Abstract
Severe bottlenecks can reduce genetic diversity and increase inbreeding as individuals are forced to mate with close relatives, but it is unknown at what minimum population size the negative fitness consequences of bottlenecks are expressed. The New Zealand avifauna contains a large number of species that have gone through bottlenecks of varying severity, providing an exceptional opportunity to test this question by using the comparative method. Using decreased hatchability as a measure of fitness costs, we found that hatching failure was significantly greater among both native and introduced species that had passed through bottlenecks of <150 individuals. Comparisons between pre- and postbottleneck populations of introduced species suggest that hatching problems arise even in populations founded by <600 individuals. Our study confirms that hatching failure is widespread and persistent among birds passing through severe bottlenecks and that the population sizes at which this fitness cost is expressed are several times greater than the number of individuals currently used to found most new populations of endangered species. We recommend that conservation managers revise the protocols they use for reintroductions or they may unwittingly reduce the long-term viability of the species they are trying to save.
Habitat destruction and exploitation are causing catastrophic population declines in many species around the world. Even if endangered populations recover, severe bottlenecks may reduce genetic diversity and increase inbreeding as survivors are forced to mate with close relatives, resulting in lowered heterozygosity, increased genetic load, and increased expression of deleterious alleles (1). Inbreeding may yield significant costs to fitness and decrease population survival (2), a process termed inbreeding depression, but its importance has been questioned (3–5), and examples of the negative fitness consequences due to inbreeding in small populations of wild animals are few (6, 7). Despite the potential importance of bottleneck size to conservation biology, the number of individuals required to avoid the fitness costs of small population size and maintain the viability of a population has been difficult to test in free-living animals (1).
Theoretical models suggest that minimum effective population sizes range from 50 to 5,000 individuals, depending on levels of acceptable loss of genetic variability and the timeframe of population persistence (8, 9). The exact number is not a trivial question because the survival of many endangered species depends on the reliability of such guidelines. It has even been suggested that severe bottlenecks may be advantageous because they reduce inbreeding depression by purging deleterious alleles (10) although whether such benefits are great enough to justify deliberate inbreeding have been questioned (11, 12). The problem for conservation biologists is to understand whether bottlenecks create fitness costs and at what population size these costs become so severe that they threaten the viability of a population.
The New Zealand avifauna provides an ideal opportunity to examine the potential fitness costs of small population size across a range of species that have experienced bottlenecks of varying severity. Human settlement brought drastic changes to the avifauna: >30% of endemic bird species became extinct and many surviving species are threatened (13). For example, the black robin (Petroica traversi) was reduced to a single breeding pair before conservation efforts increased the population to several hundred (14). A widespread program of founding new populations by transferring birds to islands free of introduced predators has similarly increased numbers of other endangered species. Despite such promising recoveries, monitoring has revealed high levels of hatching failure in some species (15). Only about half the eggs that survive the incubation period successfully hatch in the highly endangered kakapo (Strigops habroptilus; refs. 15 and 16), and similar problems have been observed in the black robin (17).
Increased hatching failure is a common outcome of inbreeding in captive and wild populations of birds and is a useful measure of the effect of an inbred genome on embryological development (18, 19). Hatching failure in out-bred birds averages ≈10% (20); thus, levels higher than this are likely to indicate an increased fitness cost to inbreeding. To determine whether severe bottlenecks result in a fitness cost, and if there is a population size below which these costs escalate, we compared hatching failure in New Zealand birds (both native and introduced species) with their population bottleneck size. Our objective was to determine whether we could estimate the minimum bottleneck size that would avoid one of the proposed fitness costs of inbreeding and therefore guide conservation managers in the establishment of new populations of endangered species.
