Abstract
Loss of E-cadherin has been associated with human cancers, and yet in the early mouse embryo and the lactating mammary gland, the E-cadherin null state results in tissue dysfunction and cell death. Here we targeted loss of E-cadherin in skin epithelium. The epidermal basal layer responded by elevating P-cadherin, enabling these cells to maintain adherens junctions. Suprabasal layers upregulated desmosomal cadherins, but without classical cadherins, terminal differentiation was impaired. Progressive hyperplasia developed with age, a possible consequence of proliferative maintenance in basal cells coupled with defects in terminal differentiation. In contrast, hair follicles lost integrity of the inner root sheath and hair cuticle without apparent elevation of cadherins. These findings suggest that, if no compensatory mechanisms exist, E-cadherin loss may be incompatible with epithelial tissue survival, whereas partial compensation can result in alterations in differentiation and proliferation.
Keywords: gene targeting, adherens junction, hair follicle, epidermis, tumorigenesis
Classical cadherins function in intercellular adhesion, polarization, and differentiation. The prototype is E-cadherin, a transmembrane protein that makes calcium-dependent homotypic adhesive interactions, known as adherens junctions (AJs) (1, 2). To establish efficient cell–cell junctions, E-cadherin uses its cytoplasmic domain to couple to catenins and the actin cytoskeleton. This association sets the classical cadherins apart from desmosomal cadherins, which form a more robust adhesive interaction (desmosome) through linking to plakoglobin, desmoplakin, and the intermediate filament cytoskeleton (3, 4).
Many cells display AJs and desmosomes, which function coordinately in epithelial sheet formation (5). Whereas desmosomes are particularly important in tissues such as muscle and epidermis that undergo substantial mechanical stress, AJs have a prominent role in remodeling epithelial cell–cell interactions. This is especially critical in early development, as reflected by the blastula defects that occur when E-cadherin is targeted for ablation in mouse (6).
AJs also participate in regulating the balance between proliferation and differentiation. Down-regulation and/or mutations in E-cadherin and α-catenin occur in a number of different tumors (7, 8), and multiple mechanisms have been uncovered in controlling this link (9–11). Thus it is surprising that loss-of-function mutations in the E-cadherin gene result in cell death in mouse blastocysts (6) and in lactating mammary gland (12). Adding to these complexities, many cell types express multiple cadherins, which in at least some cases do not seem to be functionally equivalent (7, 11–14).
To gain further insights into E-cadherin function, we have engineered and analyzed a conditional knockout (KO) of E-cadherin in epidermis and its appendages. Here, we report on the striking differences by which epidermis and hair follicles handle the loss of this critical protein. Through analyses of these different stem cell lineages in skin, we have obtained insights into our understanding of how some tissues respond to E-cadherin loss by progression to hyperproliferation, whereas others lose integrity and degenerate.
Materials and Methods
Immunofluorescence and Antibodies. Primary antibodies used were E-cadherin (M. Takeichi, Zymed), P-cadherin and p120 (Zymed), α- and β-catenin (Sigma), desmoglein 1,2 [1:100, W. Franke (German Cancer Research Center, Heidelberg)], desmoplakin (Research Diagnostic, Flanders, NJ), laminin 5 [1:100, R. Burgeson (University School of Medicine, Chiba, Japan)], β4-integrin (Pharmingen), keratin 1 (1:250, E.F.), keratin 5 (1:100, E.F.), keratin 6 [1:500, P. Coulombe (Johns Hopkins School of Medicine, Baltimore)], Ki67 (1:200, NovoCastra, Newcastle, U.K.), plakoglobin [1:50, P. Cowin (New York University School of Medicine, New York)], AE13 [1:10, T. T. Sun (New York University School of Medicine, New York)], GATA-3 (1:25, Santa Cruz Biotechnology, HCG3), involucrin (1:100, Babco, Richmond, CA), filaggrin (Covance, Berkeley, CA, PRB-417P), and loricrin (1:100, E.F.). Alexa 488 (Molecular Probes) or Texas red (The Jackson Laboratory) conjugated secondary antibodies were used for detection. Additional reagents were TRITC-Phalloidin and 4′,6-diamidino-2-phenylindole (DAPI; Sigma). Unless stated, dilutions were according to the manufacturer's recommendations.
