Abstract
We investigated parvalbumin immunoreactivity (PA-IR) in the retinas of rats maintained on a 12:12 h light:dark cycle, or after being placed in constant darkness for 24–72 h. Retinas were harvested at zeitgeber and circadian times 02:00, 06:00, 10:00, 14:00, 18:00 and 22:00 h. PA-IR was found primarily in retinal amacrine cells of the AII subtype. In a light/dark cycle, PA-IR showed a clear rhythm, with a low near zeitgeber time (ZT) 10:00 h and a peak near ZT 18:00 h. The ratio of immunofluorescence intensities at these timepoints was >15-fold. When animals were kept in complete darkness for 1–3 days, the rhythm of PA-IR was still preserved, but was progressively reduced in amplitude. The rhythm of PA-IR inferred from immunohistochemical data was confirmed by Western blots. We conclude that PA-IR in the rat retina shows an underlying circadian rhythm that is enhanced by cyclic light. The regulation may involve translocation of the protein between cell compartments and/or new protein synthesis.
Keywords: Calcium-binding proteins, Inner plexiform layer, Western blots, Immunocytochemistry, Rat
Introduction
The daily switch from rod-mediated to cone-mediated vision is accompanied by a multifaceted alteration of retinal circuits. In mammalian retinas, the second-order bipolar neurons in most cases are connected exclusively either to rods or to cones, although some rodent retinal bipolar cells may receive mixed rod/cone input (Hack et al. 1999). In contrast, amacrine and ganglion cells, the third order neurons which receive synaptic inputs from bipolar cells, typically subserve both rod and cone circuits, and undergo corresponding changes in responsiveness as a function of adaptational state. Two such examples are the weakening or loss of ganglion cell surrounds during dark adaptation (Barlow et al. 1957) and the complex change in the strength of electrical coupling between AII amacrine cells as a function of the ambient level of illumination (Bloomfield et al. 1997).
We are interested in the neuromodulatory pathways contributing to daily alterations of retinal circuitry that accompany light and dark adaptation. In a previous study we showed that in the rat retina, protein kinase Cαβ (PKCαβ), calcium-dependent isoforms of PKC, had a diurnal rhythm of expression in rod bipolar cells (GQbriel et al. 2001). An unexpected finding of the study was that, after placing the rat in constant darkness, PKCαβ immunoreactivity appeared in AII amacrine neurons and increased progressively over 72 h. Under dark-adapted conditions, the AII subclass of amacrine cell receives synaptic input from the rod bipolar cell and is an integral component of the rod pathway, whereas at higher ambient light levels it is driven by cone-connected bipolar cells (reviewed in Bloomfield and Dacheux 2001). Thus this cell class provides a good substrate in which to examine possible diurnal and circadian rhythms of calcium binding proteins.
A diurnal rhythm is responsive to environmental clues, whereas a circadian rhythm is controlled by an endogenous oscillator. Previous studies of the mammalian retinas show that they contain a number of calcium buffering proteins, whose distribution varies in a species-specific and cell class specific manner. For example, AII amacrine cells of the cat retina express calretinin (Goebel and Pourcho 1997) whereas the same AII amacrine cells in the rat retina contain parvalbumin (Wässle et al. 1993). Accordingly, in the present study we looked for a diurnal and/or circadian variation of PA-IR in the AII amacrine cells as well as in the total retinal content of parvalbumin. The results from immunocytochemistry and Western blots show both a diurnal and circadian rhythm of expression, with the highest levels found during subjective night.
Materials and methods
Animals
Male hooded rats were housed in 12:12 h light:dark cycles (LD) or, after being kept on this schedule for 3 weeks, were placed in constant darkness (DD) for 24, 48 and 72 h. Room temperature was about 233C, and a white noise generator masked environmental noise. Animals released into DD were housed in cages provided with a 17-cm-diameter running wheel to monitor locomotor activity. All procedures followed the animal care guidelines of ARVO and NIH and were approved by the local animal care committees.
