Abstract
Randomized clinical trials (RCTs) are usually the preferred strategy with which to generate evidence of comparative effectiveness, but conducting an RCT is not always feasible. Though observational studies and RCTs often provide comparable estimates, the questioning of observational analyses has recently intensified because of randomized-observational discrepancies regarding the effect of postmenopausal hormone replacement therapy on coronary heart disease. Reanalyses of observational data that excluded prevalent users of hormone replacement therapy led to attenuated discrepancies, which begs the question of whether exclusion of prevalent users should be generally recommended. In the current study, the authors evaluated the effect of excluding prevalent users of statins in a meta-analysis of observational studies of persons with cardiovascular disease. The pooled, multivariate-adjusted mortality hazard ratio for statin use was 0.77 (95% confidence interval (CI): 0.65, 0.91) in 4 studies that compared incident users with nonusers, 0.70 (95% CI: 0.64, 0.78) in 13 studies that compared a combination of prevalent and incident users with nonusers, and 0.54 (95% CI: 0.45, 0.66) in 13 studies that compared prevalent users with nonusers. The corresponding hazard ratio from 18 RCTs was 0.84 (95% CI: 0.77, 0.91). It appears that the greater the proportion of prevalent statin users in observational studies, the larger the discrepancy between observational and randomized estimates.
Keywords: bias (epidemiology), comparative effectiveness research, confounding factors (epidemiology), meta-analysis, prospective studies, selection bias
Reliable evidence on the effectiveness and safety of clinical and public health interventions is central to the ongoing discussion of health care in the United States (1–6) and other countries (7, 8). Although randomized clinical trials are usually the preferred strategy for obtaining such evidence, they are not always feasible or timely. Clearly, much evidence on the comparative effectiveness and safety of clinical and public health interventions will have to be derived from observational studies.
Observational studies often yield effect estimates comparable to those of randomized trials (9–12), but the ability of observational analyses to provide valid effect estimates has been questioned in some high-profile cases. Prominent among these is the effect of postmenopausal hormone replacement therapy (HRT) on coronary heart disease (CHD). Although some observational studies have suggested harmful effects of HRT (13), most observational studies (14, 15) have found a lower CHD risk in prevalent users of HRT compared with nonusers. This finding was interpreted as supporting the existence of a protective effect of HRT on CHD risk. However, in a large randomized clinical trial, Manson et al. (16) found a higher CHD risk in incident users of HRT compared with nonusers, especially in the early period of follow-up. Reanalyses of the observational studies (17, 18) that, like the randomized trial, compared incident users with nonusers after applying a washout period found no overall beneficial effect of HRT on CHD risk.
The above findings support previous proposals to exclude prevalent users when using observational data to assess the comparative effectiveness and safety of clinical and public health interventions (19). Here we provide further support for eliminating prevalent users in observational studies on the effectiveness of statin therapy (3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitors) in reducing CHD risk and mortality. In most of the published observational studies, investigators have compared prevalent users with nonusers.
We chose statins as a case study because the beneficial effect of statins on CHD risk and mortality has been proven beyond any doubt in more than 3 dozen randomized clinical trials and several large meta-analyses (20–32), but no systematic review and meta-analysis of observational studies of statin therapy is available. We compared estimates from randomized and observational studies according to the type of intervention (primary prevention vs. secondary prevention) and classified observational studies according to whether they included prevalent users, incident users, or both. Our goal was to draw some general conclusions to improve the analyses of observational studies for comparative effectiveness research.
MATERIALS AND METHODS
Search strategy
We searched PubMed using “hydroxymethylglutaryl-CoA reductase inhibitors” and “myocardial ischemia” or “mortality” as Medical Subject Headings. We searched separately for “epidemiologic studies” and “randomized controlled trials” and identified studies that had been published before November 2010. We limited our search to studies conducted in humans and in adults over 18 years of age (≥19 years). We imposed no restrictions on the language of the publications. We also searched Embase using the same search terms and limitations for observational studies and for randomized trials published after August 2010, which was the end-of-search date in a recent meta-analysis of randomized trials that included Embase (27). Two authors (G. D. and M. T.) screened the articles using the title and abstract and subsequently retrieved and reviewed the whole article. Any discrepancies between the two screeners were resolved by coming to consensus on whether to include or exclude the abstract or article. If a consensus could not be reached, the situation was discussed with a third author (M. A. H.). We screened the bibliographies of the selected articles to find other relevant studies.
We excluded articles comparing different statins or studying the dose-response of a single statin; randomized trials that used other interventions (such as percutaneous coronary interventions) as the control group; trials that used cerivastatin; case-control studies not nested in a prospective cohort study; studies on short-term effects of statins (periprocedural, in-hospital effects, or follow-up periods of ≤6 months); studies where the investigators had not reported clinical endpoints or had reported only cerebrovascular events; studies on patients with defibrillators, heart failure, heart transplants, familial hypercholesterolemia, or chronic kidney disease; studies that did not have 1 arm for treatment with statin only; and studies with extended follow-up or post-hoc and subgroup analyses of previously published randomized trials.
Data extraction
We divided the selected studies into studies of primary prevention and secondary prevention. When a study included subjects both with and without previous cardiovascular events, we classified it as a secondary prevention study when more than 30% of participants had previous cardiovascular events at baseline. The outcome of interest for primary prevention studies was CHD, defined as nonfatal myocardial infarction or death from CHD. For secondary prevention studies, we chose all-cause mortality as the outcome because few observational studies reported information on recurrent myocardial infarction or CHD.
