Abstract
Synaptojanin 1 is a polyphosphoinositide phosphatase concentrated in presynaptic nerve terminals, where it dephosphorylates a pool of phosphatidylinositol 4,5-bisphosphate implicated in synaptic vesicle recycling. Like other proteins with a role in endocytosis, synaptojanin 1 undergoes constitutive phosphorylation in resting synapses and stimulation-dependent dephosphorylation by calcineurin. Here, we show that cyclin-dependent kinase 5 (Cdk5) phosphorylates synaptojanin 1 and regulates its function both in vitro and in intact synaptosomes. Cdk5 phosphorylation inhibited the inositol 5-phosphatase activity of synaptojanin 1, whereas dephosphorylation by calcineurin stimulated such activity. The activity of synaptojanin 1 was also stimulated by its interaction with endophilin 1, its major binding partner at the synapse. Notably, Cdk5 phosphorylated serine 1144, which is adjacent to the endophilin binding site. Mutation of serine 1144 to aspartic acid to mimic phosphorylation by Cdk5 inhibited the interaction of synaptojanin 1 with endophilin 1. These results suggest that Cdk5 and calcineurin may have an antagonistic role in the regulation of synaptojanin 1 recruitment and activity, and therefore in the regulation of phosphatidylinositol 4,5-bisphosphate turnover at synapses.
Phosphoinositides are key regulators of cell physiology. In particular, phosphatidylinositol (PI) 4,5-bisphosphate [PI(4,5)P2] plays a major regulatory role at the cell surface, both as a precursor of important signaling molecules, as well as via interactions with cytosolic and membrane proteins (1–4). In nerve terminals, phospholipase C-mediated cleavage of PI(4,5)P2 controls the efficiency of the depolarization-exocytosis coupling of synaptic vesicles via the actions of inositol 1,4,5-trisphosphate and diacylglycerol (DAG) on Ca2+ signaling (5), and the additional action of DAG on Munc13 family proteins, key players in the “priming” of synaptic vesicle for exocytosis (6, 7). Furthermore, several proteins of the exocytic machinery, including the Ca2+ sensor synaptotagmin, bind to and are regulated directly by PI(4,5)P2 (8). Likewise, a variety of proteins implicated in synaptic vesicles endocytosis, such as the clathrin adaptor proteins AP2, AP180, and epsin, as well as the GTPase dynamin and other endocytic accessory factors, contain PI(4,5)P2 binding sites (9–12). Binding of these proteins to PI(4,5)P2 is thought to contribute to their recruitment to the plasma membrane and therefore to clathrin coat nucleation (13, 14).
Recent studies have implicated dephosphorylation of PI(4,5)P2 by the polyphosphoinositide phosphatase synaptojanin 1, a pathway that does not produce inositol 1,4,5-trisphosphate and diacylglycerol, as an additional important regulatory mechanism at synapses (15–22). This enzyme is concentrated in nerve terminals, where it is found in the cytosol, and also partially associated with endocytic intermediates (23, 24). The synaptojanin 1 molecule comprises two distinct inositol phosphatase domains and a COOH-terminal proline-rich domain (PRD). The central phosphatase domain dephosphorylates PI(4,5)P2 (and also PI 3,4,5-trisphosphate) selectively at the 5′ position (5-phosphatase domain), whereas the NH2-terminal Sac1 domain can act on several positions of the inositol ring, and its substrates in vitro include PI 4-phosphate [PI(4)P] (15, 25, 26). The PRD region functions primarily as a targeting domain via the interaction with a large number of proteins, among which endophilin 1, via its COOH-terminal Src homology 3 (SH3) domain, appears to be the most important physiological partner (9, 27, 28). In neurons of synaptojanin 1 knockout mice, which contain elevated levels of PI(4,5)P2, an abnormal accumulation of coated vesicles within a cytoskeletal cytomatrix was observed at endocytic zones of nerve terminals (16). Similar observations were made after disruption of synaptojanin function by antibody and peptide microinjection into giant lamprey synapses (17), or by genetic manipulations in Caenorhabditis elegans and Drosophila (20–22). These findings have suggested that dephosphorylation of PI(,4,5)P2 by synaptojanin on endocytic membranes may facilitate clathrin uncoating by decreasing the affinity of clathrin adaptors for the membrane. In addition, via its negative role on PI(4,5)P2 levels, synaptojanin may have additional roles in the down-regulation of actin polymerization and in signaling cascades at synapses (29).
If one of the main functions of synaptojanin 1 is the control of the synaptic vesicle cycle, its membrane recruitment and activity is likely to undergo regulation by nerve terminal stimulation. Accordingly, synaptojanin 1, like other proteins implicated in endocytosis (30–34), is constitutively phosphorylated in resting nerve terminals and dephosphorylated in a Ca2+-dependent manner upon stimulation by depolarization (23, 32, 35). Dephosphorylation of synaptojanin 1 and other endocytic proteins is blocked by calcineurin antagonists and is rapidly reversed by nerve terminal repolarization (30–32, 36). The identity of the kinase(s) responsible for rephosphorylation has been a matter of debate. Recently, strong evidence has implicated cyclin-dependent kinase 5 (Cdk5) as the main enzyme responsible for the constitutive phosphorylation of dynamin (37, 38) and amphiphysin (38, 39), and for their rephosphorylation after stimulation. Cdk5 is a proline-directed serine/threonine protein kinase highly expressed in the nervous system and present in nerve terminals together with its activator protein p35 (40). Cdk5 is known to play an important role in neuronal development, migration, survival, growth, and synaptic function (40–42). We have now investigated the role of the p35/Cdk5 complex in the regulation of synaptojanin 1 in nerve terminals.