Tissue preparation
For each timepoint examined, at least two animals (four eyes) were used. Animals were deeply anesthetized by Nembutal (200 mg/kg) then intracardially perfused with 200 ml 0.9% saline, followed by 400 ml 4% paraformaldehyde in phosphate buffer (PB, pH 7.2) at zeitgeber (ZT) and circadian (CT) times 02:00, 06:00, 10:00, 14:00, 18:00 and 22:00 h. For tissue collection during dark periods the heads of the animals were covered with a light-proof plastic hood during the anesthetic and perfusion procedures. Following perfusion, the anterior part of the eyeball was cut off, the lens removed and the posterior eyecups postfixed for 1 h in the buffered paraformaldehyde solution. After several washes in buffer and overnight cryoprotection in 20% sucrose dissolved in 0.2 M PB, 14-to 16-μm-thick frozen vertical sections were cut in a cryostat, mounted and air dried on gelatin-coated slides and processed for immunohistochemistry.
Immunohistochemistry and confocal microscopy
After blocking the non-specific binding (with a mixture of 0.25% bovine serum albumin, 0.1% Na azide, 0.3% Triton X-100 and 3% normal goat serum, all dissolved in phosphate-buffered saline, pH 7.2), we used a monoclonal mouse anti-parvalbumin antibody (Sigma). The antibodies were diluted at 1:500 and applied to the sections overnight. After several washes in phosphate-buffered saline, the secondary donkey anti-mouse antibodies conjugated to fluorescein isothiocyanate (Jackson, diluted at 1:100) were applied for 2 h at room temperature. The sections were then washed and coverslipped in Vectashield (Vector). For each experiment, a complete series of timepoints was processed with the same solutions of primary and secondary antibodies to reduce variability. As control, we omitted the primary antibody or replaced it with non-immune sera; in both cases no staining was observed.
Sections from central retina were examined in a Zeiss Axiovert 100 TV fluorescence microscope with a Zeiss LSM 410 laser scanning confocal attachment. Eight digital images of the test area were taken at the maximal resolution (1,02401,024 pixels) and then averaged. An observer blind to the experimental conditions processed the files with NIH Image 1.61 software to provide a quantitative estimate of immunoreactivity. The image was changed from the gray scale without any adjustment of brightness or contrast to pseudocolor. Then the freehand selection tool was used to delineate an area containing the PA-immunoreactive amacrine cell bodies and their processes in the inner plexiform layer. The width of the test area included at least 30 immunoreactive neurons. Over the selected area the relative optical density was measured. Next the background signal was subtracted (background tissue signal was measured over the photoreceptor nuclei and inner segments in the same section). Four measurements, each from a separate retina, were averaged for each timepoint sampled. Data are presented as means SEM.
Western blots
For each timepoint tested, two rats were anesthetized with a 20–30 s exposure to 100% CO2 and decapitated, the eyes enucleated and hemisected and the retinas removed with a fine brush. Retinas were immediately frozen on dry ice and stored at ;803C and used within 1 week. Frozen retinas were homogenized with sonication in a solution containing 100 mM TRIS-HCl buffer (pH 6.8), 4% sodium dodecyl sulfate and 20% glycerol. Protein concentrations were determined (Pierce and Suelter 1977). Western blots were prepared in 8–20% gradient polyacrylamide gels and polyvinyldifluoride membranes as described by Ausubel et al. (1987). Filters were blocked for 1 h in 2% non-fat milk in TRIS-buffered saline, containing 0.1% Tween 20, and incubated overnight at 43C with the antibody (mouse anti-parvalbumin 1:1,000, Sigma). Blots were developed with horseradish-peroxidase-conjugated secondary antibody and chemiluminescence substrate (Amersham). In every case a single immmunoreactive band was detected with an Mr of approximately 12 kDa. In control experiments, in which the secondary antibodies were applied alone, no bands were detected.
