Abstract
The circadian clock located in the suprachiasmatic nuclei (SCN) of the hypothalamus regulates daily temporal organization in behaviour and neuroendocrine function. The molecular basis for circadian rhythm generation is an interacting transcriptional/translational feedback loop comprised of several ‘clock genes’ and their respective protein products. Clock genes are expressed not only in the SCN but also in numerous other locations throughout the brain, including regions rich in neuroendocrine cells. In order to investigate whether neuroendocrine cells function as autonomous oscillators, we used female transgenic mice in which an unstable, degradable jellyfish green fluorescent protein (GFP) gene is driven by a mouse Period 1 (Per1) gene promoter. Mice were injected (s.c.) with fluorogold (FG) in order to label neuroendocrine cells and brain sections were double-labelled for either FG and Per1 mRNA (labelled by GFP immunostaining) or FG and PER1 protein using fluorescence immunocytochemistry. Mice were killed during either the day or night. Neuroendocrine cells contained Per1 mRNA and PER1 protein in several brain regions with the greatest proportion of double-labelled cells occurring in the arcuate nucleus (Arc). The number of neuroendocrine cells labelled was not affected by the stage of the estrous cycle. Fewer FG-labelled cells expressed Per1 message and protein during the night compared to the day. In the Arc, staining for tyrosine hydroxylase revealed that neuroendocrine cells expressing Per1 message and protein were dopaminergic. Together, these findings suggest that neuroendocrine cells contain the molecular machinery necessary to oscillate independently. It remains to be determined whether these cells actually function as autonomous oscillators or whether these rhythms are driven by signals from the SCN. These findings also indicate that the endocrine system represents an opportunity to study the interactions between central (SCN and neuroendocrine cells) and peripheral circadian (endocrine gland) oscillators.
Keywords: clock gene, dopamine, hormone, SCN, suprachiasmatic nucleus, TIDA
Introduction
Precise temporal coordination among numerous neuroendocrine and behavioural events is necessary for organisms to function optimally and maintain homeostasis. For example, in anticipation of waking, cortisol levels rise to mobilize energy resources while insulin levels increase in advance of food intake (Van Cauter, 1990; Czeisler & Klerman, 1999; Gillette & Tischkau, 1999; Kriegsfeld et al., 2002). The daily orchestration of these various neuroendocrine, behavioural and other physiological events is regulated by a circadian clock located in the suprachiasmatic nuclei (SCN) of the anterior hypothalamus (Turek et al., 1984; Van Cauter, 1990; Czeisler & Klerman, 1999; Gillette & Tischkau, 1999).
Lesions of the SCN abolish circadian rhythms in behaviour and physiology (Moore & Eichler, 1972; Stephan & Zucker, 1972). Transplantation of fetal SCN tissue restores rhythms in several behavioural measures but does not restore neuroendocrine rhythms (Lehman et al., 1987; Ralph et al., 1990; Meyer-Bernstein et al., 1999). As the transplanted SCN does not re-establish many of its normal neural connections with the host brain (Canbeyli et al., 1991; Lehman et al., 1998), this finding suggests that SCN neural output may be critical in the regulation of circadian endocrine rhythms. In agreement with this hypothesis, semicircular knife cuts caudal to the SCN abolish estrous cyclicity but not rhythms in drinking behaviour in rats (Nunez & Stephan, 1977). Additionally, horizontal knife cuts abolish estradiol-induced daily surges in luteinizing hormone (LH), yet leave circadian locomotor behaviour intact (Watts et al., 1989). Finally, surgical isolation of the SCN abolishes the photoperiodic gonadal response but not circadian locomotor behaviour in Syrian hamsters (Hakim et al., 1991). Taken together, these findings suggest that behavioural rhythms can be regulated by a diffusible signal from the SCN to behavioural effector areas, whereas rhythms in neuroendocrine function require neural output (Silver et al., 1996; Kramer et al., 2001; Cheng et al., 2002).
Circadian rhythms within the SCN are a cell-autonomous property produced by an autoregulatory transcriptional/translational feedback loop composed of several ‘clock’ genes and their protein products. The proteins CLOCK and BMAL1 provide positive transcriptional drive in this feedback loop, while the protein products of the Period gene (PER1, PER2, and PER3) in combination with the products of the Cryptochrome gene (CRY1 and CRY2) provide negative feedback (Welsh et al., 1995; Gekakis et al., 1998; Sangoram et al., 1998; Griffin et al., 1999; Jin et al., 1999; Kume et al., 1999). Clock genes are also present and rhythmic in numerous extra-SCN brain regions (Yamazaki et al., 2000; Abe et al., 2002) as well as in peripheral organs (Damiola et al., 2000; Yamazaki et al., 2000; Hara et al., 2001). However, the precise function of these extra-SCN clock genes and the means by which the SCN regulates clock gene expression in these brain regions and peripheral organs has not been elucidated.
The SCN projects monosynaptically to numerous brain regions containing neuroendocrine cells, including the preoptic area (POA), paraventricular nucleus of the hypothalamus (PVH), anterior hypothalamus (AH), and the arcuate nucleus (Arc) (Stephan et al., 1981; Watts & Swanson, 1987; Watts et al., 1987; Kalsbeek et al., 1993; Morin et al., 1994). Imaging studies of isolated brain regions from transgenic rats using a luciferase reporter driven by the mPer1 promoter established that average Per1-luciferase expression is rhythmic in tissue segments from these same SCN target regions (Abe et al., 2002); the specific cell type and neurochemical phenotype of these averaged rhythmic cell populations remains to be determined. Additionally, cultured fibroblasts and peripheral organs contain clock genes, express circadian rhythms in vitro, and are presumably reset daily by the SCN in vivo (Balsalobre et al., 1998; Balsalobre et al., 2000; Yamazaki et al., 2000). These findings suggest that, like fibroblasts and peripheral organs, numerous brain cell types may contain clock genes, yet require a periodic signal to temporally coordinate the combined rhythmicity of a population of autonomous oscillators. This possibility could account for the loss of circadian endocrine rhythms after transection of SCN output fibres or SCN lesions.
