Abstract
It is well established that mast cells occur within the brain of many species, and that the brain mast cell population is not static, but changes with the behavioral and physiological state of the animal. In this study, we tested whether exposure to conspecifics alters the number of brain mast cells in male rats, and then investigated the nature of stimuli influencing the changes observed in the number and localization of brain mast cells. Five days of cohabitation with an ovariectomized, estrogen–progesterone (OVX + EP)-treated female resulted in the largest number of thalamic mast cells, while pairing with such a female physically separated by a wire mesh or with a novel male produced a smaller, but significant increase over other pairings (OVX females for 5 days, OVX and OVX + EP females for 1 day, familiar or isolated males for 5 days). In all groups, mast cells were localized within specific dorsal thalamic nuclei, including the paraventricular nucleus, anterior nuclear group, or mediodorsal, ventroposterior, or medial geniculate nuclei. The results suggest that the behavioral and/or endocrine factors associated with cohabitation with conspecifics are sufficient to alter the number of brain mast cell-specific nuclei in the thalami of male rats and thus can provide targeted delivery of neuromodulators to specific regions of the brain that process information concerning the normal physiological state of the animal.
Keywords: female, thalamus, mating, aggressive, endocrine, immune, stress
Although mast cells are well known in their role as effectors of IgE-mediated allergic reactions, their physiological function(s) remains poorly understood (Wedemeyer, Tsai, and Galli, 2000; Forsberg, Pejler, Ringwall, Lunderius, Tomasini-Johansson, Kusche-Gullberg, Eriksson, Ledin, Hellman, and Kjellen, 1999; Echtenacher, Mannel, and Hultner, 1996; Malaviya, Ikeda, Ross, and Abraham, 1996). Recent evidence indicates that bacterial recognition followed by endocytosis, processing and presentation of bacterial antigen, immune cell recruitment, and elimination of parasites number among these functions (McLachlan and Abraham, 2001; Vincent-Schneider, Thery, Mazzeo, Tenza, Raposo, and Bonnerot, 2001; Abraham and Malaviya, 2000; Fox, Jewel, and Whitacre, 1994). In addition, mast cells synthesize, store, and secrete a wide variety of bioactive molecules, including neuropeptides, neurotransmitters, prostaglandins, chemotactic factors, and cytokines (Galli, 2000; Metcalfe, Baram, and Mekori, 1997; Silver, Silverman, Vitkovic, and Lederhendler, 1996; Johnson and Krenger, 1992).
Mast cells are normally present in the mammalian brain, both in the leptomeninges and within the parenchyma along the blood vessels of several dorsal thalamic nuclei (Dropp, 1972, 1976; Theoharides, 1990; Goldschmidt, Hough, Glick, and Padawer, 1984). The number of brain mast cells is modulated by physiological and behavioral state. Our demonstration that mature mast cells can migrate from the vasculature into the adult rat brain parenchyma provides a possible mechanism for the increases observed in the brain mast cell population (Silverman, Sutherland, Wilhelm, and Silver, 2000).
A plethora of endocrine and social stimuli has been shown to affect brain mast cell number in mammals. In response to immobilization stress or handling, the brain mast cell number declines in rats (Theoharides, Spanos, Pang, Alferes, Ligris, Letourneau, Rozniecki, Webster, and Chrousos, 1995; Persinger, 1980). As these studies used markers that react with mast cell granule contents, these data may reflect increased rates of brain mast cell degranulation, rather than a change in the population size (Esposito, Gheorghe, Kandere, Pang, Connolly, Jacobson, and Theoharides, 2001). Factors associated with reproduction appear to influence brain mast cells. Male mice which mate and subsequently cohabitate with females have elevated numbers of thalamic mast cells compared to male mice cohabitating with other males (Yang, Chien, and Lu, 1999). There are more mast cells in the brains of post-partum rats than in virgin rats (Silverman et al., 2000). In doves, 2 hours of courtship behavior (Zhuang, Silverman, and Silver, 1993), or treatment with estradiol (E), testosterone (T), or dihydrotestosterone (DHT) in animals housed in isolation increases the number of brain mast cells (Wilhelm, King, Silverman, and Silver, 2000).
In the present study, we extended previous work on brain mast cells in four ways. First, we tested whether cohabitation with conspecifics influences the number of mast cells in the rat brain. Having found that the conspecific could alter the population of brain mast cells, we investigated the nature of the eliciting stimuli from the conspecific. Third, we explored whether mast cells tend to accumulate in particular brain regions. Fourth, we studied whether cohabitation with conspecifics results in unique distributions of mast cells in the thalamus or whether mast cells always aggregate in particular nuclei. To address this last question we used immunocytochemistry for the calcium-binding proteins, calbindin, calretinin, and parvalbumin, as markers for subpopulations of neurons within the thalamus (Arai, Jacobowitz, and Deura, 1994; Arai, Arai, Kani, and Jacobowitz, 1992).
MATERIALS AND METHODS
Subjects and Housing
Adult male and female Long–Evans rats (Charles River Laboratories, Wilmington, MA), weighing 375–400 and 275–300 g, respectively, at the start of the study, were used. Animals were maintained in the colony room in plastic cages with cob bedding, in like-sex pairs, until tests began about 3 weeks later. Food pellets (Purina 5001, St. Louis, MO) and tap water were available ad libitum. The colony room had a 12:12 light:dark cycle (lights off 1900 h) and constant temperature (23°C).
Experimental Groups
All male subjects were sexually naïve. Of the male rats (N = 60) used as subjects, 54 were studied in behavioral experiments and 6 were used for anatomical studies. Each male was studied only once. The behavioral manipulations involved removing the male rat from its home cage and placing it in one of the following eight experimental groups: it was paired for 1 day with an ovariectomized (OVX) (n = 7) or OVX + estrogen–progesterone-treated (EP) (n = 6) female and sacrificed at the end of this interval, or placed for 5 days with either an OVX (n = 7) or OVX + EP (n = 7) female, an OVX + EP female (n = 6) separated from the male by a mesh barrier, the original cagemate (n = 7), or a novel male (n = 7), or housed alone (isolated) in the home cage (n = 7) and sacrificed at the end of this interval. At the time of pairing, the rat’s behavior was observed for 45 min. While sexual behavior was not quantified, all male rats paired with OVX + EP female rats exhibited mounting and all OVX + EP females showed lordosis and proceptive behavior. In the six animals used for anatomical studies, calcium-binding proteins served as immunocytochemical markers to determine the thalamic localization of brain mast cells. For this, males were paired with an OVX +EP female (N = 3) or with a familiar male (N = 3) for 5 days.
