Abstract
We studied the dependence of the expression of protein kinase C immunoreactivity (PKC-IR) in the rat retina on the light:dark (LD) cycle and on circadian rhythmicity in complete darkness (DD). Two anti-PKC alpha antibodies were employed: One, which we call PKCαβ recognized the hinge region; the other, here termed PKCα, recognized the regulatory region of the molecule. Western blots showed that both anti-PKC antibodies stained an identical single band at approximately 80 kD. The retinal neurons showing PKC-IR were rod bipolar cells and a variety of amacrine neurons. After 3 weeks on an LD cycle, PKCαβ-IR in both rod bipolar and certain amacrine cells manifested a clear rhythm with a peak at zeitgeber time (ZT) of 06–10 hours and a minimum at ZT 18. No rhythm in total PKC-IR was observed when using the PKCα antibody, but, at ZT 06–10 hours, rod bipolar axon terminals showed increased immunostaining. After 48 hours in DD, with either antibody, rod bipolar cells showed increased PKC-IR. The PKCα antibody alone revealed that, after 48 hours, AII amacrine neurons, which lacked PKC-IR in an LD cycle, manifested marked PKC-IR, which became stronger after 72 hours. Light administered early in the dark period greatly increased PKCαβ-IR in rod bipolar and some amacrine neurons. Our data indicate that light and darkness exert a strong regulatory influence on PKC synthesis, activation, and transport in retinal neurons.
Indexing terms: rod bipolar cell, amacrine cell, immunocytochemistry, kinase
The daily light:dark cycle (LD) is a fundamental regulator of retinal activity. Most retinas have duplex function, meaning that rod photoreceptors and their associated circuitry are specialized for nocturnal vision, whereas cone photoreceptors and their circuits govern retinal function in bright light. The change from rod to cone vision is a complex process involving diurnal and circadian rhythms. Vertebrate retinas contain a circadian clock (Cahill et al., 1991; Tosini and Menaker, 1996). Among other functions, it governs melatonin synthesis, which in turn helps to regulate the production and release of dopamine (for review see Cahill and Besharse, 1995). The rhythms of melatonin and dopamine are in counterphase (Adachi et al., 1998), and these two intrinsic retinal neurochemicals are messengers for darkness and light, respectively (Cahill et al., 1991). The production of certain retinal proteins, for example, iodopsin (Pierce et al., 1993) and tryptophan hydroxylase (Green et al., 1995), also appears to be regulated by a circadian rhythm. Perhaps only a few critical proteins are directly under the control of the circadian clock; Green and Besharse (1996) found that only four of 2,000 retinal mRNAs examined showed a circadian rhythm of expression. Several important retinal activities also manifest a circadian rhythm, including photoreceptor outer segment disk shedding (La Vail, 1976), retinomotor movements (Levinson and Burnside, 1981), visual pigment synthesis (Von Schantz et al., 1999), relative expression of rod and cone signals in the electroretinogram (Manglapus et al., 1998), and circadian rhythms of visual detection (Bassi and Powers, 1986, 1987). Other functions may be governed by a nycthemeral rhythm of light and dark, e.g., ocular length (Nickla et al., 1998).
These diverse findings imply that retinal circuits are subject to modulation through biochemical changes dependent on the LD cycle and in some cases on an endogenous clock with a free-running circadian rhythm. In this paper, we describe a diurnal rhythm and circadian alterations in the retinal expression of protein kinase C (PKC) as assessed by western blots and immunocytochemistry. PKC expression was probed using two monoclonal antibodies against either PKCα or PKC α, β1 and β2 isoforms of PKC found in the mammalian brain.
Activation of PKC involves a conformational change and a translocation to the membrane (Huang, 1989). The PKCα antibody recognizing the regulatory arm of PKC did not show a diurnal variation in total PKC immunoreactivity (PKC-IR), whereas the PKCαβ antibody whose epitope was at the hinge region of PKC revealed a prominent diurnal rhythm of PKC expression. The PKCαβ antibody we employ apparently is sensitive to the functional state of PKC, a feature we exploited to probe the dependence of PKC activation as a function of the LD cycle or following 1–3 days in complete darkness. Our findings imply that PKC synthesis, activation, and transport are increased by light and thus contribute to a growing understanding of the importance of the light:dark cycle and circadian rhythms in modulating the function of the retina.
METHODS AND MATERIALS
Animals
Male Long-Evans rats, obtained from Charles River Laboratories, were housed in transparent polyethylene cages (48 × 27 × 20 cm) and provided ad libitum access to food and water. They were kept in a 12:12 hour LD cycle. Room temperature was about 23°C, and a white noise generator masked environmental noise.
Experimental design
After 3 weeks on LD 12:12, animals were placed in one of the following photic conditions. One group was held in an LD cycle and sacrificed at zeitgeber time (ZT) 02, 06, 10, 14, 18, and 22 hours. A second group was housed in constant darkness (DD), in cages provided with a running wheel (17 cm diameter) to monitor locomotor activity. These animals were kept in DD for 1, 2, or 3 days prior to death at circadian time (CT) 02, 06, 10, 14, 18, and 22. A third group of animals was housed in LD 12:12 and received a flashing light pulse 1/second for 1 hour from ZT 14 to ZT 15 to test whether the level of PKC-IR seen during the dark phase could be up-regulated by light. The light pulse was provided by a Grass photostimulator at intensity setting 1 and situated about 25 cm from the cage. Two animals were killed 30 minutes after the light was turned off (ZT 15:30). Two additional rats raised in the same cycle but not exposed to the flashing light were sacrificed at ZT 15:30 and served as controls. For each class of experiment, two animals (four eyes) were used for each time point tested.
Surgery
All experimental procedures were reviewed and approved by the Institutional Animal Care Committee of Columbia University. Rats were deeply anesthetized by an intraperitoneal injection of Nembutal (200 mg/kg), then perfused intracardially with 200 ml of 0.9% saline, followed by 400 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2. During the anesthetic and perfusion procedures, the animal’s head was covered with a light-proof hood.
Following perfusion, the eyes were enucleated, the corneas and lenses were removed, and the posterior pole of the eye was postfixed for 1 hour in the buffered paraformaldehyde solution. After several washes in buffer and overnight cryoprotection in 20% sucrose/0.1 M phosphate buffer, pH 7.2, frozen vertical sections 14–16 µm thick were cut in a cryostat and processed for immunocytochemistry on the slide.
Immunocytochemistry
We utilized a mouse monoclonal antibody (Mc-39) against PKCα (Seikagaku America, Falmouth, MA) reported to react with the regulatory domain (Hagiwara et al., 1990) and a mouse monoclonal (OP74; Oncogene Research Products, Cambridge, MA) against PKCα/β1/β2 isoforms whose epitope is reported to be the hinge region (Young et al., 1988). Hereafter we refer to these antibodies as PKCα and PKCαβ, respectively. Secondary antibodies conjugated to rhodamine or fluorescein isothiocyanate were obtained from Jackson Immunoresearch (West Grove, PA).
