordance (>60%) was observed between self-reported analgesic use and 1H NMR-detected urinary acetaminophen and/or ibuprofen metabolites (Table 4) for all Western population samples, with concordance being lowest for Pittsburgh (60.6%, 95% CI: 54.6, 66.5) and an overall concordance of 70.5% (95% CI: 68.7, 72.2).
Table 4.
Comparison of Self-Reported Analgesic Use With Detection of Urinary Acetaminophen and/or Ibuprofen Metabolites for Participants at Western Locations, International Study of Macro-/Micro-Nutrients and Blood Pressure, 1996–1999
| Country and Location |
||||||||||||||||||||||
| United Kingdom |
United States |
United Kingdom and United States (n = 2,660) |
||||||||||||||||||||
| Belfast, Northern Ireland (n = 220) | West Bromwich, England (n = 276) | Baltimore, Maryland (n = 274) | Chicago, Illinois (n = 315) | Honolulu, Hawaii (n = 257) | Jackson, Mississippi (n = 261) | Minneapolis, Minnesota (n = 260) | Pittsburgh, Pennsylvania (n = 259) | Corpus Christi, Texas | ||||||||||||||
| Hispanics (n = 268) | Non-Hispanics (n = 270) | |||||||||||||||||||||
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Matched urinary data and self-reported data at both visits | 80.0 | 74.7, 85.2 | 69.9 | 64.5, 75.3 | 71.2 | 65.8, 76.5 | 67.6 | 62.4, 72.7 | 85.2 | 80.8, 89.5 | 67.4 | 61.7, 73.1 | 70.0 | 64.4, 75.5 | 60.6 | 54.6, 66.5 | 69.7 | 64.2, 75.2 | 65.1 | 59.5, 70.8 | 70.5 | 68.7, 72.2 |
| Discrepancy in information | ||||||||||||||||||||||
| Urinary acetaminophen and/or ibuprofen metabolites detected but participant did not report analgesic use | ||||||||||||||||||||||
| At one visit onlya | 13.6 | 9.1, 18.1 | 19.9 | 15.2, 24.6 | 13.8 | 9.8, 17.9 | 8.9 | 5.7, 12.0 | 7.8 | 4.5, 11.0 | 19.9 | 15.0, 24.7 | 11.5 | 7.7, 15.4 | 17.3 | 12.7, 21.9 | 15.6 | 11.3, 20.0 | 17.4 | 12.8, 21.9 | 14.5 | 13.2, 15.9 |
| At both visits | 1.4 | 0, 2.9 | 3.3 | 1.2, 5.4 | 7.3 | 4.2, 10.3 | 4.8 | 2.4, 7.1 | 3.5 | 1.3, 5.7 | 7.3 | 4.1, 10.4 | 4.2 | 1.8, 6.7 | 5.8 | 2.9, 8.6 | 4.9 | 2.3, 7.4 | 5.6 | 2.8, 8.3 | 4.9 | 4.0, 5.7 |
| Subtotal | 15.0 | 10.2, 19.7 | 23.2 | 18.2, 28.1 | 21.1 | 16.3, 26.0 | 13.7 | 9.9, 17.4 | 11.3 | 7.4, 15.1 | 27.2 | 21.8, 32.6 | 15.7 | 11.3, 20.1 | 23.1 | 18.0, 28.3 | 20.5 | 15.6, 25.3 | 23.0 | 17.9, 27.9 | 19.4 | 17.9, 20.9 |
| Participant reported analgesic use but urinary acetaminophen and/or ibuprofen metabolites were not detected | ||||||||||||||||||||||
| At one visit onlya | 3.2 | 0.9, 5.5 | 4.3 | 1.9, 6.8 | 3.6 | 1.4, 5.9 | 9.2 | 6.1, 12.3 | 2.3 | 0.5, 4.2 | 1.9 | 0.3, 3.6 | 6.2 | 3.2, 9.1 | 10.8 | 7.0, 14.5 | 6.3 | 3.4, 9.3 | 7.8 | 4.6, 10.9 | 5.7 | 4.8, 6.6 |
| At both visits | 1.8 | 0.1, 3.6 | 1.8 | 0.2, 3.4 | 3.6 | 1.4, 5.9 | 7.3 | 4.4, 10.1 | 1.2 | 0, 2.5 | 1.5 | 0, 3.0 | 7.3 | 4.1, 10.4 | 5.0 | 2.4, 7.7 | 2.6 | 0.7, 4.5 | 3.7 | 1.5, 6.0 | 3.7 | 3.0, 4.4 |