Females (N = 24) were used only to provide stimulus cues, and were not themselves studied. One week after arrival in the laboratory, female rats were ovariectomized (OVX) using an intrabdominal approach. OVX rats were housed singly for a week of postoperative recovery and then returned to their original cagemate. For females (N = 12) receiving hormone replacement, subcutaneous injections of 2 μg estradiol 3-benzoate (E, Sigma, St. Louis, MO) in 100 μl sesame oil were given each Tuesday and Wednesday and of 500 μg progesterone (P, Sigma) each Friday (Schumacher, Coirini, Pfaff, and McEwen, 1991). The remaining females (N = 12) received sesame oil injections alone at these times. Stimulus females were used once weekly at most.
All pairings began on Fridays. Sexual receptivity was verified in two ways: (a) tests for lordosis (Pfaff, Schwartz-Giblin, McCarthy, and Kow, 1994) followed by (b) 45 min of observation. Females were designated as sexually receptive and were used in the study if they showed lordosis for five consecutive manual tests (Zucker, 1967) and proceptive behaviors such as ear wiggling, hopping, and darting in the presence of the male rat.
Tissue Processing
Male rats were deeply anesthetized with a 1-ml injection of sodium pentobarbital (Nembutal, 50 mg/ml, Henry Schein, Melville, NY) and perfused transcardially at a rate of 21 ml/min with 100 ml of 0.9% saline, followed by 300 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (pH = 7.3). Next, the brains were removed, blocked, postfixed in 4% paraformaldehyde for 2 h at room temperature, and cryoprotected by placing them for 1 day each in 10, 20, and 30% sucrose solutions at 4°C. Coronal sections (50 μm) were cut on a freezing microtome. For the first 25 rats used in behavioral experiments, 85 sections per rat were collected. Of these, only 75 sections starting from the posterior end of the anterior commissure (AP −0.92 mm) to the posterior end of the posterior commissure (AP −5.8 mm) (Paxinos and Watson, 1986) contained mast cells. Thus, our analysis of alterations in mast cell number and mast cell localization focused on sections within these anatomical coordinates. For rats that were part of behavioral manipulations, all sections were stained with toluidine blue. For rats that were used to delineate thalamic nuclei, every sixth section was used for immunocytochemistry and every other section was stained with toluidine blue (see below).
Toluidine blue staining
Toluidine blue is an aniline dye that reacts at acidic pH with the sulfated glycosaminoglycans in the mast cell granules to reveal metachromasia. Freshly cut sections were mounted and dried overnight at room temperature, treated with 60% ethanol for 5 min, and followed by 5 min exposure to toluidine blue (0.4 mg Toluidine Blue 0 (Sigma) in 200 ml 60% ethanol, adjusted to pH 2 with hydrochloric acid). Sections were washed in tap water, dehydrated in acetone for 6 min, cleared for 30 min with HemoDe (Fisher Scientific, Piscataway, NJ), and coverslipped with Permount (Fisher Scientific).
Immunocytochemistry
Sections were processed using one of the following primary antisera: calbindin-D28k (mouse monoclonal, Sigma, 1:20,000), calretinin (rabbit polyclonal, Chemicon, 1:5000), or parvalbumin (mouse monoclonal, Sigma, 1:10,000). Antigen–antibody complexes were identified by sequential exposure to biotinylated species-specific IgG (Vector Labs, Burlington, CA) and avidin–biotin–horseradish peroxidase (HRP) complex (Vector Elite Kit). The enzyme was visualized with 3,3′-diaminobenzidine (Polysciences Inc, Warrington, PA).
Cell Counting
Observers (L.A. and E.Y.) unaware of the origin of the material quantified mast cells in serial sections through the thalamus. Interrater reliability based on the Pearson correlation coefficient was 91%. Cell counts were made using a modified optical dissector method, an unbiased stereological approach particularly appropriate for thick sections (Coggeshall and Lekan, 1996; Saper, 1996). Only cells with visible nuclei were counted. Mast cells are essentially spherical. Their diameter was measured using NIH Image (Version 1.62), scoring only cells with visible nuclei (n = 25/rat in five rats). The Abercrombie factor (Abercrombie, 1946) was applied to raw counts to correct for double counting of cells in consecutive sections, N = nT/(T + D), where N is the corrected cell number, n is the uncorrected cell number, T is the section thickness, and D is the mean diameter of cell nucleus.
Data Analysis
The number of mast cells was assessed through the entire extent of the thalamus (75 serial sections, 50 μm each). For analysis, the data were summed in bins of five sections. The thalamic distribution of mast cells deviated significantly from normality (Kolmogorov–Smirnov test, P < 0.001), and had significantly different variance (Levine median test, P < 0.001). This was true for both the raw and transformed data (square root, log, log + 1). Therefore, results were analyzed using nonparametric statistics: χ2 test (8 × 1, group × mast cell counts) to assess overall differences among experimental groups. Next, overall intergroup differences were examined using χ2 test (15 × 8, group × bins), followed by comparisons within groups (Friedman test) and comparisons across groups (Kruskall–Wallis test), corrected with modified Bonferroni tests. The null hypothesis was that mast cell counts were equally distributed across the cells of these contingency tables according to a uniform joint probability based on the numbers of counts in each row and column (Hays, 1963).