Materials and Methods
Information on hatching failure and population bottleneck size in native birds was collected from the literature, personal communication with researchers, the New Zealand nest record scheme, and our own field work. Our sample included 22 native New Zealand species with bottlenecks ranging from 5 to 5,500 individuals. Endangered populations of isolated island subspecies (e.g., Petroica macrocephala chathamensis) were used when it was clear that gene flow between the bottleneck population and the mainland subspecies was unlikely. We also included one island population of the New Zealand robin (Petroica australis) founded by five birds and that has been isolated for 23 yr (our results do not change if this species is taken out). A bottleneck was defined as the lowest number of individuals ever recorded in a species or discrete isolated population of a subspecies. Hatching failure was defined as the proportion of eggs in a nest that failed to hatch relative to the number present at hatching. Failed eggs include both unfertilized eggs as well as those that died as embryos. This measure excluded eggs that failed because of predation, desertion, or abiotic factors such as windstorms or floods. To estimate rate of hatching failure for each species or population, the proportion of eggs failing to hatch in each nest was angular transformed, and the mean and 95% confidence limits were calculated on transformed values (21). All hatching data were collected from free-living populations after each species had passed through the bottleneck. A total of 1,241 nests were used for this analysis.
Population bottleneck size was compared with rate of hatching failure by linear regression, with more than one value of y per x (21). This test allowed us to partition variance within and between species and also to test for a relationship with bottleneck size. Bottleneck size was log transformed, and hatching failure rates were angular transformed. Independent contrasts were then used on the mean values to control for phylogeny (22) by using the computer program caic (Comparative Analysis by Independent Contrasts) (23). Such controls are required because the levels of hatching failure may be similar in closely related species through inheritance from a common ancestor rather than as a consequence of bottleneck size. We used a phylogeny constructed from Sibley and Ahlquist (24) and added body mass as a third variable in a multiple regression of hatching failure contrasts on bottleneck size contrasts. Body mass is often a confounding variable in comparative studies, and a multiple regression allowed us to control for body size while comparing hatching failure rates with bottleneck size. We then calculated a series of unique linear contrasts for each node in our phylogeny for which there was variation in the independent variable. To test for relationships between taxa, the linear contrasts of one variable were correlated with those of another. All correlations were forced through the origin as recommended (22). Data on levels of consanguious matings were unavailable for most species so we assumed that inbreeding frequency increased with decreasing bottlenecks. For comparison, we also used data on hatching failure in a total of 1,319 nests in 15 native New Zealand species with populations of >10,000 individuals as nonbottleneck controls. We assumed large continuous populations would be less inbred than bottlenecked populations.
We then repeated our analysis on introduced species in New Zealand. Data on number of introduced birds released was obtained from Long (25) and Thomson (26). We summed all birds released as an estimate of bottleneck size even if individuals were not released at the same time or locality. However, if it was clear from historical records that a particular release failed, we excluded these birds from our tally. We treated the South Island release of rook (Corvus frugilegus) as separate from those released on the North Island because the former have not spread from their release site and remain in an isolated population in the central South Island. We could only obtain hatching data on this isolated population. All other introduced birds used in this study have spread across New Zealand and now form a single interbreeding population. We used 15 introduced species for which we had data on both hatching failure and number released. We then estimated hatching failure in their respective native ranges by using the files in the nest record schemes of the United Kingdom, United States, and Canada. A total of 2,147 nests from introduced species in New Zealand and 23,986 nests for the same species from their native ranges were used to calculate levels of hatching failure in this comparison.
Results and Discussion
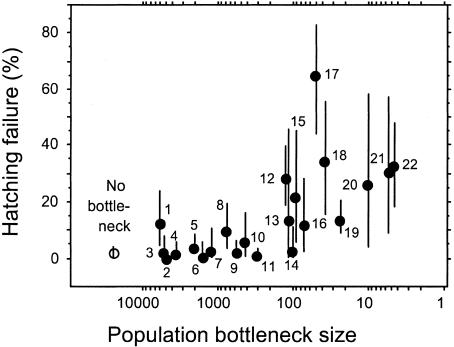
Bottleneck size had a significant effect on hatching failure in native New Zealand birds (Fig. 1). Hatching failure averaged 3.0 ± 0.6% (SE, n = 15, range 0.3–7.0%) in nonbottleneck species, but this increased to 25.3 ± 5.0% (n = 11, range 2.4–64.6%) in species subject to bottlenecks of <150 individuals. Native species that passed through bottlenecks of 300–5,500 individuals had levels of hatching failure (3.7 ± 1.2%; n = 11, range 0.1–12.5%) similar to that observed in nonendangered native species in New Zealand and elsewhere (20). Variance in hatching failure also increased with severity of bottleneck size (Fig. 1; r = 0.51, df = 21, P = 0.016). This result was not due to smaller sample sizes in more severely bottlenecked species (r = 0.24, df = 21, P = 0.28) but instead indicates that hatching failure is more variable among more bottlenecked species.