Results
In the rat retina, PA was present in two subsets of amacrine cells (Wässle et al. 1993), one of which was identified as the AII glycinergic amacrine cell. The great majority of PA-IR neurons in our study were AII amacrine cells based on the following criteria. PA-IR neurons had medium-sized round cell bodies situated at the border of the inner nuclear layer and the inner plexiform layer (IPL). PA-IR perikarya emitted a stout, vertically oriented dendrite directed towards the IPL, whose processes show a bistratified distribution in sublamina 2 and sublamina 4/5 of the IPL. These morphological features correspond to the description of AII amacrine cells in mammalian retinas (Famiglietti and Kolb 1975; Wässle et al. 1993).
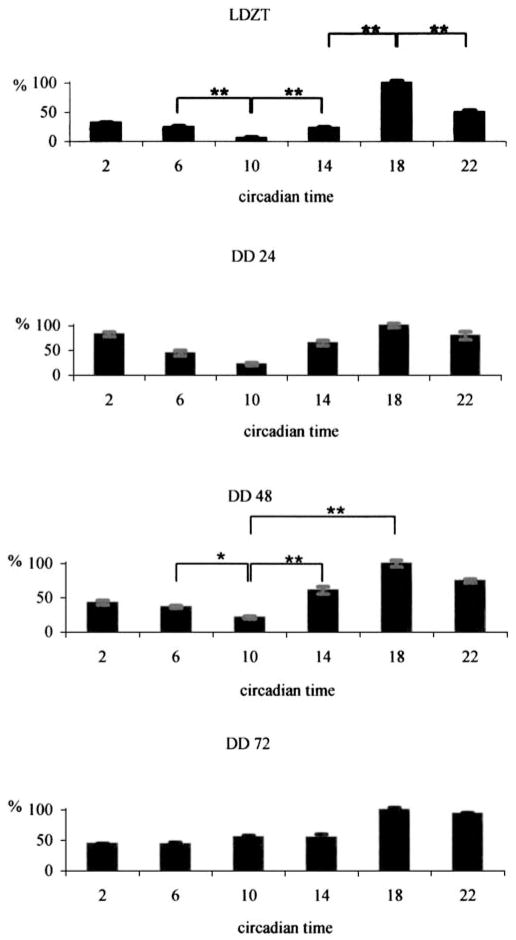
We noted systematic differences in the intensity of PAIR as a function of ZT time. In rats kept on a 12:12 h LD cycle, PA staining was weakest in both the processes and cell bodies at ZT 10:00 (Fig. 1). Labeling intensity reached its highest level at ZT 18:00 and showed intermediate values at the other examined timepoints. Changes in the intensity of PA-IR were more prominent in processes than in cell bodies (Fig. 1).
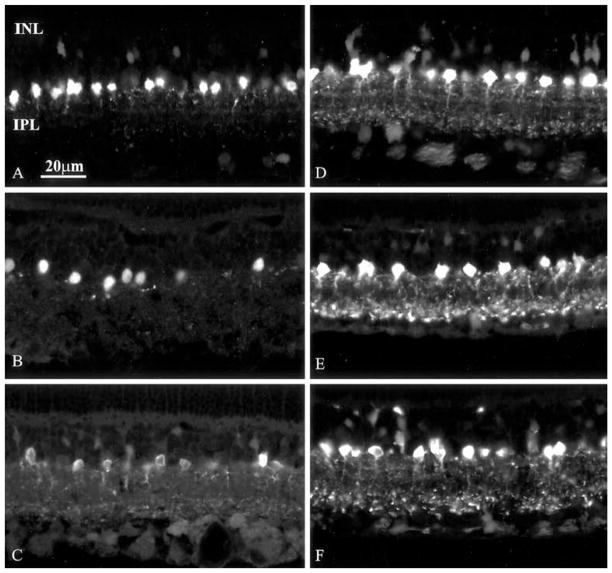
Fig. 1.