Materials and Methods
Molecular Reagents. The PRD region (amino acids 1042–1292) of the 145-kDa isoform of rat synaptojanin 1 (1,292 aa) was generated by PCR using the full-length rat synaptojanin 1 DNA (15) as a template. The DNA fragment was cloned into BamHI and XhoI sites of pGEX-6P-1 (Amersham Pharmacia) to generate the GST-fusion protein. Point mutants of the GST–PRD construct were made by a QuikChange site-directed mutagenesis kit (Promega) according to the instruction manual. GST fusion proteins were expressed in Escherichia coli strain BL21. Rabbit polyclonal antibodies directed against synaptojanin 1 and endophilin 1 and a mouse monoclonal antibody directed against amphiphysin 1 were as described (28, 43). GST fusion proteins of full-length and the SH3 domain of endophilin 1 (EndoSH3) were described (28, 44). A p35/Cdk5 complex was purified from Sf9 cells coinfected with baculovirus encoding Cdk5 and p35 (a kind gift of Shin-ichi Hisanaga and Taro Saito, Tokyo Metropolitan University, Tokyo) (45).
Purification of Synaptojanin 1 from Rat Brain. EndoSH3 was coupled to glutathione–Sepharose beads and used to affinity-purify interacting proteins from a Triton X-100 rat brain extract as described (28). The rat brain extract was prepared in buffer A [1% (vol/vol) Triton X-100/20 mM Hepes, pH 7.4/120 mM KCl/1 mM EGTA/1 mM EDTA/1 mM DTT/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml benzamidine/1 mM phenylmethylsulfonyl fluoride]. Bound material was eluted by the addition of the “PP-19 peptide” (0.5–1.0 mM) corresponding to the endophilin 1 binding site of rat synaptojanin 1 (amino acids 1,111–1,129) (17). The eluate, which contained nearly exclusively synaptojanin 1 and dynamin 1, was dialyzed overnight against buffer B (20 mM Tris, pH 8.0/1 mM DTT/1 μg/ml aprotinin/1 μg/ml leupeptin/1 μg/ml benzamidine), applied to a FPLC Mono-Q anion exchange column and then eluted at a flow rate of 1.0 ml/min with a linear NaCl gradient up to 600 mM in buffer B. As determined by SDS/PAGE and protein staining by Coomassie blue, this procedure yielded a very good separation of synaptojanin from dynamin. The synaptojanin 1 peak eluting from this column was used as “purified synaptojanin 1” for further experiments.
In Vitro Phosphorylation and Dephosphorylation of Synaptojanin 1. Purified synaptojanin 1 (0.3–0.5 μg) was mixed with a recombinant p35/Cdk5 (60–90 ng) complex. Assay mixtures (20–30 μl final volume) containing 20 mM Mops (pH 7.4), 5 mM MgCl2, 100 μM ATP, 1 mM DTT, and 10 μCi of [γ-32P]ATP were incubated for up to 1 h at 32°C and then incubations were stopped by addition of SDS/PAGE sample buffer (1 Ci = 37 GBq). For dephosphorylation experiments, the phosphorylation reactions were blocked by addition of 5 mM EDTA. Samples were then further incubated with calcineurin (40 unit) and calmodulin (0.5 μM)for1hat30°C in a buffer containing 50 mM Tris (pH 7.5), 100 mM NaCl, 0.5 mM DTT, 0.025% (vol/vol) Nonidet P-40, and 10 mM CaCl2 by using a calcineurin assay kit (Biomol, Plymouth Meeting, PA). Phosphorylation and dephosphorylation of synaptojanin 1 immunoprecipitates from rat brain extracts were performed by using the same conditions.
Catalytic Activity of Purified Synaptojanin 1. TLC. Purified synaptojanin 1 was incubated with synthetic PI(4,5)P2 [a mixture of NBD6-PI(4,5)P2 and diC8-PI(4,5)P2 (Echelon, Salt Lake City) at a 1:2 (wt/wt) ratio] in a buffer containing 30 mM Hepes (pH 7.4), 100 mM KCl, 1 mM EGTA, and 2 mM MgCl2. The final assay mixture (15–20 μl) containing 1.5 μg PI(4,5)P2 was incubated for 10–15 min at 37°C. Lipid products were separated by TLC (46) and visualized by fluorescence (due to the NBD6 probe). Fluorescence intensity was calculated by using nih image software.
Phosphate release assay. Purified synaptojanin 1 was incubated with diC8-PI(4,5)P2 [but not NBD6-PI(4,5)P2] by using the assay conditions described above. The free phosphate generated during the assay was measured by a malachite green assay (80 μl) using a microplate reader at a 620-nm wavelength (47).
In both assay methods, synaptojanin 1 exhibited only inositol 5-phosphatase (5-phosphatase) activity. Unless otherwise indicated in the figure legends, results obtained from the two methods were normalized to each other by setting the control level of each experiment as 100%, combined together and presented as bar graphs. Values shown by the bar graphs represent the average ± SD of at least two independent measurements with each of the two assays.
Catalytic Activity of Synaptojanin 1 in Synaptosomes. Rat brain synaptosomes were prepared by differential percoll gradient centrifugation and incubated as described (32). Aliquots (150 μl) of synaptosomes were diluted in a same volume of either control buffer or high K+-containing buffer and in the presence or absence of cyclosporin A. After a 1-min incubation at 37°C, synaptosomes were rapidly harvested by a brief microcentrifugation at 10,000 rpm. Some samples were directly solubilized in buffer A (see above) supplemented with 1 mM sodium vanadate, 2 μM cyclosporin A, and 2 mM sodium β-glycerophosphate, whereas other samples were resuspended in control buffer in the absence or presence of kinase inhibitors and further incubated for 10 min at 37°C before a new microcentrifugation followed by solubilization. After a 5-min incubation on ice, synaptosomal extracts were microcentrifuged and the supernatant was incubated for 1 h at 4°C with protein A-Sepharose beads precoated with anti-synaptojanin 1 antibody. Synaptojanin 1 immunoprecipitates were washed with solubilization buffer and enzymatic activity was measured by using the malachite green-based 5-phosphatase assay.
Mapping of Phosphorylation Sites. Purified 32P-labeled synaptojanin 1 phosphorylated by Cdk5 in vitro (see above) was excised and trypsin-digested from SDS/PAGE. Phosphorylated peptides were identified by HPLC, Cerenkov counting, and matrix-assisted laser desorption ionization MS.
