Abstract
Successful reproduction requires precise temporal coordination among various endocrine and behavioural events. The circadian system regulates daily temporal organization in behaviour and physiology, including neuroendocrine rhythms. The main circadian pacemaker in mammals is located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus. The SCN sends direct efferents to the reproductive axis via monosynaptic projections to gonadotropin-releasing hormone (GnRH) neurones. This communication generates circadian endocrine rhythms as well as the preovulatory luteinizing hormone (LH) surge necessary for successful ovulation. One SCN peptide thought to be important for the regulation of oestrous cycles is vasoactive intestinal polypeptide (VIP). VIP neurones from the SCN contact GnRH cells, and these cells are preferentially activated during an LH surge in rats. Unlike adult rats, prepubertal females do not exhibit oestrous cycles, nor do they exhibit an LH surge in response to oestradiol positive-feedback. The present study was undertaken to determine the extent to which the development of a ‘mature’ reproductive axis in female rats is associated with modifications in VIP contacts on GnRH neurones. The brains of diestrus adult (approximately 60 days of age) and prepubertal (21 days of age) female rats were examined using double-label fluorescence immunohistochemistry for VIP and GnRH, with light and confocal microscopy. Although the total number of GnRH-immunoreactive neurones did not differ between adult and prepubertal females, adults had a significant increase in the percentage of GnRH cells receiving VIP contacts compared to juveniles. These data suggest that the development of reproductive hormone rhythms and oestrous cyclicity may be, in part, due to modifications of VIP input to the GnRH system.
Keywords: circadian, oestrous cycle, oestrus, rhythm, hormone, endocrine, reproduction
In order to successfully reproduce, numerous physiological and behavioural events must be precisely coordinated. For example, the timing of physiological events such as sex hormone and gamete production, as well as pregnancy and parturition, must coincide with a particular time of day or night and, for natural populations of animals, a specific time of the year. The importance of the daily timing of endocrine events is underscored by the fact that there is a great deal of interaction between the neuroendocrine and circadian timing systems (1). Virtually all hormones measured to date show a pronounced circadian rhythm in production and secretion that is abolished after destruction of the bilateral suprachiasmatic nucleus (SCN), the main circadian pacemaker in mammals (1–4).
Central in this temporal organization in reproduction is the circadian regulation of the oestrous cycle in female rodents. Numerous lines of evidence indicate that the circadian system is essential for oestrous cyclicity, specifically for the generation of the preovulatory luteinizing hormone (LH) surge (5–8). For example, the LH surge in rats requires a neuronal signal that is only present during a specific limited daily interval (5). If this neuronal signal is blocked (by barbiturate administration) the LH surge is delayed by 24 h, presumably due to blocking of the time-specific neuronal signal initiating the LH surge (5). Evidence that this daily neuronal signal is under circadian control comes from studies in which animals that are ovariectomized and treated with oestradiol exhibit daily LH surges (7, 8). Lesion studies and those severing neural output from the SCN confirm that this daily signal is generated by neural efferents from the circadian clock (9, 10).
The precise mechanisms regulating the timing of the preovulatory LH surge and generation of oestrous cycles have not been fully elucidated. However, several lines of evidence suggest that direct monosynaptic projections from the SCN to GnRH neurones may play an important role in the temporal coordination of the reproductive axis necessary for successful ovulation (11–13). Numerous neuro-active substances are localized to different subregions within the SCN (14). Neurones in the ventral SCN contain vasoactive intestinal polypeptide (VIP), and VIP cells regularly contact GnRH cell bodies and dendrites (11, 15). Importantly, lesions of the SCN virtually abolish VIP contacts on GnRH neurones in adult female rats, thereby establishing the SCN as the major source for this VIP input (11). These VIPergic neurones synapse in a sex differentiated manner in the medial preoptic area, with females exhibiting a greater number of contacts per neurone (13). GnRH neurones in female rats contain VIP2 receptors (16), and manipulations of VIP lead to alterations in LH release (17–19). Finally, GnRH neurones receiving innervation from VIP cells in the SCN are preferentially activated (i.e. express Fos) during the LH surge (20), further suggesting a role for VIP regulation of the timing of oestrus.
Puberty in female mammals is associated with a number of neuroendocrine developments, including increased secretion of hormones of the reproductive axis and the onset of a diurnal rhythm in GnRH and LH secretion and/or mRNA production with peaks occurring during the night (21–26). Because VIP from the SCN has been established as an important source of input to the neuroendocrine system to regulate oestrus, and oestrous cyclicity does not develop until after puberty, the present study was undertaken to determine whether or not the development of a ‘mature’, functional reproductive axis of female rats is associated with alterations in VIP input to the GnRH neuronal system.