The present study was undertaken to determine if neuroendocrine cells contain the mPer1 gene, one of the core circadian clock components. Because the pattern of circadian endocrine rhythms is critical for females in the organization of the hormonal milieu necessary for successful ovulation and mating, female mice were investigated in these studies. Transgenic mice with a degradable green fluorescent protein (GFP) reporter driven by a Per1 gene promoter were used (Kuhlman et al., 2000). This mouse model allowed for the indirect detection of Per1 mRNA in neuroendocrine cells using double-label fluorescence immunocytochemistry (ICC) permitting both conventional and confocal microscopy. The results of the present investigation indicate that several neuroendocrine cell populations show daily variation in Per1 mRNA and PER1 protein, particularly neuroendocrine dopaminergic neurons in the arcuate nucleus.
Materials and methods
Experiment 1
Animals and housing
Twelve heterozygous transgenic mice were used in the present experiment (a generous gift of Dr Douglas McMahon, University of Kentucky). All animals were housed in translucent polypropylene cages (48 × 27 × 20 cm) and provided with access to food and water ad libitum for the duration of the study. Animals were maintained in a colony room with a 24 h light–dark cycle (LD 12: 12). The rooms were maintained at 23 ± 1 °C. All animals were allowed a minimum of a 1-week acclimation period prior to the onset of the experiment. All animal research in this report was approved by Columbia University’s Animal Care and Use Committee.
Experimental manipulation and tissue collection
In order to label neuroendocrine cells in the CNS, all animals were injected (s.c.) with 0.05 cc of 4% fluorogold (FG; Fluorochrome, Inc., Denver, CO) in 0.9% saline and killed 5 days later with an injection of sodium pentobarbital (see below). Because FG does not cross the blood–brain barrier, this procedure only labels cells in the brain that come into contact with fenestrated capillaries (Merchenthaler, 1991). One set (n =4) of the animals was killed during the day at Zeitgeber time 10 (ZT10; 10 h after light onset) without regard to the stage of the estrous cycle. GFP protein peaks at approximately ZT7.5–ZT8 (D. McMahon, personal communication) while PER1 protein is elevated between ZT10 and ZT16 (Hastings et al., 1999). Thus, ZT10 was chosen as a time point during which both GFP and PER1 could be investigated in the same animal. Because activation or appearance of clock components may be important for the onset of estrous behaviour, a second set (n =4) of the females was killed during vaginal proestrus (i.e. behavioural estrus). Finally, because previous studies in Per1-luciferase rats have reported that maximal clock gene expression in some extra-SCN brain regions is in antiphase (i.e. 12 h out of phase) with expression in the SCN (Abe et al., 2002), an additional set of animals (n =4) was killed during the night at ZT22.
To collect the brains, animals were deeply anaesthetized with sodium pentobarbital (200 mg/kg) and perfused transcardially with 50 mL of 0.9% saline followed by 100 mL of 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were postfixed in 4% paraformaldehyde for 2 h at 4 °C, then cryoprotected in 20% sucrose in 0.1 M PBS overnight at 4 °C. Coronal sections (30 μm) were cut on a cryostat and collected into 0.1 M PBS. The brain sections were processed as free-floating sections. Every fourth section was processed for FG and a different protein (i.e. GFP or PER1).
Double-label GFP::Per1 fluorescence immunocytochemistry
For simultaneous visualization of GFP and FG, every 4th, 30 μm section was double-labelled (4 brains for each condition; ZT10, n =4; ZT10 vaginal proestrus, n =4; ZT22, n =4) using fluorescence immunocytochemistry. For visualization of GFP, sections were washed in PBS, incubated in 0.5% H2O2, and then incubated in normal goat serum in 0.1% Triton X-100 (PBT) for 1 h. Sections were then incubated for 48 h at 4 °C in rabbit anti-GFP (Molecular Probes, Eugene, OR) diluted at 1: 800 000 with 0.1% PBT. Following incubation in anti-GFP, brains were incubated for 1 h in biotinylated goat anti-rabbit (1: 300; Vector, Burlingame, CA), followed by incubation in avidin–biotin-horseradish peroxidase complex (ABC Elite Kit, Vector, Burlingame, CA). Brains were then incubated in a biotinylated tyramide solution (0.6%) for 30 min This protocol allowed for the amplification of the highly diluted anti-GFP antibody required for double-labelling with two antibodies generated in the same species. Cells were then labelled using Cy-2 conjugated to streptavidin (Jackson Laboratories, West Grove, PA) as the fluorophore. Following labelling for GFP, sections were incubated for 48 h in a rabbit anti-FG antibody (Chemicon, Temecula, California) diluted 1: 2000 with 0.1% PBT. FG cells were labelled with Cy-2 donkey anti-rabbit (Jackson Laboratories, West Grove, PA) as the secondary antibody/fluorophore. Sections were mounted onto gelatin-coated slides and coverslips were applied following dehydration in a graded series of alcohols. For control experiments, one antibody was eliminated (i.e. GFP or FG) and all other steps were followed as previously described.
Double-label PER1 protein fluorescence immunocytochemistry
To determine if Per1 mRNA seen in neuroendocrine cells is being translated into a biologically significant protein, one series of 30 μm sections from the brains of all animals from each condition were stained for PER1 protein and FG. Immunocytochemistry was performed as described above for GFP and FG. PER1 was the first antibody labelled using a rabbit anti-PER1 antibody (1: 50 000; generous gift of Dr Stephen Reppert and Dr David Weaver, University of Massachusetts, Worcester, MA). PER1 labelling was subsequently amplified using the biotinylated tyramide protocol as described above. Staining for FG was also performed as described above. For control experiments, one antibody was eliminated (i.e. PER1 or FG) and all other steps were followed as previously described.