| Subtotal | 5.0 | 2.1, 7.9 | 6.1 | 3.3, 9.0 | 7.2 | 4.2, 10.3 | 16.5 | 12.4, 20.6 | 3.5 | 1.3, 5.7 | 3.4 | 1.2, 5.7 | 13.5 | 9.3, 17.6 | 15.8 | 11.3, 20.2 | 8.9 | 5.5, 12.3 | 11.5 | 7.7, 15.2 | 9.4 | 8.3, 10.5 |
| Mismatch of information at one visit but information was matched at another visit | 0 | 0, 0 | 0.7 | 0, 1.7 | 0.4 | 0, 1.1 | 2.2 | 0.6, 3.9 | 0 | 0, 0 | 1.9 | 0.3, 3.6 | 0.8 | 0, 1.8 | 0.4 | 0, 1.1 | 0.8 | 0, 1.8 | 0.4 | 0, 1.1 | 0.8 | 0.5, 1.1 |
Abbreviation: CI, confidence interval.
Indicates concordance for another visit.
Three main types of discrepancy were also observed. The first group was persons who did not report use of any analgesics but for whom acetaminophen and/or ibuprofen metabolites were detected in the urine specimen at 1 or more visits (putative underreporters) (19.4%, 95% CI: 17.9, 20.9). This discrepancy varied across population samples, being lowest for Honolulu (11.3%, 95% CI: 7.4, 15.1) and highest for Jackson, Mississippi (27.2%, 95% CI: 21.8, 32.6). Jackson (7.3%, 95% CI: 4.1, 10.4) and Baltimore, Maryland (7.3%, 95% CI: 4.2, 10.3) showed the highest rates of potential underreporting at both visits. Overall, 129 persons (4.9%, 95% CI: 4.0, 5.7) underreported analgesic use at both visits, while 387 (14.5%, 95% CI: 13.2, 15.9) underreported analgesic use at 1 visit only. The second group was persons who reported analgesic use but for whom acetaminophen and/or ibuprofen metabolites were not detected in urine specimens (due to putative underdetection of acetaminophen/ibuprofen by 1H NMR, use of an alternative analgesic not detected by the prediction models, or time-frame differences in urine collection and self-reported data). The overall rate for this discrepancy was 9.4% (95% CI: 8.3, 10.5), and rates were higher for Chicago (16.5%, 95% CI: 12.4, 20.6), Pittsburgh (15.8%, 95% CI: 11.3, 20.2), Minneapolis (13.5%, 95% CI: 9.3, 17.6), and Corpus Christi (non-Hispanics) (11.5%, 95% CI: 7.7, 15.2). The third group was persons with a mixture of the above two types of discrepancy—for example, a person who reported use of an analgesic at the first visit but not the third visit, but the 1H NMR urine spectra revealed the presence of either acetaminophen and/or ibuprofen or both metabolites in the second urine specimen though not the first. The percentage in this group was typically low (∼2% or less) across all Western population samples, being highest in the Chicago sample (2.2%, 95% CI: 0.6, 3.9).
For East Asian participants overall, both urinary levels and self-reported analgesic use were low (∼4%) and gender/age differences in analgesic use were not observed (Web Tables 3 and 4). Comparisons of reported analgesic use and 1H NMR-detected urinary acetaminophen and ibuprofen metabolites were not made for the East Asian samples because of a low prevalence of analgesic use in those population samples.