Miscellaneous Procedures. Rat brain cytosol was prepared by using buffer A (without Triton X-100) as described (35). For synaptojanin 1 immunoprecipitation, Triton X-100 rat brain extracts containing 1 mM sodium vanadate, 2 μM cyclosporin A, and 10 mM sodium β-glycerophosphate were incubated with antisynaptojanin 1 antibody for2hat4°C and then further incubated with protein A-Sepharose beads for 1 h. SDS/PAGE, Western blotting, protein quantification, and production of GST fusion proteins were carried out by standard procedures.
Results
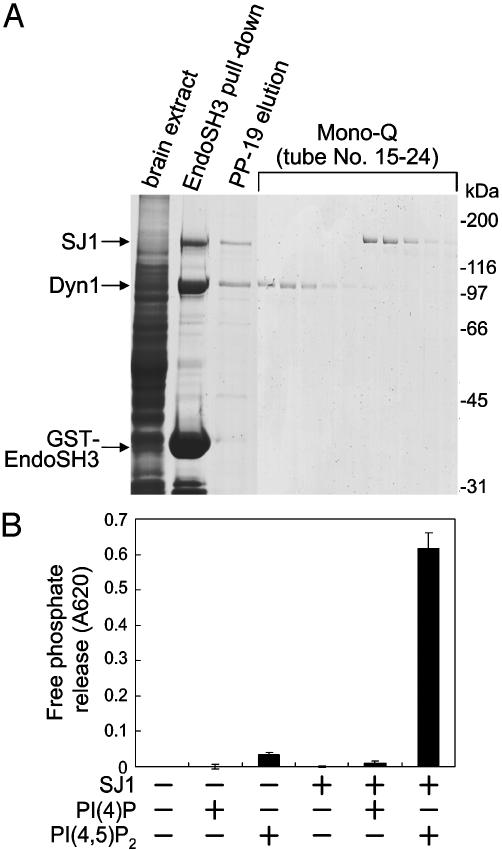
Synaptojanin Is Phosphorylated by Cdk5 in Vitro. To obtain synaptojanin 1 for in vitro assays, the protein was purified from rat brain extracts by SH3 domain affinity chromatography (Fig. 1A). A Triton X-100 brain extract was purified by using a GST fusion protein of the SH3 domain of endophilin 1 (EndoSH3). The procedure, as expected, resulted in the affinity purification of two major proteins, synaptojanin 1 and dynamin 1, both of which are known to interact with EndoSH3 via their PRD regions (28). A peptide corresponding to the endophilin 1-binding site in the PRD of synaptojanin 1 (PP-19) (17) was used for the elution of bound proteins, and the eluate was injected into a Mono-Q anion exchange column to separate synaptojanin 1 from dynamin 1. The two proteins eluted at a concentration of 180 mM and 240 mM NaCl, respectively. The fractions containing synaptojanin 1 were pooled and used as purified synaptojanin 1. Synaptojanin 1 was catalytically active as demonstrated by its ability to dephosphorylate water-soluble PI(4,5)P2, by using either a TLC (data not shown, see Figs. 3A or 4B) or a malachite green-based assay (Fig. 1B). No dephosphorylation of water-soluble PI(4)P was observed with either assay method, which involved water-soluble phosphoinositides [diC8-PI(4)P and/or diC8-PI(4,5)P2]. Therefore, under these conditions, synaptojanin 1 functions exclusively as a 5-phosphatase.
Fig. 1.
Purification of synaptojanin 1 from rat brain extract. (A) An immobilized GST fusion protein of the SH3 domain of endophilin 1 was used to affinity-purify binding proteins from a Triton X-100 rat brain extract. Bound proteins, represented primarily by synaptojanin 1 (SJ1) and dynamin 1 (Dyn1), were eluted by the PP-19 peptide (corresponding to the endophilin binding peptide of synaptojanin 1). Mono-Q chromatography of the PP-19 eluate with a linear gradient of NaCl resulted in the separation of the two proteins. Each fraction was examined by SDS/PAGE followed by protein staining. (B) Purified synaptojanin 1 has 5-phosphatase activity when tested against water-soluble phosphoinositides [diC8-PI(4)P or diC8-PI(4,5)P2] by using a malachite green-based assay for the detection of free phosphate.
Fig. 3.
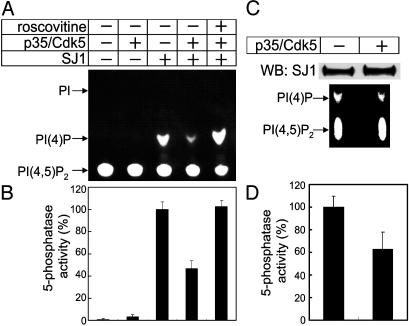
Phosphorylation of synaptojanin 1 by Cdk5 inhibits its 5-phosphatase activity. (A) Synaptojanin 1 was incubated with the p35/Cdk5 complex in phosphorylation conditions (see Fig. 2) in the presence or absence of the Cdk5 inhibitor roscovitine (20 μM). Phosphorylation mixtures and their controls were then added to assay tubes containing water-soluble PI(4,5)P2 substrates [diC8–PI(4,5)P2 and NBD6-PI(4,5)P2] and further incubated for 10–15 min at 37°C. PI(4,5)P2 cleavage was monitored by analysis of NBD6 fluorescence after TLC chromatography. Note that only PI(4)P is generated. In separate experiments (data not shown), the 5-phosphatase activity of synaptojanin 1 was monitored by the release of free phosphate from diC8-PI(4,5)P2 using a malachite green-based assay (47). (B) Pooled results of 5-phosphatase activity assays involving the TLC and the malachite green-based assay. (C) Antisynaptojanin 1 immunoprecipitates from rat brain extracts were incubated in the presence or absence of the p35/Cdk5 complex in phosphorylation conditions, and the resulting mixtures were analyzed for 5-phosphatase activity as described for A. The Western blot (Upper) indicates that the same amount of synaptojanin 1 was present in the two samples. (D) A result of 5-phosphatase activity measured by malachite green-based assay.