We extracted data on characteristics of the study population, including age, proportion of male participants, sample size, eligibility criteria, intervention or comparison groups, follow-up duration, compliance with or adherence to treatment, outcome definition and variables, and methods used to adjust for confounding. For randomized trials, we also extracted the point estimate for the intention-to-treat hazard ratio comparing treated persons with controls (and its confidence interval) for primary and secondary endpoints. If investigators had only reported results for several subgroups separately (e.g., men and women), we extracted the effect estimates for each subgroup. We excluded studies for which the article did not include enough information to calculate incidence rates and their confidence intervals.
For observational studies, we extracted the crude and adjusted hazard ratio estimates (and their 95% confidence intervals) comparing users with nonusers for the primary and secondary outcomes considered in each study. Statin users were defined as prevalent users if they had initiated statin therapy prior to their inclusion in the study and incident users if they had initiated statin therapy at or after their inclusion in the study. We classified observational studies into 3 categories based on these definitions: 1) studies that compared prevalent (current) users with nonusers, 2) studies that compared incident users with nonusers, and 3) studies that compared a combination of prevalent and incident users with nonusers. The latter group mostly included hospital-based studies that had assigned an initiation time (usually at discharge from the hospital) but did not exclude patients who were taking statins prior to admission. We excluded studies that compared persistent (long-term prevalent) users with nonpersistent users because the definitions of persistent or long-term use were not consistent across these studies.
Statistical analysis
We pooled the reported hazard ratio estimates and computed their 95% confidence intervals separately for studies of primary and secondary prevention and also separately for randomized trials and observational studies. Pooled estimates were similar regardless of whether we used a fixed-effects model or a random-effects model (33). We used results from the latter in all meta-analyses. We evaluated the role of outliers among secondary prevention studies by dropping the two studies with the smallest and largest effect estimates and pooling the other studies. This analysis showed that our results were robust to the presence of outliers (results not shown). We used a funnel plot and a regression asymmetry test (34) to assess small-study bias. When the estimates differed substantially among the pooled studies, we used meta-regression (35) to explore the following potential predictors of heterogeneity: duration of follow-up (mean or median), mean age of participants at baseline, proportion of male participants, context (hospital-based vs. community- or population-based), location (United States or elsewhere), and adjustment methods (multivariate outcome models vs. propensity score methods). All analyses were conducted using Stata, version 10.1 (StataCorp LP, College Station, Texas).
RESULTS
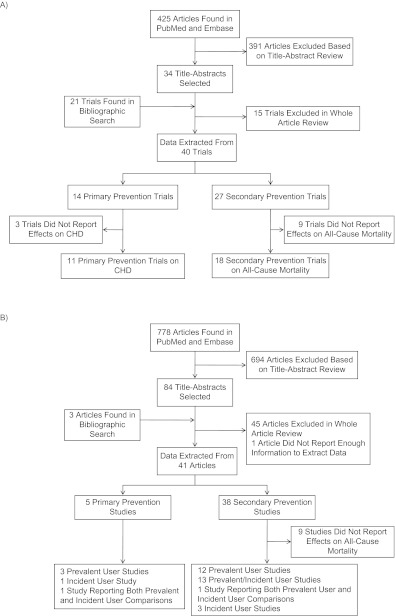
Our search identified 425 randomized clinical trials and 778 observational studies, of which we selected 34 randomized trials and 84 observational studies after reviewing their titles and abstracts. A bibliographic search on the selected articles identified 21 additional randomized trials and 3 additional observational studies that matched our inclusion criteria. One observational study did not provide enough information to estimate the variance of the effect estimate and was excluded (36). We extracted information for at least 1 disease outcome from 40 randomized trials and 41 observational studies. See Figure 1 for a flowchart of studies processed in this review and Web Tables 1 and 2 (which appear on the Journal’s Web site (http://aje.oxfordjournals.org/)) for characteristics of the studies that contributed to one or more of the pooled estimates.
Figure 1.
Processing of A) randomized trials and B) observational studies in a review and meta-analysis of observational studies of statin therapy. One randomized trial and 2 observational studies included comparisons of both primary and secondary prevention. (CHD, coronary heart disease).
Secondary prevention
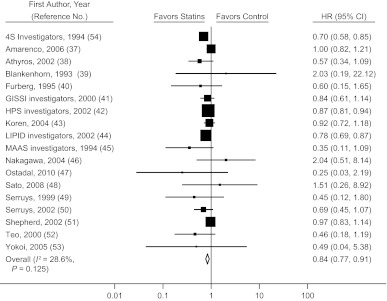
Eighteen randomized trials (37–54) estimated the mortality hazard ratio for statin initiation versus no initiation, either after a cardiovascular event or in a population with a high prevalence of cardiovascular disease. The pooled mortality hazard ratio was 0.84 (95% confidence interval (CI): 0.77, 0.91) (Figure 2). The pooled hazard ratio for recurrent CHD in 17 randomized trials of secondary prevention (37, 40–46, 50–52, 54–58) was 0.75 (95% CI: 0.70, 0.80) (Web Figure 1).
Figure 2.
Hazard ratio (HR) for mortality (squares) according to initiation of statin use in secondary prevention randomized clinical trials and pooled HR for treatment versus control status (diamond). Bars, 95% confidence interval (CI). (GISSI, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico; HPS, Heart Protection Study; LIPID, Long-term Intervention with Pravastatin in Ischaemic Disease; MAAS, Multicentre Anti-Atheroma Study; 4S, Scandinavian Simvastatin Survival Study).