DISCUSSION
Our findings showed the efficacy of a novel method for objective assessment of xenobiotic use, to validate epidemiologic questionnaire data, via 1H NMR spectroscopy of urine specimens. We found high levels of concordance for self-reported acetaminophen and/or ibuprofen use with urinary acetaminophen and/or ibuprofen metabolites detected by 1H NMR spectroscopy. Although detailed self-reported acetaminophen and ibuprofen data were available only for 496 Belfast and Chicago participants, confidence intervals for concordance, underreporting, and underdetection showed acceptable levels of precision. Furthermore, similar levels of concordance and underreporting were observed with self-reported unspecified analgesic use among 2,660 Western INTERMAP participants.
Detection of underreporting was generally higher in women than in men. Urinary rates of acetaminophen/ibuprofen detection were mostly higher than rates of self-reported analgesic use, indicating an overall rate of underreporting of analgesic use among Western INTERMAP participants of approximately 20%. Underreporting at both clinic visits, which were made on average 3 weeks apart, was detected for 5% of Western participants. Such “serial” underreporting could potentially reflect intentional underreporting by the participant. Unintentional underreporting, which could occur at one or both visits, could be due to memory lapse and/or ingestion of analgesics after the clinic visit but during the 24-hour urine collection period. It is likely that the scale of underreporting of total analgesic use is greater, since only acetaminophen and ibuprofen metabolites were detected by the present method.
For 9.4% of Western participants, analgesic use was reported but acetaminophen and/or ibuprofen metabolites were not detected in urine specimens. This discrepancy may have been due to underdetection by 1H NMR of acetaminophen/ibuprofen metabolites in the urinary spectra. However, this is unlikely to have been a major factor, since we previously demonstrated that the acetaminophen and ibuprofen prediction models each had a sensitivity greater than 98% (27); furthermore, detailed data on analgesic use from Belfast and Chicago participants indicated an underdetection rate of approximately 1%. It is also possible that the rate of analgesic use was underestimated by 1H NMR because of differences in the time frame between collection of self-reported data and collection of urine specimens. Both acetaminophen and ibuprofen are rapidly excreted in the urine after oral ingestion, and the elimination half-lives are approximately 4 hours and 2 hours, respectively (29). Thus, analgesics taken during the 24 hours prior to the first and third clinic visits could have been reported by the participants but fully excreted before the start of the 24-hour urine collections. It is likely that the major contributor to the apparent “underdetection” by 1H NMR is self-reported use of common alternative analgesics not detected by the current prediction models. This is supported by our detailed data from Belfast and Chicago, where alternative analgesics (predominantly aspirin) accounted for approximately one-third of total reported analgesic use. Given these limitations, we can assume that the overall concordance between reported and 1H NMR-detected acetaminophen/ibuprofen use for Western participants will be higher than the 70% observed between unspecified analgesic use and 1H NMR-detected acetaminophen/ibuprofen.
Our data indicate low rates of analgesic use in East Asian population samples compared with Western samples and higher rates in older participants and women. The latter finding is consistent with other epidemiologic data, including data from the Third National Health and Nutrition Examination Survey (30) and the Tromsø Study (31). Observed gender and age differences in analgesic use probably reflect gender- and age-related differences in disease rates—for example, a higher prevalence of rheumatoid arthritis in women and higher prevalences of both rheumatoid arthritis and osteoarthritis in older persons (32, 33). The level of concordance observed here was similar to that in other studies, such as studies comparing self-reported questionnaire data with electronic monitoring systems (34, 35) and general practitioner records (36). However, our method offers several advantages compared with self-report validation tools that rely on pharmacy or general practitioner records (34–40). First, pharmacy or general practitioner records may be prone to incomplete information, particularly if patients use multiple pharmacies or physicians. Second, analgesics purchased over the counter, such as acetaminophen and ibuprofen, may not be recorded in general practitioner/pharmacy databases. Third, general practitioner records document prescriptions only; the prescriptions may or may not have been filled (41–43). Even if a patient filled the prescription, he or she may not have actually taken the drug; nonadherence to medication regimens has been widely reported (44–46). In contrast, urinary 1H NMR data reflect actual ingestion of the analgesic.