RESULTS
Exposure to Conspecifics Increases the Number of Thalamic Mast Cells in Male Rats
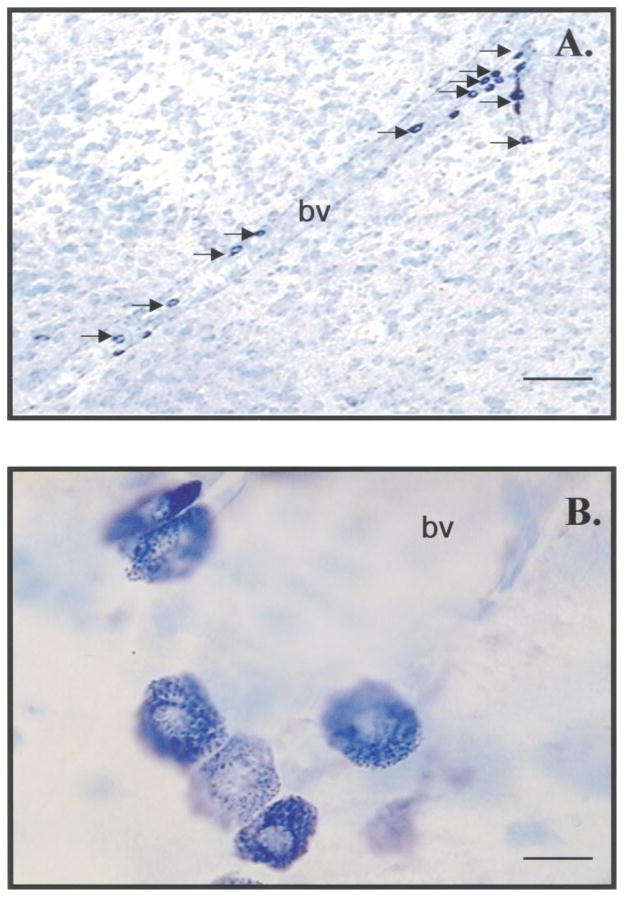
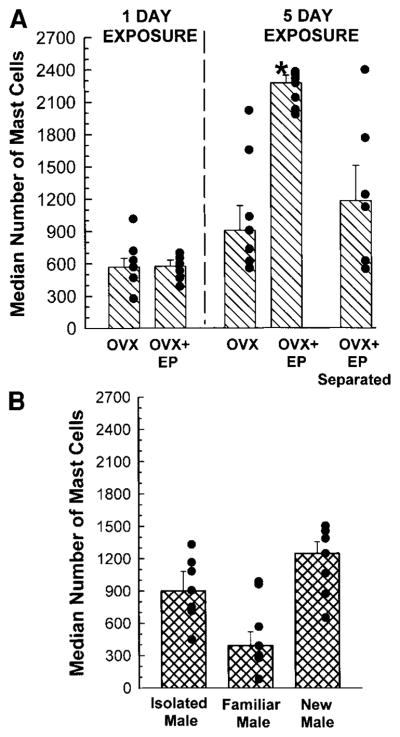
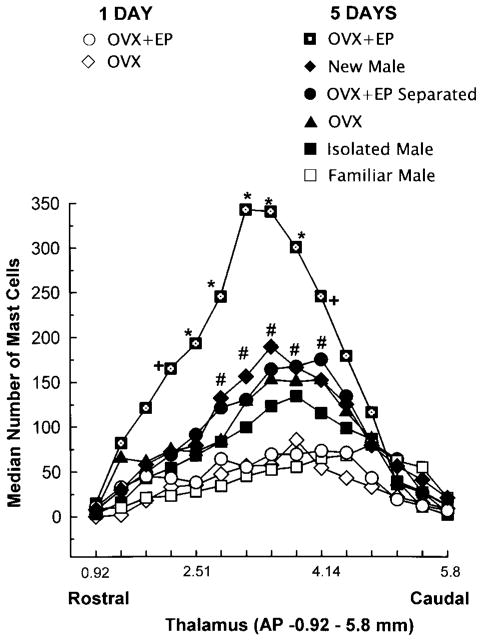
Mast cells were identified by their metachromatic granules (Fig. 1) and were often found adjacent to blood vessels. Mast cell diameter was 10.7 ± 0.4 μm, with a nuclear diameter of 6.5 ± 0.5 μm. The number of mast cells varied significantly among experimental groups (Fig. 2). The overall (8 × 1) χ2 analysis revealed that there was a significant variation in the total number of thalamic mast cells (χ2 (7) = 16.2, P < 0.02). The Kruskall–Wallis test indicated that the number of mast cells in male rats that were paired for 5 days with OVX + EP rats was greater than all other groups (χ2 (7) = 23.21, P < 0.009) (Fig. 2). The increases in mast cell number were greatest at the same anteroposterior levels of the thalamus in all experimental groups. The 8 × 15 χ2 analysis, which took into account the thalamic location of the mast cells, revealed that in the midthalamic region (AP −2.51–4.1 mm), there were more mast cells in males paired for 5 days with OVX + EP females or with novel males, or males physically separated from OVX + EP females compared with all other groups (χ2 (98) = 503.2, P < 0.001) (Fig. 3).
FIG. 1.
Appearance of mast cells in thalamic sections stained with acidic toluidine blue. (A) Perivascular mast cells (arrows) in the anterior thalamic artery. Bar = 125 μm. (B) Intense staining of mast cell granules reflecting the metachromasia of the mast cell granular content. Bar = 10 μm. bv, blood vessel.
FIG. 2.
Median (± interquartile range) numbers of mast cells in the thalami of male rats in various pairing conditions. Filled black circles indicate individual data. (A) Male rats were paired with OVX or OVX + EP female rats for 1 day (top) or 5 days (top, right), or physically separated from OVX + EP females for 5 days (top, right). (B) Male rats were isolated (bottom right and paired with familiar (middle) or novel males (right) for 5 days. *Significantly different from any other group in panel, as indicated by Kruskall–Wallis following overall χ2 test (P < 0.01).
FIG. 3.
Rostral–caudal distribution of mast cells in the thalami of male rats in various pairing conditions. Each data point represents the median number of mast cells in five adjacent 50-μm sections. Thalamic coordinate refer to the atlas of Paxinos and Watson (1986). *Significantly different from all groups, P < 0.01. + Significantly different from isolated males, males paired with familiar males, or 1-day pairing only, P < 0.05. #Significantly different from familiar males and 1-day pairing only, P < 0.01.