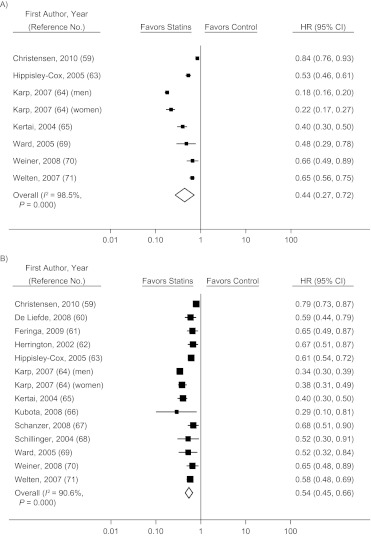
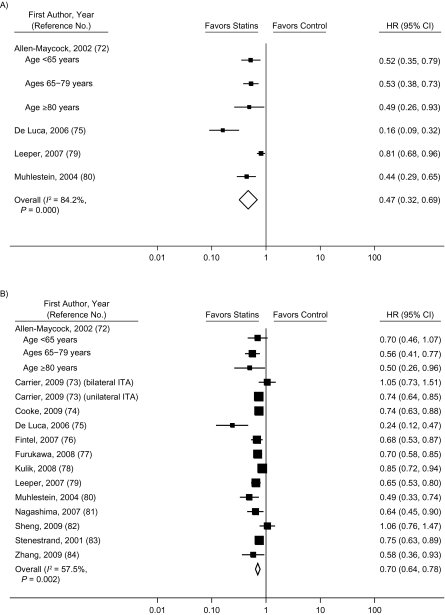
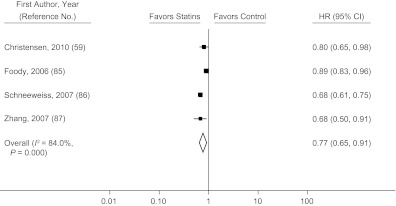
The pooled hazard ratio for all-cause mortality in observational studies comparing prevalent users with nonusers (59–71) was 0.44 (95% CI: 0.27, 0.72) before adjustment for potential confounders and 0.54 (95% CI: 0.45, 0.66) after adjustment (Figure 3). Not all observational studies reported the unadjusted hazard ratios, but when we restricted the analysis to those which did, our findings were not materially affected (Web Figure 2). Studies that compared a combination of prevalent and incident users with nonusers (72–84) had a pooled hazard ratio for all-cause mortality of 0.47 (95% CI: 0.32, 0.69) before adjustment for confounders and 0.70 (95% CI: 0.64, 0.78) after adjustment (Figure 4). Only 4 studies (59, 85–87) compared incident users with nonusers, and the pooled hazard ratio from these studies was 0.77 (95% CI: 0.65, 0.91) after adjustment for confounders (Figure 5). There was substantial heterogeneity among these groups of studies, as evidenced by large I2 values (91% for studies of prevalent users, 58% for studies of prevalent and incident users combined, and 84% for studies of incident users). The meta-regression analyses did not find any strong predictors of heterogeneity in studies of prevalent users. In studies that compared a combination of prevalent and incident users with nonusers, the log hazard ratio increased by 0.09 (95% CI: 0.02, 0.16) per additional year of mean/median follow-up and decreased by 0.038 (95% CI: 0.018, 0.074) per 10% increase in proportion of male participants at baseline.
Figure 3.
Hazard ratio (HR) for mortality (squares) according to statin use in secondary-prevention observational studies comparing prevalent users with nonusers and pooled HR for users versus nonusers (diamond). A) unadjusted results; B) adjusted results. Bars, 95% confidence interval (CI).
Figure 4.
Hazard ratio (HR) for mortality (squares) according to statin use in secondary-prevention observational studies comparing a combination of prevalent and incident users with nonusers and pooled HR for users versus nonusers (diamond). A) unadjusted results; B) adjusted results. Bars, 95% confidence interval (CI). (ITA, internal thoracic artery graft).
Figure 5.
Multivariate-adjusted hazard ratio (HR) for mortality (squares) according to statin use in secondary-prevention observational studies comparing incident users with nonusers and pooled HR for users versus nonusers (diamond). Bars, 95% confidence interval (CI).
Primary prevention
Eleven randomized trials of primary prevention of CHD (88–98) estimated the hazard ratio for statin initiation versus no initiation. The pooled hazard ratio was 0.69 (95% CI: 0.60, 0.79) (Web Figure 3). There were only 2 observational studies (99, 100) comparing incident users with nonusers, and the pooled adjusted hazard ratio for CHD from these studies was 0.80 (95% CI: 0.63, 1.02). Of the 4 studies that compared prevalent users with nonusers, 2 studies reported only the hazard ratio for all-cause mortality (79) or cardiovascular mortality (101), and 2 studies reported hazard ratios for myocardial infarction, with considerable heterogeneity (the adjusted hazard ratio was 0.35 in one study (102) and 1.41 in the other one (99)); thus, we did not pool data from those 2 studies.
Small-study bias
The funnel plots for randomized trials and observational studies (Web Figure 4) showed little evidence of small-study bias. The P values from the asymmetry test were 0.11 and 0.24 for primary- and secondary-prevention randomized trials, respectively; 0.30 for secondary-prevention observational studies of prevalent users; 0.15 for studies that combined prevalent and incident users; and 0.46 for studies of incident users. Furthermore, exclusion of angiographic trials (4 on primary prevention and 6 on secondary prevention) did not change the results of our meta-analyses (results not shown).