To our knowledge, our study provides the only objective data on population exposure to acetaminophen and ibuprofen currently available. We were able to account for discrepancies with self-reported unspecified analgesic data, and this approach could be used in future studies to validate self-reported analgesic data and improve exposure classification. Moreover, the generality of metabolic profiling methods makes it more efficient than most biologic assays, which tend to be calibrated for the measurement of specific biochemical compounds. The approach demonstrated here could be adapted for validation of self-reported dietary supplements and food consumption. Using a 1H NMR-based approach, Heinzmann et al. (47) identified proline betaine as a biomarker of self-reported citrus fruit consumption. Others have applied mass spectrometry-based approaches to nutritional biomarker discovery (48, 49).
In terms of a general metabonomics-based biomarker discovery strategy, we recommend global untargeted 1H NMR spectroscopy or untargeted mass spectrometry with multivariate chemometric analyses for hypothesis generation, complemented as necessary by targeted mass spectrometry or other biochemical analyses for validation. This approach could be used to validate self-reported data on drug use or to retrospectively estimate the prevalence of (for example) analgesic use, in population samples where urine specimens were collected but data on analgesic use were not available. It is essential for validation studies that the time frames of self-reported data and urine specimens be aligned appropriately to account for the excretion rates of the biomarker metabolites.
In conclusion, in our large-scale, multinational epidemiologic study, we demonstrated the efficacy of an objective 1H NMR-based method for validation of self-reported data on analgesic use, detecting an underreporting rate of approximately 15% or more. The analytical strategy offers benefits over traditional methods for validating drug-use data (e.g., general practitioner or pharmacy records) and may be applied to a wide range of xenobiotics, including pharmaceuticals, dietary supplements, and foods.
Supplementary Material
Acknowledgments
Author affiliations: Clinical and Professional Practice Division, Medway School of Pharmacy, Universities of Kent and Greenwich, Kent, United Kingdom (Ruey Leng Loo); Biomolecular Medicine Section, Department of Surgery and Cancer, Faculty of Medicine, Imperial College London, London, United Kingdom (Ruey Leng Loo, Jeremy K. Nicholson, Elaine Holmes); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, St. Mary’s Campus, London, United Kingdom (Queenie Chan, Ian J. Brown, Paul Elliott); Department of Human and Health Sciences, School of Biosciences, University of Westminster, London, United Kingdom (Claire E. Robertson); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Jeremiah Stamler); and MRC-HPA Centre for Environment and Health, Imperial College London, London, United Kingdom (Jeremy K. Nicholson, Elaine Holmes, Paul Elliott).
This work was supported by the US National Heart, Lung, and Blood Institute, Bethesda, Maryland (grants R01-HL50490 and R01-HL84228); the Chicago Health Research Foundation, Chicago, Illinois; the West Midlands National Health Service Research and Development Programme; the Northern Ireland Chest, Heart and Stroke Association, Belfast, Northern Ireland (grant R2019EPH); and national agencies in China and Japan (Ministry of Education, Science, Sports, and Culture, Grant-in-Aid for Scientific Research [A] 090357003). Prof. Paul Elliott received funding from the United Kingdom National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre, Imperial College Healthcare NHS Trust. He is an NIHR Senior Investigator.