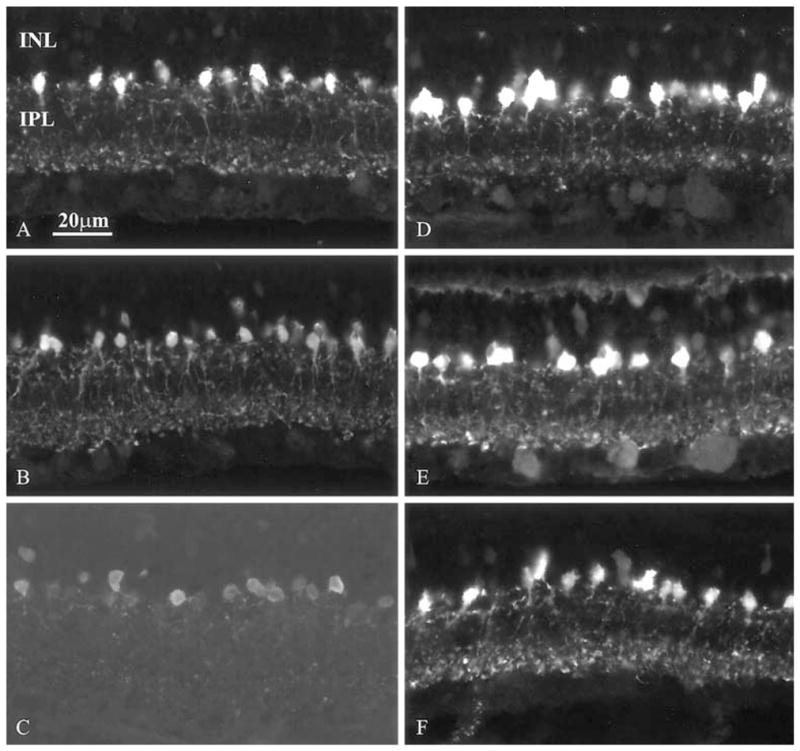
A–F Changes in PA immunoreactivity in the retina of rats kept under 12:12 h light/dark cycles. A–C Light phase, ZT 2, 6 and 10 h, respectively; D–F dark phase, ZT 14, 18 and 22 h, respectively (INL inner nuclear layer, IPL inner plexiform layer)
A quantitative estimate of the relative intensity of immunofluorescence was carried out using the NIH Image program 1.61 (see “Materials and methods”). The results were that the ratio of PA-IR of processes in the IPL between ZT 10:00 h and ZT 18:00 h differed more than 15-fold (Fig. 2, uppermost graph).
Fig. 2.
Estimation of relative fluorescence intensity in the IPL. All values are given as percentages of the maximum value within 1 day (LD animals kept under 12:12 h light/dark cycles, DD animals released into total darkness for 24, 48 and 72 h)
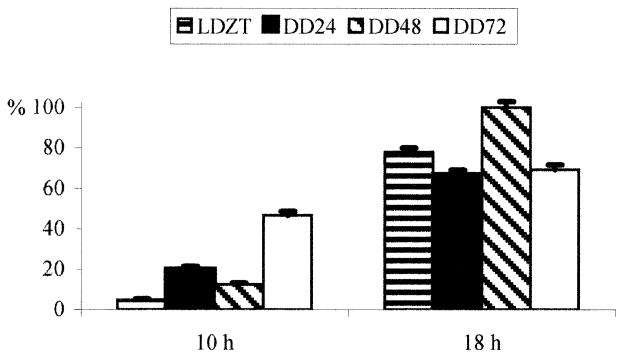
When animals were maintained in DD, the cyclic nature of changes in PA immunoreactivity was still evident (Fig. 2, lower three graphs, Fig. 3); however, the amplitude of the changes after 72 h DD diminished to threefold (Fig. 4), primarily as a consequence of an increase in PA-IR at CT 10:00.