Sections were first incubated for 1 hour in a blocking solution consisting of 0.25% bovine serum albumin, 0.1% Na azide, 0.3% Triton-X 100, and 3% normal goat serum, all dissolved in PBS, pH 7.2. The primary antibodies were diluted 1:400 (Seikagaku) or 1:200 (Oncogene Research Products) in PBS and left on the slides for 16 hours, followed by six 10 minute washes in PBS. Secondaries were diluted 1:100 in PBS, and the slides were incubated for 2 hours, followed by six 10 minute washes in PBS, then mounted in Vectashield (Vector, Burlingame, CA). For each experiment, a complete series of time points was processed with the same solutions of primary and secondary antibodies to reduce variability. Control experiments were the following: Omitting either of the primary antibodies resulted in no immunostaining. Pretreatment with a blocking peptide provided by Oncogene Research Products prevented immunostaining with their primary antibody (not illustrated).
Western blots
For each time point tested, two rats were anesthetized with a 20–30 second exposure to 100% CO2 and decapitated, eyes enucleated and hemisected, and retinas removed with a fine brush. Retinas (4/test point) were immediately frozen on dry ice, stored at −80°C, and used within 1 week. Frozen retinas were sonicated in 0.4 ml solution containing 25 mM Tris HCl, pH 8.0, 2% sodium dodecyl sulfate, and 2 mM EDTA. Protein concentration was determined using the bicinchoninic acid method (Smith et al., 1985). Western blots were prepared in 7.5% polyacrylamide gels and polyvinyldifluoridine membranes as described by Ausubel et al. (1987). Filters were blocked 1 hour in 2% nonfat milk in Tris-buffered saline (100 mM Tris HCl, pH 7.5, in 150mMNaCl) containing 0.1% Tween 20, and incubated overnight at 4°C with the antibodies (PKCα, 1:1,000; PKCαβ, 1:500). Blots were developed with horseradish peroxidase-conjugated secondary antibody (Amersham, Piscataway, NJ) and chemiluminescent substrate (Super Signal West Femto Maximum Sensitivity Substrate; Pierce, Rockford, IL). We performed control experiments in which the primary antibody was omitted and the secondaries were applied alone at the same concentration. With regard to the PKCα antibody, no bands were revealed. For the PKCαβ antibody, the secondaries revealed staining bands, but not at 80 kD. By matching experimental and control runs, it was determined that only the 80 kD band was specific to PKCαβ (not illustrated), so both anti-PKC antibodies reacted only with a protein band at 80 kD.
Microscopy and data processing
Sections from central retina were examined in a Zeiss Axiovert 100TV fluorescence microscope (Carl Zeiss, Thornwood, NY) with a Zeiss LSM 410 laser scanning confocal attachment. Eight digital images of the test area were taken at the maximal resolution (1,024 × 1,024 pixels) and then averaged. They were processed in Adobe Photoshop 5.0 as follows: For the set of images corresponding to a given experiment (e.g., diurnal variation in PKC immunoreactivity, tested with one anti-PKC antibody), the most immunoreactive section had its brightness and contrast set to be visually bright but without noticeable image bloom. The same brightness and contrast settings were then applied to all other images in the set. An observer blind to the experimental condition of the animals utilized NIH Image, version 1.61, to provide a quantitative estimate of immunoreactivity. The image was changed from gray scale, without any adjustment of brightness or contrast, to pseudocolor, using the [options/color tables/system] menu. The [freehand selection] tool was used to delineate an area containing the rod bipolar cells, extending from the outer plexiform layer (OPL) to the rod bipolar cell axon terminals in the proximal portion of the inner plexiform layer (IPL). The width of the area included at least 50 immunoreactive neurons. With the [Analyze/Options] command, the delineated area was selected, and the [Analyze/Measure] tool gave the area measured and its relative optical density (ROD). Next, background ROD was measured in the layer of photoreceptor nuclei and inner segments, a retinal region not showing PKC-IR. The background measurement was subtracted from the ROD of the rod bipolar cells to provide a relative value of PKC-IR. Four measurements, each from a separate retina were averaged for each time point sampled. Data are presented as means ± SEM.
RESULTS
Neurons showing PKC immunoreactivity in the rat retina
The presence and location of α and β PKC isoenzymes in mammalian retinas have been reported by several groups (Negishi et al., 1988; Greferath et al., 1990; Osborne et al., 1991, 1992; Kosaka et al., 1998). These studies demonstrated that, in the rat retina, rod bipolar cells and a heterogeneous population of amacrine cells show PKC-IR. The rod bipolar cell is characterized by a tight cluster of dendrites in the OPL, an oval cell body located in the distal portion of the inner nuclear layer (INL), and a vertically directed axon, which expands into clusters of terminals in the proximal portion of the IPL.
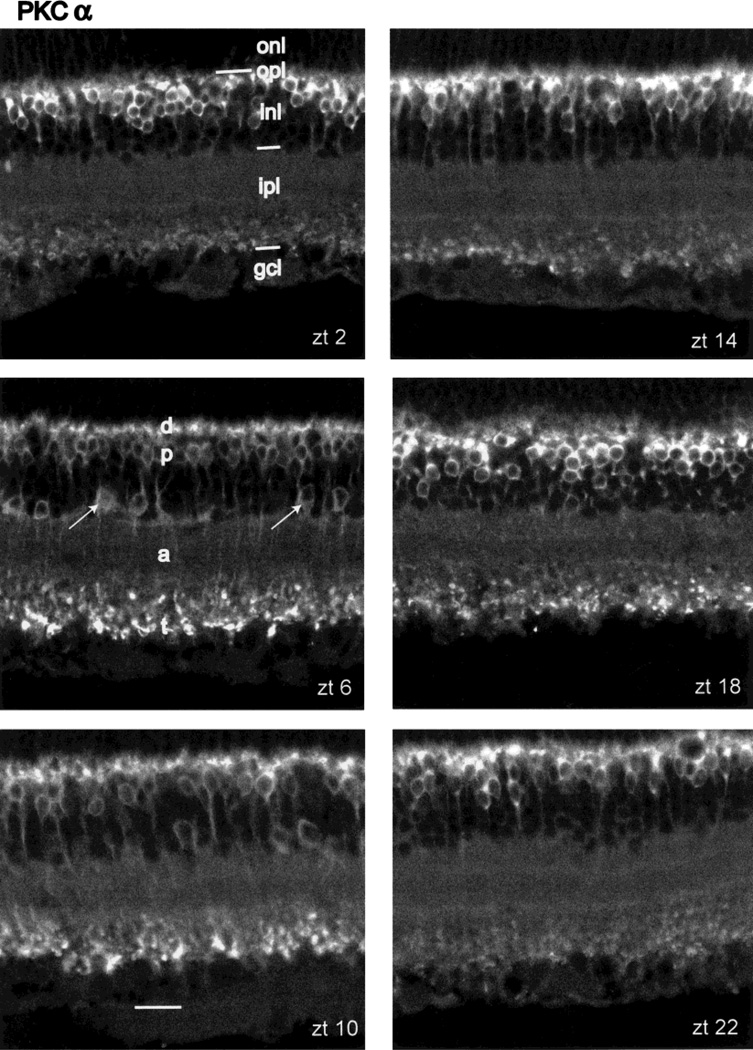
Figure 1 presents vertical sections of central retina reacted with the PKCα antibody. Figure 1 shows PKC-IR at ZT 02, 06, 10, 14, 18, and 22 hours of the LD cycle. It is clear that most of the PKC-IR resides in cells corresponding to the description of rod bipolar cells. They are present at high density and so constitute most of the PKC-IR signal. In addition, however, a morphologically diverse group of amacrine cell perikarya, whose cell bodies are located at or near the INL/IPL border, also shows PKC-IR (Fig. 1, arrows). In some PKC-IR amacrine cells, processes extended horizontally from the perikaryon, but PKC-IR could be followed for only a short distance from the cell body. Negishi et al. (1988) reported that the PKC-IR amacrine cells colocalized tyrosine hydroxylase (TH) in retinas of various vertebrates but that, in the rat retina, TH cells were only weakly reactive to PKC and that some non-TH+ amacrines showed PKC-IR.