Fig. 1.
Increase in hatching failure with increasing severity of population bottleneck in native New Zealand birds (n = 22). Circles are means ± 95% confidence intervals. Open circle shows mean hatching failure in 15 species that did not pass through a bottleneck. Hatching failure increased when bottlenecks dropped below ≈150 individuals. A linear regression between bottleneck size (excluding nonbottleneck species) and rate of hatching failure was significant (y = –0.155X + 0.67; F = 10.8, df = 1,19, P < 0.01). This result did not change when controlled for body mass and phylogeny (multiple regression on contrasts forced through origin: y =–0.181x1 + 0.119x2; partial F for bottleneck size (x1) = 24.7, df = 1,19, P < 0.0001; partial F for body mass (x2) = 2.8, df = 1,19, P = 0.11). Species are (number of nests in parentheses): 1, Eudyptes pachyrhynchus (37); 2, Megadyptes antipodes (36); 3, Anarhynchus frontalis (29); 4, Procellaria parkinsoni (66); 5, Haematopus unicolor (80); 6, Coenocorypha pusilla (11); 7, Charadrius obscurus aquilonius (24); 8, Petroica macrocephala chathamensis (40); 9, Anas aucklandica (32); 10, Philesturnus rufusater (17); 11, Pterodroma axillaris (203); 12, Porphyrio hochstetteri (111); 13, Ha. chathamensis (11); 14, Pt. magentae (30); 15, Thinornis novaeseelandiae (21); 16, Anas nesiotis (13); 17, Strigops habroptilus (37); 18, Ph. carunculatus (29); 19, Himantopus novaezelandiae (181); 20, Sterna nereis (19); 21, Pe. australis (25); and 22, Pe. traversi (189).
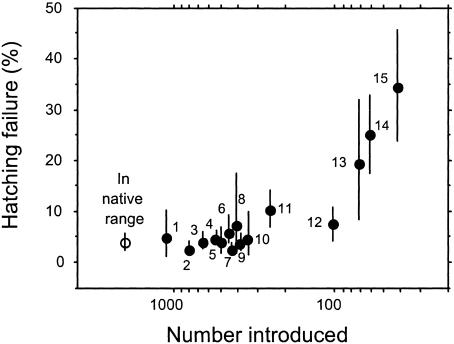
An examination of hatching failure in birds introduced to New Zealand confirms that the pattern we found is not the result of unusual demographic or genetic traits of island birds (27). In the 19th century, >30 species of exotic birds were established in New Zealand by acclimatization societies, and most are now more widespread and abundant than native species (28). The number released, and hence the severity of their bottleneck, varied across species from ≈10 to 1,000 individuals. As with native birds, introduced species that passed through bottlenecks of <150 birds showed higher levels of hatching failure (21.6 ± 5.6%, n = 4, range 7.5–34.4%) compared with introduced species passing through less severe bottlenecks (Fig. 2). This finding is not significantly different from the level of hatching failure in native birds that passed through bottlenecks of <150 individuals (t test: t = 0.42, df = 13, P = 0.68). In contrast, introduced species founded by populations of >150 birds showed similar levels of hatching failure (4.9 ± 0.7%, n = 11, range 2.4–10.3%) to that in their native ranges (4.1 ± 0.7%, n = 15, range 1.2–11.7%; Fig. 2). As with native species, variance in hatching failure in introduced species increased with severity of bottleneck (Fig. 1; r = 0.67, df = 14, P = 0.006). This pattern was not due to smaller sample sizes in more bottlenecked species (r = 0.10, df = 14, P = 0.73).