Materials and methods
Animals
Sprague–Dawley rats (Rattus norvegicus) were used in the present experiment. Animals were purchased from Charles River (Wilmington, MA, USA). Immature females (n=5) were purchased at 14 days of age together with their dams (n=5). Adult animals were purchased at approximately 40 days of age (n=5). All animals were housed in translucent propylene cages (48×27×20 cm) and provided with access to food and water ad libitum for the duration of the study. Animals were maintained in a colony room with a 24-h light/dark cycle (12 : 12 h light/dark). The rooms were maintained at 23±1 °C. All animals were allowed a minimum of a 1-week acclimation period prior to the onset of the experiment. All animal research in this report was approved by Columbia University’s Animal Care and Use Committee.
Experimental procedure
Brains from juvenile rats were collected when the animals were 21 days of age. For adult females, vaginal smears were taken daily for at least two consecutive cycles. Because oestradiol concentrations throughout the oestrous cycle can affect neuronal structure (and potentially connectivity between neurones) (27–29), adult females were killed on the morning of diestrus I when oestradiol concentrations are at a minimum, allowing a more valid comparison between adult and prepubertal female rats with low oestradiol concentrations and adults (approximately 60 days of age).
To collect the brains, animals were deeply anaesthetized with sodium pentobarbital (200 mg/kg) and perfused transcardially with 150 ml of 0.9% saline followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS) (pH 7.3). Brains were postfixed in 4% paraformaldehyde for 2 h at 4 °C, then cryoprotected in 20% sucrose in 0.1 M PBS overnight at 4 °C. Coronal sections (30 μm) were cut on a cryostat and collected into 0.1 M PBS. The brain sections were processed as free-floating sections.
For simultaneous visualization of VIP and GnRH, every fourth, 30 μm section was double-labelled using fluorescence immunohistochemistry. For visualization of GnRH, sections were washed in PBS, incubated in 1% H2O2, and then incubated in normal donkey serum in 0.1% Triton X-100 (PBT) for 1 h. Sections were then incubated for 48 h at 4 °C in guinea-pig anti-mammalian GnRH (antigenic determinants are amino acids 6–10; Advanced Chemtech, Louisville, KY, USA) diluted at 1 : 10 000 with 0.1% PBT. Following incubation in the GnRH antibody, cells were labelled using Cy-3 donkey anti-guinea-pig (Jackson Laboratories, West Grove, PA, USA) as the secondary antibody/fluorophore. Following labelling for GnRH, sections were incubated for 48 h in a rabbit anti-VIP antibody (Peninsula, San Carlos, CA, USA) diluted 1 : 20 000 with 0.1% PBT. VIP cells were labelled with Cy-2 donkey antirabbit (Jackson Laboratories) as the secondary antibody/fluorophore. Sections were mounted onto gelatin-coated slides and coverslips were applied. For control experiments, one antibody was eliminated (i.e. VIP or GnRH) and all other steps were followed as previously described.
Light microscopy
GnRH cell counts and VIP contacts on GnRH cell bodies were investigated using a Nikon Eclipse E800 microscope. Sections were examined using the standard wavelengths for Cy-2 (488 nm) and Cy-3 (568 nm). Every fourth section from the medial septum to the caudal aspect of the anterior hypothalamus was evaluated. Specifically, the medial septum/diagonal band (MS/DBB), medial and lateral preoptic areas (POA) and the anterior hypothalamus, were investigated. Cells were counted beginning with the septal population (i.e. evaluating sequential sections and beginning counts when the first cells were seen). The beginning of the POA was defined as the first brain section in which the OVLT was apparent. The transition from the POA to the anterior hypothalamus was defined as the first section in which VIP fibre staining was seen in the SCN. GnRH cells counts were made and putative axosomatic contacts of VIP fibres on GnRH soma were screened at ×200. Contacts were assessed at ×400 and ×1000. A contact was scored only if a VIP bouton-like structure was observed in close proximity to a GnRH cell body (with both the bouton and cell body being in the same plane of focus), and with examination of the fine focal plane revealing the continuity of the VIP fibre. All contacts were digitally captured to further confirm VIP contacts in 8 bit greyscale using a cooled CCD camera (SPOT; Morrel, Meville, NY, USA). Each image was captured as a single image without moving the position of the stage or plane of focus between captures. Images were superimposed digitally using SPOT software (Morrel). All measurements were performed by an experimenter unaware of the experimental group to which the animal belonged.