The authors thank the many colleagues who collected and processed the INTERMAP data (for a listing of many of them, see reference 28). They also thank Drs. T. Ebbels and H. Keun for use of the phasing and referencing algorithm. In addition, the authors thank F. Crespo for her assistance in obtaining records for the INTERMAP Chicago center.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- 1H NMR
hydrogen-1 nuclear magnetic resonance
- INTERMAP Study
International Study of Macro-/Micro-Nutrients and Blood Pressure
References
- 1.Consumer Healthcare Products Association. Briefing Book. Joint Meeting of the Drug Safety and Risk Management Advisory Committee, Nonprescription Drugs Advisory Committee, and the Anesthetic and Life Support Drugs Advisory Committee. June 29 and 30, 2009. Washington, DC: Consumer Healthcare Products Association; 2009. (Docket no. FDA-2009-N-0138). (Accessed November 11, 2010) [Google Scholar]
- 2.Sheen CL, Dillon JF, Bateman DN, et al. Paracetamol pack size restriction: the impact on paracetamol poisoning and the over-the-counter supply of paracetamol, aspirin and ibuprofen. Pharmacoepidemiol Drug Saf. 2002;11(4):329–331. doi: 10.1002/pds.701. [DOI] [PubMed] [Google Scholar]
- 3.Wood DM, English E, Butt S, et al. Patient knowledge of the paracetamol content of over-the-counter (OTC) analgesics, cough/cold remedies and prescription medications. Emerg Med J. 2010;27(11):829–833. doi: 10.1136/emj.2009.085027. [DOI] [PubMed] [Google Scholar]
- 4.French DP, James DH. Reasons for the use of mild analgesics among English students. Pharm World Sci. 2008;30(1):79–85. doi: 10.1007/s11096-007-9146-7. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar H, Stewart A, Mitchell E, et al. The role of paracetamol in the pathogenesis of asthma. Clin Exp Allergy. 2010;40(1):32–41. doi: 10.1111/j.1365-2222.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 6.McKeever TM, Lewis SA, Smit HA, et al. The association of acetaminophen, aspirin, and ibuprofen with respiratory disease and lung function. Am J Respir Crit Care Med. 2005;171(9):966–971. doi: 10.1164/rccm.200409-1269OC. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen SO, Newson RB, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57(11):958–963. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fosbøl EL, Folke F, Jacobsen S, et al. Cause-specific cardiovascular risk associated with nonsteroidal antiinflammatory drugs among healthy individuals. Circ Cardiovasc Qual Outcomes. 2010;3(4):395–405. doi: 10.1161/CIRCOUTCOMES.109.861104. [DOI] [PubMed] [Google Scholar]
- 9.Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17(6):275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 10.Patel TN, Goldberg KC. Use of aspirin and ibuprofen compared with aspirin alone and the risk of myocardial infarction. Arch Intern Med. 2004;164(8):852–856. doi: 10.1001/archinte.164.8.852. [DOI] [PubMed] [Google Scholar]
- 11.Kang EM, Lundsberg LS, Illuzzi JL, et al. Prenatal exposure to acetaminophen and asthma in children. Obstet Gynecol. 2009;114(6):1295–1306. doi: 10.1097/AOG.0b013e3181c225c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lesko SM, Louik C, Vezina RM, et al. Asthma morbidity after the short-term use of ibuprofen in children. Pediatrics. 2002;109(2):E20. doi: 10.1542/peds.109.2.e20. [DOI] [PubMed] [Google Scholar]
- 13.Cotterchio M, Kreiger N, Darlington G, et al. Comparison of self-reported and physician-reported antidepressant medication use. Ann Epidemiol. 1999;9(5):283–289. doi: 10.1016/s1047-2797(98)00072-6. [DOI] [PubMed] [Google Scholar]
- 14.Boudreau DM, Daling JR, Malone KE, et al. A validation study of patient interview data and pharmacy records for antihypertensive, statin, and antidepressant medication use among older women. Am J Epidemiol. 2004;159(3):308–317. doi: 10.1093/aje/kwh038. [DOI] [PubMed] [Google Scholar]