Tissues were frozen, embedded in OCT compound, and sectioned (10 μm). Fixation was with 4% formaldehyde in PBS for 8 min. Slides were blocked with PBS, 0.2% Triton X-100, 1% BSA, 5% normal goat serum, 5% normal donkey serum, or MOM Basic kit (Vector Laboratories).
Electron Microscopy. Tissues were fixed for ≥1 h in 2% glutaraldehyde, 4% formaldehyde, 2 mM CaCl2 in 0.05 M sodium cacodylate buffer, and then processed for Epon embedding (16). Samples were visualized with a Tecnai (Hillsboro, OR) G2 transmission electron microscope.
Protein Analysis. Newborn backskin was incubated in dispase for 30 min at 37°C. Epidermis was flash-frozen in liquid nitrogen and then crushed. Typically, proteins were then extracted and processed for Western blot analysis (5, 10). For detection of E-cadherin, crushed tissue was resuspended in 1 ml of radioimmunoprecipitation assay (RIPA) buffer with 1 mM DTT and protease inhibitors, and after 30 min, extracts were centrifuged at 14,000 × g for 15 min. Anti-E-cadherin-coated protein G-Sepharose beads were incubated with extracts for 2 h, washed three times with RIPA buffer, and then boiled in Laemmli buffer to release bound proteins, which were analyzed by SDS/PAGE and anti-E-cadherin Western blotting.
Results
Mice lacking E-cadherin in the skin epithelium were made by mating keratin 14-Cre recombinase mice (15) with E-cadherin mice harboring loxP sites flanking exons 6–10 (12). Newborn pups were smaller and displayed reduced activity as well as alterations in whisker size and number (Fig. 1A; see below). Pups with these phenotypic aberrations were genotypically null for the normal E-cadherin allele, which resulted in near quantitative loss of protein (Fig. 1B). Immunofluorescence revealed the absence of anti-E-cadherin labeling in skin epithelium (Fig. 1C). These data corroborated the efficacy of the targeting event.
Fig. 1.
Generation of mice conditionally null for E-cadherin in skin epithelium. (A) Animals whose epidermis is either heterozygous or homozygous for the WT E-cadherin allele are referred to as WT; those whose epidermis is homozygous for the K14-Cre-recombined mutant E-cadherin allele are called KO. Shown are newborn pups. (B) PCR confirmation of genotype (Top and Middle) and anti-E-cadherin (EC) Western blot of epidermal proteins (Bottom). (C and C′) Immunofluorescence of skin sections labeled with Abs indicated (color coding is according to secondary antibodies). * denotes nonspecific staining of cornified layer with the secondary FITC-conjugated Ab. (D–E′) Toluidine blue-stained semithin sections (1 μm) of newborn backskin. BL, basal layer; SL, spinous layer; GL, granular layer; CL, cornified layer. (E and E′ are 5× the magnification of D and D′.) Arrows denote regions where intercellular spaces are widened. epi, epidermis, de, dermis; hf, hair follicle.
Toluidine blue-stained semithin sections of newborn KO skin revealed only minor changes in epidermal architecture (Fig. 1 D and D′). All four morphologically distinct differentiation stages were visible: a single basal layer of proliferative cells, differentiating desmosome-rich spinous layers, a keratohyalin granule-rich granular layer, and the dead enucleated stratum corneum layers at the skin surface. In some regions of newborn KO skin (e.g., those shown here), the epidermis appeared similar to WT, but in others, it was thickened. In addition, intercellular membrane gaps were sometimes seen between basal and spinous layers and often near downgrowths of developing KO follicles (arrows in Fig. 1E′). However, most cells within the basal layer appeared to establish normal contacts even at the ultrastructural level (not shown).