Fig. 2.
Increase in hatching failure of introduced species (n = 15) with decreased numbers of individuals released by 19th century New Zealand acclimatization societies. Circles are means ± 95% confidence intervals. Open circle shows mean hatching failure in the same species in their native range. A linear regression between number introduced (excluding data from native range) and rate of hatching failure was significant (y =–0.27x + 0.93; F = 43.3, df = 1,13, P < 0.001). The relationship between hatching failure and decrease in number released held when controlled for phylogeny and body mass (multiple regression on contrasts forced through origin: y = –0.288x1 –0.05x2; partial F for number introduced (x1) = 33.6, df = 1,12, P < 0.0001; partial F for body mass (x2) = 0.9, df = 1,12, P = 0.37). Species are (number of nests in parentheses): 1, Callipepla californica (10); 2, Turdus merula (303); 3, Sturnus vulgaris (173); 4, Fringilla coelebs (99); 5, Carduelis carduelis (420); 6, Car. flammea (68); 7, Passer domesticus (212); 8, Alauda arvensis (44); 9, T. philomelos (197); 10, Emberiza citrinella (22); 11, Prunella modularis (120); 12, Car. chloris (109); 13, Acridotheres tristis (14); 14, Branta canadensis (141); and 15, Corvus frugilegus (441).
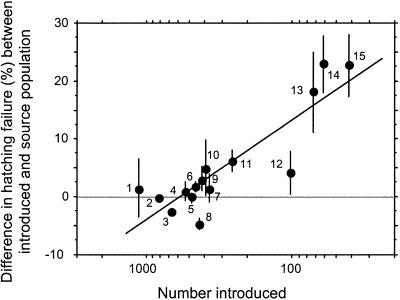
Our analyses of current levels of hatching failure suggest that a bottleneck of ≈150 individuals seems to be a critical size below which the negative fitness consequences of small bottlenecks increases. A more direct test for an effect of bottleneck size would be to compare hatching failure both before and after the bottleneck event. This test is not possible with native birds, but introduced species have large extant populations in their source countries (primarily the United Kingdom). Thus, a comparison between introduced species in their native range (“before” a bottleneck) with that in New Zealand (“after” a bottleneck) provides a matched-pair experiment of bottleneck size on level of hatching failure. Consistent with the hypothesis that small population size increases fitness costs such as hatching failure, we found an increasing difference in hatching failure rates between populations in their native and introduced range with severity of bottleneck (Fig. 3). The greatest differences in hatching failure rates were observed in introduced species that passed through the most severe bottlenecks. The line in this regression intercepts the x axis at 606 individuals (95% confidence limit: 490–1,585 individuals). This is the number of founders in which hatching success in a postbottleneck population does not differ from that in its prebottleneck population.
Fig. 3.
Increase in differences in rate of hatching failure between each introduced population in New Zealand (postbottleneck) and their source population (prebottleneck) for 15 species of introduced birds with data in both localities. Circles are differences ± 95% confidence intervals. Positive values indicate that hatching failure is greater in the introduced populations. A linear regression between number introduced and difference in rate of hatching failure was significant (y = –0.271x + 0.75; F = 28.1, df = 1,14, P < 0.001). This relationship was significant when controlled for phylogeny and body mass (multiple regression on contrasts forced through origin: y = –12.29x1 + 4.14x2; partial F for number introduced (x1) = 42.1, df = 1,12, P < 0.0001; partial F for body mass (x2) = 3.9, df = 1,12, P = 0.07). The intercept on the x axis (606) indicates the number of individuals in a founding population where hatching failure does not differ from that in source population. Numbers refer to species listed in Fig. 2.