Confocal microscopy
Brain sections used for light microscopy were also used for the confocal scans in order to confirm that the close contacts were on the same 0.5 μm plane. To this end, GnRH-immunoreactive (GnRH-ir) cells from adult brains (n=8 cells from five animals) and cells from prepubertal females (n=7 cells from five animals) with VIP contacts identified at the light level were evaluated. An additional 24 GnRH cells (seven juvenile and 17 adult) with no VIP contacts identified at the light level were investigated to determine if any of these cells contained VIP contacts not identified at the light level. Cells were observed under a Zeiss Axiovert 100TV fluorescence microscope (Carl Zeiss, Thornwood, NY) with a Zeiss LSM 410 laser scanning confocal attachment. The sections were excited with an Argon-Krypton laser using the standard excitation wavelengths for Cy-2 and Cy-3. Stacked images were collected as 0.5 μm multitract optical sections (with sequential excitation by each laser to avoid ‘cross-talk’ between the two wavelengths). Using the LSM 3.95 software (Zeiss), red and green images of the sections were superimposed. Each cell was examined through its entirety in 0.5 μm steps and axosomatic appositions were assessed.
Statistical analysis
GnRH cell count data and the percentage of GnRH cells contacted by VIP fibres were analysed using 2×3 (age×brain region) analysis of variance (ANOVA). Because percentage data are nonparametric, these statistics were performed on arcsin transformed values. For the purposes of clarity, percent data (rather than transformed values) are depicted and discussed. Age was treated as a between-group factor while brain region was analysed as a within subject variable. Post-hoc comparisons for significant main effects were evaluated using the Tukey-HSD test. P<0.05 was considered statistically significant.
Results
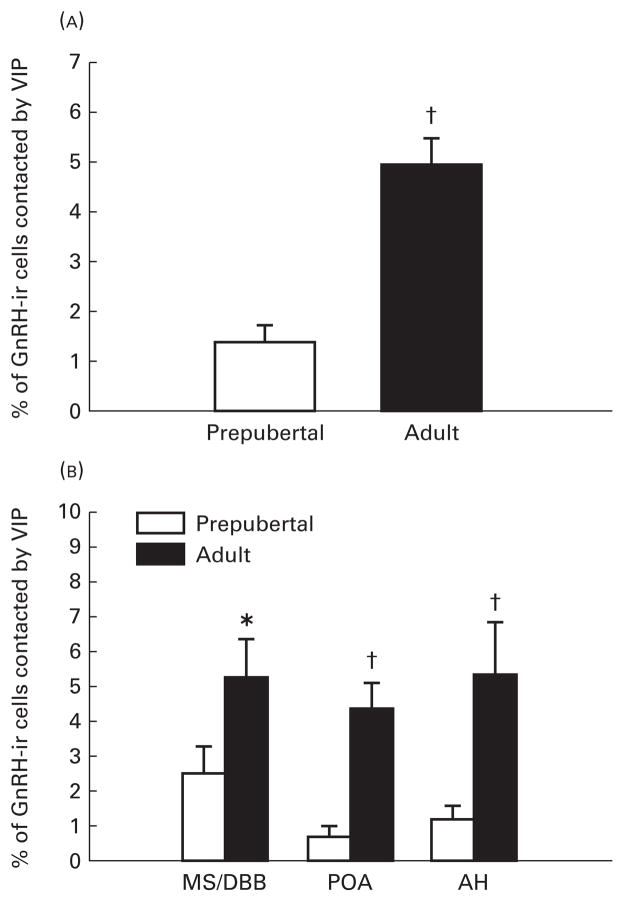