Overall, the morphological defects in E-cadherin null epidermis were significantly less severe than those of α-catenin null epidermis, conditionally targeted with the same K14-Cre animals (10). To probe more deeply into the molecular explanation underlying this difference, we first tested for possible expression of other classical cadherin proteins. Anti-N-cadherin immunofluorescence was not detected in either WT or KO newborn skin epithelium (data not shown). Intriguingly, however, whereas anti-P-cadherin immunoreactivity is normally strong only in hair follicle downgrowths (17), it was strong throughout the KO basal epidermal layer (Fig. 2A′). Although overexposure revealed only very weak anti-P-cadherin in the KO suprabasal cells, this was not seen in WT skin. Western blot analyses verified that this change was not merely a reflection of antigen unmasking as a consequence of the loss of E-cadherin but rather a bona fide increase in P-cadherin protein (Fig. 2I′).
Fig. 2.
Up-regulation of P-cadherin in basal but not suprabasal cells results in a selective loss of AJ components in the differentiating cells of E-cadherin null epidermis. (A–H′) Sections of newborn backskin were processed for indirect immunofluorescence microscopy using the Abs indicated. In some cases, 4′,6-diamidino-2-phenylindole was used to identify the nuclei. Markers are specific for: P-cadherin (PC), AJs; Laminin 5 (Lam5), basement membrane; β4 integrin (β4) and keratin 5 (K5), basal layer (BL); α-catenin (αcat), β-catenin (βcat), and p120-catenin (p120), AJs; desmogleins 1 and 2 (Dsg1,2), desmoplakin (DP), and plakoglobin (PG), desmosomes; keratin 6 (K6), suprabasal cells of hyperproliferative epidermis and the companion layer of hair follicles (hf). (I) Western blot analyses of epidermal proteins. Monospecific Abs used to probe the blots are indicated (Right).
The paucity of P-cadherin and absence of E-cadherin in the suprabasal layers led us to wonder whether AJ formation might be compromised in spinous cells. To test this possibility, we conducted immunofluorescence with antibodies against other AJ proteins. As shown in Fig. 2 B–D, the basal layer of both WT and KO epidermis displayed similar fluorescence intensities with antibodies against α-, β-, and p120-catenin. In contrast, suprabasal layers of KO, but not WT, epidermis exhibited a marked decrease in AJ antibody fluorescence. As judged by Western blot analysis, α- and β-catenin levels were consistently decreased in KO epidermis (Fig. 2I). The reduction was always more striking for α-catenin, consistent with the facts that (i) β- but not α-catenin can associate with desmosomes (18), and (ii) the cytoplasmic pools of β-catenin are larger (9). These factors may also explain why p120-catenin protein levels were largely similar between WT and KO epidermis, despite a reduction in intensity of anti-p120 immunoreactivity in suprabasal KO cells.
Although overall AJ protein levels were down-regulated suprabasally, desmosomal cadherins were up-regulated in KO spinous layers. This was true at the levels of both immunofluorescence (Fig. 2E) and Western blot (Fig. 2I). Although overall levels of desmoplakin and plakoglobin were largely similar between KO and WT epidermis (Fig. 2 F and G), these proteins are not components of the core plaque but rather are involved in linkage of desmosomes to the intermediate filament cytoskeleton (3, 4).
In addition to the decrease in AJ proteins and increase in desmosomal cadherins, anti-keratin 6 immunoreactivity was seen in some suprabasal cells of E-cadherin null epidermis (Fig. 2 H and I). Characteristic of hyperproliferative skin, this feature correlated with the increased thickness of KO epidermis, which became more pronounced with age (see below).