Fig. 1.
Diurnal variation in PKCα-IR. Panels illustrate vertical sections through central retina taken from rats sacrificed at ZT 02, 06, 10, 14, 18, and 22 hours, as labeled. All sections in this figure and the other figures are oriented with the photoreceptor layer up and the vitreous body below. The main neuronal class exhibiting PKC-IR is the rod bipolar cell. Its dendrites (d), cell bodies (p), axons (a), and axon terminals (t) are labeled in panel ZT 06. Amacrine cells showing PKC-IR are indicated by arrows in panel ZT 06. The layers of the retina are indicated in panel ZT 02 as follows: outer nuclear layer (onl), outer plexiform layer (opl), inner nuclear layer (inl), inner plexiform layer (ipl), and combined ganglion cell and optic fiber layer (gcl). Scale bar = 20 µm.
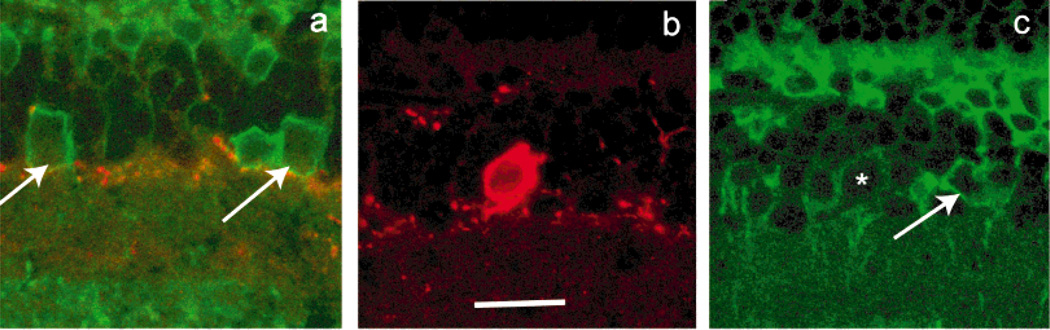
We repeated the experiment of Negishi et al. (1988) and obtained the same results (Fig. 2); that is, TH+ cells showed weak or no PKC-IR, and other amacrines, which did not show TH-IR, manifested PKC-IR. These PKC+, TH− amacrine cells were of different sorts, judging exclusively on the basis of perikaryal size and shape. One of the PKC-IR amacrines illustrated in Figure 2c has a small, round cell body with a vertically directed primary dendrite, which are morphological characteristics of an AII amacrine cell (Wässle et al., 1993). Further data on PKC-IR in AII amacrine cells are discussed below.
Fig. 2.
Colocalization of PKCα and TH-IR. a: Certain amacrine cells (arrows) at the border of inl/ipl show PKCα-IR (green) but not tyrosine hydroxylase (TH)-IR (red). A few TH-IR processes running in the distal most sublamina of the ipl are visible. In b, the cell body of a large TH-IR neuron is visible. c: The same neuron (asterisk) shows weak PKCα-IR, whereas adjacent amacrines not showing TH-IR (arrows) have pronounced PKCα-IR. Scale bar = 20 µm for a–c.
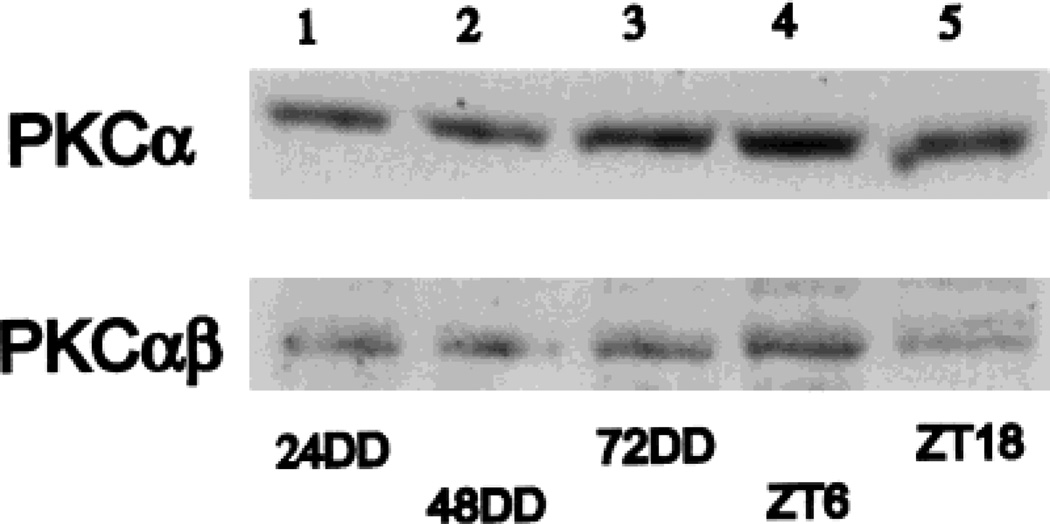
Western blots
Figure 3 summarizes the results obtained with the two anti-PKC antibodies employed. In each trial with either antibody, a single band at approximately 80 kD was stained. The five lanes illustrated in Figure 3 correspond to 1) 24 hours DD, 2) 48 hours DD, 3) 72 hours DD, 4) ZT 06 in LD, and 5) ZT 18 in LD. For the data in DD (lanes 1–3), the CT 02 time point was used. For the DD data, there is an increase in total PKC staining revealed by the PKCα antibody, which is not so clearly reflected in the results with the PKCαβ antibody. In contrast, for the LD data, the PKCαβ antibody reveals a clear increase in staining at ZT 06 compared with ZT 18, a difference that is present but less pronounced with the PKCα antibody. Further interpretation of the Western blot data is considered below in relation to the histological findings.
Fig. 3.
Western blot analysis of PKC-IR in rat retinas. Each lane represents a 100 µg protein sample derived from four sonicated retinas (see Materials and Methods). Lanes 1–3 illustrate, respectively, the CT 02 time point at 24, 48, and 72 hours DD. Note a progressive increase in staining for PKCα but not for PKCαβ. Lanes 4 and 5 illustrate, respectively, the times points ZT 06 and ZT 18 from animals in LD. For both antibodies a greater staining is seen at ZT 06 than at ZT 18.