Alternative explanations cannot account for our findings. For example, pesticide pollution has been implicated in hatching failure among endangered birds elsewhere (29). However, pollution-induced hatching failure cannot explain the results here because most native species in our sample occur in remote areas, and populations declined before the introduction of persistent pesticides. Levels of pesticides in species most susceptible to bioaccumulation (e.g., New Zealand falcon Falco novaeseelandiae) are also not high enough to affect hatching success (30). Instead, the primary cause threatening the survival of native New Zealand birds is predation by introduced mammals (31). The rapid recovery of many species after the removal of exotic predators also argues against pesticides as the cause of bottlenecks.
It is possible that species with high levels of hatching failure before a bottleneck (for reasons unrelated to inbreeding) were more likely to experience a bottleneck, and this bias could explain the pattern we found. In this case, high initial levels of hatching failure may have increased the severity of the bottleneck when such species were exposed to other negative demographic factors such as habitat fragmentation or the introduction of exotic predators. The lack of information on levels of hatching failure in native species before they declined precludes any test of this hypothesis. However, the levels of hatching failure in introduced species in their native range was not significantly correlated with the number of individuals subsequently released in New Zealand (F = 1.65, df = 1,14, P = 0.65). In other words, introduced species with a small number of founders did not by chance have high levels of hatching failure in their native range. This finding suggests that high levels of hatching failure were caused by the severe bottlenecks that these species passed through and were not the cause of the bottlenecks in the first place.
Another possible explanation for our results is that the most endangered species are now confined to marginal or degraded habitat, and this environment leads to greater hatching failure because of poor adult condition. For example, hatching failure of takahe (Porphyrio hochstetteri) translocated to offshore islands is higher than in their source population in mainland alpine habitats (17). However, this hypothesis cannot account for increased hatching failure in introduced species that also passed through severe bottlenecks (Fig. 2). Introduced species are common and widespread, and hatching failure should not therefore be the result of confinement to marginal environments. Differences in environmental conditions between the source and transplanted ranges in introduced species could still account for their increased hatching failure in New Zealand even if these differences do not limit population size. For example, dietary deficiencies in the introduced range could lead to increased hatching failure. Detailed studies of diet differences and other potentially stressful environmental factors are lacking to test this idea, but such a problem would have to disproportionately affect the most severely bottlenecked species to explain the pattern we found.
Our comparison of introduced species between their native and introduced ranges suggests that as many as 600 individuals may be needed to avoid increased hatching failure when founding a new population. This is 4-fold higher than the level of ≈150 individuals we found when comparing birds within New Zealand. It is unlikely that the higher level is due entirely to genetic effects such as inbreeding because differences in environmental conditions might also induce higher hatching failure for species transplanted outside their range (1). This adjustment would not change the slope in Fig. 3, but it would increase the intercept and thus overestimate the number of founders required to avoid increased hatching failure. Conservation managers should nonetheless be cautious of relying exclusively on our lower estimate of 150 individuals as a minimum population size because environmental effects are likely to interact with genetic effects in ways difficult to predict on a species by species basis. It may be prudent to use our upper estimate of bottleneck size to ensure that future changes in environmental conditions (e.g., global warming) do not induce higher levels of hatching failure at a later date. This guideline may be especially important for species transplanted outside of their natural range for conservation purposes.
We found no support for the hypothesis that deleterious alleles are purged during bottlenecks (10). Introduced species have been established in New Zealand for over a century (≈50–100 generations for most species), and most native birds declined even earlier, yet levels of hatching failure remain high. This result suggests that the aftermath of severe bottlenecks may last for hundreds of generations. Our results also argue against the widespread view that the apparent health of some bottlenecked populations (e.g., black robin) is evidence against the deleterious effects of small population size (3, 32). Although some populations of birds have been founded successfully by small numbers of individuals, higher hatching failure in these species indicate that they are not immune from its fitness consequences. Indeed, we found increased hatching failure to be nearly universal among species (both native and introduced) passing through severe bottlenecks, which suggests that the fitness costs of small population size are not compensated for by the proposed benefits of purging.