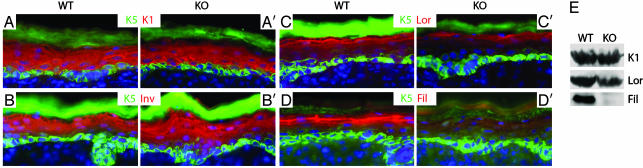
To assess whether the changes in adhesive components of KO skin epidermis might influence other aspects of differentiation, we examined normal differentiation-specific markers of the tissue. Because basal cells withdraw from the cell cycle and commit to differentiate, they switch off expression of keratins 5 and 14 and switch on K1 and K10. KO epidermis still executed this switch at the proper stage in differentiation (Fig. 3 A and A′). Similarly, the spinous cell marker involucrin was also expressed in a pattern similar to WT epidermis (Fig. 3 B and B′).
Fig. 3.
Reduced differentiation in the granular layer of E-cadherin null epidermis. (A–D′) Immunofluorescence microscopy of sections of newborn backskins labeled with the Abs indicated: K5, specific for the basal epidermal layer and follicle ORS; K1, specific for suprabasal layers; Inv, involucrin, expressed in the spinous layer but an early marker of the cornified envelope; Lor, loricrin, a granular marker of the cornified envelope; Fil, filaggrin, expressed as profilaggrin in the granular layer and then processed near or at the end of terminal differentiation. (E) Western blot analyses of epidermal proteins. Abs used to probe the blots are indicated on the right.
As cells move toward the skin surface, they remain transcriptionally active, and in the granular layer, they express profilaggrin, which is processed to filaggrin as keratinohyalin granules form. Granular cells also express loricrin, a major constituent of the cornified envelope that serves as a scaffold to organize the lipids of the epidermal barrier. Despite the morphological presence of a granular layer, anti-filaggrin and anti-loricrin immunoreactivity were down-regulated in KO relative to WT epidermis (Fig. 3 C and D′). Western blot analyses revealed very little processed filaggrin (27 kDa) as well as reduced loricrin levels (Fig. 3E). These granular layer perturbations were suggestive of a defect in the epidermal barrier. The defect was likely to be mild, because animals with overt barrier function defects dehydrate and die shortly after birth (19), whereas E-cadherin conditionally null mice sometimes survived to adulthood. We return to this point below.
In the adult, the most dramatic defect of E-cadherin conditionally null animals was not at the skin surface, but rather in their hair coat (Fig. 4). Animals also tended to be smaller and displayed abnormally short and misshapen whiskers and sparse pelage hairs. Such abnormalities made mosaic animals distinctive.
Fig. 4.
Hair and epidermal abnormalities in adult E-cadherin conditionally null mice. (A and B) Backlit images of adult snouts. (C and D) Example of a full adult KO (C) and mosaic (D) mice from a postnatal day 10 litter. (E–H) Hematoxylin/eosin-stained sections of frozen backskins of the ages indicated. Arrows denote hairs that broke through the skin surface (rare in KO skin). Arrowheads denote misangled KO follicles; asterisks, follicle remnants. epi, epidermis; de, dermis, sf, subcutaneous fat. (I and J) Immunofluorescence of sections of adult backskins labeled with Abs against the proliferation marker, Ki67. Nuclei were counterlabeled with 4′,6-diamidino-2-phenylindole (DAPI). Dotted line indicates basement membrane.
The defects in hair coat were not due to an inability to initiate placodes or progress through the early stages of follicle morphogenesis. By postnatal day 10, mature follicles had formed in normal numbers and spacing and at first glance, WT and KO skin appeared similar (Fig. 4 E and F). Closer inspection revealed that hairs lacking E-cadherin were abnormally short and fragile, and the orifices were not always properly formed (arrows in Fig. 4 E and F). Additionally, whereas the long WT follicles of the growth (anagen) phase were readily captured within the plane of sectioning, this was not the case for KO follicles, reflecting their bent irregular shape (arrowheads in Fig. 4 F and G).