Fig. 4.
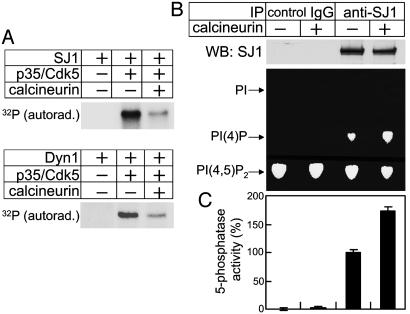
Synaptojanin 1 is a substrate for calcineurin, and its dephosphorylation by calcineurin increases its 5-phosphatase activity. (A) Purified synaptojanin 1 (Upper) or dynamin 1 (Lower) were first phosphorylated by p35/Cdk5 in the presence of [γ-32P]ATP, then further incubated with calcineurin, and finally analyzed by SDS/PAGE and autoradiography. (B) Immunoprecipitates generated from rat brain extracts with control IgG and anti-synaptojanin 1 antibody were incubated with calcineurin and then analyzed for 5-phosphatase activity by TLC as described in the legend of Fig. 3A. Anti-synaptojanin 1 Western blotting (Upper) indicates that the similar amount of synaptojanin 1 was present in the “plus” and “minus” calcineurin assay conditions. (C) Pooled results of 5-phosphatase activity assays as described in the legend of Fig. 3B.
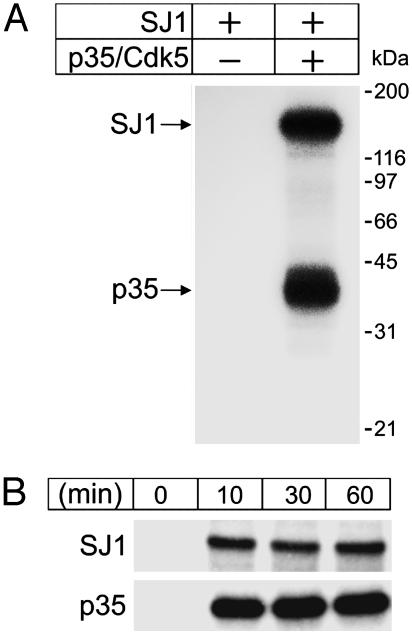
Incubation of purified synaptojanin 1 with recombinant p35/Cdk5 in an ≈1:1 stoichiometric ratio resulted in a robust phosphorylation of synaptojanin 1 (Fig. 2A). As expected, p35 was strongly autophosphorylated during the incubation (48). Phosphorylation of both synaptojanin 1 and p35 occurred rapidly and reached a plateau after 10 min, suggesting that synaptojanin 1 is an effective substrate for Cdk5 in vitro (Fig. 2B).
Fig. 2.
In vitro phosphorylation of synaptojanin 1 by Cdk5. Purified synaptojanin 1 was incubated with the p35/Cdk5 complex and [γ-32P]ATP in phosphorylation assay buffer, and samples were analyzed by SDS/PAGE and autoradiography. (A) Autoradiogram showing phosphorylation of synaptojanin 1 (SJ1) and p35 by Cdk5 after 60-min incubation. (B) γ-32P autoradiogram showing a time-course of synaptojanin 1 and p35 phosphorylation.
Phosphorylation by p35/Cdk5 Inhibits the 5-Phosphatase Activity of Synaptojanin 1. The effect of Cdk5 phosphorylation on the ability of synaptojanin 1 to dephosphorylate PI(4,5)P2 was investigated (Fig. 3). Purified synaptojanin 1 was preincubated in phosphorylation buffer in the presence or absence of p35/Cdk5. Synaptojanin 1 was then incubated for 10–15 min with water-soluble PI(4,5)P2, and the lipid mixture resulting from this incubation was analyzed by TLC. The production of PI(4)P was strikingly decreased by prior Cdk5 phosphorylation (Fig. 3A). This effect was blocked by roscovitine (49), a specific Cdk5 inhibitor. No PI(4)P was detectable in the absence of synaptojanin 1. The same effects of p35/Cdk5 phosphorylation on the enzymatic activity of synaptojanin 1 was observed by using the malachite green-based assay to measure the release of free phosphate from PI(4,5)P2 (data not shown). The pooled results obtained from the two assays are illustrated in the bar graphs shown in Fig. 3B. Similar results were obtained by using synaptojanin 1 immunoprecipitates generated from rat brain extracts by an antibody directed against the PRD of synaptojanin 1. Incubation of these immunoprecipitates with Cdk5 in phosphorylation buffer decreased the 5-phosphatase activity of synaptojanin 1 relative to control, as determined by both the TLC analysis (Fig. 3C) and the malachite green-based assay (Fig. 3D).
Dephosphorylation by Calcineurin Stimulates the 5-Phosphatase Activity of Synaptojanin 1. The stimulation-dependent dephosphorylation of synaptojanin 1 occurring in synaptosomes is inhibited by FK-506 and cyclosporin A (32), suggesting the involvement of calcineurin. Calcineurin dephosphorylates Cdk5 phosphorylation sites of several proteins such as protein phosphatase inhibitor-1, Tau, and synapsin I (50–52). Based on these considerations, the effect of calcineurin on the phosphorylation state of synaptojanin 1 previously phosphorylated by p35/Cdk5 was tested. As assessed by autoradiography, calcineurin strikingly reversed the incorporation of 32P induced by Cdk5 (Fig. 4A). A similar effect was produced by calcineurin on the Cdk5-mediated phosphorylation of dynamin 1, as expected (Fig. 4A).
No increase in inositol 5-phosphatase activity was observed when purified synaptojanin 1 was incubated with calcineurin, most likely due to the dephosphorylation state of synaptojanin 1 after purification (data not shown). However, preincubation of calcineurin with synaptojanin 1 immunoprecipitated from rat brain in the presence of phosphatase inhibitors resulted in activation of the 5-phosphatase activity (Fig. 4B). PI(4)P was not generated in control IgG immunoprecipitates irrespective of the presence of calcineurin, indicating that PI(4)P is produced by the inositol 5-phosphatase activity of synaptojanin 1 but not by calcineurin. The stimulatory effect of calcineurin on the 5-phosphatase activity of synaptojanin 1 immunoprecipitates was confirmed by measurements of free phosphate release by the malachite green-based assay (data not shown). The pooled results obtained from the two assays are illustrated in the bar graphs shown in Fig. 4C.