Light microscopy
GFP or PER1 proteins and FG double-label was investigated using a Nikon Eclipse E800 microscope. Sections were examined using the standard wavelengths for Cy-2 (488 nm) and Cy-3 (568 nm). Every 4th section (for each protein) from the medial septum to the caudal aspect of the anterior hypothalamus was assessed, and those areas expressing GFP or PER1 were investigated more extensively for coexpression with FG using confocal microscopy (see below). Areas identified as having double-labelled cells were digitally captured at 200 x in 8 bit greyscale using a cooled CCD camera (SPOT; Morrel, Meville, NY). Each label was captured as a single image without moving the position of the stage or plane of focus between captures. Images were superimposed digitally using SPOT software (Morrel, Meville, NY). Brain areas were examined for double-labelling using conventional light microscopy and were scanned at the confocal level for quantification.
Confocal microscopy
Brain sections used for light microscopy were also used for the confocal scans in order to characterize FG and GFP or FG and PER1 double-labelling. Regions of the brain with putative double-label identified at the light level were scanned at 400 × using confocal microscopy. Cells were observed under a Zeiss Axiovert 100TV fluorescence microscope (Carl Zeiss, Thornwood, NY) with a Zeiss LSM 410 laser scanning confocal attachment. The sections were excited with an Argon-Krypton laser using the standard excitation wavelengths for Cy-2 and Cy-3. Stacked images were collected as 1.0 μm multitract optical sections (with sequential excitation by each laser to avoid ‘cross-talk’ between the two wavelengths). Using the LSM 3.95 software (Zeiss), red and green images of the sections were superimposed. Neuroendocrine cells in a given brain region were examined through their entirety in 1.0 μm steps. The number of scans through each brain region was a function of the rostral–caudal width of a particular population of cells within a nucleus, and therefore differed for each brain region. The following brain areas expressing double-labelled cells were investigated (other brain regions did not have double-labelled cells, see Results section): anterodorsal preoptic nucleus (ADP; one brain section per animal per label), anteroventral periventricular nucleus (AVPV; 1 brain section per animal per label), PVH, (three brain sections per animal per label), and Arc (three brain sections per animal per label). Counts from the brains of three animals from each experimental condition [for GFP labelling ZT10 (n =3), ZT10 proestrus (n =3), ZT22 (n =3); for PER1 labelling, ZT10 (n =3), ZT10 proestrus (n =3), ZT22 (n =3)] were quantified at the confocal level.
Experiment 2
Treatment and immunocytochemistry
Because the Arc contains a large number of dopaminergic neuroendocrine cells, and this region shows robust rhythms of Per1-luciferase expression (Abe et al., 2002), one series of 30 μm sections from the brains of animals from Experiment 1 was stained for GFP or PER1 protein as well as tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine production. Immunocytochemistry was performed on 4 additional animals as described in Experiment 1. In alternate sections, brains were labelled for either GFP and TH (TH from Diasorin Inc; diluted 1: 5000) or PER1 and TH as described in Experiment 1. For control experiments, one antibody was eliminated and all other steps were followed as previously described.
Statistics
All reported analyses and statistics were performed on data collected at the confocal level. For each brain region within an animal, results were averaged over individual brain sections to yield a single value for each brain region for each animal. Total cell count data for Experiments 1 and 2 were analysed using 2 × 3 (time × brain region) analyses of variance (ANOVA). Because percentage data from Experiments 1 and 2 were nonparametric, all percentage data was analysed using 2 × 3 (time × brain region) analyses of variance on ranks. Data from Experiment 2 were also nonparametric percentage data analysed using a 2 × 2 (time × staining type) ANOVA on ranks. Group comparisons were made following a significant main effect using the Tukey HSD test. Results were considered significant when P <0.05.
Results
Experiment 1
Characterization of FG and Per1::GFP and PER1 labeling
Followings.c. injectionof FG,neuroendocrine cells inthemedial septum (MS), diagonal band of Broca (DBB), ADP, POA, AVPVand hypothalamic areas including the PVH, dorsomedial hypothalamus (DMH), supraoptic nucleus (SON), Arc were labelled by the FG antibody.
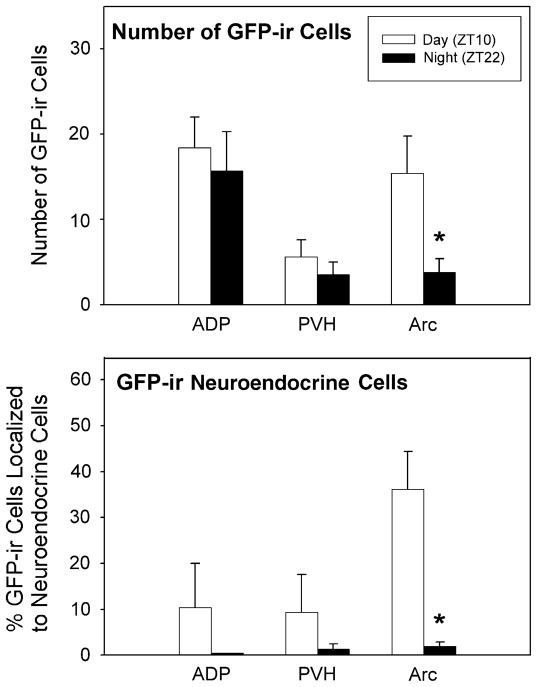
Comparisons of the expression of GFP in the SCN at two different times of day indicated that, during the day, GFP and PER1 protein expression were extensive throughout the rostral–caudal extent of the SCN, whereas staining was virtually absent during the night (Fig. 1). In addition to the SCN, the expression of GFP and PER1 was also observed in the following brain regions that contained neuroendocrine cells: septal areas, the DBB, ADP, POA, AVPV, PVH, DMH and Arc. The total number of GFP cells seen was significantly reduced in the Arc at night compared to the day (P <0.05; Fig. 2, top), while there were no significant differences seen in the ADP and PVH with regard to the time that the animal was killed. Results for PER1 staining are presented below.