The second hair cycle normally initiates at ≈3 wk of age. Thereafter, follicles cycle regularly although less synchronously, leading to a mixture of cycling and resting follicles in adult backskin. At these ages, it was readily apparent that with each postnatal hair cycle, the E-cadherin conditionally null follicles became progressively more aberrant. By 1.5 mo, the divergence in angling was striking (Fig. 4G, arrowheads). In addition, whereas 1.5-mo WT skin displayed regularly spaced hair follicles (not shown), KO skin exhibited expanded interfollicular gaps, and by 4 mo, only remnants of follicles were detected (Fig. 4 G and H). In addition, increased cellularity was detected in the dermis along with a reduction in subcutaneous fat.
Normal adult skin is typically thinner than newborn skin, a difference manifested primarily in the suprabasal layers (data not shown; see ref. 20). As E-cadherin null epidermis aged, however, many regions remained thickened (Fig. 4H). Adult KO epidermis displayed suprabasal anti-K6 immunoreactivity (not shown; see Fig. 2H), as well as a marked increase in the number of basal cell nuclei positive for the proliferating nuclear antigen Ki67 (Fig. 4 I and J). Overall, these differences were indicative of a hyperproliferative state.
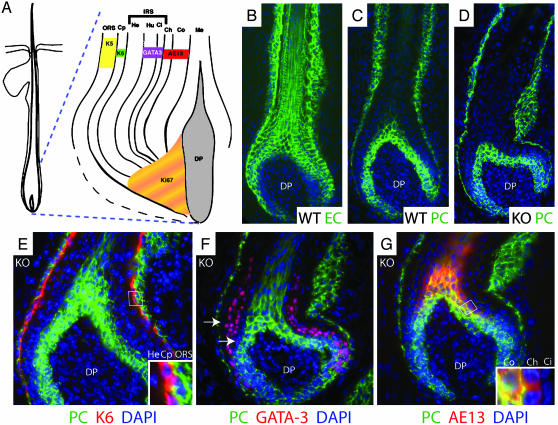
To begin to understand how a loss of E-cadherin could lead to progressive hyperproliferation in epidermis but progressive loss of hair follicles, we analyzed the expression patterns of cadherins and differentiation-specific markers in the hair follicle. Fig. 5A provides a schematic of the concentric rings of differentiating hair follicle cells that arise from the proliferative compartment (matrix), which is in contact with the specialized mesenchyme (dermal papilla) at the base of the follicle. Because the program of differential cadherin expression is comparable in whisker and backskin follicles, we show data for whiskers, where layers are more readily distinguished.
Fig. 5.
Expression of cadherins and differentiation markers in hair follicles. (A) Diagram of a hair follicle highlighting the different cell layers and their associated differentiation markers (16, 22). ORS, outer root sheath; Cp, companion layer; IRS, inner root sheath; He, Henle layer; Hu, Huxley layer; Ci, cuticle of the IRS; Cu, cuticle of the hair shaft; Co, cortex; Me, medulla; DP, dermal papilla. (B–G) Immunofluorescence of sections of newborn whisker follicles labeled with the indicated Abs: EC, E-cadherin; PC, P-cadherin; AE13, specific for the hair shaft keratins; GATA-3 and K6, 4′,6-diamidino-2-phenylindole (DAPI) in blue. (Insets) Magnified views of boxed regions. Arrows denote P-cadherin-negative cells layers flanking the GATA-3 positive Huxley and IRS cuticle layers. [A is reproduced with permission from ref. 22 (Copyright 2003, The Rockefeller University Press).]
In normal skin, matrix, outer root sheath (ORS), cortex, and medulla express both P- and E-cadherin (Fig. 5 B and C) (21). In contrast, other follicle regions express E- and not P-cadherin (17). In E-cadherin null follicles, the pattern and levels of P-cadherin appeared largely unchanged (Fig. 5D), as did that of Dsg1,2 (data not shown). In this regard, KO follicles behaved differently than KO epidermis.
Colabeling with anti-P-cadherin and antibodies against the differentiation-specific markers enabled us to assess differentiation and identify precisely which KO follicle layers lacked both E- and P-cadherin. In contrast to the epidermis, the programs of differentiation were largely indistinguishable for KO and WT follicles, at least at early stages, which permitted assessment of primary rather than secondary effects (Fig. 5 E–G; data shown for KO follicles).