Diurnal variability in PKC-IR
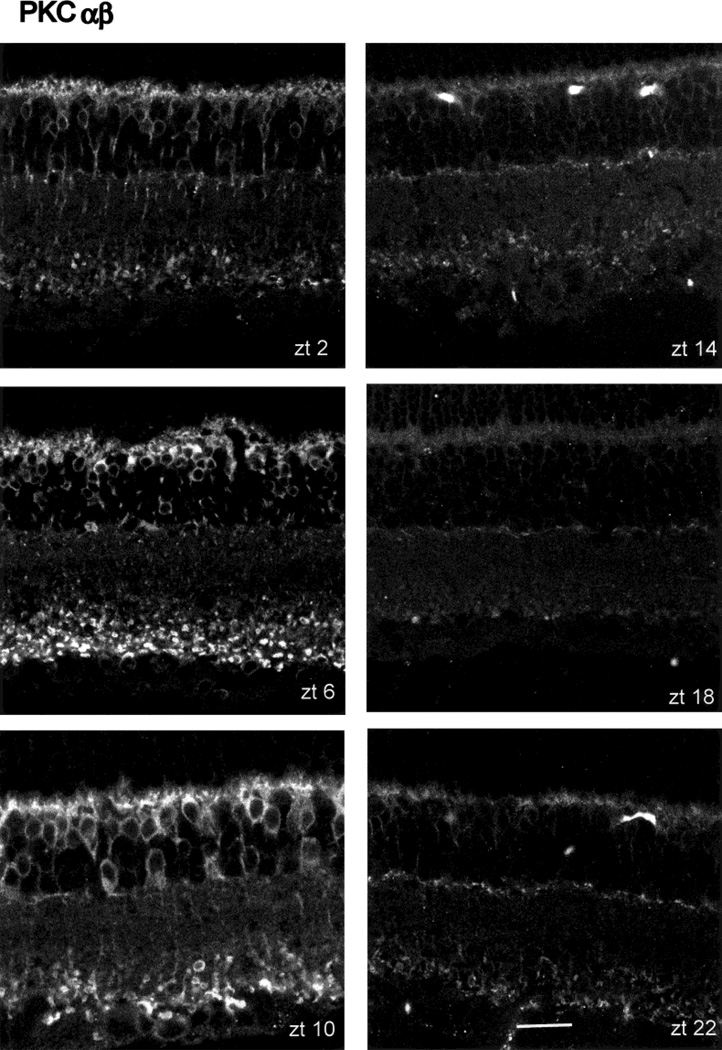
When the PKCα antibody was employed, no marked changes in PKC-IR as a function of ZT were noted (Fig. 1), although the axon terminals of the rod bipolar cells showed greater immunoreactivity at ZT 06 and 10 than at other times tested. Quite different results were obtained, however, when identically treated retinas were reacted with the PKCαβ antibody (Fig. 4). The presentation of the data in Figure 4 is identical to that shown in Figure 1. Visual inspection reveals that PKC-IR is relatively high during the subjective day at ZT 06 and 10 and falls to much lower levels during subjective night (ZT 14–22). Both the rod bipolar cells and the immunoreactive amacrine cells showed a similar cycling of PKC-IR.
Fig. 4.
Diurnal variation in PKCαβ-IR. The arrangement of this figure is identical to that of Figure 1. PKCαβ-IR is strongest at ZT 06 and 10, falling to lower levels at other times, with a trough at ZT 18. Scale bar = 20 µm.
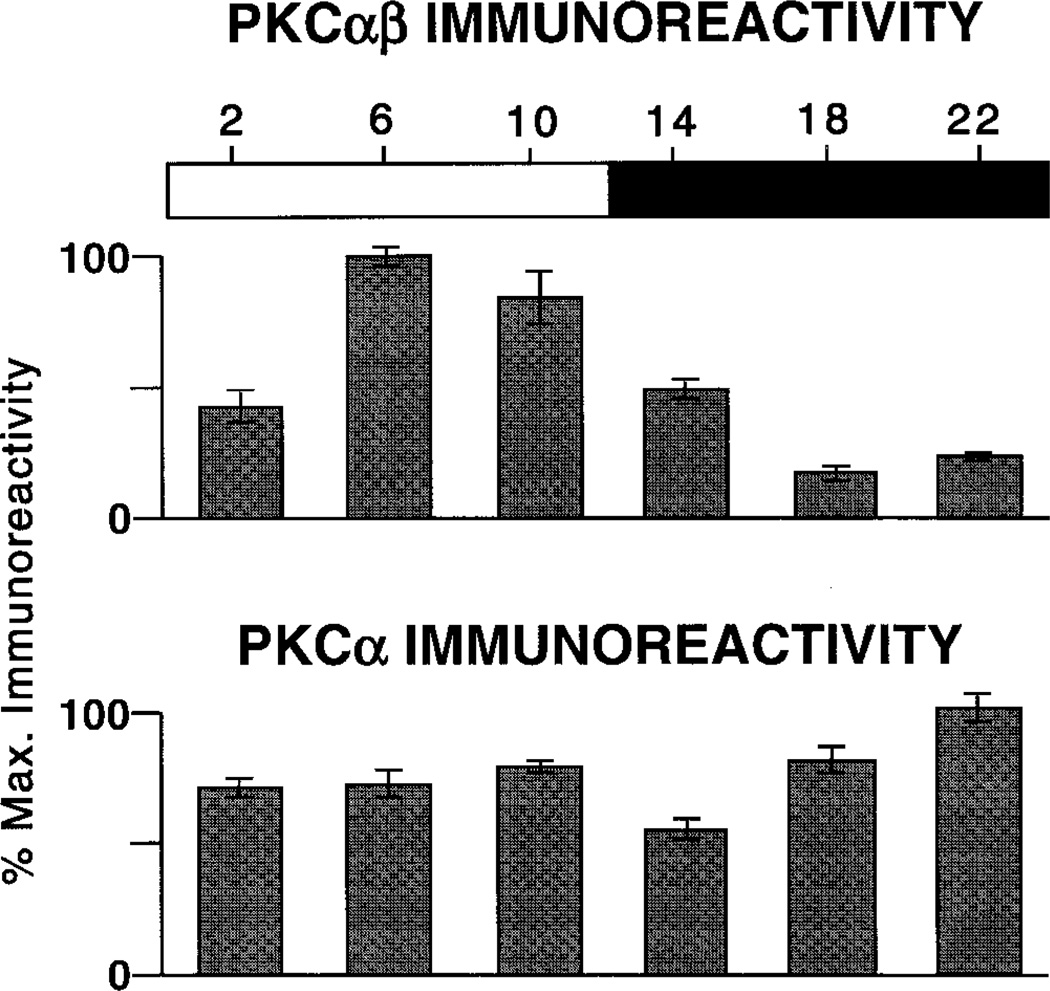
The visual impression of a diurnal expression of PKC-IR, when measured with the PKCαβ antibody, is borne out by measurements of the IR signal, as described in Materials and Methods and graphed in Figure 5. It is clear from Figures 1 and 4 that, because of the numerical superiority of rod bipolar cells and their radial geometry, the spatially averaged light signals reflect almost exclusively their PKC-IR, not that of the immunoreactive amacrine cells. The upper panel of Figure 5 provides measures of immunoreactivity as a function of ZT. The values are normalized to peak PKC-IR, which in this case occurred at ZT 06. Error bars are the SEM for four measurements, each taken from a different eye. The statistical significance of ZT-dependent differences were evaluated by ANOVA, followed by a Sheffe analysis. The results show that the values at ZT 06 and 10 were significantly different from the values at all other ZT, with P < 0.01.