Fig. 1.

Photomicrographs of GFP, (panels A and B) and PER1, (panels C and D) immunoreactive neurons in the SCN of animals killed during the day (ZT10, panels A and C) and during the night (ZT22, panels B and D).
Fig. 2.
Mean (+SEM) number of GFP cells in the ADP, PVH and Arc in animals that were killed during the day and night (ZT10 and ZT22, respectively; top panel). Mean (+SEM) percentage of GFP cells that are expressed in FG-positive neurons. Cells were evaluated in the ADP, PVH, and Arc in animals that were killed during the day and night (ZT10 and ZT22, respectively; bottom panel). *Significantly less than animals killed during the day within the same brain region (P <0.05).
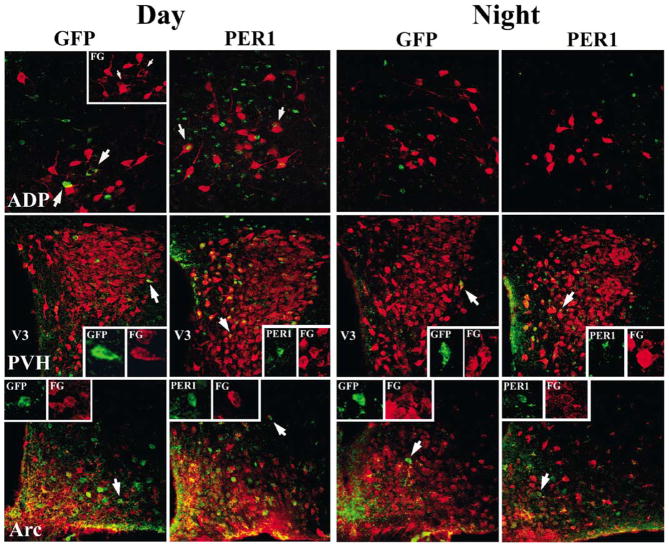
In order to determine whether or not Per1 mRNA expression occurred within neuroendocrine cells, all brain regions in which the FG and GFP labelling was observed (i.e. MS, DBB, ADP, POA, AVPV, PVH, DMH, and Arc), were first examined at the light microscopic level. Double-labelled cells were observed at the light level only in the ADP, PVH and Arc during both the day and night. The percentage of GFP cells double-labelled for FG was quantified in 1 μm confocal scans (Fig. 2, bottom). Double-labelling was confirmed and quantified at the confocal microscopic level in the ADP, PVH and Arc (Figs 2 and 3). Confocal scans of the POA and AVPV failed to reveal any double-labelled cells. As there were no significant differences between animals in proestrus vs. animals at random stages of the reproductive cycle (P >0.05 for each brain region), data were collapsed over reproductive stage within each time point. The percentage of GFP cells also labelled with FG was reduced in the Arc at night compared to the day (P <0.05). A similar trend, that did not reach statistical significance was observed in the ADP and PVH (P >0.05).
Fig. 3.
Photomicrographs showing 1 μm confocal optical scans through the ADP (top panels), paraventricular nucleus of the hypothalamus (PVH; middle panels), and the arcuate nucleus (Arc; bottom panels) from animals killed during the day and night (ZT10 and ZT22, respectively). Brains were double-labelled for GFP or PER1 protein (green) and FG (red) using immunofluorescence. Arrows for GFP staining in the ADP in row 1 point to cells for which the red channel is shown separately in the insert. Arrows for PER1 staining in the ADP in row 1 point to representative double-labelled cells in the insert. V3, 3rd ventricle. Arrows in rows 2 and 3 point to cells that are displayed as separate red and green channels.
To establish whether Per1 mRNA was being translated into protein in neuroendocrine cells, PER1 expression was examined. PER1 was localized to the same brain regions listed for GFP but PER1-ir cells were greater in number than GFP-ir cells. Overall, the mean number of PER1-ir cells was higher during the day than at night (P <0.05), with significant differences in the PVH and Arc (P <0.05 in each case; Fig. 4). As with GFP, PER1 protein expression was seen in FG cells in the ADP, PVH, and Arc at both the light and confocal microscope levels (Fig. 3). The percentage of double-labelled PER1-ir was significantly reduced in the Arc at night compared to the day (P <0.05), with a similar trend that did not reach significance in the ADP and PVH (P >0.05 in each case; Fig. 4). PER1-ir in cells in the AVPV was not consistent among animals. In one animal, about 25% of the cells (of the 69 cells evaluated at the confocal level) in the AVPV contained PER1 protein. Staining was absent in the AVPV of other animals during the day and night.
Fig. 4.
Mean (+SEM) number of PER1-ir cells in the ADP, PVH, and Arc in animals that were killed during the day and night (ZT10 and ZT22, respectively; top panel). Mean (+SEM) percentage of PER1-labelled nuclei that were expressed in FG-positive neurons (bottom panel). Cells were evaluated in the ADP, PVH, and Arc in animals that were killed during the day and night (ZT10 and ZT22, respectively). *Significantly less than animals killed at ZT10 within the same brain region (P <0.05).