- 15.Van den Brandt PA, Petri H, Dorant E, et al. Comparison of questionnaire information and pharmacy data on drug use. Pharm Weekbl Sci. 1991;13(2):91–96. doi: 10.1007/BF01974987. [DOI] [PubMed] [Google Scholar]
- 16.Holmes E, Loo RL, Stamler J, et al. Human metabolic phenotype diversity and its association with diet and blood pressure. Nature. 2008;453(7193):396–400. doi: 10.1038/nature06882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barton RH, Nicholson JK, Elliott P, et al. High-throughput 1H NMR-based metabolic analysis of human serum and urine for large-scale epidemiological studies: validation study. Int J Epidemiol. 2008;37(suppl 1):i31–i40. doi: 10.1093/ije/dym284. [DOI] [PubMed] [Google Scholar]
- 18.Suhre K, Meisinger C, Döring A, et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS One. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953. (doi:10.1371/journal.pone.0013953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19(6):443–449. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 20.Catlin DH, Ahrens BD, Kucherova Y. Detection of norbolethone, an anabolic steroid never marketed, in athletes’ urine. Rapid Commun Mass Spectrom. 2002;16(13):1273–1275. doi: 10.1002/rcm.722. [DOI] [PubMed] [Google Scholar]
- 21.Hatton CK, Catlin DH. Detection of androgenic anabolic steroids in urine. Clin Lab Med. 1987;7(3):655–668. [PubMed] [Google Scholar]
- 22.Moshkovska T, Stone MA, Clatworthy J, et al. An investigation of medication adherence to 5-aminosalicylic acid therapy in patients with ulcerative colitis, using self-report and urinary drug excretion measurements. Aliment Pharmacol Ther. 2009;30(11-12):1118–1127. doi: 10.1111/j.1365-2036.2009.04152.x. [DOI] [PubMed] [Google Scholar]
- 23.Holmes E, Loo RL, Cloarec O, et al. Detection of urinary drug metabolite (xenometabolome) signatures in molecular epidemiology studies via statistical total correlation (NMR) spectroscopy. Anal Chem. 2007;79(7):2629–2640. doi: 10.1021/ac062305n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bales JR, Nicholson JK, Sadler PJ. Two-dimensional proton nuclear magnetic resonance “maps” of acetaminophen metabolites in human urine. Clin Chem. 1985;31(5):757–762. [PubMed] [Google Scholar]
- 25.Bales JR, Sadler PJ, Nicholson JK, et al. Urinary excretion of acetaminophen and its metabolites as studied by proton NMR spectroscopy. Clin Chem. 1984;30(10):1631–1636. [PubMed] [Google Scholar]
- 26.Spraul M, Hofmann M, Dvortsak P, et al. High-performance liquid chromatography coupled to high-field proton nuclear magnetic resonance spectroscopy: application to the urinary metabolites of ibuprofen. Anal Chem. 1993;65(4):327–330. doi: 10.1021/ac00052a004. [DOI] [PubMed] [Google Scholar]
- 27.Loo RL, Coen M, Ebbels T, et al. Metabolic profiling and population screening of analgesic usage in nuclear magnetic resonance spectroscopy-based large-scale epidemiologic studies. INTERMAP Research Group. Anal Chem. 2009;81(13):5119–5129. doi: 10.1021/ac900567e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamler J, Elliott P, Dennis B, et al. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). INTERMAP Research Group. J Hum Hypertens. 2003;17(9):591–608. doi: 10.1038/sj.jhh.1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Association of the British Pharmaceutical Industry. Medicine Compendium 2010. London, United Kingdom: Datapharm Communications Ltd; 2010. [Google Scholar]
- 30.Paulose-Ram R, Hirsch R, Dillon C, et al. Prescription and non-prescription analgesic use among the US adult population: results from the third National Health and Nutrition Examination Survey (NHANES III) Pharmacoepidemiol Drug Saf. 2003;12(4):315–326. doi: 10.1002/pds.755. [DOI] [PubMed] [Google Scholar]
- 31.Eggen AE. The Tromsø Study: frequency and predicting factors of analgesic drug use in a free-living population (12–56 years) J Clin Epidemiol. 1993;46(11):1297–1304. doi: 10.1016/0895-4356(93)90098-l. [DOI] [PubMed] [Google Scholar]