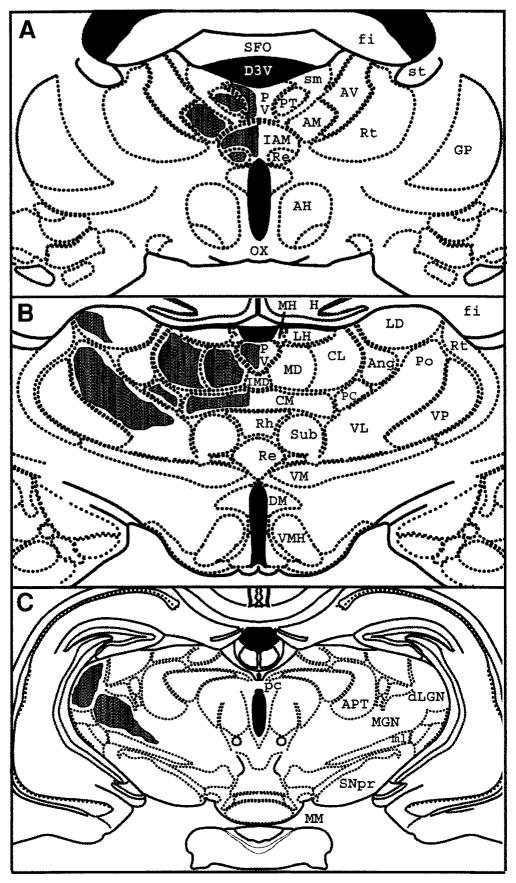
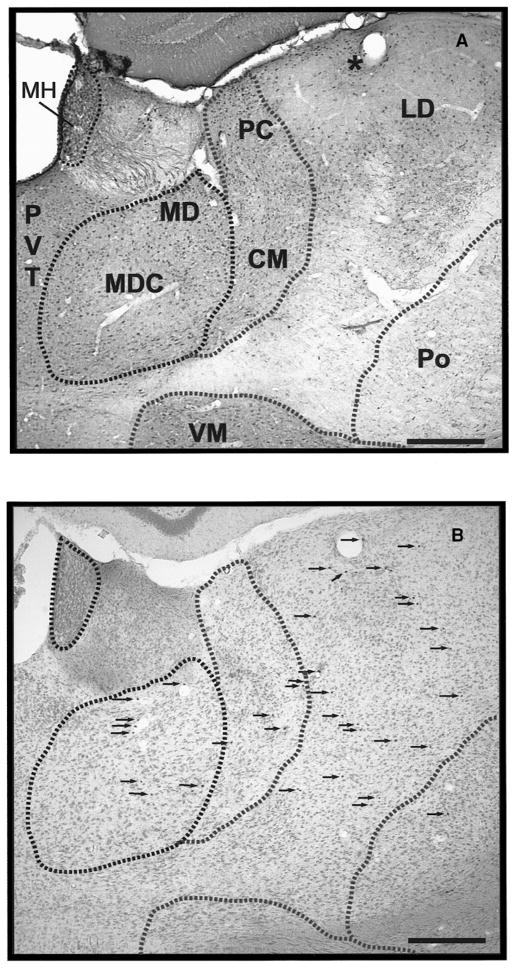
To delineate thalamic nuclei with the greatest concentration of mast cells, we used calbindin-D28k, calretinin, and parvalbumin as markers (Arai et al., 1992, 1994). Mast cells were concentrated in the dorsal thalamus, occurred rarely in the median eminence and epithalamus, and were largely absent from the rest of the diencephalon and overlying telencephalon. Figure 4 is a schematic of the location of mast cells along the rostral–caudal extent of the thalamus of male rats paired with stimulus females for 5 days. Rats paired with familiar cagemates for 5 days showed similar anatomical distribution of mast cells in the thalamus. In the anterior aspect of the thalamus (AP −0.92–2.51 mm) (Fig. 4A), mast cells are located in midline thalamic nuclei (PVT and paratenial, PT) identified by calbindin-D28k immunocytochemistry (Fig. 5). More laterally, in the anterior nuclear group (ANG), calbindin cell bodies were found in the anteromedial (AM) nucleus while the anterodorsal (AD), anteroventral dorsomedial (AVDM), and anteroventral ventrolateral (AVVL) nuclei were void of cell bodies. Calbindin-ir fibers were found in AD. Calretinin and parvalbumin fibers were found in AD, AVDM, and AM (Fig. 5 for calbindin-D28k). Mast cells were detected in the AM nucleus and its medial extension, the interanteromedial (IAM) nucleus. Few mast cells occur in the Reuniens (Re) nucleus, identified by staining of both calbindin and calretinin cell bodies. In the MD nucleus, identified by its rich calbindin-ir cell population except for the central part of the nucleus (Fig. 5), mast cells were present in both the inner and outer layers.
FIG. 4.
Schematic representations of anatomical distributions of mast cells in representative coronal sections through anterior (A, AP −1.4 mm), middle (B, AP −3.14 mm), or caudal (C, AP −4.8 mm) thalamus. Shaded areas on the left indicate occurrence of mast cells in thalamic nuclei labeled on the right. Anatomical coordinates are from Paxinos and Watson (1986). AD, anterodorsal; AH, anterior thalamus; AM, anteromedial; ANG, anterior nuclear group; APT, anterior pretectal; AR, acoustic radiations; AVDM, anteroventral dorsomedial part; AVVL, anteroventral ventrolateral part; CL, centrolateral; CM, centromedial; D3V, third ventricle; DM, dorsomedial hypothalamus; fi, fimbria; FR, fasciculus retroflexus; GP, globus pallidus; H, hippocampus; IAM, interanteromedial; IMD, inter-anteromedial; LD, laterodorsal; dLGN, dorsal lateral geniculate nucleus; LH, lateral habenula; LNG, lateral nuclear group; MD, mediodorsal; MGN, medial geniculate nucleus; MH, medial habenula; ml, medial lemniscus; MM, mammillary bodies; OX, optic chiasm; PC, paracentral; pc, posterior commissure; Po, posterior; PT, paratenial; PVT, paraventricular thalamic; Re, reunions; Rh, rhomboid; Rt, reticular; SFO, subfornical organ; sm, stria medularis; SNpr, substantia nigra pars reticulate; st, stria terminalis; Sub, subthalamic; VL, ventrolateral; VM, ventromedial; VMH, ventromedial hypothalamus; VP, ventroposterior.