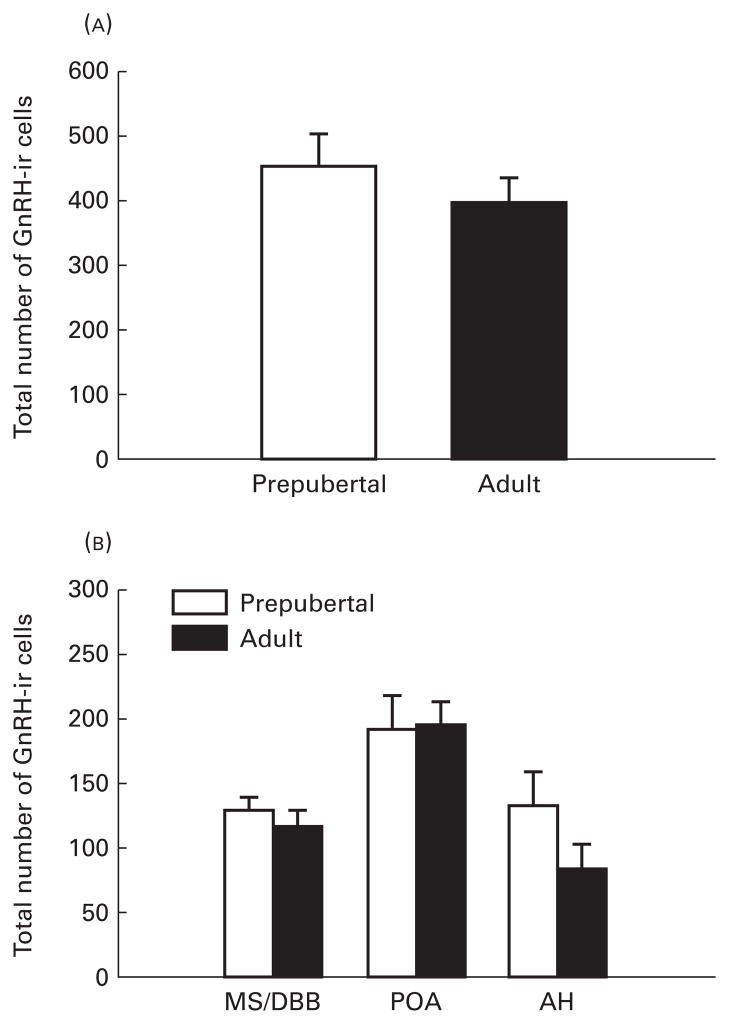
VIP contacts on GnRH neurones were readily identifiable at the light microscopic level. Representative contacts for both adult and prepubertal animals are shown in Fig. 1. Overall, adult animals exhibited a pronounced increase in the percentage (5.01±0.48) of individual GnRH-ir neurones receiving VIP contacts compared to prepubertal female rats (1.41±0.33, P<0.001) (Fig. 2). When the percent of GnRH neurones receiving contacts was analysed by brain region, adult females had a greater percent of VIP contacts on GnRH neurones in all brain areas investigated, including the MS/DBB (5.26±1.07 versus 2.49±0.77, P<0.05; for adult and prepubertal females, respectively), POA (4.37±0.71 versus 0.69±0.27, P<0.01) and anterior hypothalamus (5.33±1.51 versus 1.23±0.34, P<0.01) (Fig. 2). The total number of GnRH-ir neurones, in every fourth section from the septum to the caudal aspect of the median eminence, did not differ between adult (397.37±37.00), and prepubertal (454±49), females (P>0.05) (Fig. 3). When analysed by brain region, the number of GnRH-ir cells did not differ between adult and immature female rats in any brain region investigated (P>0.05 in each case) (Fig. 3).
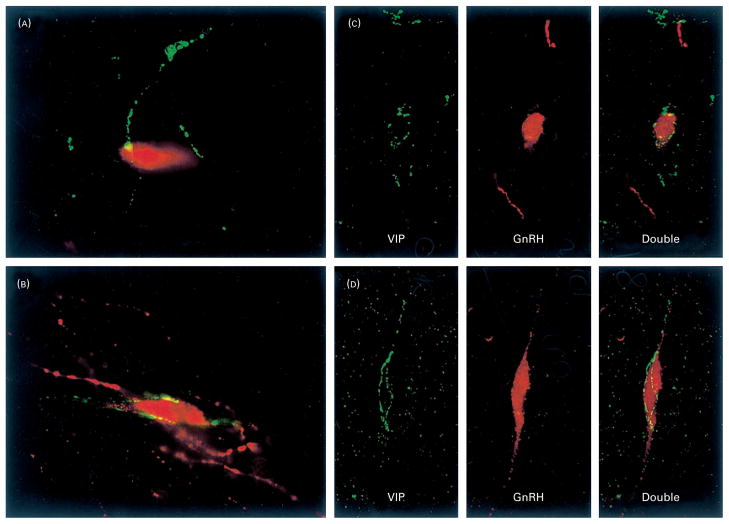
Fig. 1.
High-power light photomicrographs of representative interactions between vasoactive intestinal polypeptide (VIP) and gonadotropin-releasing hormone (GnRH) neurones in both (A) prepubertal and (B) adult female rats (section thickness=30 μm). Confocal microscopy photomicrographs of 0.5 μm optical sections of representative interactions between VIP and GnRH neurones in both (C) prepubertal and (D) adult female rats. Red (GnRH) and Green (VIP) channels are separated in the confocal image (and shown together) to clearly indicate the extent of VIP fibres on the same 0.5 μm plane as the GnRH cell body. Green=VIP; Red=GnRH in all images.