- 32.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeller A, Schroeder K, Peters TJ. An adherence self-report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol. 2008;61(3):282–288. doi: 10.1016/j.jclinepi.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Zeller A, Ramseier E, Teagtmeyer A, et al. Patients’ self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31(11):2037–2043. doi: 10.1291/hypres.31.2037. [DOI] [PubMed] [Google Scholar]
- 36.Grimaldi-Bensouda L, Rossignol M, Aubrun E, et al. Agreement between patients’ self-report and physicians’ prescriptions on cardiovascular drug exposure: the PGRx database experience. Pharmacoepidemiol Drug Saf. 2010;19(6):591–595. doi: 10.1002/pds.1952. [DOI] [PubMed] [Google Scholar]
- 37.Klungel OH, de Boer A, Paes AH, et al. Agreement between self-reported antihypertensive drug use and pharmacy records in a population-based study in the Netherlands. Pharm World Sci. 1999;21(5):217–220. doi: 10.1023/a:1008741321384. [DOI] [PubMed] [Google Scholar]
- 38.Heerdink ER, Leufkens HG, Koppedraaijer C, et al. Information on drug use in the elderly: a comparison of pharmacy, general-practitioner and patient data. Pharm World Sci. 1995;17(1):20–24. doi: 10.1007/BF01875554. [DOI] [PubMed] [Google Scholar]
- 39.West SL, Savitz DA, Koch G, et al. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142(10):1103–1112. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 40.Uiters E, van Dijk L, Devillé W, et al. Ethnic minorities and prescription medication; concordance between self-reports and medical records. BMC Health Serv Res. 2006;6:115. doi: 10.1186/1472-6963-6-115. (doi:10.1186/1472-6963-6-115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of Medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553–560. doi: 10.18553/jmcp.2008.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esposito D, Schone E, Williams T, et al. Prevalence of unclaimed prescriptions at military pharmacies. J Manag Care Pharm. 2008;14(6):541–552. doi: 10.18553/jmcp.2008.14.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ekedahl A, Månsson N. Unclaimed prescriptions after automated prescription transmittals to pharmacies. Pharm World Sci. 2004;26(1):26–31. doi: 10.1023/b:phar.0000013464.09197.41. [DOI] [PubMed] [Google Scholar]
- 44.Valeberg BT, Miaskowski C, Hanestad BR, et al. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24(7):627–636. doi: 10.1097/AJP.0b013e31816fe020. [DOI] [PubMed] [Google Scholar]
- 45.Christensen A, Christrup LL, Fabricius PE, et al. The impact of an electronic monitoring and reminder device on patient compliance with antihypertensive therapy: a randomized controlled trial. J Hypertens. 2010;28(1):194–200. doi: 10.1097/HJH.0b013e328331b718. [DOI] [PubMed] [Google Scholar]
- 46.Higgins PD, Rubin DT, Kaulback K, et al. Systematic review: impact of non-adherence to 5-aminosalicylic acid products on the frequency and cost of ulcerative colitis flares. Aliment Pharmacol Ther. 2009;29(3):247–257. doi: 10.1111/j.1365-2036.2008.03865.x. [DOI] [PubMed] [Google Scholar]
- 47.Heinzmann SS, Brown IJ, Chan Q, et al. Metabolic profiling strategy for discovery of nutritional biomarkers: proline betaine as a marker of citrus consumption. Am J Clin Nutr. 2010;92(2):436–443. doi: 10.3945/ajcn.2010.29672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altmaier E, Kastenmüller G, Römisch-Margl W, et al. Questionnaire-based self-reported nutrition habits associate with serum metabolism as revealed by quantitative targeted metabolomics. Eur J Epidemiol. 2011;26(2):145–156. doi: 10.1007/s10654-010-9524-7. [DOI] [PubMed] [Google Scholar]
- 49.Legido-Quigley C, Stella C, Perez-Jimenez F, et al. Liquid chromatography-mass spectrometry methods for urinary biomarker detection in metabonomic studies with application to nutritional studies. Biomed Chromatogr. 2010;24(7):737–743. doi: 10.1002/bmc.1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.