FIG. 5.
Localization of brain mast cells in thalamic nuclei. (A) Thalamic nuclei identified with calbindin-D28K immunocytochemistry. (B) Localization of mast cells (arrows) identified with toluidine blue staining in adjacent sections. Note correspondence of anatomic features such as blood vessel indicated (*). Bar = 250 μm. CM, centromedial; LD, laterodorsal; MD, mediodorsal; MDC, mediodorsal (central); MH, medial habenula; PC, paracentral; Po, posterior.
In the middle aspect of the thalamus (AP −2.51–4.1 mm) (Fig. 4B), mast cells were identified in the nuclei of the intralaminar group, the centromedial (CM), centrolateral (CL), and the paracentral (PC) nuclei, which contain almost exclusively calbindin cell bodies and neurons. Mast cells were also present laterally in the posterior (Po) nucleus stained with calbindin cell bodies and fibers and the ventrolateral (VL) nucleus, which was devoid of calbindin cell body staining, but stained with calretinin and parvalbumin fibers. Proceeding caudad, mast cells were also noted in the medial habenula (MH) of the epithalamus, adjacent to the fasciculus retroflexus (FR), as well as in the VP nuclear group. The epithalamus stains very richly with calbindin cell bodies and fibers, while the VP is devoid of calbindin cell bodies, but stained with calretinin. Few mast cells were found in the lateral nuclear group (LNG) which was identified by staining with both calbindin and calretinin cell bodies with the exception of the caudal lateral dorsal (LD) nucleus, which is stained only with calbindin cell bodies (Fig. 5).
In the caudal aspect of the thalamus (AP −4.1–5.8 mm) (Fig. 4C), mast cells were noted predominantly in the MGN along the acoustic radiations (AR). Very few were observed in the dorsal lateral geniculate nucleus (dLGN). The dLGN was identified by a plexus of parvalbumin-positive fibers and also by strong labeling of calbindin neurons. The MGN was void of calbindin or calretinin cell bodies and fibers. The MGN was located medial and caudal to dLGN.
DISCUSSION
The present results demonstrate that in male rats, the brain mast population is influenced by the conspecific with which the animal is housed. The behavioral and endocrine conditions under which thalamic mast cell number is altered, cues leading to mast cell aggregation in these brain regions, where are mast cells localized in the thalamus, and the possible function of these immune system cells are discussed in turn.
Behavioral and Endocrine Conditions Affecting Brain Mast Cell Number
Sexually receptive females present a very complex set of stimuli. The various control groups indicate that several dimensions of this complex set of stimuli affect the increases in brain mast cell number. First, the alterations in behavioral and hormonal status of the female are key to obtaining the full response as exposure to OVX females was an insufficient stimulus. Second, physical contact with the OVX + EP female is necessary for the full response. However, olfactory, acoustic, or other sensory stimuli presented by OVX + EP females in the absence of physical contact were sufficient to produce a statistically significant increase in brain mast cell number. Finally, the duration of exposure of the male rat to an OVX + EP female is an important variable as pairing for 1 day did not increase brain mast cell number.
Our results extend previous studies showing that behavioral and/or endocrine factors alter the number of brain mast cells. A brief period (2 h) of courtship triggers the appearance of mast cells in the medial habenula of the epithalamus of ring doves (Silver, Ramos, and Silverman, 1992; Zhuang et al., 1993). This phenomenon also occurs following treatment of doves with either T, DHT, or E (Wilhelm et al., 2000). It is known that plasma levels of LH, DHT, and T increase dramatically in male ring doves within hours of courtship (Feder, Storey, Goodwin, Reboulleau, and Silver, 1977; Silver, Goldsmith, and Follett, 1980); hence, these steroid secretions may be the essential element in increasing the brain mast cell population. Similar increases in LH and T are induced by mating in male rats (Kamel and Frankel, 1978; Graham and Dejardins, 1980). One hypothesis, therefore, is that the increases in brain mast cells observed here were triggered, at least in part, by changes in LH and T.
Yang, Chien, and Lu (1999) demonstrated that the number of brain mast cells increased in male mice housed with females for up to 19 days, during which time pregnancy was confirmed. This increase in brain mast cells was observed in comparison with non-mated male mice. Our result that brain mast cells increase after 5 days of pairing with OVX + EP females, but not after 1 day of pairing is consistent with this progressive change. Yang et al. (1999) speculate that in this situation, brain mast cells help prepare the male for postparturition parenting behavior. Thus, it is interesting that Silverman et al. (2000) showed that 4-day-postpartum female rats had significantly higher mast cell numbers in the brain, than did virgin, age-and weight-matched controls.
In the present study, 5 days of continuous exposure to a novel male rat increased the number of brain mast cells, perhaps due to the fact that this pairing was a stressor due to the potential for aggressive attacks and territorial disputes. Thus, behavioral manipulations that are independent of reproductive function are also associated with changes in the number of brain mast cells. In mice, 20-min daily exposures to the same aggressive partner for 3 weeks increased the number of thalamic mast cells in the submissive mouse as compared to daily exposure to odors from the aggressive partner or placement in a new cage (Cirulli, Pistillo, De Acetis, Aleva, and Aloe, 1998). In another study, 30 min of immobilization stress was shown to induce intracranial rat mast cell degranulation and elevate the mast cell protease levels in the cerebrospinal fluid (Theoharides et al., 1995). This paradigm also increased the permeability of the blood–brain barrier (Esposito et al., 2001). Breakdown in the blood–brain barrier integrity induced by brain mast cell activation has been documented to precede the onset of clinical symptoms in multiple sclerosis (Goodin, Ebers, Johnson, Rodriguez, Sibley, and Wolinsky, 1999; Kermode, Thompson, Tofts, MacMannus, Kendall, Kingsley, Moseley, Rudge, and McDonald, 1990). Thus, it becomes important to determine the role of brain mast cells in various behavioral models such as immobilization stress, aggression, or isolation and to further our understanding of the role of brain mast cells in physiological or pathophysiological neural–immune interactions.