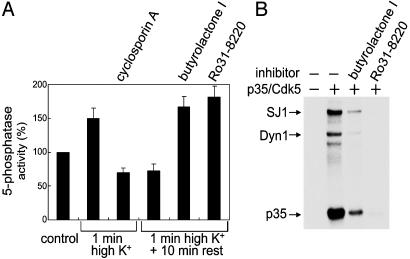
Cdk5 and Calcineurin Regulate Synaptojanin 1 Activity in Intact Synaptosomes. Next, we investigated whether a cycle of dephosphorylation and phosphorylation mediated by calcineurin and Cdk5 could affect the inositol 5-phosphatase activity of synaptojanin 1 in intact nerve terminals. For this purpose, rat brain synaptosomes were incubated in control or depolarizing (55 mM K+) conditions for 1 min, and anti-synaptojanin 1 immunoprecipitates were obtained from these samples in the presence of protein phosphatase inhibitors (Fig. 5).
Fig. 5.
Regulation of the 5-phosphatase activity of synaptojanin 1 in synaptosomes. (A) Synaptosomes were stimulated for 1 min with high K+ in the absence or presence of 2 μM cyclosporin A. Aliquots of synaptosomes stimulated without cyclosporin A were returned for 10 min to control medium or to control medium containing butyrolactone I (10 μM) or Ro31–8220 (20 μM). After solubilization of synaptosomes and anti-synaptojanin 1 immunoprecipitation, the 5-phosphatase activity of the immunoprecipitates was assayed with the malachite green-based assay. Bar graphs show average and range of two experiments. (B) A mixture of purified synaptojanin and dynamin was phosphorylated by p35/Cdk5 in the presence of [32P]ATP and in the absence and presence of butyrolactone I or Ro31–8220 (20 μM each) and then analyzed by SDS/PAGE and autoradiography. Note that Ro31–8220 is more potent than butyrolactone I in inhibiting Cdk5 activity.
Depolarization of synaptosomes resulted in an increase in synaptojanin 5-phosphatase activity (Fig. 5A). Addition of cyclosporin A to the synaptosome buffer not only blocked the stimulation caused by depolarization, but reduced the 5-phosphatase activity to less than that of the control sample. Together with the in vitro assays using calcineurin and immunoprecipitated synaptojanin 1 (Fig. 4B), these studies support the conclusion that calcineurin dephosphorylates and activates synaptojanin 1 in vivo. The depolarization-dependent dephosphorylation of synaptosomal proteins was previously shown to be rapidly followed by rephosphorylation upon removal of high K+ and repolarization (32, 36), and Cdk5 was shown to be involved in this rephosphorylation in the case of dynamin 1 (37, 38) and amphiphysin 1 (38). Accordingly, returning synaptosomes to control buffer for 10 min after the 1-min depolarization resulted in a reversal of the activation of the 5-phosphatase activity of synaptojanin 1 induced by depolarization (Fig. 5A). This reduction was inhibited strikingly by butyrolactone I (10 μM), a specific Cdk5 inhibitor. Ro31-8220 (20 μM), a drug initially characterized as a protein kinase C inhibitor but recently shown to also block Cdk5 (37), had similar effect. Ro31-8220 and butyrolactone I also potently blocked the p35/Cdk5-dependent phosphorylation of synaptojanin 1, dynamin 1, and p35 when using purified components in vitro (Fig. 5B). These results are consistent with Cdk5 playing a major role in the rephosphorylation and inhibition of synaptojanin 1 in vivo, although an additional role of protein kinase C and possibly other kinases should also be considered.
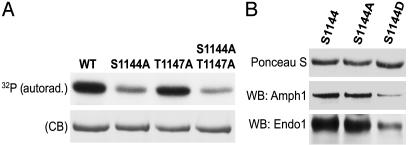
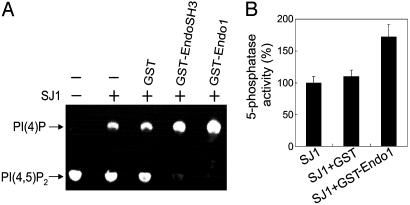
Phosphorylation of Serine 1144 by Cdk5 May Influence the Interaction of Synaptojanin 1 with Endophilin and Amphiphysin. To identify phosphorylation sites for Cdk5 in synaptojanin 1, the purified protein was phosphorylated by p35/Cdk5 in vitro and peptides generated by tryptic digestion were analyzed by HPLC and matrix-assisted laser desorption ionization MS. The masses of peptide fragments containing significant radioactivity were then searched against the sequence of rat synaptojanin 1 (1,292 aa) (15). A strongly radioactive peptide had the expected mass of the amino acid sequence 1,138–1,155, EFGAPKSPGTARKDNIGR, plus the mass of a phosphate group. This peptide contains both a serine (position 1144) and a threonine (position 1147), but only serine 1144 is in the context of a putative Cdk5 phosphorylation site. To confirm the identification of this site, GST fusion proteins of the PRD region of synaptojanin 1 (1,042–1,292 aa), the region that includes serine 1144, and of mutants harboring S → A and T → A substitutions at position 1144 and 1147 respectively, were generated. Incubation of these fusion proteins with p35/Cdk5 revealed a strikingly reduced phosphorylation of the two mutants containing the S1144A mutation relative to wild type, but only a minor reduction in the phosphorylation of the mutant containing only the T1147A mutation (Fig. 6A). The presence of the Cdk5 phosphorylation site at serine 1144 within the PRD region raised the possibility that phosphorylation of this site might affect the interaction of with SH3 domains in proteins such as endophilin. Interestingly, serine 1144 is localized in close proximity of the binding site for endophilin, the preferred binding partner of synaptojanin 1 (53, 54). To test for a possible effect of phosphorylation, serine 1144 was mutated to aspartic acid in an attempt to mimic phosphorylation at this residue. GST-fusion proteins of the wild-type PRD or of the PRD containing S1144D or S1144A mutations were expressed and used in pull-down assays with rat brain cytosol. Affinity purification not only of endophilin 1, but also of amphiphysin 1 (28), was significantly reduced by the S1144D mutation but not by the S1144A mutation (Fig. 6B). This finding suggests that phosphorylation of serine at position 1144 is likely to interfere with the interaction of synaptojanin 1 with amphiphysin 1 or endophilin 1. Finally, we tested whether the binding of endophilin 1 to synaptojanin 1 has any effect on the inositol 5-phosphatase activity of synaptojanin 1. Both full-length endophilin 1 and its SH3 domain alone stimulated the activity of synaptojanin 1 (Fig. 7). Thus, the stimulation of synaptojanin 1 activity produced by its dephosphorylation in vivo, may be further potentiated by its interaction with endophilin.