Fig. 2.
Mean±SEM percentage of gonadotropin-releasing hormone (GnRH) neurones contacted by vasoactive intestinal polypeptide (VIP)-immunoreactive (ir) axons in prepubertal female rats and adult females in diestrus. (A) Total percentage of brain GnRH-ir neurones contacted by VIP-ir axons and (B) regional distribution of the percentage of GnRH-ir cells contacted. *P<0.05, †P<0.01 prepubertal.
Fig. 3.
Mean±SEM number of gonadotropin-releasing hormone (GnRH) cells in prepubertal and adult females rats. (A) Total number of brain GnRH-immunoreactive (ir) neurones and (B) regional distribution of the number of GnRH-ir cells.
Results from the confocal analysis confirmed that all cells with close contacts identified at the light level (n=15 cells) were on the same 0.5 μm plane captured optically using confocal laser scanning, thereby supporting the findings at the light microscopic level. Random GnRH cells (n=24 cells) without VIP contacts identified at the light level did not have recognizable contacts when investigated at the confocal level at any 0.5 μm plane throughout the course of the cells, suggesting that contacts were likely not overlooked at the light microscopic level. Representative photomicrographs of the confocal scans in both adult and juvenile animals are depicted in Fig. 1.
Discussion
The results of the present study suggest that the maturation of the reproductive axis following puberty in female rats may be due, at least in part, to modifications in neuronal input to the GnRH system. The present study provides evidence that VIP may play a role in this process. More specifically, adult female rats have a pronounced increase in VIP contacts on GnRH neurones compared to prepubertal females. This increase is not specific to a particular subgroup of GnRH neurones, but a global change in the entire rostro-caudal extent of the continuum in which GnRH perikarya are located (subdivided into the MS/DBB, POA and anterior hypothalamus in the present study). These findings, combined with extensive evidence that the SCN is critical for the generation of reproductive cycles in female rodents (1, 6, 9, 10, 15) and VIP from the SCN communicates extensively with the GnRH system (11, 15, 20), suggest that increased VIP input from neurones, likely originating in the SCN, contribute to the development of a functional reproductive axis in female rats.
Confocal microscopy was used in the current study because it allowed us to scan the same material investigated at the light level, but at a finer level of analysis. It should be noted that laser scanning confocal microscopy is not adequate to identify true VIP synapses on GnRH neurones; this type of analysis requires electron microscopy. However, the confocal analysis allows optical scanning of 0.5 μm of tissue in a given image. As a result, high power confocal scans showing a presumptive bouton contacting a cell body in the same 0.5 μm plane provide strong support for a functional relationship between the fibre and the cell body it is contacting.
The present findings are in agreement with previous studies demonstrating alterations in synaptogenesis during or shortly after puberty, or as a result of hormonally accelerated puberty. For example, oestrogen administration resulting in precocious puberty causes a pronounced increase in synaptic area density in the arcuate nucleus of the hypothalamus (30–32). Similarly, female rats exhibit an increase in spine density around the time of puberty, with a dramatic decline in spine density occurring at 75 days of age (33), suggesting that this change may be necessary for the induction of puberty but not the maintenance of adult reproductive function. The present findings expand upon these previous results by showing the involvement of a specific peptidergic phenotype communicating with the GnRH system.
A smaller percentage of GnRH-ir cells receiving VIP contacts was detected in the present study than previously reported (11, 15). Several methodological differences between this and previous studies may account for this apparent discrepancy. First, the present study used fluorescence immunohistochemistry, while previous studies used diamidobenzide (DAB) combined with nickel-enhanced DAB to label the two antibodies. Fluorescence immunohistochemistry has the advantage of allowing the visualization of one label at a time (or both together), allowing the observer to more easily determine whether or not the GnRH neurone and VIP bouton are in the same focal plane. In addition, previous studies counted both axodendritic as well as axosomatic contacts (11, 15), while the present study evaluated only axosomatic contacts. Because previous studies did not differentiate between the relative contribution of axosomatic versus axodendritic contacts to total VIP contacts on GnRH cells, it cannot be determined if this difference in contact criterion accounts for difference between results of the present study and previous studies of VIP contacts on GnRH cells. Our confocal analysis extends previous light microscopy studies of VIP-GnRH contacts, by lending further support to the fact that the cell and the fibre are in the same plane.