Adherence to treatment
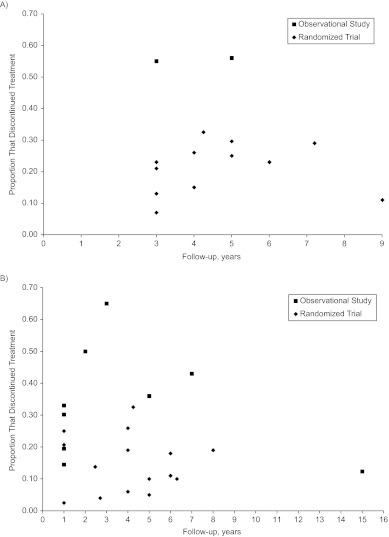
Figure 6 presents findings for treatment discontinuation among statin initiators/users in primary and secondary prevention studies by year of follow-up and type of study. In primary prevention studies, on average, 21% of statin initiators discontinued treatment in randomized trials versus 41% in observational studies. In secondary prevention studies, the corresponding proportions were 15% in randomized trials and 32% in observational studies. In only 2 observational studies did investigators report the proportion of nonusers who started treatment during follow-up, so we could not make any meaningful comparison of imperfect adherence among nonusers.
Figure 6.
Proportion of patients stopping statin treatment in A) primary prevention studies and B) secondary prevention studies, by type of study and duration of follow-up.
DISCUSSION
According to our meta-analysis of randomized trials of statins for secondary prevention, the mortality hazard ratio for initiation of statin therapy versus no initiation was 0.84 (95% CI: 0.77, 0.91). That is, statin therapy reduces mortality by 16% in patients with a history of cardiovascular disease. These results are generally consistent with prior meta-analyses of randomized trials (20–31). In contrast, our meta-analysis of observational studies of prevalent statin users found a hazard ratio of 0.54 (95% CI: 0.45, 0.66).
The difference between the pooled hazard ratio for randomized trials and that for observational studies with prevalent users is unlikely to be due to chance (because their 95% confidence intervals do not overlap), use of different drugs across studies (because commonly used statins have similar effects (25)), differences in adherence to treatment (because lower adherence in observational studies should have resulted in a bias towards the null), or different distributions of effect modifiers (because subgroup analyses of many randomized trials have rarely found differences (23, 25, 103)). On the other hand, the randomized-observational discrepancy may be due to selection bias or residual confounding, as discussed below.
A comparison of prevalent users of statins with nonusers is subject to selection bias (18, 104) because prevalent users have by definition survived under treatment. If treatment decreases the risk of the outcome, the group of prevalent users will be progressively enriched with susceptible patients as compared with nonusers or never users (and conversely more resilient patients if treatment increases the risk of the outcome). In addition, including prevalent users in the analysis often implies that the confounders are measured after treatment initiation. If confounders are affected by prior treatment, adjustment for confounding will introduce selection bias (105).
To eliminate these selection biases, one can restrict the analysis to patients who have not used the drug for some period of time before the start of follow-up. Then the analysis would compare incident users (or initiators) with nonusers of treatment (19, 99). Our meta-analysis suggested that the hazard ratio for observational studies approaches that of randomized trials (hazard ratio (HR) = 0.84) as the proportion of incident users increases (although the 95% confidence intervals overlapped). Further, we recently showed that an observational comparison involving incident statin users resulted in more reasonable estimates than one involving prevalent users (99).
Residual confounding is always a potential source of discrepancy between randomized trials and observational studies. In the secondary-prevention observational studies included in our analysis, statin users were consistently younger than nonusers (64, 65, 71, 106), had undergone more revascularizations (62, 70, 71, 106), and had more use of antihypertensive and antithrombotic drugs (62, 64, 65, 71), which implies better access to health care and possibly a better prognosis. As a result, statin use may be a marker for healthy status and/or high-quality medical care in secondary-prevention observational studies. This confounding might partly explain why the pooled crude hazard ratios (HR = 0.44 in studies with prevalent users only; HR = 0.47 in studies with a combination of prevalent and incident users) were slightly smaller than the fully adjusted ones (HR = 0.54 and HR = 0.70, respectively) (Figures 3 and 4).
Our main findings are focused on secondary prevention studies, and we had to focus on all-cause mortality because very few studies reported effects on recurrent CHD. Unfortunately, the number of primary prevention observational studies was too small for a meaningful analysis. The pooled hazard ratio from 2 observational studies of incident users suggested a smaller protective effect than in randomized trials (HR = 0.80 vs. HR = 0.69), possibly because of residual confounding by indication. A comparison of the crude and adjusted estimates from these 2 studies (HR = 1.67 vs. HR = 0.80) showed that the participants with a higher risk of the disease were more likely to receive treatment in primary prevention settings, which suggests that the pattern of confounding varies between the primary and secondary prevention settings.
Our study had several limitations. First, few observational studies had compared incident users of statin therapy with nonusers, which limited the precision of the estimates for these studies. Second, our search was limited to PubMed and Embase. To minimize the inadvertent exclusion of small studies, we conducted a comprehensive bibliographic search and reviewed previously published meta-analyses of randomized trials. Our funnel plots for secondary-prevention observational studies did not show any evidence of small-study bias. Third, the primary-prevention randomized trials used slightly different definitions of CHD, which may have increased the heterogeneity of the hazard ratios.
Mean follow-up time and proportion of male participants were the main determinants of heterogeneity in secondary-prevention observational studies that compared a combination of prevalent and incident users with nonusers. A larger protective effect for men was also observed in a recent meta-analysis of randomized trials (32). The smaller hazard ratio estimates with longer follow-up times may be partly explained by increased nonadherence over time (see Figure 6) or by increased selection bias due to inclusion of prevalent users.