Mechanisms for Increases in Thalamic Mast Cell Number
Mast cell identification by their metachromasia to acidic toluidine blue was due to recognition of distinctive constituents of mast cells granules such as sulfated heparan and heparin proteoglycans. This is a technique previously used by us and others, in both rats and doves, which ensures proper detection of mast cells in the brain (Zhuang, Silverman, and Silver, 1999; Wilhelm et al., 2000; Florenzano and Bentivoglio, 2000).
The increases in brain mast cell numbers reported here could be due to in situ cell division of resident brain mast cells or they could migrate from the periphery in response to chemoattractants. Previous studies using BrDU in doves indicate that there was no discernible mitosis within the mast cell population in the MH (Silver, Kirwan, and Silverman, unpublished). We have found that mast cells labeled with a vital dye and injected intravascularly can enter the brain parenchyma, on the brain side of the capillary basal lamina (Silverman et al., 2000).
What mechanisms determine the specificity of the pattern of brain loci in which mast cells aggregate under the conditions studied here? Numerous mast cell chemoattractants have been described, including MCP-1, Rantes, and TGF-β; however, none have shown to be specific to the thalamic regions where we report increases in mast cell number. A chemokine mechanism has recently been described that may account for the aggregation of mast cells in the dorsal thalamus, but not other brain areas. Endothelial cells in blood vessels that supply the dorsal thalamus and choroid plexus (Banisadr, Dicou, Berbar, Rostene, Lombet, and Haour, 2000) have been shown to express CXCR4 receptors, which avidly bind to the chemokine, stromal cell-derived factor 1α (SDF-1). SDF-1 is a CXC chemokine originally isolated from a bone marrow stromal cell line (Nagasawa, Kikutani, and Kishimoto, 1994; Tashiro, Tada, Heilker, Shirozu, Nakano, and Honjo, 1993). In the brain, SDF-1 was shown to induce the migration of microglial cells and astrocytes (Tanabe, Heesen, Yoshizawa, Berman, Luo, Bleul, Springer, Okuda, Gerard, and Dorf, 1997). However, this ligand has also been shown to be a potent chemoattractant for mast cells and to facilitate mast cell transmigration through the umbilical cord blood vessel lamina without inducing degranulation (Lin, Issekutz, and Marshall, 2000). Only two arteries, the dorsal thalamic artery and the anterior choroidal artery, supply the dorsal thalamus (Scremin, 1995), in particular the nuclei in which mast cells increased in number during the pairing situations in our study. Thus, a chemokine specialization of the vasculature of the dorsal thalamus might attract mast cells. What other specialized mechanisms regulate their subsequent entry into the brain parenchyma or otherwise affect their function in these areas are currently unknown.
Localization and Function of Thalamic Mast Cells
Another goal of this study was to analyze the anatomical localization of brain mast cells. We concentrated on the diencephalon as previous reports indicated that in unstimulated rats, brain mast cells occur mainly in the dorsal thalamic nuclei, although some are also found in the leptomeninges, olfactory bulb, hypothalamus, and mesencephalon (Dropp, 1976; Persinger, 1977; Ibrahim, Wirr, and Bahuth, 1979; Goldschmidt et al., 1984; Theoharides, 1996).
Increases in brain mast cell number following exposure of male rats to stimulus females were limited to specific thalamic areas, including the anterior nuclear group, the VPN, the intralaminar nuclei, the VL, MGN and dLGN. Mast cells therefore increased in nuclei of each of the functional subcategories of the thalamus, i.e., sensory (e.g., VP, Po, dLGN, MGN), motor (e.g., VM), and limbic (e.g., MD, PVT, PT, IAM) areas (Price, 1995). This may reflect the fact that reproductive pairing involves numerous somatosensory, visual, and auditory stimuli, complicated motor responses, and motivational and emotional (i.e., “limbic”) processes. In regard to the latter, two examples are noteworthy. The ANG receives dense projections from the hippocampal formation and the mammillary nuclei and projects to the cingulate, medial prefrontal, and orbital cortical areas. In addition, the MD receives inputs from the primary olfactory cortex, the amygdala, the entorhinal cortex, and the brainstem monoaminergic nuclei and projects to the anterior cingulate cortex and several hypothalamic areas (Price, 1995; Bentivoglio, Kultas-Ilinsky, and Ilinsky, 1993). Investigations into the neural control of male copulatory behavior have been focused on forebrain pathways (Meisel and Sachs, 1994). Based on our anatomical localization, it appears that these pathways send or receive projections to thalamic nuclei that have shown an increase in the number of mast cells in our study. For example, damage to the olfactory system produces severe copulatory deficits, and lesions of the amygdala or the stria terminalis disrupt copulation in male rats by altering the ejaculation latencies.
It is of interest to speculate on the functions of mast cells. Rat mast cells located in the bladder, upper airways, and dura mater and in the peritoneum are positive for estrogen receptor α (Pang, Marchand, Sant, Kream, and Theoharides, 1995; Vliagoftis, Dimitriadou, Boucher, Rozniecki, Correia, Raam, and Theoharides, 1992). We have now documented that rat thalamic mast cells express estrogen receptor α (Asarian, Silverman, and Silver, unpublished). As mentioned above, gonadal steroids modulate mast cell activation in vivo (Wilhelm et al., 2000) and estradiol augments histamine secretion in response to substance P, which is a potent mast cell degranulator. It is thus possible that gonadal steroids or other neuromodulators such as substance P may act on brain mast cells to modulate physiological responses underlying social behaviors. Mast cells secrete powerful vasodilators including substance P, calcitonin gene-related peptide, and nitric oxide (reviewed in Silver et al., 1996). One can speculate that these serve to increase vasodilation of blood vessels in specific thalamic regions, thus increasing the flow of specific neuroendocrine factors into these regions.