Experiment 2
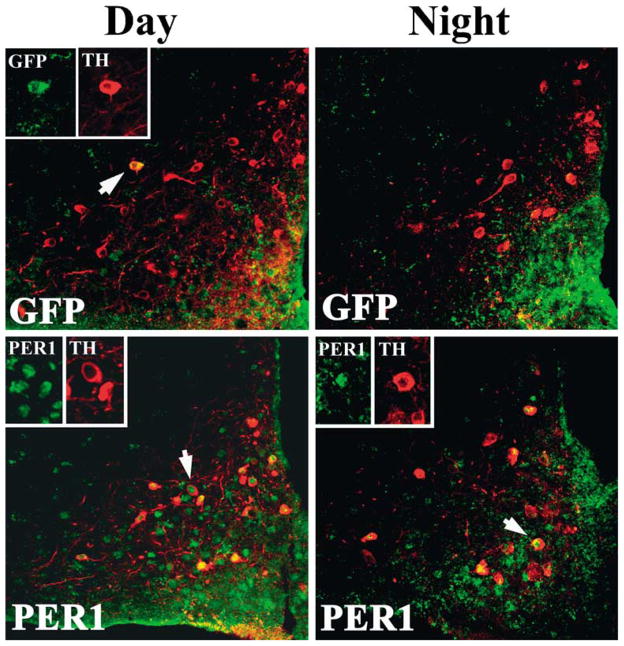
The results of Experiment 1 indicated that a large number of neuroendocrine cells in the Arc expressed both Per1 mRNA and protein. The rostral Arc contains neuroendocrine dopaminergic neurons that project to the anterior pituitary (tuberohypophyseal dopaminergic neurons; THDA) (Bjorklund et al., 1973), while more caudal aspects of the Arc contain neuroendocrine dopaminergic neurons that project to the infundibulum (tuberoinfundibular dopaminergic neurons; TIDA) (Fuxe, 1964); both populations of cells regulate prolactin secretion (Ben-Jonathan, 1985). To determine if the cell phenotype of GFP- and PER1-expressing FG cells labelled was dopaminergic, TH cells were visualized in the Arc. A subset of TH cells was labelled with both GFP and PER1 proteins, with the latter marker revealing more cells (P <0.05; Figs 5 and 6). Significantly more TH cells were labelled with PER1 during the day than the night (P <0.05; Fig. 6). A similar trend that did not reach statistical significance was seen with GFP labelling (P >0.05).
Fig. 5.
Photomicrographs showing a 1 μm confocal optical scan through the arcuate nucleus of animals killed during the day (ZT10; left) and night (ZT22; right). Brains were double-labelled for either GFP or PER1 protein (green) and TH (red) using immunofluorescence. Arrows in each figure point to cells that are displayed as separate red and green channels
Fig. 6.
Percentage of TH-ir cells containing GFP or PER1 in animals killed during either the day or night (ZT10 and ZT22, respectively).
Discussion
The present results are the first to reveal that specific neuroendocrine cell populations (i.e. the ADP, PVH, and Arc) express Per1, with several exhibiting a diurnal rhythm. These findings extend previous studies examining isolated brain regions from Per1-luciferase rats, showing that mean Per1 expression is rhythmic in brain regions outside of the SCN (e.g. Abe et al., 2002). Overall, Per1 expression in neuroendocrine cells showed a diurnal rhythm paralleling that of the SCN. In a number of brain regions (septum, DBB, POA, AVPV, and DMH), both neurosecretory neurons and Per1-expressing neurons were seen, but no double-labelled cells were detected. In contrast, in other brain regions (ADP, PVH, Arc), neuroendocrine cells expressed Per1 mRNA and protein. Additionally, neuroendocrine dopaminergic neurons in the Arc exhibited a diurnal rhythm in Per1 expression. Dopamine neurons in the Arc are the major regulators of prolactin secretion (Fuxe, 1964; Bjorklund et al., 1973; Ben-Jonathan, 1985), suggesting that daily rhythms in prolactin secretion may be regulated locally by a circadian mechanism in dopaminergic neurosecretory cells. Together with data from previous studies of SCN regulation of neuroendocrine rhythms, the findings reveal the hierarchical organization by which circadian endocrine rhythms may be regulated by SCN input.
In neuroendocrine cells, Per1mRNA and protein exhibit the same diurnal pattern of expression as the SCN. Most hormones exhibit a circadian rhythm but, importantly, the phase of peak production and secretion differs among hormonal systems (reviewed in Kriegsfeld et al., 2002). That the general pattern of Per1 expression in several neuroendocrine cell populations in the present investigation mirrored that of the SCN (i.e. high during the day and low at night) suggests that differences in the rhythmic pattern among hormonal systems may ultimately be regulated at the level of the target endocrine gland. Further studies are necessary to determine if this daily rhythm will persist in populations of neuroendocrine cells (or in individual cells) in the absence of the SCN or if input from the main circadian pacemaker is necessary to sustain this rhythm in vivo.
Because dopamine is the only catecholamine expressed in the Arc, the present results with tyrosine hydroxylase staining suggest that circadian clock function in neuroendocrine dopaminergic neurons in this brain region are important for the regulation of reproduction in females. Female rodents exhibit a surge of prolactin on the afternoon of proestrus (Butcher et al., 1974; Smith et al., 1975). As mentioned above, the primary regulator of prolactin secretion is dopamine (Ben-Jonathan, 1985), and the SCN projects to dopamine neurons in the Arc (Gerhold et al., 2001). Lesions of the SCN abolish the proestrus surge of prolactin, resulting in constant basal release of this hormone (Palm et al., 2001). One SCN neurohormone, vasopressin, is secreted in a circadian fashion with low levels at night and high levels during the day (Schwartz et al., 1983). Administration of vasopressin into the 3rd venticle inhibits prolactin release in a time-dependent fashion (Palm et al., 2001). Together, these findings suggest that projections from the SCN communicate critical timing information to dopaminergic neurons in the Arc, but time-dependent sensitivity to this SCN signal may be regulated or gated locally by clocks within these dopaminergic cells.