Fig. 6.
Cdk5 phosphorylation of the serine 1144 of rat synaptojanin 1 affects its interaction with endophilin and amphiphysin. (A) Serine 1144 and/or threonine 1147 were mutated to alanine, and GST fusions of the wild-type and mutant PRDs were incubated with p35/Cdk5 and [32P]ATP. Incubation mixtures were then analyzed by SDS/PAGE followed by Coomassie blue (CB) staining and autoradiography. (B) GST fusion proteins of the wild-type PRD of synaptojanin 1, or of two mutants harboring a S → A or S → D mutation at position 1144, were used in pull-down assays from rat brain cytosol. Western blotting of the bound material revealed that the “phosphomimetic mutation” (S1144D) inhibited the recovery of both amphiphysin 1 and endophilin 1 in the bound material.
Fig. 7.
Stimulatory effect of endophilin 1 on the 5-phosphatase of synaptojanin 1. Purified synaptojanin 1 (0.2 μg) was preincubated for 30 min at 37°C with GST alone, GST-EndoSH3, or GST-endophilin 1 (GST-Endo1) at a molar concentration of 1:10 (synaptojanin 1/GST fusion proteins). The 5-phosphatase of this material was then assayed by either the TLC assay (A) or the malachite green-based assay (see Fig. 3A). The bar graphs shown in B represent the combined results from the two assays.
Discussion
The results of this study demonstrate that synaptojanin 1 is regulated by the protein kinase Cdk5 at synapses. Cdk5-mediated phosphorylation appears to be responsible, at least in part, for the constitutive phosphorylation of synaptojanin 1 observed in resting synaptosomes. Recently, two other proteins enriched in nerve terminals, dynamin 1 and amphiphysin were found to be physiological substrates for Cdk5 (37–39), pointing to Cdk5 as a major player in the rephosphorylation of endocytic proteins after their stimulation-dependent dephosphorylation (37, 38). Like dynamin 1 and amphiphysin, synaptojanin 1 undergoes a stimulation-dependent dephosphorylation, and this reaction is inhibited by calcineurin antagonists (23, 32). Accordingly, we show here that purified calcineurin can dephosphorylate synaptojanin 1 previously phosphorylated in vitro by Cdk5.
Synaptojanin 1 is partially soluble, indicating that it shuttles between the cytosol and the membrane (23). Its putative role in the dephosphorylation of a pool of phosphoinositides implicated in synaptic vesicle recycling (13) implies its recruitment in response to nerve terminal stimulation, when the vesicle cycle is activated. Results from cell-free studies and from peptide microinjection experiments in the giant axon of the lamprey indicate a major role for endophilin in the recruitment of synaptojanin 1 to membranes (17, 27, 28, 44). This possibility was recently strongly corroborated by the phenotypic analysis of endophilin mutants of C. elegans and Drosophila (21, 22). It is therefore of interest that a Cdk5 phosphorylation site (S1144) identified in synaptojanin 1 by in vitro experiments is close to the binding site for endophilin and that a phosphomimetic mutation at this position (S1144D) inhibits endophilin binding. This finding provides a plausible mechanistic explanation for the regulation of the interaction between synaptojanin 1 and endophilin mediated by nerve terminal stimulation at mammalian synapses.
In addition to regulating membrane recruitment, Cdk5 and calcineurin also have a direct effect on the catalytic activity of synaptojanin 1. Cdk5-mediated phosphorylation inhibits synaptojanin 1 activity, whereas calcineurin-mediated dephosphorylation stimulates it. Although serine 1144 was identified as a major phosphorylation site, the HPLC/matrix-assisted laser desorption ionization MS analysis indicates that other sites remain to be identified. Thus, additional studies are required to determine the precise mechanism through which Cdk5 and calcineurin regulate the 5-phosphatase activity of synaptojanin 1. Finally, binding of endophilin to synaptojanin1 through its SH3 domain stimulates the catalytic activity of synaptojanin 1. Thus, synaptojanin 1 dephosphorylation in response to depolarization-dependent Ca2+ entry may facilitate its catalytic action at the membrane via multiple mechanisms that include its increased recruitment by endophilin, the stimulation of its catalytic activity as a result of the binding to endophilin, and the dephosphorylation of a site(s) that inhibit(s) its enzymatic activity.
In conclusion, our study indicates that Cdk5 and calcineurin have opposite actions in the regulation of PI(4,5)P2 degradation at synapses via their phosphorylation and dephosphorylation, respectively, of synaptojanin 1. Besides dynamin 1, amphiphysin 1, and synaptojanin 1, additional proteins implicated in nerve terminal endocytosis undergo constitutive phosphorylation in resting nerve terminals and Ca2+-dependent dephosphorylation upon nerve terminal depolarization (33, 34, 46). Generally, phosphorylation inhibits, whereas dephosphorylation promotes, their assembly into endocytic complexes (34, 35, 37, 38). Based on these considerations, Cdk5 and calcineurin may play a general and antagonistic role in the transition of nerve terminals from a “resting mode” to a “stimulated mode.”