Although a relatively low percentage of GnRH neurones were found to receive VIP contacts in the present study, this finding is consistent with previous studies of the GnRH system (34–36), suggesting that alterations in input to a small number of GnRH cells is biologically meaningful. One study of GnRH synaptic input reported one or fewer synapses per GnRH cell in the rostral hypothalamus and POA of rats (36). In a subsequent study from the same laboratory, axodendritic synapses were quantified, with approximately four synapses per GnRH dendrite found in female rats; approximately three synapses were seen on each GnRH cell body (37). In addition to the GnRH system being regulated by sparse neuronal input, studies of hypogonadal mice (animals unable to produce GnRH), suggest that very few neurones are required to regulate the reproductive axis (38, 39). For example, POA grafts from wild-type mice, placed into the third ventricle of hypogonadal animals integrate with the host brain, extend to the median eminence and restore reproductive function (i.e. maintain gonadal size and gonadal steroid synthesis) (38, 39). Importantly, only a small number of GnRH neurones are required to maintain reproductive function; successful grafts contained as few as three GnRH neurones. Taken together, these findings demonstrate that the GnRH system is modulated by minimal synaptic input and only requires a small number of GnRH neurones to regulate and maintain reproductive function. Thus, the findings from the present study showing a significant increase in VIP contacts upon GnRH neurones suggests meaningful functional implications for developmental changes in the regulation of the reproductive axis. However, these findings do not rule out the possibility that VIP may play a more substantial role in regulating the reproductive axis indirectly by acting on other systems that provide substantial modulation of the GnRH system.
To our knowledge, only one previous study has investigated age differences in VIP contacts on GnRH neurones (20). In this work, no significant differences were seen in the percentage of VIP contacts on GnRH cells in 29-day-old females compared to adult females. However, at 29 days of age, although there is no apparent diurnal rhythm in LH in female rats (24), oestradiol can have positive-feedback effects on the reproductive system (40). The finding that there is positive-feedback to oestradiol in 29-day-old female rats suggests that the timing signal coordinating the sensitivity of the GnRH system to oestradiol positive-feedback is functioning at this age. The present study compared younger (i.e. 21 days old) female rats to adult animals. Thus, the present findings, in combination with previous findings in 29-day-old female rats, suggest that VIP contacts on GnRH cells form primarily between 21 and 29 days of age.
Recent findings suggest that vasopressin, another SCN peptide localized primarily to the dorsomedial SCN, may also be involved in the regulation of the preovulatory LH surge (41, 42). For example, in organotypic cocultures containing both the SCN and the medial preoptic area, GnRH exhibits a daily rhythm that is in phase with the rhythm of vasopressin, but not VIP (42). Similarly, in SCN-lesioned rats, vasopressin administration into the MPOA causes an LH surge, while SCN-lesioned animals not given vasopressin exhibit constant basal levels of LH (41). Although these data suggest an important role for SCN-derived vasopressin in the modulation of the LH surge, these findings do not rule out a role for VIP. As mentioned previously, GnRH neurones receiving innervation from VIP cells in the SCN are preferentially activated (i.e. express Fos) during the LH surge (20). Likewise, application of VIP antisense into the SCN, or peripheral administration of antiserum directed against VIP, leads to a suppression of the LH surge in ovariectomized, oestradiol-treated rats (17). The findings of a role for VIP in regulating the LH surge, combined with data indicating a stimulatory role for vasopressin, suggest that both of these SCN peptides may normally act in concert to precisely coordinate the timing and generation of the LH surge. Together with the results from the present study, these findings suggest that the development of the reproductive axis in female rats may be, at least in part, a result of alterations in VIP (and possibly vasopressin) input to the GnRH system.
Acknowledgments
The authors thank Ruslan Korets and William G. M. Janssen for technical assistance. We also thank Dr Joseph LeSauter for valuable comments and suggestions on an earlier version of this manuscript. Supported by NIH Grants DK-07328 and MH-12408 (L.J.K.), NS-37919 (R.S.), P01AG16765 and P50ES09584 (A.C.G.) and MH-41770 (D.C.).