Fig. 5.
Quantitative measure of the diurnal variation in PKC-IR. Top: PKCαβ. Each bar is the average signal from four identically treated retinas (see Materials and Methods for details). Responses are scaled; error bars are ± 1 SE. Horizontal bar indicates times of subjective day (open bar) and night (solid bar). PKCαβ-IR shows a clear diurnal rhythm of expression. Bottom: PKCα. There is no detectable diurnal rhythm of PKCα-IR.
An identical set of experiments was repeated, utilizing the PKCα antibody (Fig. 5, lower panel). It is important to note that the two antibodies recognize the same two cell groups: rod bipolar cells and some types of amacrine cell. Nevertheless, compared with the rhythm of PKC-IR revealed by the PKCαβ antibody, the PKCα antibody did not reveal a significant diurnal variation in PKC expression. For the latter antibody, maximal and minimal PKC-IR differ by less than a factor of two (Fig. 5, lower panel), and the differences between the various time points did not vary in any systematic way.
PKC-IR in darkness
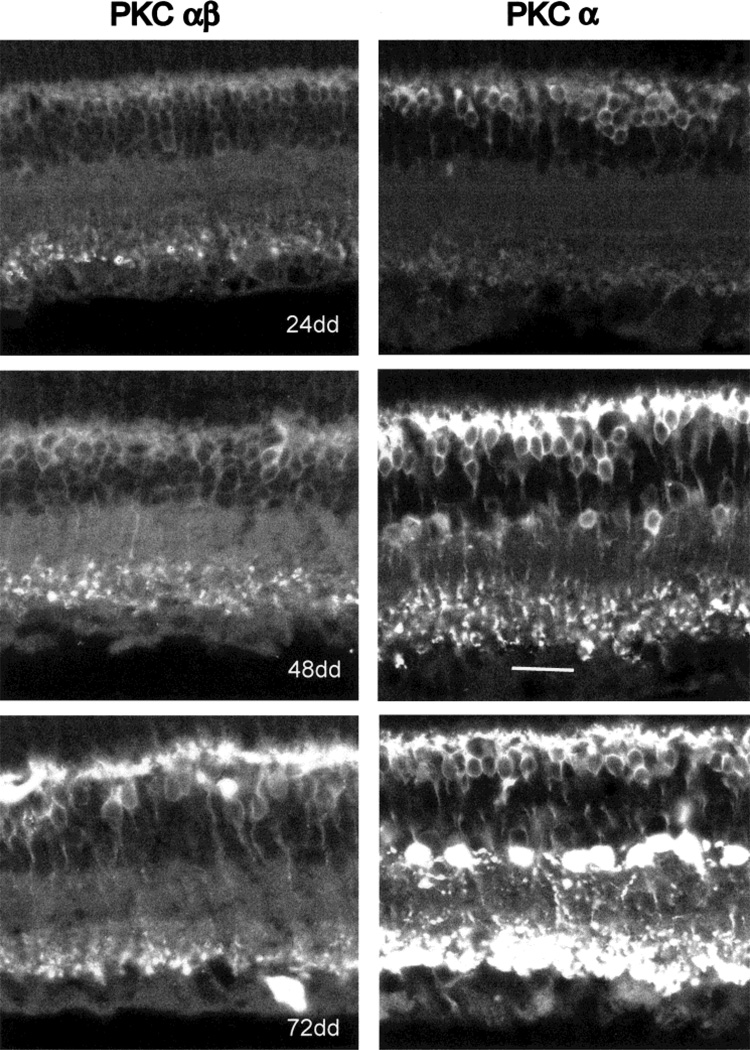
Figure 6 illustrates a comparison of immunocytochemical results obtained with the two antibodies at CT 02, after 24, 48, or 72 hours in DD. The retinas were processed exactly as described above and are illustrated in Figures 1 and 3. Both antibodies reveal a progressive increase in the PKC-IR of rod bipolar cells at 48 and 72 hours DD compared with 24 hours DD. For both anti-PKC antibodies, there is a suggestion of increased immunostaining of the rod bipolar cell axon terminals relative to that of cell bodies and dendrites after 48 or 72 hours DD. With respect to PKC-IR in amacrine cells, the results obtained with the two antibodies differed dramatically. The PKCαβ antibody stained amacrines only faintly at any of the time points tested (Fig. 6, left side). In contrast, beginning at 48 hours DD, the PKCα antibody revealed PKC-IR in a variety of amacrine cells, one subgroup of which was identified as AII amacrines based on perikaryal size, shape, and location and the presence of a vertically directed primary dendrite. The identity of the cell was confirmed by treating some sections with antiparvalbumin and finding that the parvalbumin-positive amacrines also showed PKC-IR (not illustrated). Chun et al. (1993) have shown that, in rodent retina, the antiparvalbumin Ab reacts almost exclusively with AII amacrine cells.
Fig. 6.
PKC-IR after 1–3 days in darkness. Left side illustrates results with the PKCαβ antibody, right side with the PKCα antibody. Vertical retinal sections were made from animals sacrificed at CT 02 in each case. At 24 hours of DD, weak PKC-IR is seen in rod bipolar cell bodies and dendrites. No amacrine cells show PKC-IR. After 48 hours of DD, all parts of rod bipolar cells are immunoreactive. Only with the PKCα antibody is PKC-IR revealed in amacrine cells. The smaller, round amacrine neurons whose cell bodies show intense PKC-IR are AII amacrines. After 72 hours of DD, both rod bipolar and AII amacrine cell PKC-IR have increased over that at 48 hours DD. Scale bar = 20 µm.