The single layer of companion cells is readily identified by anti-K6 antibodies (Fig. 5E). This layer marked the boundary between P-cadherin-positive ORS and -negative inner root sheath (IRS). The transcription factor GATA-3, which identifies the Huxley layer and IRS cuticle (16), revealed a two-layer gap between GATA-3-positive cells and P-cadherin-positive ORS (Fig. 5F). These two P-cadherin-negative layers are the Henley and companion layers (Fig. 5A). Finally, colabeling with the hair keratin marker AE13 showed that all but the outermost (cuticle) layer of the shaft was also positive for P-cadherin (G). Taken together, these data established a zone of P- and E-cadherin-negative cells that encompassed the companion layer, IRS, and hair shaft cuticle of the KO follicle.
Ultrastructural analyses revealed the structural consequences associated with the loss of both P- and E-cadherin. As expected, the bulb of the E-cadherin null follicle displayed intact intercellular adhesion with normal AJs (Fig. 6A; for extensive data on WT follicles, see refs. 16 and 22). Just above the pocket of dermal papilla cells, however, adhesive defects were obvious. Although the largest intercellular gaps (Fig. 6A, asterisks) were in the Huxley layer of the IRS, AJs were missing between the companion layer and the electron-dense Henle layer and between the Henle layer and the trichohyalin-rich Huxley layer (Fig. 6B). The finger-like connections between these layers were desmosomes, which were intact and in comparable numbers to WT follicles. Desmosomes were abundant in KO Henle–Henle cell junctions as they are in WT, and correspondingly, adhesion between Henle cells was largely intact (Fig. 6 B and C). A paucity of AJs was also seen in intercellular contacts between the trichohyalin-rich IRS cuticle and the hair shaft cuticle (Fig. 6D; compare with Fig. 6E). Thus, layers that lacked both P- and E-cadherin exhibited significant defects in AJs, leading to a loss of integrity and a corresponding distortion of follicle structure.
Fig. 6.
Ultrastructural abnormalities in E-cadherin null follicles. Backskins of postnatal day 11 animals were processed for transmission electron microscopy. Sagittal sections of follicles are oriented with the skin surface toward the top of each frame. Asterisks denote intercellular gaps, reflective of a loss of membrane sealing (opposing arrows). Mx, matrix; IRS, inner root sheath; Hu, Huxley; He, Henle; Ci, IRS cuticle; Cp, companion layer; Ch, hair shaft cuticle; Co, cortex; De, desmosomes; Th, trichohyalin. [Bars = 10 μm(A); 500 nm (B and C); 500 nm (D and E).]
Discussion
Defects in skin epithelium have been described for loss-of-function mutations in α- and β-catenin (10, 23). However, in those cases, E-cadherin remained at cell–cell borders, and either the Ras-MAPK pathway (α-catenin) or Lef1/β-catenin-mediated signaling (β-catenin) was affected. This has made it difficult to assess the extent to which catenin-related defects arise from alterations in cell–cell junctions per se. Moreover, null mutations in P-cadherin resulted in no overt abnormalities in skin, and even when desmoglein 3 was also missing, no new skin defects surfaced (24). Thus, although genetic studies have documented the importance of desmosomes in skin (4, 5, 25), the role of AJs in this tissue has remained elusive.
Our studies now reveal the deleterious consequences of reducing AJs in the IRS, hair cuticle, and suprabasal epidermal layers. Interestingly, although normal cellular function was compromised in all these compartments, the outcomes were markedly different. In the lower IRS and hair cuticle, desmosomes were too few to maintain membrane sealing and prevent structural aberrations. Although additional explanations are possible, the selective loss of IRS and hair cuticle integrity is sufficient to cause a secondary loss of hair follicles, because ORS, cortex, and medulla alone cannot sustain follicle structure (26).