Acknowledgments
We are deeply indebted to Paul Greengard for generous support, to Shin-ichi Hisanaga and Taro Saito for providing a critical reagent, and to the Yale Keck Foundation Biotechnology Resource Laboratory for assistance in a phosphorylation site analysis. This work was supported in part by National Institutes of Health Grants NS36251 and CA46128 (to P.D.C.) and DA10044 (to A.C.N.).
Abbreviations: PI, phosphatidylinositol; PI(4,5)P2, PI 4,5-bisphosphate; PI(4)P, PI 4-phosphate; Cdk5, cyclin-dependent kinase 5; PRD, proline-rich domain; SH3, Src homology 3.
References
- 1.De Camilli, P., Emr, S. D., McPherson, P. S. & Novick, P. (1996) Science 271, 1533–1539. [DOI] [PubMed] [Google Scholar]
- 2.Martin, T. F. J. (1998) Annu. Rev. Cell Dev. Biol. 14, 231–264. [DOI] [PubMed] [Google Scholar]
- 3.Takenawa, T. & Itoh, T. (2001) Biochim. Biophys. Acta 1533, 190–206. [DOI] [PubMed] [Google Scholar]
- 4.Yin, H. L. & Janmey, P. A. (2003) Annu. Rev. Physiol. 65, 761–789. [DOI] [PubMed] [Google Scholar]
- 5.Audigier, S. M., Wang, J. K. & Greengard, P. (1988) Proc. Natl. Acad. Sci. USA 85, 2859–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brose, N. & Rosenmund, C. (2002) J. Cell Sci. 115, 4399–4411. [DOI] [PubMed] [Google Scholar]
- 7.Lackner, M. R., Nurrish, S. J. & Kaplan, J. M. (1999) Neuron 24, 335–346. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O., Gao, Y., Anderson, R. G., Sudhof, T. C. & Rizo, J. (2001) Neuron 32, 1057–1069. [DOI] [PubMed] [Google Scholar]
- 9.Slepnev, V. I. & De Camilli, P. (2000) Nat. Rev. Neurosci. 1, 161–172. [DOI] [PubMed] [Google Scholar]
- 10.Itoh, T., Koshiba, S., Kigawa, T., Kikuchi, A., Yokoyama, S. & Takenawa, T. (2001) Science 291, 1047–1051. [DOI] [PubMed] [Google Scholar]
- 11.Evans, P. R. & Owen, D. J. (2002) Curr. Opin. Struct. Biol. 12, 814–821. [DOI] [PubMed] [Google Scholar]
- 12.Lemmon, M. A. (2003) Traffic 4, 201–213. [DOI] [PubMed] [Google Scholar]
- 13.Cremona, O. & De Camilli, P. (2001) J. Cell Sci. 114, 1041–1052. [DOI] [PubMed] [Google Scholar]
- 14.Ford, M. G., Mills, I. G., Peter, B. J., Vallis, Y., Praefcke, G. J., Evans, P. R. & McMahon, H. T. (2002) Nature 419, 361–366. [DOI] [PubMed] [Google Scholar]
- 15.McPherson, P. S., Garcia, E. P., Slepnev, V. I., David, C., Zhang, X., Grabs, D., Sossin, W. S., Bauerfeind, R., Nemoto, Y. & De Camilli, P. (1996) Nature 379, 353–357. [DOI] [PubMed] [Google Scholar]
- 16.Cremona, O., Di Paolo, G., Wenk, M. R., Luthi, A., Kim, W. T., Takei, K., Daniell, L., Nemoto, Y., Shears, S. B., Flavell, R. A., et al. (1999) Cell 99, 179–188. [DOI] [PubMed] [Google Scholar]
- 17.Gad, H., Ringstad, N., Low, P., Kjaerulff, O., Gustafsson, J., Wenk, M., Di Paolo, G., Nemoto, Y., Crun, J., Ellisman, M. H., et al. (2000) Neuron 27, 301–312. [DOI] [PubMed] [Google Scholar]
- 18.Luthi, A., Di Paolo, G., Cremona, O., Daniell, L., De Camilli, P. & McCormick, D. A. (2001) J. Neurosci. 21, 9101–9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, W. T., Chang, S., Daniell, L., Cremona, O., Di Paolo, G. & De Camilli, P. (2002) Proc. Natl. Acad. Sci. USA 99, 17143–17148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris, T. W., Hartwieg, E., Horvitz, H. R. & Jorgensen, E. M. (2000) J. Cell Biol. 150, 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verstreken, P., Koh, T. W., Schulze, K. L., Zhai, R. G., Hiesinger, P. R., Zhou, Y., Mehta, S. Q., Cao, Y., Roos, J. & Bellen, H. J. (2003) Neuron 40, 733–748. [DOI] [PubMed] [Google Scholar]
- 22.Schuske, K. R., Richmond, J. E., Matthies, D. S., Davis, W. S., Runz, S., Rube, D. A., van der Bliek, A. M. & Jorgensen, E. M. (2003) Neuron 40, 749–762. [DOI] [PubMed] [Google Scholar]
- 23.McPherson, P. S., Takei, K., Schmid, S. L. & De Camilli, P. (1994) J. Biol. Chem. 269, 30132–30139. [PubMed] [Google Scholar]
- 24.Haffner, C., Takei, K., Chen, H., Ringstad, N., Hudson, A., Butler, M. H., Salcini, A. E., Di Fiore, P. P. & De Camilli, P. (1997) FEBS Lett. 419, 175–180. [DOI] [PubMed] [Google Scholar]
- 25.Woscholski, R., Finan, P. M., Radley, E., Totty, N. F., Sterling, A. E., Hsuan, J. J., Waterfield, M. D. & Parker, P. J. (1997) J. Biol. Chem. 272, 9625–9628. [DOI] [PubMed] [Google Scholar]
- 26.Guo, S., Stolz, L. E., Lemrow, S. M. & York, J. D. (1999) J. Biol. Chem. 274, 12990–12995. [DOI] [PubMed] [Google Scholar]