In all cases, GFP expression in neuroendocrine cells was lower than that of PER1. This discrepancy is most likely due to the different time course of GFP and PER1 translation following the transcription of their respective genes, with the former peaking approximately ZT7.5–ZT8 (McMahon, personal communication) while the latter is elevated between ZT10–ZT16 (Hastings et al., 1999). The present study investigated Per1 expression during both the day (ZT10) and the night (ZT22). Insertion of the Per1::GFP transgene may not insure transcription in all cell types. For example, adjacent chromatin could silence the transgene in some cells or cell types (Walters et al., 1995; Festenstein et al., 1996; Graubert et al., 1998; Festenstein & Kioussis, 2000). Thus, GFP staining in the present study may underestimate the number of neuroendocrine cells expressing Per1. Nonetheless, results with PER1 protein expression, demonstrating the same basic pattern of findings in the present study, indicate that GFP provides a useful tool for evaluating which populations of neuroendocrine cells express Per1.
The results of the present study suggest a hierarchical organization by which some circadian endocrine rhythms are generated. This theoretical model is based on the assumption that neuroendocrine cells containing clock genes can oscillate independently, although the present findings do not rule out the possibility that Per1 is driven by the SCN in some neuroendocrine cells. At the top of this hierarchy, oscillator cells within the SCN communicate rhythmic information via neural efferents to putative ‘slave’ oscillators in neuroendocrine cells. Although capable of oscillating independently, these neuroendocrine cells require periodic SCN input to maintain a synchronized rhythm in the local cell population. Such a mechanism could account for the loss of circadian endocrine rhythms following lesions or transection of outputs from the SCN (Moore & Eichler, 1972; Nunez & Stephan, 1977; Meyer-Bernstein et al., 1999). Coordinated rhythmic output from neuroendocrine cells can then be communicated to the pituitary, which also contains circadian clock genes (Messager et al., 2001; von Gall et al., 2002). Finally, rhythmic information from the pituitary can be communicated humorally to target glands in the periphery that themselves contain clock genes (Zylka et al., 1998; Tong et al., 2002). This hierarchy, with multiple levels of circadian regulation, may allow the anticipation of the reception of specific daily signals at each level of the axis. Consistent with this logic, multisynaptic projections from the SCN to several endocrine glands have been identified using viral tracers (Buijs et al., 1998; Buijs et al., 1999; Gerendai et al., 2000; Kalsbeek et al., 2000), suggesting a mechanism by which the SCN could coordinate timing among glands to optimize responses to endocrine signals (Fig. 7).
Fig. 7.
Theoretical model based on the present and previous studies suggesting a means by which circadian regulation of neuroendocrine function may be hierarchically organized such that each level of the axis can anticipate signals from other levels of the axis. In order for neuroendocrine rhythms to be synchronized to local environmental time, retinal input is first transmitted to the SCN to synchronize the endogenous oscillators in this nucleus. Given the present findings of a core clock component in neuroendocrine neurons, and previous studies showing direct SCN innervation of these neurons, the 2nd link in this hierarchy is a direct neural connection from the SCN to potentially autonomous oscillating neuroendocrine cells which theoretically have rhythms of different periods and phases until coordinated by SCN input. This SCN input synchronizes rhythms among independent cells and sets their phase in these brain regions. In turn, rhythmic neurohormone secretion communicates to downstream targets of the neuroendocrine axis (some of which contain clock genes).
A hierarchical organization requires that the SCN communicate directly or indirectly with neuroendocrine cells containing clock genes. There is substantial evidence of direct projections from the SCN to neuroendocrine cells. The SCN projects monosynaptically to corticotropin-releasing hormone (CRH) and spinal-projecting cell populations in the PVH (Buijs et al., 1993; Vrang et al., 1995; Teclemariam-Mesbah et al., 1997). Likewise, direct SCN projections to neuroendocrine dopamine neurons in the Arc have also been identified (Gerhold et al., 2001). Expression of the clock gene, Per1, has been shown in both the PVH and Arc (Asai et al., 2001; Takahashi et al., 2001; Abe et al., 2002); Per1 is rhythmically expressed in the Arc (Abe et al., 2002) and exhibits a stress-induced increase in the PVH (Takahashi et al., 2001; Abe et al., 2002). Per1 has also been localized to CRH-ir cells in the PVH (Takahashi et al., 2001), while the neurochemical phenotype of Per1-expressing cells in the Arc has been identified as dopaminergic in the present study. In addition, results from the present study extend these previous findings by showing a diurnal rhythm in PER1 protein in the PVH and Arc, providing evidence for the rhythmic translation of Per1 mRNA into its functional protein product in discrete brain regions.
In summary, results from the present study along with previous findings demonstrate that the neuroendocrine axis is hierarchically organized with circadian clock gene regulation at multiple levels of the hypothalamo–pituitary–endocrine gland axis. Circadian regulation at multiple levels of the neuroendocrine system suggests that this redundancy is required to anticipate the reception of daily signals from each higher level of the axis. This organization suggests that the endocrine system provides an ideal system by which to study the significance of the interactions between central and peripheral oscillators in circadian regulation.
Acknowledgments
The authors thank Dr Joseph LeSauter and Ilia Karatsoreos for valuable comments on an earlier version of this manuscript. We also thank Eliza Bobek for technical assistance. This work was supported by NIH Grant NS-37919 (RS) and MH-12408 (LJK).