Mast cells have been implicated in the acute-phase response following bacterial infection. Mast cell-deficient mice have up to 80% mortality (compared with no mortality in the wild-type mice), and their rate of bacterial clearance is 20-fold less than that of wild-type mice (Echtenacher et al., 1996; Malaviya et al., 1996). While the foregoing studies did not attempt to localize the site of mast cell actions, our work on peripheral bacterial lipopolysaccharide-injected rats support a role for brain mast cells in the behavioral aspect of the acute-phase response in rats (Asarian, Silverman, and Silver, unpublished data). It will be of interest to determine whether or not the brain locus of mast cell action is in the hypothalamus, a site known to be involved in nervous system responses to infection. In the present study, we did not observe any hypothalamic mast cells, though small numbers of hypothalamic mast cells have been reported in other studies (Pang et al., 35 mast cells/rat; Cocchiara et al., 5 mast cells/rat). No evidence is available, to our knowledge, on whether the neural localization of mast cells is unique to each behavioral/endocrine/infectious state.
In summary, brain mast cell numbers increase in the thalami of male rats in response to behavioral and/or endocrine stimuli. The presence of mast cells in some but not all dorsal thalamic nuclei suggests that the migration of mast cells depends on chemotactic factors produced therein. Given their rapid ability to de-granulate and release mediators, the presence of mast cells in the brain following normal behavioral manipulations represents a highly significant neural–immune control mechanism modulating normal brain function.
Acknowledgments
We thank Dr. David Ruggerio and Dr. Lique Coolen for helpful discussions. This work was supported by NIMH Grants 29380 (R.S.), 54088 (A.J.S.), and NRSA 528831 (L.A.).
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Abraham SN, Malaviya R. Mast cell modulation of the innate immune response to enterobacterial infection. Adv Exp Med Biol. 2000;479:91–105. doi: 10.1007/0-306-46831-X_8. [DOI] [PubMed] [Google Scholar]
- Arai M, Arai R, Kani K, Jacobowitz DM. Immunohistochemical localization of calretinin in the rat lateral geniculate nucleus and its retino-geniculate projection. Brain Res. 1992;596:215–222. doi: 10.1016/0006-8993(92)91550-x. [DOI] [PubMed] [Google Scholar]
- Arai R, Jacobowitz DM, Deura S. Distribution of calretinin, calbindin-D28k, and parvalbumin in the rat thalamus. Brain Res Bull. 1994;33:595–614. doi: 10.1016/0361-9230(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Dicou E, Berbar T, Rostene W, Lombet A, Haour F. Characterization and visualization of [125I] stromal cell-derived factor-1 alpha binding to CXCR4 receptors in rat brain and human neuroblastoma cells. J Neuroimmunol. 2000;110:151–160. doi: 10.1016/s0165-5728(00)00338-6. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M, Kultas-Ilinsky K, Ilinsky I. Limbic thalamus: Structure, intrinsic organization, and connections. In: Vogt BA, Gabriel M, editors. Neurobiology of Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Birkhauser; Boston: 1993. pp. 71–122. [Google Scholar]
- Cirulli F, Pistillo L, De Acetis L, Alleva E, Aloe L. Increased number of mast cells in the central nervous system of adult male mice following chronic subordination stress. Brain Behav Immunol. 1998;12:123–133. doi: 10.1006/brbi.1998.0505. [DOI] [PubMed] [Google Scholar]
- Cocchiara R, Albeggiani G, Lampiasi N, Bongiovanni A, Azzolina A, Geraci D. Histamine and tumor necrosis factor-alpha production from purified rat brain mast cells mediated by substance P. NeuroReport. 1998;10:575–578. doi: 10.1097/00001756-199902250-00024. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the central nervous system of several rodents. Anat Rec. 1972;174:227–237. doi: 10.1002/ar.1091740207. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in mammalian brain. Acta Anat (Basel) 1976;94:1–21. doi: 10.1159/000144540. [DOI] [PubMed] [Google Scholar]
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood–brain-barrier through activation of brain mast cells. Brain Res. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Feder HH, Storey A, Goodwin D, Reboulleau C, Silver R. Testosterone and “5alpha-dihydrotestosterone” levels in peripheral plasma of male and female ring doves (Streptopelia risoria) during and reproductive cycle. Biol Reprod. 1977;16:666–677. doi: 10.1095/biolreprod16.5.666. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density and distribution of mast cells in the rat thalamus: A light and electron microscopic study in basal conditions and after intrace-rebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–669. [PubMed] [Google Scholar]
- Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- Fox CC, Jewell SD, Whitacre CC. Rat peritoneal mast cells present antigen to a PPD-specific T cell line. Cell Immunol. 1994;158:253–264. doi: 10.1006/cimm.1994.1272. [DOI] [PubMed] [Google Scholar]
- Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;1:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- Goldschmidt RC, Hough LB, Glick SD, Padawer J. Mast cells in rat thalamus: Nuclear localization, sex difference and left–right asymmetry. Brain Res. 1984;323:209–217. doi: 10.1016/0006-8993(84)90291-9. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Ebers GC, Johnson KP, Rodriguez M, Sibley WA, Wolinsky JS. The relationship of MS to physical trauma and psychological stress. Neurology. 1999;52:1737–1745. doi: 10.1212/wnl.52.9.1737. [DOI] [PubMed] [Google Scholar]
- Graham JM, Desjardins C. Classical conditioning: Induction of luteinizing hormone and testosterone secretion in anticipation of sexual activity. Science. 1980;210:1039–1041. doi: 10.1126/science.7434016. [DOI] [PubMed] [Google Scholar]
- Hays WL. Statistics for Psychologists. Holt, Rinehart & Winston; New York: 1963. pp. 336–356. [Google Scholar]
- Ibrahim MZ, Al Wirr ME, Bahuth N. The mast cells of the mammalian central nervous system. III. Ultrastructural characteristics in the adult rat brain. Acta Anat (Basel) 1979;104:134–154. doi: 10.1159/000145062. [DOI] [PubMed] [Google Scholar]