The hyperproliferative defects in epidermis were distinct not only from the IRS but also from the lactating mammary gland (12) and blastocyst (6), all of which exhibited cell degeneration and/or loss of tissue integrity. They also differed from P-cadherin null mice, which displayed precocious differentiation in mammary gland and no skin defects (14, 24). The molecular explanation underlying this paradoxical difference is complex. In the suprabasal layers, the loss of E-cadherin was accompanied by a reduction in α- and β-catenins, reflective of a corresponding reduction in AJs. However, the concomitant increase in desmosomal cadherins coupled with the natural abundance of desmosomes in spinous layers seemed to explain the preservation of KO spinous layer integrity. This said, these adhesive changes were not fully compensatory, as best exemplified by the failure to properly execute terminal differentiation. Although quantitative loss of epidermal barrier results in dehydration and death, mild defects are often counterbalanced by hyperproliferation and thickening (26, 27), similar to what we observed in older E-cadherin null skin.
Precisely how hyperproliferation arises in aging E-cadherin null epidermis is likely to be important in understanding why decreases in cadherin expression are widely associated with human cancers. It has been speculated that by uncoupling β-catenin from its partner cadherin, Wnt signaling could be activated, contributing to tumorigenesis (8). In skin epidermis, constitutively stabilized β-catenin can lead to hyperproliferation and thickening, but it is also accompanied by follicle-like down-growths (28) not observed in our KO animals. An alternative possibility is that by up-regulating P-cadherin, KO basal cells maintain AJs, survive, and counterbalance the terminal differentiation defects by hyperproliferating.
Our results are interesting in light of the many cancers involving alterations in the expression of classical cadherins (7, 8). Although future studies will be necessary to fully appreciate the functional relation between E- and P-cadherins, our studies suggest that if mutations in E-cadherin are accompanied by cadherin compensation in progenitor cells but not their differentiating offspring, the prognosis can be prolonged hyperplasia and/or dysplasia, which could in turn lead to tumor progression.
Acknowledgments
We thank R. Kemler (Max Planck Institute, Freiburg, Germany) for the E-cadherin fl/fl mice; P. Coulombe, M. Takeichi, R. Burgeson, T.-T. Sun, P. Cowin, and W. Franke for Abs; and L. Polak and R. Huang for assistance in the LARC animal facility. E.F. is an Investigator of the Howard Hughes Medical Institute, and T.L. is a Jane Coffin Childs Postdoctoral Fellow. This work was supported by the National Institutes of Health (Grant AR27883 to E.F.) and the Medical Science Training Program of the National Institutes of Health (C.L.T.).
Abbreviations: AJ, adherens junctions; KO, knockout; ORS, outer root sheath; IRS, inner root sheath.
Note Added in Proof. A recent E-cadherin conditional KO using Krox20-Cre resulted in postnatal defects in adult mice. Defects included perturbations in epidermal adhesion and hair follicles (29).