- 27.de Heuvel, E., Bell, A. W., Ramjaun, A. R., Wong, K., Sossin, W. S. & McPherson, P. S. (1997) J. Biol. Chem. 272, 8710–8716. [DOI] [PubMed] [Google Scholar]
- 28.Ringstad, N., Nemoto, Y. & De Camilli, P. (1997) Proc. Natl. Acad. Sci. USA 94, 8569–8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakisaka, T., Itoh, T., Miura, K. & Takenawa, T. (1997) Mol. Cell. Biol. 17, 3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu, J. P., Sim, A. T. & Robinson, P. J. (1994) Science 265, 970–973. [DOI] [PubMed] [Google Scholar]
- 31.Marks, B. & McMahon, H. T. (1998) Curr. Biol. 8, 740–749. [DOI] [PubMed] [Google Scholar]
- 32.Bauerfeind, R., Takei, K. & De Camilli, P. (1997) J. Biol. Chem. 272, 30984–30992. [DOI] [PubMed] [Google Scholar]
- 33.Chen, H., Slepnev, V. I., Di Fiore, P. P. & De Camilli, P. (1999) J. Biol. Chem. 274, 3257–3260. [DOI] [PubMed] [Google Scholar]
- 34.Cousin, M. A. & Robinson, P. J. (2001) Trends Neurosci. 24, 659–665. [DOI] [PubMed] [Google Scholar]
- 35.Slepnev, V. I., Ochoa, G.-C., Butler, M. H., Grabs, D. & De Camilli, P. (1998) Science 281, 821–824. [DOI] [PubMed] [Google Scholar]
- 36.Cousin, M. A., Tan, T. C. & Robinson, P. J. (2001) J. Neurochem. 76, 105–116. [DOI] [PubMed] [Google Scholar]
- 37.Tan, T. C., Valova, V. A., Malladi, C. S., Graham, M. E., Berven, L. A., Jupp, O. J., Hansra, G., McClure, S. J., Sarcevic, B., Boadle, R. A., et al. (2003) Nat. Cell Biol. 5, 701–710. [DOI] [PubMed] [Google Scholar]
- 38.Tomizawa, K., Sunada, S., Lu, Y.-F., Oda, Y., Kinuta, M., Ohshima, T., Saito, T., Wei, F.-Y., Matsushita, M., Li, S.-T., et al. (2003) J. Cell Biol. 163, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Floyd, S. R., Porro, E. B., Slepnev, V. I., Ochoa, G.-C., Tsai, L. H. & De Camilli, P. (2001) J. Biol. Chem. 276, 8104–8110. [DOI] [PubMed] [Google Scholar]
- 40.Dhavan, R. & Tsai, L. H. (2001) Nat. Rev. Mol. Cell Biol. 2, 749–759. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka, T., Veeranna, Ohshima, T., Rajan, P., Amin, N. D., Cho, A., Sreenath, T., Pant, H. C., Brady, R. O. & Kulkarni, A. B. (2001) J. Neurosci. 21, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie, Z., Sanada, K., Samuels, B. A., Shih, H. & Tsai, L. H. (2003) Cell 114, 469–482. [DOI] [PubMed] [Google Scholar]
- 43.Floyd, S., Butler, M. H., Cremona, O., David, C., Freyberg, Z., Zhang, X., Solimena, M., Tokunaga, A., Ishizu, H., Tsutsui, K., et al. (1998) Mol. Med. 4, 29–39. [PMC free article] [PubMed] [Google Scholar]
- 44.Ringstad, N., Nemoto, Y. & De Camilli, P. (2001) J. Biol. Chem. 276, 40424–40430. [DOI] [PubMed] [Google Scholar]
- 45.Saito, T., Onuki, R., Fujita, Y., Kusakawa, G., Ishiguro, K., Bibb, J. A., Kishimoto, T. & Hisanaga, S. (2003) J. Neurosci. 23, 1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenk, M. R., Pellegrini, L., Klenchin, V. A., Di Paolo, G., Chang, S., Daniell, L., Arioka, M., Martin, T. F. & De Camilli, P. (2001) Neuron 32, 79–88. [DOI] [PubMed] [Google Scholar]
- 47.Harder, K. W., Owen, P., Wong, L. K., Aebersold, R., Clark-Lewis, I. & Jirik, F. R. (1994) Biochem. J. 298, 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patrick, G. N., Zhou, P., Kwon, Y. T., Howley, P. M. & Tsai, L. H. (1998) J. Biol. Chem. 273, 24057–24064. [DOI] [PubMed] [Google Scholar]
- 49.Norrholm, S. D., Bibb, J. A., Nestler, E. J., Ouimet, C. C., Taylor, J. R. & Greengard, P. (2003) Neuroscience 116, 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bibb, J. A., Nishi, A., O'Callaghan, J. P., Ule, J., Lan, M., Snyder, G. L., Horiuchi, A., Saito, T., Hisanaga, S., Czernik, A. J., et al. (2001) J. Biol. Chem. 276, 14490–14497. [DOI] [PubMed] [Google Scholar]
- 51.Mandelkow, E. M., Biernat, J., Drewes, G., Gustke, N., Trinczek, B. & Mandelkow, E. (1995) Neurobiol. Aging 16, 355–362. [DOI] [PubMed] [Google Scholar]
- 52.Jovanovic, J. N., Sihra, T. S., Nairn, A. C., Hemmings, H. C., Jr., Greengard, P. & Czernik, A. J. (2001) J. Neurosci. 21, 7944–7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ringstad, N., Gad, H., Low, P., Di Paolo, G., Brodin, L., Shupliakov, O. & De Camilli, P. (1999) Neuron 24, 143–154. [DOI] [PubMed] [Google Scholar]
- 54.Cestra, G., Castagnoli, L., Dente, L., Minenkova, O., Petrelli, A., Migone, N., Hoffmuller, U., Schneider-Mergener, J. & Cesareni, G. (1999) J. Biol. Chem. 274, 32001–32007. [DOI] [PubMed] [Google Scholar]