Fig. 3.
A–F Representative sections from retinas of rats released into DD. A–C Animals kept in darkness for 24, 48 and 72 h at the lowest (circadian time 10 h) point of PA immunoreactivity; D–F animals kept in darkness for 24, 48 and 72 h at the highest (circadian time 18 h) point of PA immunoreactivity. The cyclic nature of immunofluorescence intensity is evident
Fig. 4.
Comparison of values of relative intensity of immunofluorescence in LD and DD 24, 48 and 72 h at circadian times 10 h and 18 h. There is an overall increase in intensity of PA labeling and a decrease in difference between the lowest and highest values
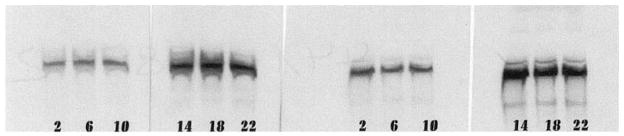
The conclusion that circadian and diurnal rhythms exist in PA-IR was supported by data obtained from Western blots. In both LD and DD, a clear daily variation in staining intensity of blots was noted (Fig. 5). These same data also show an increase in total retinal PA with time spent in DD, which is in line with the results obtained from immunocytochemistry (Figs. 3, 4). Measurements made from computer-scanned Western blots show that the estimated staining intensity difference within LD timepoints (highest/lowest=5.3) was slightly greater than within 48-h DD timepoints (highest/lowest = 5.0).
Fig. 5.
Western blots of PA immunoreactivity in the rat retina (left six lanes LD timepoints, right six lanes DD48 timepoints)
Discussion
Our main finding was that the intensity of PA-IR within AII amacrine neurons varied cyclically whether the rat was kept in LD or in DD. These rhythmic changes were more prominent in AII amacrine cell processes than in their perikarya, suggesting a transport of PA from its site of synthesis in the cell body to the processes. The finding that peak to trough differences in PA-IR or in total retinal PA were most prominent in LD, although still maintained in DD after 72 h, indicates that there is a circadian component to PA expression, but that it also has a dependence on light-driven retinal activity. The parvalbumin data differ from those relating to rhythmic expression of PKCαβ (GQbriel et al. 2001) in that although PKCαβ immunoreactivity in AII amacrine cell increases progressively with time in constant darkness, the phase of the rhythm of expression found in LD is completely lost in DD.
Circadian rhythms in the retina
Recent evidence shows that biological clock components are present in retinal cells (Anderson and Green 2000) and a retinal circadian cycle of melatonin synthesis is maintained in cultured eyes freed from their connections to the brain (Cahill and Besharse 1995; Tosini and Menaker 1996). These studies prove that the retina has the molecular machinery to regulate circadian rhythms, using the same molecular components identified in cells of the suprachiasmatic nucleus (Zylka et al. 1998; Liu et al. 1997). Melatonin and dopamine are in a reciprocal relationship such that melatonin is a signal for dark adaptation and dopamine a chemical messenger for light adaptation (Cahill and Besharse 1995). Several important retinal activities are regulated by these two molecules, including retinomotor movements (Levinson and Burnside 1981), photoreceptor outer segment disk shedding (La Vail 1976), visual pigment synthesis (Von Schantz et al. 1999), and retinal sensitivity as measured by the electroretinographic b-wave (Manglapus et al. 1998). The pathways and proteins which mediate biological clock-dependent rhythms in the retina are little understood; only a very small number of retinal proteins, however, have been shown to manifest a circadian or diurnal rhythm of expression (Green and Besharse 1996; Anderson and Green 2000; GQbriel et al. 2001).