References
- 1.Perez-Moreno, M., Jamora, C. & Fuchs, E. (2003) Cell 112, 535–548. [DOI] [PubMed] [Google Scholar]
- 2.Yap, A. S. & Kovacs, E. M. (2003) J. Cell Biol. 160, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowalczyk, A. P., Bornslaeger, E. A., Norvell, S. M., Palka, H. L. & Green, K. J. (1999) Int. Rev. Cytol. 185, 237–302. [DOI] [PubMed] [Google Scholar]
- 4.Huber, O. (2003) Cell Mol. Life Sci. 60, 1872–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasioukhin, V., Bowers, E., Bauer, C., Degenstein, L. & Fuchs, E. (2001) Nat. Cell Biol. 3, 1076–1085. [DOI] [PubMed] [Google Scholar]
- 6.Ohsugi, M., Larue, L., Schwarz, H. & Kemler, R. (1997) Dev. Biol. 185, 261–271. [DOI] [PubMed] [Google Scholar]
- 7.Cavallaro, U. & Christofori, G. (2001) Biochim. Biophys. Acta 1552, 39–45. [DOI] [PubMed] [Google Scholar]
- 8.Conacci-Sorrell, M., Zhurinsky, J. & Ben-Ze'ev, A. (2002) J. Clin. Invest. 109, 987–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottardi, C. J. & Gumbiner, B. M. (2001) Curr. Biol. 11, 792–794. [DOI] [PubMed] [Google Scholar]
- 10.Vasioukhin, V., Bauer, C., Degenstein, L., Wise, B. & Fuchs, E. (2001) Cell 104, 605–617. [DOI] [PubMed] [Google Scholar]
- 11.Wong, A. S. T. & Gumbiner, B. M. (2003) J. Cell Biol. 161, 1191–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boussadia, O., Kutsch, S., Hierholzer, A., Delmas, V. & Kemler, R. (2002) Mech. Dev. 115, 53–62. [DOI] [PubMed] [Google Scholar]
- 13.Hirai, Y., Nose, A., Kobayashi, S. & Takeichi, M. (1989) Development (Cambridge, U.K.) 105, 271–277. [DOI] [PubMed] [Google Scholar]
- 14.Radice, G. L., Rayburn, H., Matsunami, H., Knudsen, K. A., Takeichi, M. & Hynes, R. O. (1997) Dev. Biol. 181, 64–78. [DOI] [PubMed] [Google Scholar]
- 15.Vasioukhin, V., Degenstein, L., Wise, B. & Fuchs, E. (1999) Proc. Natl. Acad. Sci. USA 96, 8551–8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman, C. K., Zhou, P., Pasolli, H. A., Rendl, M., Bolotin, D., Lim, K. C., Dai, X., Alegre, M. L. & Fuchs, E. (2003) Genes Dev. 17, 2108–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muller-Rover, S., Tokura, Y., Welker, P., Furukawa, F., Wakita, H., Takigawa, M. & Paus, R. (1999) Exp. Dermatol. 8, 237–246. [DOI] [PubMed] [Google Scholar]
- 18.Bierkamp, C., Schwarz, H., Huber, O. & Kemler, R. (1999) Development (Cambridge, U.K.) 126, 371–381. [DOI] [PubMed] [Google Scholar]
- 19.Segre, J. A., Bauer, C. & Fuchs, E. (1999) Nat. Genet. 22, 356–360. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs, E. & Raghavan, S. (2002) Nat. Rev. Genet. 3, 199–209. [DOI] [PubMed] [Google Scholar]
- 21.Jamora, C., DasGupta, R., Kocieniewski, P. & Fuchs, E. (2003) Nature 422, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobielak, K. Pasolli, H. A., Alonso, L., Polak L. & Fuchs, E. (2003) J. Cell Biol. 163, 609–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huelsken, J. & Behrens, J. (2002) J. Cell Sci. 115, 3977–3988. [DOI] [PubMed] [Google Scholar]
- 24.Lenox, J. M., Koch, P. J., Mahoney, M. G., Lieberman, M., Stanley, J. R. & Radice, G. L. (2000) J. Invest. Dermatol. 114, 948–952. [DOI] [PubMed] [Google Scholar]
- 25.Kljuic, A., Bazzi, H., Sundberg, J. P., Martinez-Mir, A., O'Shaughnessy, R., Mahoney, M. G., Levy, M., Montagutelli, X., Ahmad, W., Aita, V. M., et al. (2003) Cell 113, 249–260. [DOI] [PubMed] [Google Scholar]
- 26.Byrne, C., Hardman, M. & Nield, K. (2003) J. Anat. 202, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madison, K. C. (2003) J. Invest. Dermatol. 121, 231–241. [DOI] [PubMed] [Google Scholar]
- 28.Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. (1998) Cell 95, 605–614. [DOI] [PubMed] [Google Scholar]
- 29.Young, P., Boussadia, O., Halfter, H., Grose, R., Berger, P., Leone, D. P., Robenek, H., Charnay, P., Kemler, R. & Suter, U. (2003) EMBO J. 22, 5723–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]