References
- 1.Turek FW, Swann J, Earnest DJ. Role of the circadian system in reproductive phenomena. Recent Prog Horm Res. 1984;40:143–183. doi: 10.1016/b978-0-12-571140-1.50009-8. [DOI] [PubMed] [Google Scholar]
- 2.Kriegsfeld LJ, LeSauter JL, Hamada T, Pitts SM, Silver R. Circadian rhythms in the endocrine system. In: Pfaff D, Etgen A, editors. Hormones, Brain, and Behavior. New York: Academic Press; 2002. [Google Scholar]
- 3.Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Horm Res. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- 4.Gillette MU, Tischkau SA. Suprachiasmatic nucleus: the brain’s circadian clock. Recent Prog Horm Res. 1999;54:33–58. [PubMed] [Google Scholar]
- 5.Everett JW, Sawyer CH. A 24-hour periodicity in the ‘LH-release apparatus’ of female rat disclosed by barbiturate administration. Endocrinology. 1950;47:198–218. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald K, Zucker I. Circadian organization of the estrous cycle of the golden hamster. Proc Natl Acad Sci USA. 1976;73:2923–2927. doi: 10.1073/pnas.73.8.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- 8.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology. 1975;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- 9.Gray GD, Soderstein P, Tallentire D, Davidson JM. Effects of lesions in various structures of the suprachiasmatic-preoptic region on LH regulation and sexual behavior in female rats. Neuroendocrinology. 1978;25:174–191. doi: 10.1159/000122739. [DOI] [PubMed] [Google Scholar]
- 10.Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977;20:224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- 11.van der Beek EM, Wiegant VM, van der Donk HA, van den Hurk R, Buijs RM. Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptide-containing projection to gonadotrophin-releasing hormone neurons in the female rat. J Neuroendocrinol. 1993;5:137–144. doi: 10.1111/j.1365-2826.1993.tb00373.x. [DOI] [PubMed] [Google Scholar]
- 12.van der Beek EM, Wiegant VM, van Oudheusden HJ, van der Donk HA, van den Hurk R, Buijs RM. Synaptic contacts between gonadotropin-releasing hormone-containing fibers and neurons in the suprachiasmatic nucleus and perichiasmatic area: an anatomical substrate for feedback regulation? Brain Res. 1997;755:101–111. doi: 10.1016/s0006-8993(97)00086-3. [DOI] [PubMed] [Google Scholar]
- 13.Horvath TL, Cela V, van der Beek EM. Gender-specific apposition between vasoactive intestinal peptide-containing axons and gonadotrophin-releasing hormone-producing neurons in the rat. Brain Res. 1998;795:277–281. doi: 10.1016/s0006-8993(98)00208-x. [DOI] [PubMed] [Google Scholar]
- 14.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 15.van der Beek EM, Horvath TL, Wiegant VM, van den Hurk R, Buijs RM. Evidence for a direct neuronal pathway from the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. J Comp Neurol. 1997;384:569–579. doi: 10.1002/(sici)1096-9861(19970811)384:4<569::aid-cne6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Smith MJ, Jennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141:4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- 17.Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- 18.Kimura F, Mitsugi N, Arita J, Akema T, Yoshida K. Effects of preoptic injections of gastrin, cholecystokinin, secretin, vasoactive intestinal peptide and PHI on the secretion of luteinizing hormone and prolactin in ovariectomized estrogen-primed rats. Brain Res. 1987;410:315–322. doi: 10.1016/0006-8993(87)90330-1. [DOI] [PubMed] [Google Scholar]
- 19.van der Beek EM, Swarts HJ, Wiegant VM. Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology. 1999;69:227–237. doi: 10.1159/000054423. [DOI] [PubMed] [Google Scholar]
- 20.van der Beek EM, van Oudheusden HJ, Buijs RM, van der Donk HA, van den Hurk R, Wiegant VM. Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptide-innervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology. 1994;134:2636–2644. doi: 10.1210/endo.134.6.8194489. [DOI] [PubMed] [Google Scholar]
- 21.Gore AC. Diurnal rhythmicity of gonadotropin-releasing hormone gene expression in the rat. Neuroendocrinology. 1998;68:257–263. doi: 10.1159/000054373. [DOI] [PubMed] [Google Scholar]
- 22.Gore AC, Roberts JL, Gibson MJ. Mechanisms for the regulation of gonadotropin-releasing hormone gene expression in the developing mouse. Endocrinology. 1999;140:2280–2287. doi: 10.1210/endo.140.5.6711. [DOI] [PubMed] [Google Scholar]