Calcium regulation in retinal neurons
Cytoplasmic free calcium is a highly regulated, diffusible messenger in retinal neurons. Calcium influx occurs through voltage- and ligand-gated channels and may be released from intracellular stores. Maintenance of a low (submicromolar) basal concentration of free calcium depends on a variety of calcium buffers, of which some, like parvalbumin, function solely as passive calcium stores, whereas others, e.g., calmodulin, are active participants in neuromodulatory pathways (reviewed in Akopian and Witkovsky 2002). The findings that AII amacrines in different mammalian retinas have different calcium buffer proteins (parvalbumin, calretinin) and yet have a similar physiology (reviewed in Bloomfield and Dacheux 2001) suggest that parvalbumin and calretinin play similar passive roles in controlling intracellular calcium. Wässle et al. (1998) studied a mutant mouse in which calbindin gene was deleted. The horizontal cells which normally contain calbindin lacked it in mutant mice but did not substitute another calcium binding protein, nor was the horizontal cell morphology altered. On the other hand, Airaksinen et al. (1999) report that mice lacking calbindin D28 showed severe motor deficits, suggesting cerebellar dysfunction, a conclusion bolstered by the finding of abnormal synaptically induced calcium transients in cerebellar Purkinje cells.
One calcium-sensitive process is cell to cell coupling (deVries and Schwartz 1989). Bloomfield et al. (1997) showed that the degree of AII-AII coupling was low in absolute darkness, increased in moderate light but was decreased by bright light. (In the present study it was found that parvalbumin levels fall during early morning hours, possibly favoring an increase in free Ca2+ level.) One consequence would be an increased activation of nitric oxide synthase which activates soluble guanylate cyclase, leading to a decrease in coupling (Mills and Massey 1995). However, we made no test of the possible effect of different light levels on PA-IR, and other calcium buffer systems present in the AII amacrine cells, e.g., calmodulin, also regulate calcium-dependent processes.
As the circadian clock prepares the retinal signal transduction system for night, the sensitivity of the rod pathway is increased to make weak light signals perceptible. An increase in Ca2+-buffering capacity in AII cells, peaking near ZT 18:00 h, may participate in this process. Calcium-calmodulin dependent kinase, which upregulates cone pathway signals (Ko et al. 2001), might be expected to have decreased activity at this time in the rod pathway. In conclusion, circadian and diurnal regulation of parvalbumin in the retina appears to be part of a multifaceted program underlying dark and light adaptation, through which the neuromodulatory systems up- or downregulate rod and cone pathways to conform to the level of ambient illumination.
Acknowledgments
This study was supported by an OTKA grant (T 34160), NIH grants NS 37919 (R.S.) and ET 03570, NSF grant IBN-96418886 (R.S.), and grants from the Helen Hoffritz Charitable Trust and Research to Prevent Blindness, Inc. R.G. was also in receipt of a János Bolyai fellowship
Contributor Information
Robert Gábriel, Email: gabriel@ttk.pte.hu, Department of General Zoology and Neurobiology, University of Pécs, 7601 Pécs, u. 6. Ifjúság, Hungary, Tel.: +36-72-503600 ext. 4116, Fax: +36-72-501517. MTA-PTE Adaptational Biology Research Group, University of Pécs, 7601 Pécs, u. 6. Ifjúság, Hungary.
Joseph Lesauter, Department of Psychology, Barnard College, New York, NY 10027, USA.
Tamás Bánvölgyi, Department of General Zoology and Neurobiology, University of Pécs, 7601 Pécs, u. 6. Ifjúság, Hungary, Tel.: +36-72-503600 ext. 4116, Fax: +36-72-501517.
György Petrovics, Uniformed Services, University of Health Sciences, Department of Surgery, Center for Prostate Diseases Research, Bethesda, MD 20814, USA.
Rae Silver, Department of Psychology, Barnard College, New York, NY 10027, USA. Department of Psychology, Columbia University, New York, NY 10027, USA.
Paul Witkovsky, Department of Ophthalmology, New York University School of Medicine, 550 First Ave., New York, NY 10016, USA. Department of Physiology and Neuroscience, New York University School of Medicine, 550 First Ave., New York, NY 10016, USA.