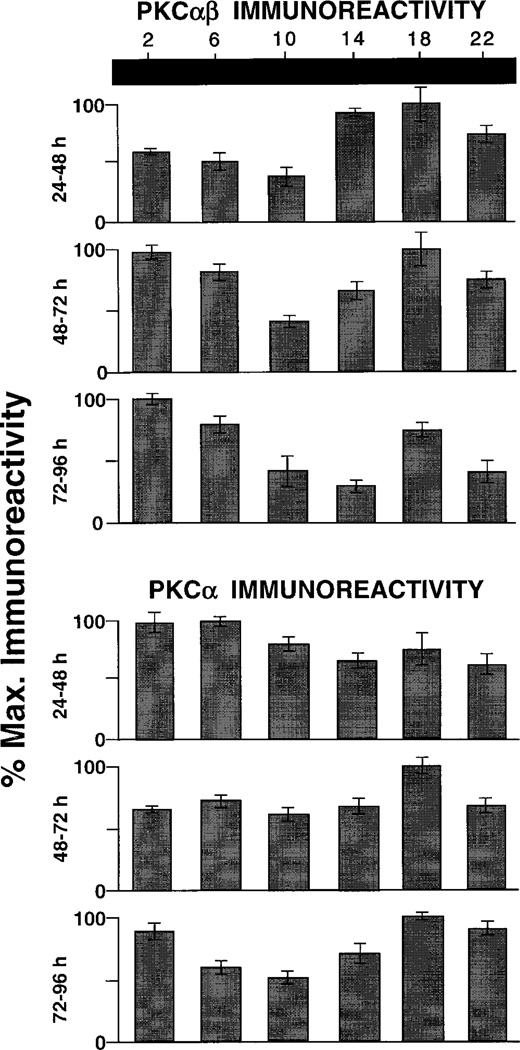
The results obtained for rod bipolar cells with the two anti-PKC antibodies after 1–3 days in DD are illustrated graphically in Figure 7. With the PKCαβ antibody, the pattern of PKC-IR was reversed from what was noted in LD (Fig. 6, upper part); i.e., the highest levels of PKC-IR were noted at CT 14 and 18, with relatively low values found at ZT 02 and 06. In the two succeeding days of DD, the pattern of PKC-IR was somewhat variable but never resembled the rhythm observed in LD (cf. Fig. 4). The data obtained using the PKCα antibody are presented in Figure 7 (lower part). Just as was observed in LD, DD did not induce a prominent rhythm of PKC-IR in rod bipolar cells. Although the data showed variation in PKC expression as a function of the time points sampled, there was always less than a twofold variation in PKC-IR over any 24 hour period.
Fig. 7.
Circadian variation in PKC-IR. Bar graphs are organized as for Figure 3. Top: PKCαβ antibody. Upper row: after 24 hours of DD, PKCαβ-IR is relatively low at CT 06–10 and relatively high at CT 14–18, the opposite of what was seen in LD (cf. Fig. 3). The pattern of rhythmic variation is not maintained after 48 (middle row) or 72 (lower row) hours of DD. Bottom: PKCα antibody. There is no clear time-dependent variation in PKCα-IR after 24 or 48 hours of DD. A weak (less than twofold) rhythmic pattern is seen after 72 hours of DD.
Light-induced increase in PKC-IR
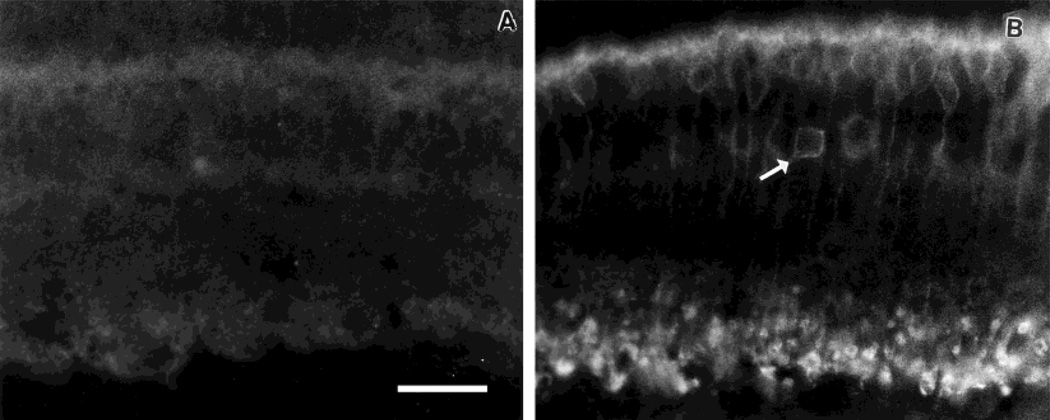
Studies of oncogenes such as c-fos have shown that light administered during the dark phase of the diurnal cycle is an effective stimulator of oncogene expression. We used this strategy to test whether the low level of PKC-IR seen during the dark phase of an LD cycle (cf. Fig. 3) could be up-regulated by light. For this group of experiments, only the PKCαβ antibody was employed, because it revealed a rhythm of PKC expression in LD (cf. Figs. 3, 4). Accordingly, at ZT 14, rats were stimulated for 1 hour with flashing light. Thereafter, the rats remained for 30 minutes in darkness before sacrifice. A group of control rats was kept on the same light cycle and sacrificed at the same time as the light-treated animals but without having been exposed to flashing light. The results illustrated in Figure 8 show that this treatment resulted in a very large increase in PKC-IR in both rod bipolar cells and a subpopulation of amacrine cells. All four eyes exposed to light showed increased expression of PKC-IR; none of the four control eyes did.
Fig. 8.
Light-induced increase in PKCαβ-IR. A: Control eye showing PKCαβ-IR in a vertical section of retina taken from a rat on an LD cycle and killed at ZT 15:30. B: PKCαβ-IR in vertical section of retina taken from rat exposed to 1 hour of flashing light (ZT 14–15) and then killed at ZT 15:30. Note increased immunoreactivity of rod bipolar cells and certain amacrine neurons (arrow). Scale bar = 20 µm.
DISCUSSION
Our main finding is that PKC-IR in retinal rod bipolar and certain subtypes of amacrine neuron varies as a function of the LD cycle and of time spent in DD. Both anti-PKC antibodies employed in our study were shown on western blots to react with a single protein band at about 80 kD, which is characteristic for the α and β isoenzymes of PKC (Osborne et al., 1991; Huwiler et al., 1992). Nevertheless, the two antibodies gave different results, whose interpretation requires prior discussion of activity-dependent changes in PKC.
Activity related structural alterations in PKC
In its inactive form PKC is in a folded configuration such that the regulatory domain occludes the catalytic domain. Inactive PKC is found primarily in the cytoplasm; activation results in translocation of PKC from the cytosol to the plasma membrane and is accompanied by a change in the conformation of the molecule to an extended form. The PKCαβ antibody is reported (Young et al., 1988) to bind in the hinge region, a portion of the PKC molecule that is most completely exposed when PKC is in the active state. Thus the greater PKCαβ-IR during ZT 06–10 (Fig. 3) indicates that PKC is more active in rod bipolar cells and certain amacrine cells during exposure to light. Support for this conclusion was provided by Germain et al. (2001), who found that, in rat retina, daylight induced a translocation of PKCα from cytosolic to membrane compartments. Our finding (Fig. 1) that rod bipolar cell terminals showed greatest PKCα-IR during the period ZT 06–10 also indicates increased transport of PKC during daylight hours.
PKC has many isoforms, most of which are found in retina and brain (Huwiler et al., 1992; Tanaka and Nishizuka, 1994). The antibodies we utilized recognize isoforms of the PKC subgroup activated by Ca2+ and/or diacylglycerol (Huang and Huang, 1986). Osborne et al. (1991) reported that phorbol esters, which mimicked the action of diacylglycerol, stimulated transport of PKC from cell body to terminal regions in rod bipolar cells of the rabbit retina. Feigenspan and Bormann (1994) noted that, in rat retinas, PKC-stimulating phorbol esters decrease GABA-induced currents in bipolar cells. A metabotropic glutamate agonist, 2-amino-4-phosphonobutyric acid (L-AP4), elicited similar results, but whether L-AP4 acted through the PKC pathway is an open question with particular relevance for rod bipolar cell function, because, at its synapse with rod photoreceptors, this neuron responds to glutamate through a metabotropic receptor (mGluR6), which is AP-4 sensitive (Nakajima et al., 1993).