- 23.Ojeda SR, Aguado LI, Smith S. Neuroendocrine mechanisms controlling the onset of female puberty: the rat as a model. Neuroendocrinology. 1983;37:306–313. doi: 10.1159/000123565. [DOI] [PubMed] [Google Scholar]
- 24.Urbanski HF, Ojeda SR. The juvenile-peripubertal transition period in the female rat: establishment of a diurnal pattern of pulsatile luteinizing hormone secretion. Endocrinology. 1985;117:644–649. doi: 10.1210/endo-117-2-644. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. doi: 10.1210/endo-125-1-92. [DOI] [PubMed] [Google Scholar]
- 26.Sisk CL, Richardson HN, Chappell PE, Levine JE. In vivo gonadotropin-releasing hormone secretion in female rats during peripubertal development and on proestrus. Endocrinology. 2001;142:2929–2936. doi: 10.1210/endo.142.7.8239. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto A, Arai Y. Synaptogenic effect of estrogen on the hypothalamic arcuate nucleus of the adult female rat. Cell Tissue Res. 1979;198:427–433. doi: 10.1007/BF00234187. [DOI] [PubMed] [Google Scholar]
- 28.Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen BS, Woolley CS. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol. 1994;29:431–436. doi: 10.1016/0531-5565(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 30.Arai Y, Matsumoto A. Synapse formation of the hypothalamic arcuate nucleus during post-natal development in the female rat and its modification by neonatal estrogen treatment. Psychoneuroendocrinology. 1978;3:31–45. doi: 10.1016/0306-4530(78)90039-2. [DOI] [PubMed] [Google Scholar]
- 31.Clough RW, Rodriguez-Sierra JF. Synaptic changes in the hypothalamus of the prepuberal female rat administered estrogen. Am J Anat. 1983;167:205–214. doi: 10.1002/aja.1001670206. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto A, Arai Y. Precocious puberty and synaptogenesis in the hypothalamic arcuate nucleus in pregnant mare serum gonadotropin (PMSG) treated immature female rats. Brain Res. 1977;129:375–378. doi: 10.1016/0006-8993(77)90019-1. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CH. Changes in dendritic spine density in the preoptic area of the female rat at puberty. Brain Res Bull. 1982;8:261–265. doi: 10.1016/0361-9230(82)90057-0. [DOI] [PubMed] [Google Scholar]
- 34.Witkin JW, Silverman AJ. Synaptology of luteinizing hormone-releasing hormone neurons in rat preoptic area. Peptides. 1985;6:263–271. doi: 10.1016/0196-9781(85)90050-6. [DOI] [PubMed] [Google Scholar]
- 35.Witkin JW, O’Sullivan H, Silverman AJ. Novel associations among gonadotropin-releasing hormone neurons. Endocrinology. 1995;136:4323–4330. doi: 10.1210/endo.136.10.7664651. [DOI] [PubMed] [Google Scholar]
- 36.Witkin JW. Synaptology of luteinizing hormone-releasing hormone neurons in the preoptic area of the male rat: effects of gonadectomy. Neuroscience. 1989;29:385–390. doi: 10.1016/0306-4522(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 37.Chen WP, Witkin JW, Silverman AJ. Sexual dimorphism in the synaptic input to gonadotropin releasing hormone neurons. Endocrinology. 1990;126:695–702. doi: 10.1210/endo-126-2-695. [DOI] [PubMed] [Google Scholar]
- 38.Silverman AJ, Zimmerman EA, Gibson MJ, Perlow MJ, Charlton HM, Kokoris GJ, Krieger DT. Implantation of normal fetal preoptic area into hypogonadal mutant mice: temporal relationships of the growth of gonadotropin-releasing hormone neurons and the development of the pituitary/testicular axis. Neuroscience. 1985;16:69–84. doi: 10.1016/0306-4522(85)90048-x. [DOI] [PubMed] [Google Scholar]
- 39.Silverman AJ, Kokoris GJ, Gibson MJ. Quantitative analysis of synaptic input to gonadotropin-releasing hormone neurons in normal mice and hpg mice with preoptic area grafts. Brain Res. 1988;443:367–372. doi: 10.1016/0006-8993(88)91635-6. [DOI] [PubMed] [Google Scholar]
- 40.Andrews WW, Mizejewski GJ, Ojeda SR. Development of estradiol-positive feedback on luteinizing hormone release in the female rat: a quantitative study. Endocrinology. 1981;109:1404–1413. doi: 10.1210/endo-109-5-1404. [DOI] [PubMed] [Google Scholar]
- 41.Palm IF, van Der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A. Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience. 1999;93:659–666. doi: 10.1016/s0306-4522(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 42.Funabashi T, Shinohara K, Mitsushima D, Kimura F. Gonadotropin-releasing hormone exhibits circadian rhythm in phase with arginine-vasopressin in co-cultures of the female rat preoptic area and suprachiasmatic nucleus. J Neuroendocrinol. 2000;12:521–528. doi: 10.1046/j.1365-2826.2000.00481.x. [DOI] [PubMed] [Google Scholar]