References
- Airaksinen MS, Eilers J, Garaschuk O, Thoenen H, Konnerth A, Meyer M. Ataxia and altered dendritic calcium signaling in mice carrying a targeted null mutation of the calbindin D28 gene. Proc Natl Acad Sci U S A. 1997;94:1488–1493. doi: 10.1073/pnas.94.4.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;25:113–132. doi: 10.1385/MN:25:2:113. [DOI] [PubMed] [Google Scholar]
- Anderson FE, Green CB. Symphony of rhythms in the Xenopus laevis retina. Microsc Res Techn. 2000;50:360–372. doi: 10.1002/1097-0029(20000901)50:5<360::AID-JEMT5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl H. Current protocols in molecular biology. Green Publ Assoc and Wiley Interscience; New York: 1987. [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Dacheux RF. Rod vision: pathways and processing in the mammalian retina. Prog Retin Res. 2001;20:351–384. doi: 10.1016/s1350-9462(00)00031-8. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Xin D, Osborne T. Light-induced modulation between AII amacrine cells in the rabbit retina. Vis Neurosci. 1997;14:565–576. doi: 10.1017/s0952523800012220. [DOI] [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Prog Ret Eye Res. 1995;14:267–291. [Google Scholar]
- deVries SH, Schwartz EA. Modulation of an electrical synapse between solitary pairs of catfish horizontal cells by dopamine and second messengers. J Physiol. 1989;414:351–375. doi: 10.1113/jphysiol.1989.sp017692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Gabriel R, LeSauter J, Garcia-Espana T, Silver R, Witkovsky P. Diurnal and circadian rhythms of protein kinase C expression in rat retina. J Comp Neurol. 2001;439:140–150. doi: 10.1002/cne.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel DJ, Pourcho RG. Calretinin in the cat retina: co-localization with other calcium-binding proteins. Vis Neurosci. 1997;14:311–322. doi: 10.1017/s0952523800011445. [DOI] [PubMed] [Google Scholar]
- Green CB, Besharse J. Use of a high stringency differential display screen for identification of retinal mRNAs that are regulated by a circadian clock. Mol Brain Res. 1996;37:157–165. doi: 10.1016/0169-328x(95)00307-e. [DOI] [PubMed] [Google Scholar]
- Hack I, Peichl L, Brandstätter JH. An alternative pathway for rod signals in the rodent retina: rod photoreceptors, cone bipolar cells and the localization of glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:14130–14135. doi: 10.1073/pnas.96.24.14130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channel of chick retinal cones. Erk MAP kinase and Ca2+/camodulin dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- La Vail MM. Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1076. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Levinson G, Burnside B. Circadian rhythms in teleost retinomotor movements: a comparison of the effect of circadian rhythm and light condition on cone length. Invest Ophthalmol Vis Sci. 1981;20:431–438. [PubMed] [Google Scholar]
- Liu C, Weaver DR, Strogatz SH, Reppert SM. Cellular construction of a circadian clock: period determination in the suprachiasmatic nucleus. Cell. 1997;91:855–860. doi: 10.1016/s0092-8674(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills SL, Massey SC. Differential properties of two gap junctional pathways made by AII amacrine cells. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- Pierce J, Suelter CH. An evaluation of the Coomassie brilliant blue G-250 dye-binding method for quantitative protein determination. Anal Biochem. 1977;81:478–480. doi: 10.1016/0003-2697(77)90723-0. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythm in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Von Schantz M, Lucas RJ, Foster RG. Circadian oscillation of photopigment transcript levels in the mouse retina. Mol Brain Res. 1999;72:108–114. doi: 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J. Immunocytochemical staining of AII amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;322:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Airaksinen MS, Meyer M. Calcium-binding proteins in the retina of a calbindin-null mutant mouse. Cell Tissue Res. 1998;292:211–218. doi: 10.1007/s004410051052. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside the brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]