Abbreviations
- AH
anterior hypothalamus
- ADP
anterodorsal preoptic nucleus
- Arc
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- DBB
diagonal band of Broca
- DMH
dorsomedial hypothalamus
- FG
fluorogold
- GFP
green fluorescent protein
- ICC
immunocytochemistry
- MS
medial septum
- POA
preoptic area
- PVH
paraventricular nucleus of the hypothalamus
- SCN
suprachiasmatic nuclei
- SON
supraoptic nucleus
- TH
tyrosine hydroxylase
- THDA
tuberohypophyseal dopaminergic neurons
References
- Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD. Circadian rhythms in isolated brain regions. J Neurosci. 2002;22:350–356. doi: 10.1523/JNEUROSCI.22-01-00350.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M, Yoshinobu Y, Kaneko S, Mori A, Nikaido T, Moriya T, Akiyama M, Shibata S. Circadian profile of Per gene mRNA expression in the suprachiasmatic nucleus, paraventricular nucleus, and pineal body of aged rats. J Neurosci Res. 2001;66:1133–1139. doi: 10.1002/jnr.10010. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol. 2000;10:1291–1294. doi: 10.1016/s0960-9822(00)00758-2. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N. Dopamine: a prolactin-inhibiting hormone. Endocr Rev. 1985;6:564–589. doi: 10.1210/edrv-6-4-564. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Moore RY, Nobin A, Stenevi U. The organization of tubero-hypophyseal and reticulo-infundibular catecholamine neuron systems in the rat brain. Brain Res. 1973;51:171–191. doi: 10.1016/0006-8993(73)90371-5. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Hermes MH, Kalsbeek A. The suprachiasmatic nucleus–paraventricular nucleus interactions: a bridge to the neuroendocrine and autonomic nervous system. Prog Brain Res. 1998;119:365–382. doi: 10.1016/s0079-6123(08)61581-2. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Markman M, Nunes-Cardoso B, Hou YX, Shinn S. Projections of the suprachiasmatic nucleus to stress-related areas in the rat hypothalamus: a light and electron microscopic study. J Comp Neurol. 1993;335:42–54. doi: 10.1002/cne.903350104. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Van Heerikhuize JJ, Feenstra MG, Ter Horst GJ, Romijn HJ, Kalsbeek A. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci. 1999;11:1535–1544. doi: 10.1046/j.1460-9568.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol- 17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Canbeyli RS, Lehman M, Silver R. Tracing SCN graft efferents with Dil. Brain Res. 1991;554:15–21. doi: 10.1016/0006-8993(91)90166-s. [DOI] [PubMed] [Google Scholar]
- Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130. [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festenstein R, Kioussis D. Locus control regions and epigenetic chromatin modifiers. Curr Opin Genet Dev. 2000;10:199–203. doi: 10.1016/s0959-437x(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Festenstein R, Tolaini M, Corbella P, Mamalaki C, Parrington J, Fox M, Miliou A, Jones M, Kioussis D. Locus control region function and heterochromatin-induced position effect variegation. Science. 1996;271:1123–1125. doi: 10.1126/science.271.5252.1123. [DOI] [PubMed] [Google Scholar]
- Fuxe K. Cellular localization of monoamines in the median eminence and in the infundibular stem of some mammals. Acta Physiol Scand. 1964;58:383–384. doi: 10.1111/j.1748-1716.1963.tb02662.x. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nature Neurosci. 2002;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gerendai I, Toth IE, Boldogkoi Z, Medveczky I, Halasz B. CNS structures presumably involved in vagal control of ovarian function. J Auton Nerv Syst. 2000;80:40–45. doi: 10.1016/s0165-1838(00)00071-0. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Tischkau SA. Suprachiasmatic nucleus: the brain’s circadian clock. Recent Prog Horm Res. 1999;54:33–58. [PubMed] [Google Scholar]
- Graubert TA, Hug BA, Wesselschmidt R, Hsieh CL, Ryan TM, Townes TM, Ley TJ. Stochastic, stage-specific mechanisms account for the variegation of a human globin transgene. Nucl Acids Res. 1998;26:2849–2858. doi: 10.1093/nar/26.12.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- Hakim H, DeBernardo AP, Silver R. Circadian locomotor rhythms, but not photoperiodic responses, survive surgical isolation of the SCN in hamsters. J Biol Rhythms. 1991;6:97–113. doi: 10.1177/074873049100600201. [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, Shibata S. Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus. Genes Cells. 2001;6:269–278. doi: 10.1046/j.1365-2443.2001.00419.x. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Field MD, Maywood ES, Weaver DR, Reppert SM. Differential regulation of mPER1 and mTIM proteins in the mouse suprachiasmatic nuclei: new insights into a core clock mechanism. J Neurosci. 1999;19(RC11):1–7. doi: 10.1523/JNEUROSCI.19-12-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Shearman LP, Weaver DR, Zylka MJ, de Vries GJ, Reppert SM. A molecular mechanism regulating rhythmic output from the suprachiasmatic circadian clock. Cell. 1999;96:57–68. doi: 10.1016/s0092-8674(00)80959-9. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Fliers E, Franke AN, Wortel J, Buijs RM. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology. 2000;141:3832–3841. doi: 10.1210/endo.141.10.7709. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Teclemariam-Mesbah R, Pevet P. Efferent projections of the suprachiasmatic nucleus in the golden hamster (Mesocricetus auratus) J Comp Neurol. 1993;332:293–314. doi: 10.1002/cne.903320304. [DOI] [PubMed] [Google Scholar]