In summary, our findings support the hypothesis that the greater the proportion of prevalent statin users in observational studies, the larger the discrepancy between observational and randomized estimates. In future observational studies of comparative effectiveness, investigators may reduce the potential for bias by attempting to emulate the design and analysis of a hypothetical trial by imposing the same eligibility criteria and comparing incident users of treatment with nonusers (99).
Supplementary Material
Acknowledgments
Author affiliations: Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (Miguel A. Hernán, Goodarz Danaei); Department of Global Health and Population, Harvard School of Public Health, Boston, Massachusetts (Goodarz Danaei, Mohammad Tavakkoli); and Harvard-MIT Division of Health Sciences and Technology, Harvard University and Massachusetts Institute of Technology, Boston, Massachusetts (Miguel A. Hernán).
This work was funded by National Institutes of Health grant R01 HL080644 to Dr. Miguel A. Hernán.
Conflict of interest: none declared.
Glossary
Abbreviations
- CHD
coronary heart disease
- CI
confidence interval
- HR
hazard ratio
- HRT
hormone replacement therapy
References
- 1.Alexander GC, Stafford RS. Does comparative effectiveness have a comparative edge? JAMA. 2009;301(23):2488–2490. doi: 10.1001/jama.2009.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avorn J. Debate about funding comparative-effectiveness research. N Engl J Med. 2009;360(19):1927–1929. doi: 10.1056/NEJMp0902427. [DOI] [PubMed] [Google Scholar]
- 3.Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361(4):325–328. doi: 10.1056/NEJMp0904133. [DOI] [PubMed] [Google Scholar]
- 4.Kuehn BM. Institute of Medicine outlines priorities for comparative effectiveness research. JAMA. 2009;302(9):936–937. doi: 10.1001/jama.2009.1186. [DOI] [PubMed] [Google Scholar]
- 5.Mushlin AI, Ghomrawi H. Health care reform and the need for comparative-effectiveness research. N Engl J Med. 2010;362(3):e6. doi: 10.1056/NEJMp0912651. (doi:10.1056/NEJMp0912651) [DOI] [PubMed] [Google Scholar]
- 6.Naik AD, Petersen LA. The neglected purpose of comparative-effectiveness research. N Engl J Med. 2009;360(19):1929–1931. doi: 10.1056/NEJMp0902195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng GC. NHS Evidence: better and faster access to information. Lancet. 2009;373(9674):1502–1504. doi: 10.1016/S0140-6736(09)60786-8. [DOI] [PubMed] [Google Scholar]
- 8.Rawlins MD. The decade of NICE. Lancet. 2009;374(9686):351–352. doi: 10.1016/S0140-6736(09)60616-4. [DOI] [PubMed] [Google Scholar]
- 9.MacLehose RR, Reeves BC, Harvey IM, et al. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health Technol Assess. 2000;4(34):1–154. [PubMed] [Google Scholar]
- 10.Furlan AD, Tomlinson G, Jadad AA, et al. Methodological quality and homogeneity influenced agreement between randomized trials and nonrandomized studies of the same intervention for back pain. J Clin Epidemiol. 2008;61(3):209–231. doi: 10.1016/j.jclinepi.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NS, Byrne CJ, Young JM, et al. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J Clin Epidemiol. 2010;63(3):238–245. doi: 10.1016/j.jclinepi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons RJ, Gardner TJ, Anderson JL, et al. The American Heart Association’s principles for comparative effectiveness research: a policy statement from the American Heart Association. Circulation. 2009;119(22):2955–2962. doi: 10.1161/CIRCULATIONAHA.109.192518. [DOI] [PubMed] [Google Scholar]
- 13.Wilson PW, Garrison RJ, Castelli WP. Postmenopausal estrogen use, cigarette smoking, and cardiovascular morbidity in women over 50. The Framingham Study. N Engl J Med. 1985;313(17):1038–1043. doi: 10.1056/NEJM198510243131702. [DOI] [PubMed] [Google Scholar]
- 14.Varas-Lorenzo C, García-Rodríguez LA, Perez-Gutthann S, et al. Hormone replacement therapy and incidence of acute myocardial infarction. A population-based nested case-control study. Circulation. 2000;101(22):2572–2578. doi: 10.1161/01.cir.101.22.2572. [DOI] [PubMed] [Google Scholar]
- 15.Grodstein F, Stampfer MJ, Manson JE, et al. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. Women’s Health Initiative Investigators. N Engl J Med. 2003;349(6):523–534. doi: 10.1056/NEJMoa030808. [DOI] [PubMed] [Google Scholar]
- 17.Hernán MA, Robins JM. García Rodríguez LA. Discussion on “Statistical issues arising in the Women’s Health Initiative.”. Biometrics. 2005;61(4):922–930. doi: 10.1111/j.0006-341X.2005.454_1.x. [DOI] [PubMed] [Google Scholar]
- 18.Hernán MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 20.Ross SD, Allen IE, Connelly JE, et al. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Intern Med. 1999;159(15):1793–1802. doi: 10.1001/archinte.159.15.1793. [DOI] [PubMed] [Google Scholar]
- 21.Cheung BM, Lauder IJ, Lau CP, et al. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br J Clin Pharmacol. 2004;57(5):640–651. doi: 10.1111/j.1365-2125.2003.02060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simes J, Furberg CD, Braunwald E, et al. Effects of pravastatin on mortality in patients with and without coronary heart disease across a broad range of cholesterol levels. The Prospective Pravastatin Pooling Project. Eur Heart J. 2002;23(3):207–215. doi: 10.1053/euhj.2001.2775. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 24.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 25.Wilt TJ, Bloomfield HE, MacDonald R, et al. Effectiveness of statin therapy in adults with coronary heart disease. Arch Intern Med. 2004;164(13):1427–1436. doi: 10.1001/archinte.164.13.1427. [DOI] [PubMed] [Google Scholar]
- 26.Ray KK, Seshasai SR, Erqou S, et al. Statins and all-cause mortality in high-risk primary prevention: a meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch Intern Med. 2010;170(12):1024–1031. doi: 10.1001/archinternmed.2010.182. [DOI] [PubMed] [Google Scholar]