Studies on a variety of central nervous system (CNS) synapses indicate that group I glutamate metabotropic receptors (for review see Cartmell and Schoepp, 2000) can stimulate diacylglycerol and inositol trisphosphate formation, which in turn activate PKC (Sladeczek et al., 1985; Sigel and Baur, 1988). On the other hand, L-AP4, which activates group III glutamate metabotropic receptors, usually is associated with inhibition of calcium currents and transmitter release (Trombley and Westbrook, 1992), actions that are independent of the PKC pathway but that often are opposed by PKC activated by some other means (Herrero et al., 1996).
The rod bipolar cell responds to light by a depolarization, which increases the probability of Ca2+ entry through the L-type Ca channels concentrated in its axonal terminals (Tachibana et al., 1993). Ca2+ entry in turn activates PKC (Vaquero et al., 1996). An additional potential mechanism of PKC activation in rod bipolar cells is through serotonin. The rod bipolar cell axon terminal receives synaptic input from a serotoninergic amacrine cell (Kolb, 1997), and serotonin, acting through a 5-HT2 receptor, mimics the PKC-induced inhibition of GABAC responses in rod bipolar cells (Feigenspan and Bormann, 1994).
Changes in PKC expression during darkness
The observation that the rhythm of PKC expression peaks at ZT 06–10, then falls off, even during the light phase of the LD cycle, suggests an interplay between light and an underlying circadian rhythm. A similar interaction is reported for dopamine synthesis and release in the rat retina (Iuvone et al., 1978; Iuvone, 1984). Other workers have reported that prolonged light or darkness did not affect PKC expression in rabbit or rat retinas (Osborne et al., 1991; Huwiler et al., 1992), but in those studies the time of day was not controlled.
With constant darkness, we observed that both antibodies revealed an increased PKC-IR in rod bipolar cells between 24 and 72 hours DD. It is important to note that in this experiment the time when the animals were killed was held constant at CT 02. These data are therefore consistent with the hypothesis that the total amount of PKC protein in rod bipolar cells increases with time in constant darkness. We observed that PKC-IR increased in all parts of the rod bipolar cell between 24 and 72 hours DD (cf. Fig. 6), indicating that, if an increased PKC-IR in rod bipolar cell axon terminals was due to transport of PKC from the cell body, it must have been accompanied by increased PKC synthesis.
With regard to the AII amacrine cells, their increasing PKC-IR in constant darkness was seen only with the PKCα antibody. We interpret this to mean that the epitope for the PKCαβ antibody is completely masked under our experimental conditions. An alternative possibility—that the isoenzyme of PKC expressed in AII amacrines is recognized only by the PKCα antibody—is very unlikely in that it is a monoclonal antibody for PKCα, an isoenzyme also recognized by the PKCαβ antibody.
The western blot data are in good agreement with the above-mentioned hypothesis. Both PKCαβ and PKCα antibodies revealed increased staining between 24 and 72 hours DD, although the increase was more dramatic for the PKCα antibody. These data suggest that the increase inPKCαβ immunostaining reflects only the augmented PKC-IR of rod bipolar cells, whereas, for the PKCα antibody, both the rod bipolar cell and the AII amacrine cell PKC-IR contribute to the increase.
Changes in PKC expression induced by light presented in the dark phase
In addition to its activation by glutamate, PKC expression is increased when light is presented in the dark phase of the LD cycle (Fig. 7). Similar results are reported for the oncogene c-fos (Sagar and Sharp, 1990). The mechanism of activation by light has been studied in relation to the activity of TH, the rate-limiting enzyme for dopamine synthesis. In the short term, meaning on the time scale of a few minutes, light stimulates phosphorylation of TH and dopamine synthesis (Iuvone et al., 1978; Witkovsky et al., 2000), but, in the long term (i.e., ~1 hour), it results in synthesis of new TH molecules (reviewed in Iuvone, 1984). Our data do not reveal the mechanism underlying increased light-induced expression of PKC during the dark period.
PKC-IR in retinal amacrine cells
If either PKC antibody is used, and retinas from eyes maintained on an LD cycle are examined, the reactive amacrines can be identified only on the basis of perikaryal characteristics, in that PKC-IR is observed only in the initial portions of some dendrites but does not extend to the terminal processes as it does for the rod bipolar cell. For the rat retina, we found that the TH+ dopaminergic amacrine was only very weakly reactive to PKC. Our results are in perfect agreement with the data of Negishi et al. (1988). They reported that, in the rat retina, TH+ amacrines were only weakly reactive to PKC but that other non-TH amacrines showed PKC-IR.
A smaller amacrine cell showing PKC-IR colocalized parvalbumin (PV). In a study of the rat retina, Wässle et al. (1993) determined that PV+ amacrines are almost exclusively AII amacrine cells. AII amacrines are extremely numerous (about 13% total amacrine cell population; MacNeil et al., 1999) and have a characteristic morphology consisting of an approximately round perikaryon 6–9 µm in diameter and a vertically directed primary dendrite (Wässle et al., 1993). Many of the PKC+ amacrines we visualized in preparations subjected to 2–3 days in total darkness had this morphology.
PKC and the rod pathway
Our data indicate that PKC expression is most evident in neurons of the rod pathway (Wässle and Boycott, 1991), the chain of information flow beginning with rods, through the rod bipolar cells to the AII amacrine cell. The finding that PKC-IR in AII amacrines is up-regulated by constant darkness suggests that light-induced modulation of rod bipolar input is the controlling signal for PKC expression in AII amacrine neurons.
In a general way, our data demonstrate that PKC expression in retinal neurons is not fixed but rather is controlled by the LD cycle. PKC is involved in the control of transmitter release at retinal bipolar cells, for which glutamate is the transmitter, and at retinal amacrine cells, which employ the neurotransmitter GABA. PKC thus appears to play a role in glutamate–GABA interactions at the bipolar cell terminal, which are an important component of information processing in the inner retina (Euler and Masland, 2000). AII amacrines utilize glycine as their neurotransmitter (Kolb, 1997), suggesting that PKC may also contribute to the control of glycine release, both by modifying rod bipolar cell (glutamatergic) input to the AII amacrine cell and through an as yet unidentified direct neuromodulatory action of its intrinsic, inducible PKC following prolonged, constant darkness.
ACKNOWLEDGMENTS
Contract grant sponsor: Research to Prevent Blindness, Inc.; Contract grant sponsor: The Helen Hoffritz Foundation.
This work was supported by a Bolyai Fellowship (R.G.) and NIH grants NS 37919 and NSF IBN-96418886 (R.S.) and EY 03570 (P.W.). We thank Eleazer Yousefadeh for excellent technical assistance and the Confocal Microscope Facility of Columbia University for the use of their equipment.