- Kramer A, Yang FC, Snodgrass P, Li X, Scammell TE, Davis FC, Weitz CJ. Regulation of daily locomotor activity and sleep by hypothalamic EGF receptor signaling. Science. 2001;294:2511–2515. doi: 10.1126/science.1067716. [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, LeSauter JL, Hamada T, Pitts SM, Silver R. Circadian rhythms in the endocrine system. In: Pfaff D, Etgen A, editors. Hormones, Brain, and Behavior. Academic Press; New York: 2002. pp. 33–91. [Google Scholar]
- Kuhlman SJ, Quintero JE, McMahon DG. GFP fluorescence reports Period 1 circadian gene regulation in the mammalian biological clock. Neuroreport. 2000;11:1479–1482. [PubMed] [Google Scholar]
- Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell. 1999;98:193–205. doi: 10.1016/s0092-8674(00)81014-4. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Lesauter J, Silver R. Fiber outgrowth from anterior hypothalamic and cortical xenografts in the third ventricle. J Comp Neurol. 1998;391:133–145. doi: 10.1002/(sici)1096-9861(19980202)391:1<133::aid-cne11>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immuno-cytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I. Neurons with access to the general circulation in the central nervous system of the rat: a retrograde tracing study with fluoro-gold. Neuroscience. 1991;44:655–662. doi: 10.1016/0306-4522(91)90085-3. [DOI] [PubMed] [Google Scholar]
- Messager S, Garabette ML, Hastings MH, Hazlerigg DG. Tissue-specific abolition of Per1 expression in the pars tuberalis by pine-alectomy in the Syrian hamster. Neuroreport. 2001;12:579–582. doi: 10.1097/00001756-200103050-00029. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Jetton AE, Matsumoto SI, Markuns JF, Lehman MN, Bittman EL. Effects of suprachiasmatic transplants on circadian rhythms of neuroendocrine function in golden hamsters. Endocrinology. 1999;140:207–218. doi: 10.1210/endo.140.1.6428. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Stephan FK. The effects of hypothalamic knife cuts on drinking rhythms and the estrus cycle of the rat. Behav Biol. 1977;20:224–234. doi: 10.1016/s0091-6773(77)90786-6. [DOI] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Swarts HJ, van der Vliet J, Wiegant VM, Buijs RM, Kalsbeek A. Control of the estradiol-induced prolactin surge by the suprachiasmatic nucleus. Endocrinology. 2001;142:2296–2302. doi: 10.1210/endo.142.6.8219. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Sangoram AM, Saez L, Antoch MP, Gekakis N, Staknis D, Whiteley A, Fruechte EM, Vitaterna MH, Shimomura K, King DP, Young MW, Weitz CJ, Takahashi JS. Mammalian circadian autoregulatory loop: a timeless ortholog and mPer1 interact and negatively regulate CLOCK-BMAL1-induced transcription. Neuron. 1998;21:1101–1113. doi: 10.1016/s0896-6273(00)80627-3. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Coleman RJ, Reppert SM. A daily vasopressin rhythm in rat cerebrospinal fluid. Brain Res. 1983;263:105–112. doi: 10.1016/0006-8993(83)91205-2. [DOI] [PubMed] [Google Scholar]
- Silver R, LeSauter J, Tresco PA, Lehman MN. A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature. 1996;382:810–813. doi: 10.1038/382810a0. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Berkley KJ, Moss RL. Efferent connections of the rat suprachiasmatic nucleus. Neuroscience. 1981;6:2625–2641. doi: 10.1016/0306-4522(81)90108-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Rat drinking rhythms: central visual pathways and endocrine factors mediating responsiveness to environmental illumination. Physiol Behav. 1972;8:315–326. doi: 10.1016/0031-9384(72)90379-4. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, Shibata S. Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology. 2001;142:4910–4917. doi: 10.1210/endo.142.11.8487. [DOI] [PubMed] [Google Scholar]
- Teclemariam-Mesbah R, Kalsbeek A, Pevet P, Buijs RM. Direct vasoactive intestinal polypeptide-containing projection from the suprachiasmatic nucleus to spinal projecting hypothalamic paraventricular neurons. Brain Res. 1997;748:71–76. doi: 10.1016/s0006-8993(96)01246-2. [DOI] [PubMed] [Google Scholar]
- Tong Y, Lee H, Lehman MN, Bittman EL. The Testes Are Different. (Conference Abstract.). Society for Research in Biological Rhythms; Anelia Island, FL, USA. 2002. [Abstract number 100] [Google Scholar]
- Turek FW, Swann J, Earnest DJ. Role of the circadian system in reproductive phenomena. Recent Prog Horm Res. 1984;40:143–183. doi: 10.1016/b978-0-12-571140-1.50009-8. [DOI] [PubMed] [Google Scholar]
- Van Cauter E. Diurnal and ultradian rhythms in human endocrine function: a minireview. Horm Res. 1990;34:45–53. doi: 10.1159/000181794. [DOI] [PubMed] [Google Scholar]
- Vrang N, Larsen PJ, Mikkelsen JD. Direct projection from the suprachiasmatic nucleus to hypophysiotrophic corticotropin-releasing factor immunoreactive cells in the paraventricular nucleus of the hypothalamus demonstrated by means of Phaseolus vulgaris-leucoagglutinin tract tracing. Brain Res. 1995;684:61–69. doi: 10.1016/0006-8993(95)00425-p. [DOI] [PubMed] [Google Scholar]
- Walters MC, Fiering S, Eidemiller J, Magis W, Groudine M, Martin DI. Enhancers increase the probability but not the level of gene expression. Proc Natl Acad Sci U S A. 1995;92:7125–7129. doi: 10.1073/pnas.92.15.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG, Sheward WJ, Whale D, Fink G. The effects of knife cuts in the sub-paraventricular zone of the female rat hypothalamus on oestrogen-induced diurnal surges of plasma prolactin and LH, and circadian wheel-running activity. J Endocrinol. 1989;122:593–604. doi: 10.1677/joe.0.1220593. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus. II Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258:230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus. I Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light responses in the suprachiasmatic circadian clock and oscillating transcripts outside of brain. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]