- 27.Mills EJ, Wu P, Chong G, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104(2):109–124. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 28.Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52(22):1769–1781. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 29.Brugts JJ, Yetgin T, Hoeks SE, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. (doi: 10.1136/bmj.b2376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mora S, Glynn RJ, Hsia J, et al. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: results from the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation. 2010;121(9):1069–1077. doi: 10.1161/CIRCULATIONAHA.109.906479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor F, Ward K, Moore TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;(1):CD004816. doi: 10.1002/14651858.CD004816.pub4. (doi:10.1002/14651858.CD004816.pub4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petretta M, Costanzo P, Perrone-Filardi P, et al. Impact of gender in primary prevention of coronary heart disease with statin therapy: a meta-analysis. Int J Cardiol. 2010;138(1):25–31. doi: 10.1016/j.ijcard.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18(20):2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Chikamori T, Sugimoto K, Hamada T, et al. Efficacy of cholesterol-lowering treatment in Japanese elderly patients with coronary artery disease and normal cholesterol level using 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. J Cardiol. 2000;35(2):95–101. [PubMed] [Google Scholar]
- 37.Amarenco P, Bogousslavsky J, Callahan A, III, et al. High-dose atorvastatin after stroke or transient ischemic attack. Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 38.Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus ‘usual’ care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18(4):220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 39.Blankenhorn DH, Azen SP, Kramsch DM, et al. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS) Ann Intern Med. 1993;119(10):969–976. doi: 10.7326/0003-4819-119-10-199311150-00002. [DOI] [PubMed] [Google Scholar]
- 40.Furberg CD, Pitt B, Byington RP, et al. Reduction in coronary events during treatment with pravastatin. PLAC I and PLAC II Investigators. Pravastatin Limitation of Atherosclerosis in the Coronary Arteries. Am J Cardiol. 1995;76(9):60C–63C. doi: 10.1016/s0002-9149(99)80472-x. [DOI] [PubMed] [Google Scholar]
- 41.GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? Ital Heart J. 2000;1(12):810–820. [PubMed] [Google Scholar]
- 42.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 43.Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the ALLIANCE study. J Am Coll Cardiol. 2004;44(9):1772–1779. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 44.LIPID Study Group (Long-term Intervention with Pravastatin in Ischaemic Disease) Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359(9315):1379–1387. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 45.Effect of simvastatin on coronary atheroma: the Multicentre Anti-Atheroma Study (MAAS) Lancet. 1994;344(8923):633–638. [PubMed] [Google Scholar]
- 46.Nakagawa T, Kobayashi T, Awata N, et al. Randomized, controlled trial of secondary prevention of coronary sclerosis in normocholesterolemic patients using pravastatin: final 5-year angiographic follow-up of the Prevention of Coronary Sclerosis (PCS) study. Int J Cardiol. 2004;97(1):107–114. doi: 10.1016/j.ijcard.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Ostadal P, Alan D, Vejvoda J, et al. Fluvastatin in the first-line therapy of acute coronary syndrome: results of the multicenter, randomized, double-blind, placebo-controlled trial (the FACS-trial) Trials. 2010;11:61. doi: 10.1186/1745-6215-11-61. (doi: 10.1186/1745-6215-11-61) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato H, Kinjo K, Ito H, et al. Effect of early use of low-dose pravastatin on major adverse cardiac events in patients with acute myocardial infarction: the OACIS-LIPID Study. Circ J. 2008;72(1):17–22. doi: 10.1253/circj.72.17. [DOI] [PubMed] [Google Scholar]
- 49.Serruys PW, Foley DP, Jackson G, et al. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty: final results of the Fluvastatin Angiographic Restenosis (FLARE) Trial. Eur Heart J. 1999;20(1):58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 50.Serruys PW, de Feyter P, Macaya C, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;287(24):3215–3222. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 51.Shepherd J, Blauw GJ, Murphy MB, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 52.Teo KK, Burton JR, Buller CE, et al. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: The Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT) Circulation. 2000;102(15):1748–1754. doi: 10.1161/01.cir.102.15.1748. [DOI] [PubMed] [Google Scholar]
- 53.Yokoi H, Nobuyoshi M, Mitsudo K, et al. Three-year follow-up results of Angiographic Intervention Trial using an HMG-CoA Reductase Inhibitor to Evaluate Retardation of Obstructive Multiple Atheroma (ATHEROMA) study. Circ J. 2005;69(8):875–883. doi: 10.1253/circj.69.875. [DOI] [PubMed] [Google Scholar]
- 54.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 55.Colivicchi F, Guido V, Tubaro M, et al. Effects of atorvastatin 80 mg daily early after onset of unstable angina pectoris or non-Q-wave myocardial infarction. Am J Cardiol. 2002;90(8):872–874. doi: 10.1016/s0002-9149(02)02711-x. [DOI] [PubMed] [Google Scholar]
- 56.Flaker GC, Warnica JW, Sacks FM, et al. Pravastatin prevents clinical events in revascularized patients with average cholesterol concentrations. Cholesterol and Recurrent Events CARE Investigators. J Am Coll Cardiol. 1999;34(1):106–112. doi: 10.1016/s0735-1097(99)00145-x. [DOI] [PubMed] [Google Scholar]