LITERATURE CITED
- Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Smith JA, Seidman JG, Struhl H. Current protocols in molecular biology. New York: Greene Publ Assoc and Wiley-Interscience; 1987. [Google Scholar]
- Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- Bassi CJ, Powers MK. Circadian rhythm in goldfish visual sensitivity. Invest Ophthalmol Vis Sci. 1987;28:1811–1815. [PubMed] [Google Scholar]
- Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas: regulation by a photoreceptor oscillator. Progr Ret Eye Res. 1995;14:267–291. [Google Scholar]
- Cahill GM, Grace MS, Besharse JC. Rhythmic regulation of retinal melatonin: metabolic pathways, neurochemical mechanisms, and the ocular circadian clock. Cell Mol Neurobiol. 1991;11:529–560. doi: 10.1007/BF00734814. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chun M-H, Han S-H, Chung J-W, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- Euler T, Masland RH. Light-evoked responses of bipolar cells in mammalian retina. J Neurophysiol. 2000;83:1817–1829. doi: 10.1152/jn.2000.83.4.1817. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Bormann J. Modulation of GABAc receptors in rat retinal bipolar cells by protein kinase C. J Physiol. 1994;481:325–330. doi: 10.1113/jphysiol.1994.sp020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain F, Cisneros E, De la Villa P, Bragado J. Protein kinase C in the rat retina. Functional translocation from cytosolic to membrane compartments. Invest Ophthalmol Vis Sci. 2001;42:S671. [Google Scholar]
- Green CB, Besharse JC. Use of a high stringency differential display screen for identification of retinal mRNAs that are regulated by circadian clock. Mol Brain Res. 1996;37:157–165. doi: 10.1016/0169-328x(95)00307-e. [DOI] [PubMed] [Google Scholar]
- Green CB, Cahill GM, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995;677:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Hagiwara M, Hachiya T, Watanabe M, Usuda N, Iida F, Tamai K, Hidaka H. Assessment of protein kinase C isozymes by enzyme immunoassay and overexpression of type II in thyroid adenocarcinoma. Cancer Res. 1990;50:5515–5519. [PubMed] [Google Scholar]
- Herrero I, Vazquez E, Miras-Portugal MT, Sanchez-Prieto J. A decrease in [Ca2+]c but not in cAMP mediates L-AP4 inhibition of glutamate release: PKC-mediated suppression of this inhibitory pathway. Eur J Neurosci. 1996;8:700–709. doi: 10.1111/j.1460-9568.1996.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Huang K. The mechanism of protein kinase C activation. Trends Neurosci. 1989;12:425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Huang KP, Huang FL. Immunochemical characterization of rat brain protein kinase. J Biol Chem. 1986;261:14781–14787. [PubMed] [Google Scholar]
- Huwiler A, Jung HH, Pfelschifter J, Remé CE. Protein kinase C in the rat retina: immunocharacterization of calcium-independent δ, ε, and ζ isoenzymes. Mol Brain Res. 1992;16:360–364. doi: 10.1016/0169-328x(92)90247-9. [DOI] [PubMed] [Google Scholar]
- Iuvone PM. Regulation of retinal dopamine biosynthesis and tyrosine hydroxylase activity by light. Fed Proc. 1984;43:2709–2713. [PubMed] [Google Scholar]
- Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- Kolb H. Amacrine cells of the mammalian retina: neurocircuitry and functional roles. Eye. 1997;11:904–933. doi: 10.1038/eye.1997.230. [DOI] [PubMed] [Google Scholar]
- Kosaka J, Suzuki A, Morii E, Nomura S. Differential localization and expression of α and β isoenzymes of protein kinase C in the rat retina. J Neurosci Res. 1998;54:655–663. doi: 10.1002/(SICI)1097-4547(19981201)54:5<655::AID-JNR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- La Vail MM. Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1076. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Levinson G, Burnside B. Circadian rhythms in teleost retinomotor movements: a comparison of the effects of circadian rhythm and light condition on cone length. Invest Ophthalmol Vis Sci. 1981;20:431–438. [PubMed] [Google Scholar]
- MacNeil M, Heussy J, Dacheux R, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413:305–326. [PubMed] [Google Scholar]
- Manglapus MK, Uchiyama H, Buelow NF, Barlow RB. Circadian rhythms of rod-cone dominance in the Japanese quail retina. J Neurosci. 1998;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem. 1993;268:11368–11373. [PubMed] [Google Scholar]
- Negishi K, Kato S, Ternaishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163–181. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Broyden NJ, Barnett NL, Morris NJ. Protein kinase C (α and β) immunoreactivity in rabbit and rat retina: effect of phorbol esters and transmitter agonists on immunoreactivity and the translocation of the enzyme from cytosolic to membrane compartments. J Neurochem. 1991;57:594–604. doi: 10.1111/j.1471-4159.1991.tb03790.x. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Barnett NL, Morris NJ, Huang FL. The occurrence of three isoenzymes of protein kinase C (α, β, and γ) in retinas of different species. Brain Res. 1992;570:161–166. doi: 10.1016/0006-8993(92)90577-v. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR. Light induces a Fos-like nuclear antigen in retinal neurons. Mol Brain Res. 1990;7:17–21. doi: 10.1016/0169-328x(90)90068-o. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R. Activation of protein kinase C differentially modulates neuronal Na+, Ca2+, and gamma-aminobutyrate type A channels. Proc Natl Acad Sci USA. 1988;85:6192–6196. doi: 10.1073/pnas.85.16.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladeczek F, Pin J-P, Recasens M, Bockaert J, Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurons. Nature. 1985;317:717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurements of protein using bichinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Okada T, Arimura T, Kobayashi K, Piccolino M. Dihydropyridine-sensitive calcium current mediates neurotransmitter release from bipolar cells of the goldfish retina. J Neurosci. 1993;13:2898–2909. doi: 10.1523/JNEUROSCI.13-07-02898.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka C, Nishizuka Y. The protein kinase C family for neuronal signaling. Annu Rev Neurosci. 1994;17:551–567. doi: 10.1146/annurev.ne.17.030194.003003. [DOI] [PubMed] [Google Scholar]
- Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- Trombley PQ, Westbrook GL. L-AP4 inhibits calcium currents and synaptic transmission via a G-protein-coupled glutamate receptor. J Neurosci. 1992;12:2043–2050. doi: 10.1523/JNEUROSCI.12-06-02043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquero CF, Velasco A, de la Villa P. Protein kinase C localization in the synaptic terminal of rod bipolar cells. Neuroreport. 1996;7:2176–2180. doi: 10.1097/00001756-199609020-00024. [DOI] [PubMed] [Google Scholar]
- Von Schantz M, Lucas RJ, Foster RG. Circadian oscillation of photopigment transcript levels in the mouse retina. Mol Brain Res. 1999;72:108–114. doi: 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Gabriel R, Haycock JW, Meller E. Influence of light and neural circuitry on tyrosine hydroxylase phosphorylation in the rat retina. J Chem Neuroanat. 2000;19:105–116. doi: 10.1016/s0891-0618(00)00055-7. [DOI] [PubMed] [Google Scholar]
- Young S, Rothbard J, Parker PJ. A monoclonal antibody recognising the site of limited proteolysis of protein kinase C. Eur J Biochem. 1988;173:247–252. doi: 10.1111/j.1432-1033.1988.tb13991.x. [DOI] [PubMed] [Google Scholar]