- Johnson D, Krenger W. Interactions of mast cells with the nervous system: Recent advances. Neurochem Res. 1992;17:939–951. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- Kamel F, Frankel AI. The effect of medial preoptic area lesions on sexually stimulated hormone release in the male rat. Horm Behav. 1978;10:10–21. doi: 10.1016/0018-506x(78)90020-x. [DOI] [PubMed] [Google Scholar]
- Kermode AG, Thompson AJ, Tofts P, MacManus DG, Kendall BE, Kingsley DP, Moseley IF, Rudge P, McDonald WI. Breakdown of the blood–brain barrier precedes symptoms and other MRI signs of new lesions in multiple sclerosis: Pathogenetic and clinical implications. Brain. 1990;113(Pt. 5):1477–1489. doi: 10.1093/brain/113.5.1477. [DOI] [PubMed] [Google Scholar]
- Lin T-J, Issekutz TB, Marshall JS. Human mast cells transmigrate through human umbilical vein endothelial monolayers and selectively produce IL-8 in response to stromal cell-derived factor-1. α J Immunol. 2000;165:211–220. doi: 10.4049/jimmunol.165.1.211. [DOI] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham S. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–79. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Abraham SN. Studies of the multi-faceted mast cell response to bacteria. Curr Opin Microbiol. 2001;4:260–266. doi: 10.1016/s1369-5274(00)00200-9. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Sachs BD. The physiology of male sexual behavior. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 3–105. [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC. Definitive characterization of rat hypothalamic mast cells. Neuroscience. 1996;73:889–902. doi: 10.1016/0306-4522(95)00606-0. [DOI] [PubMed] [Google Scholar]
- Pang X, Marchand J, Sant GR, Kream RM, Theoharides TC. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744–750. doi: 10.1111/j.1464-410x.1995.tb07384.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Stereotaxic Coordinates. 2. Academic Press; Sydney: 1986. [Google Scholar]
- Persinger MA. Mast cells in the brain: Possibilities for physiological psychology. Physiol Psychol. 1977;5:166–176. [Google Scholar]
- Persinger MA. Handling factors not body marking influence thalamic mast cell numbers in the preweaned albino rat. Behav Neural Biol. 1980;30:448–459. doi: 10.1016/s0163-1047(80)91283-2. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow L-M. Cellular and molecular mechanisms of female reproductive behaviors. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 107–220. [Google Scholar]
- Price JL. Thalamus. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney: 1995. pp. 629–648. [Google Scholar]
- Saper CB. Any way you cut it: A new journal policy for the use of unbiased counting methods. J Comp Neurol. 1996;364:5. doi: 10.1002/(SICI)1096-9861(19960101)364:1<5::AID-CNE1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS. Light–dark differences in behavioral sensitivity to oxytocin. Behav Neurosci. 1991;105:487–492. doi: 10.1037//0735-7044.105.3.487. [DOI] [PubMed] [Google Scholar]
- Scremin OU. Cerebral vascular system. In: Paxinos G, editor. The Rat Nervous System. Academic Press; Sydney: 1995. pp. 3–35. [Google Scholar]
- Silver R, Goldsmith AR, Follett BK. Plasma luteinizing hormone in male ring doves during the breeding cycle. Gen Comp Endocrinol. 1980;42:19–24. doi: 10.1016/0016-6480(80)90252-x. [DOI] [PubMed] [Google Scholar]
- Silver R, Ramos CL, Silverman AJ. Sexual behavior triggers the appearance of non-neuronal cells containing gonadotropin-releasing hormone-like immunoreactivity. J Neuroendocrinol. 1992;4:207–210. doi: 10.1111/j.1365-2826.1992.tb00160.x. [DOI] [PubMed] [Google Scholar]
- Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: Evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen M, Muller C, Arnold M, Hultner L, Klein-Hessling S, Neudorfl C, Reineke T, Serfling E, Schmitt E. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-kappa B is decisively involved in the expression of IL-9. J Immunol. 2001;166:4391–4398. doi: 10.4049/jimmunol.166.7.4391. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol. 1997;159:905–911. [PubMed] [Google Scholar]
- Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: A cloning strategy for secreted protein and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: The immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. The mast cell: A neuroimmunoendocrine master player. Int J Tissue React. 1996;18:1–21. [PubMed] [Google Scholar]
- Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: A corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Thery C, Mazzeo D, Tenza D, Raposo G, Bonnerot C. Secretory granules of mast cells accumulate mature and immature MHC class II molecules. J Cell Sci. 2001;114:323–334. doi: 10.1242/jcs.114.2.323. [DOI] [PubMed] [Google Scholar]
- Vliagoftis H, Dimitriadou V, Boucher W, Rozniecki JJ, Correia I, Raam S, Theoharides TC. Estradiol augments while tamoxifen inhibits rat mast cell secretion. Int Arch Allergy Immunol. 1992;98:398–409. doi: 10.1159/000236217. [DOI] [PubMed] [Google Scholar]
- Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, King B, Silverman AJ, Silver R. Gonadal steroids regulate the number and activational state of mast cells in the medial habenula. Endocrinology. 2000;141:1178–1186. doi: 10.1210/endo.141.3.7352. [DOI] [PubMed] [Google Scholar]
- Yang M, Chien C, Lu K. Morphological, immunohistochemical and quantitative studies of murine brain mast cells after mating. Brain Res. 1999;846:30–39. doi: 10.1016/s0006-8993(99)01935-6. [DOI] [PubMed] [Google Scholar]
- Yano H, Wershil BK, Arizono N, Galli SJ. Substance P-induced augmentation of cutaneous vascular permeability and granulocyte infiltration in mice is mast cell dependent. J Clin Invest. 1989;84:1276–1286. doi: 10.1172/JCI114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Reproductive behavior, endocrine state, and the distribution of GnRH- like immunoreactive mast cells in dove brain. Horm Behav. 1993;27:283–295. doi: 10.1006/hbeh.1993.1021. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the fore-brain. J Comp Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Zucker I. Progesterone in the experimental control of the behavioural sex cycle in the female rat. J Endocrinol. 1967;38:269–277. doi: 10.1677/joe.0.0380269. [DOI] [PubMed] [Google Scholar]