- 57.Riegger G, Abletshauser C, Ludwig M, et al. The effect of fluvastatin on cardiac events in patients with symptomatic coronary artery disease during one year of treatment. Atherosclerosis. 1999;144(1):263–270. doi: 10.1016/s0021-9150(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 58.Waters D, Higginson L, Gladstone P, et al. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantitative arteriography. The Canadian Coronary Atherosclerosis Intervention Trial. Circulation. 1994;89(3):959–968. doi: 10.1161/01.cir.89.3.959. [DOI] [PubMed] [Google Scholar]
- 59.Christensen S, Thomsen RW, Johansen MB, et al. Preadmission statin use and one-year mortality among patients in intensive care—a cohort study. Crit Care. 2010;14(2):R29. doi: 10.1186/cc8902. (doi:10.1186/cc8902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Liefde II, Hoeks SE, van Gestel YR, et al. Usefulness of hypertensive blood pressure response during a single-stage exercise test to predict long-term outcome in patients with peripheral arterial disease. Am J Cardiol. 2008;102(7):921–926. doi: 10.1016/j.amjcard.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 61.Feringa HH, Bax JJ, Karagiannis SE, et al. Elderly patients undergoing major vascular surgery: risk factors and medication associated with risk reduction. Arch Gerontol Geriatr. 2009;48(1):116–120. doi: 10.1016/j.archger.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 62.Herrington DM, Vittinghoff E, Lin F, et al. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS) Circulation. 2002;105(25):2962–2967. doi: 10.1161/01.cir.0000019406.74017.b2. [DOI] [PubMed] [Google Scholar]
- 63.Hippisley-Cox J, Coupland C. Effect of combinations of drugs on all cause mortality in patients with ischaemic heart disease: nested case-control analysis. BMJ. 2005;330(7499):1059–1063. doi: 10.1136/bmj.330.7499.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Karp I, Chen SF, Pilote L. Sex differences in the effectiveness of statins after myocardial infarction. CMAJ. 2007;176(3):333–338. doi: 10.1503/cmaj.060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kertai MD, Boersma E, Westerhout CM, et al. Association between long-term statin use and mortality after successful abdominal aortic aneurysm surgery. Am J Med. 2004;116(2):96–103. doi: 10.1016/j.amjmed.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Kubota N, Kasai T, Miyauchi K, et al. Therapy with statins and aspirin enhances long-term outcome of percutaneous coronary intervention. Heart Vessels. 2008;23(1):35–39. doi: 10.1007/s00380-007-1007-8. [DOI] [PubMed] [Google Scholar]
- 67.Schanzer A, Hevelone N, Owens CD, et al. Statins are independently associated with reduced mortality in patients undergoing infrainguinal bypass graft surgery for critical limb ischemia. J Vasc Surg. 2008;47(4):774–781. doi: 10.1016/j.jvs.2007.11.056. [DOI] [PubMed] [Google Scholar]
- 68.Schillinger M, Exner M, Mlekusch W, et al. Statin therapy improves cardiovascular outcome of patients with peripheral artery disease. Eur Heart J. 2004;25(9):742–748. doi: 10.1016/j.ehj.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 69.Ward RP, Leeper NJ, Kirkpatrick JN, et al. The effect of preoperative statin therapy on cardiovascular outcomes in patients undergoing infrainguinal vascular surgery. Int J Cardiol. 2005;104(3):264–268. doi: 10.1016/j.ijcard.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 70.Weiner MG, Xie D, Tannen RL. Replication of the Scandinavian Simvastatin Survival Study using a primary care medical record database prompted exploration of a new method to address unmeasured confounding. Pharmacoepidemiol Drug Saf. 2008;17(7):661–670. doi: 10.1002/pds.1585. [DOI] [PubMed] [Google Scholar]
- 71.Welten GM, Chonchol M, Hoeks SE, et al. Statin therapy is associated with improved outcomes in vascular surgery patients with renal impairment. Am Heart J. 2007;154(5):954–961. doi: 10.1016/j.ahj.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 72.Allen Maycock CA, Muhlestein JB, Horne BD, et al. Statin therapy is associated with reduced mortality across all age groups of individuals with significant coronary disease, including very elderly patients. Intermountain Heart Collaborative Study. J Am Coll Cardiol. 2002;40(10):1777–1785. doi: 10.1016/s0735-1097(02)02477-4. [DOI] [PubMed] [Google Scholar]
- 73.Carrier M, Cossette M, Pellerin M, et al. Statin treatment equalizes long-term survival between patients with single and bilateral internal thoracic artery grafts. Ann Thorac Surg. 2009;88(3):789–795. doi: 10.1016/j.athoracsur.2009.04.097. [DOI] [PubMed] [Google Scholar]
- 74.Cooke CA, Kirkland SA, Sketris IS, et al. The impact of statins on health services utilization and mortality in older adults discharged from hospital with ischemic heart disease: a cohort study. BMC Health Serv Res. 2009;9:198. doi: 10.1186/1472-6963-9-198. (doi:10.1186/1472-6963-9-198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Luca G, Suryapranata H, Ottervanger JP, et al. Impact of statin therapy at discharge on 1-year mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty. Atherosclerosis. 2006;189(1):186–192. doi: 10.1016/j.atherosclerosis.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 76.Fintel D, Joyce A, Mackell J, et al. Reduced mortality rates after intensive statin therapy in managed-care patients. Value Health. 2007;10(2):161–169. doi: 10.1111/j.1524-4733.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- 77.Furukawa Y, Taniguchi R, Ehara N, et al. Better survival with statin administration after revascularization therapy in Japanese patients with coronary artery disease: perspectives from the CREDO-Kyoto registry. Circ J. 2008;72(12):1937–1945. doi: 10.1253/circj.cj-08-0293. [DOI] [PubMed] [Google Scholar]
- 78.Kulik A, Brookhart MA, Levin R, et al. Impact of statin use on outcomes after coronary artery bypass graft surgery. Circulation. 2008;118(18):1785–1792. doi: 10.1161/CIRCULATIONAHA.108.799445. [DOI] [PubMed] [Google Scholar]
- 79.Leeper NJ, Ardehali R, deGoma EM, et al. Statin use in patients with extremely low low-density lipoprotein levels is associated with improved survival. Circulation. 2007;116(6):613–618. doi: 10.1161/CIRCULATIONAHA.107.694117. [DOI] [PubMed] [Google Scholar]
- 80.Muhlestein JB, Anderson JL, Horne BD, et al. Early effects of statins in patients with coronary artery disease and high C-reactive protein. Intermountain Heart Collaborative Study Group. Am J Cardiol. 2004;94(9):1107–1112. doi: 10.1016/j.amjcard.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 81.Nagashima M, Koyanagi R, Kasanuki H, et al. Effect of early statin treatment at standard doses on long-term clinical outcomes in patients with acute myocardial infarction (the Heart Institute of Japan, Department of Cardiology Statin Evaluation Program) Am J Cardiol. 2007;99(11):1523–1528. doi: 10.1016/j.amjcard.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 82.Sheng X, Wei L, Murphy MJ, et al. Statins and total (not LDL) cholesterol concentration and outcome of myocardial infarction: results from a meta-analysis and an observational study. Eur J Clin Pharmacol. 2009;65(11):1071–1080. doi: 10.1007/s00228-009-0720-x. [DOI] [PubMed] [Google Scholar]
- 83.Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1-year survival. Swedish Register of Cardiac Intensive Care (RIKS-HIA) JAMA. 2001;285(4):430–436. doi: 10.1001/jama.285.4.430. [DOI] [PubMed] [Google Scholar]
- 84.Zhang ZJ, Marroquin OC, Weissfeld JL, et al. Beneficial effects of statins after percutaneous coronary intervention. Eur J Cardiovasc Prev Rehabil. 2009;16(4):445–450. doi: 10.1097/HJR.0b013e32832a4e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foody JM, Rathore SS, Galusha D, et al. Hydroxymethylglutaryl-CoA reductase inhibitors in older persons with acute myocardial infarction: evidence for an age-statin interaction. J Am Geriatr Soc. 2006;54(3):421–430. doi: 10.1111/j.1532-5415.2005.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneeweiss S, Patrick AR, Stürmer T, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med Care. 2007;45(10 suppl 2):S131–S142. doi: 10.1097/MLR.0b013e318070c08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Q, Safford M, Miller D, et al. Short-term statin exposure is associated with reduced all-cause mortality in persons with diabetes. Med Care. 2007;45(4):308–314. doi: 10.1097/01.mlr.0000250227.94196.f0. [DOI] [PubMed] [Google Scholar]
- 88.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) JAMA. 2002;288(23):2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 89.Anderssen SA, Hjelstuen AK, Hjermann I, et al. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis. 2005;178(2):387–397. doi: 10.1016/j.atherosclerosis.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 90.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 91.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 92.Furberg CD, Adams HP, Jr, Applegate WB, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90(4):1679–1687. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 93.Knopp RH, d’Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN) Diabetes Care. 2006;29(7):1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura H, Arakawa K, Itakura H, et al. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 95.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 96.Salonen R, Nyyssönen K, Porkkala E, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation. 1995;92(7):1758–1764. doi: 10.1161/01.cir.92.7.1758. [DOI] [PubMed] [Google Scholar]
- 97.Sever PS, Dahlöf B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 98.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 99.Danaei G, Garcıa Rodriguez LA, Cantero OF, et al. Observational data for comparative effectiveness research: an emulation of randomized trials of statins for primary prevention of coronary heart disease [published online ahead of print October 19, 2011] Stat Methods Med Res. doi: 10.1177/0962280211403603. (doi: 10.1177/0962280211403603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf. 2005;14(7):465–476. doi: 10.1002/pds.1062. [DOI] [PubMed] [Google Scholar]
- 101.Gardette V, Bongard V, Dallongeville J, et al. Ten-year all-cause mortality in presumably healthy subjects on lipid-lowering drugs (from the Prospective Epidemiological Study of Myocardial Infarction [PRIME] prospective cohort) Am J Cardiol. 2009;103(3):381–386. doi: 10.1016/j.amjcard.2008.09.092. [DOI] [PubMed] [Google Scholar]
- 102.Lemaitre RN, Psaty BM, Heckbert SR, et al. Therapy with hydroxymethylglutaryl coenzyme A reductase inhibitors (statins) and associated risk of incident cardiovascular events in older adults: evidence from the Cardiovascular Health Study. Arch Intern Med. 2002;162(12):1395–1400. doi: 10.1001/archinte.162.12.1395. [DOI] [PubMed] [Google Scholar]
- 103.Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Cholesterol Treatment Trialists’ (CTT) Collaboration. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hernán MA, Robins JM. Authors’ response, part I: observational studies analyzed like randomized experiments. Best of both worlds. Epidemiology. 2008;19(6):789–792. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 106.Horne BD, Muhlestein JB, Carlquist JF, et al. Statin therapy, lipid levels, C-reactive protein and the survival of patients with angiographically severe coronary artery disease. J Am Coll Cardiol. 2000;36(6):1774–1780. doi: 10.1016/s0735-1097(00)00950-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.