In theory, a population bottleneck of 150 individuals (N) should increase inbreeding only by <1% (1). However, the effective population size (Ne) is unlikely to be the same as the population bottleneck size (1, 8). Ratios of effective population size to bottleneck size (Ne/N) suggest values around 0.1, which would suggest an effective population size of only 15 individuals (8). This finding yields an inbreeding coefficient of ≈3%, which may be enough to increase some of the fitness consequences of inbreeding such as hatching failure. Inbreeding may be more severe if Ne/N ratios are even lower when populations are transplanted to another country (i.e., < 0.1), or if population growth is slow and the bottleneck lasts for more than a few generations (33). A low ratio also could explain the higher threshold value of 600 individuals observed in our sample of introduced species (Fig. 3). A review of genetic variation in introduced bird species between their native and introduced ranges (including five species from New Zealand) found that more severe bottlenecks indeed reduced genetic variability in the resulting populations (34). Further genetic studies are required to confirm this pattern in both the native and introduced species in this study, but our results suggest that severe bottlenecks result in measurable fitness costs and that this result is consistent with that expected by increased inbreeding and the loss of genetic diversity when populations drop to small numbers.
How does our finding, that hatching failure increases if populations pass through bottlenecks of <150 individuals, compare with current practice in founding new populations for conservation purposes? In New Zealand, conservationists typically release ≈40 individuals when transferring birds to found a new population although some releases have involved as few as 5 individuals (35). A worldwide review of ≈700 translocations revealed that 72% involved <75 animals and 46% involved founding populations of <30 animals (36). Our study shows that these levels will impair reproductive success and hinder the recovery of endangered species unless steps are taken to reduce the loss of genetic diversity and the increased risk of inbreeding in the resulting populations. At worst, the current practice of founding new populations of endangered species with such small numbers of founders may be inducing widespread reproductive failure and hastening their extinction.
Acknowledgments
We thank the Ornithological Society of New Zealand, the British Trust for Ornithology, and the Canadian and American nest record schemes for access to their data files. We especially thank the many individuals who answered our requests for information on their study species. Our paper benefited greatly from the advice of F. Allendorf, R. Didham, N. Gemmell, D. Kelly, B. Kempenaers, B. Lyon, T. Martin, R. Montgomerie, L. Shorey, and two reviewers. Funding for this study was provided by the Brian Mason Scientific and Technical Trust.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Frankham, R., Ballou, J. D. & Briscoe, D. A. (2002) Introduction to Conservation Genetics (Cambridge Univ. Press, Cambridge, U.K.).
- 2.Saccheri, I., Kuussaari, M., Kankare, M., Vikman, P., Fortelius, W. & Hanski, I. (1998) Nature 392, 491–494. [Google Scholar]
- 3.Shields, W. M. (1993) in The Natural History of Inbreeding and Outbreeding, ed. Thornhill, N. W. (Univ. of Chicago Press, Chicago), pp. 143–169.
- 4.Lande, R. (1988) Science 241, 1455–1460. [DOI] [PubMed] [Google Scholar]
- 5.Elgar, M. A. & Clode, D. (2001) Conserv. Biol. 15, 284–286. [Google Scholar]
- 6.Jimenez, J. A., Hughes, K. A., Alaks, G., Graham, L. & Lacy, R. C. (1994) Science 264, 271–273. [DOI] [PubMed] [Google Scholar]
- 7.Crnokrak, P. & Roff, D. A. (1999) Heredity 83, 260–270. [DOI] [PubMed] [Google Scholar]
- 8.Frankham, R. (1995) Genet. Res. Camb. 66, 95–107. [Google Scholar]
- 9.Franklin, I. R. & Frankham, R. (1998) Anim. Conserv. 1, 69–71. [Google Scholar]
- 10.Templeton, A. R. & Read, B. (1984) Zoo Biol. 3, 177–199. [Google Scholar]
- 11.Willis, K. & Wiese, R. J. (1997) Zoo Biol. 16, 9–16. [Google Scholar]
- 12.Lacy, R. C. & Ballou, J. D. (1998) Evolution 52, 900–909. [DOI] [PubMed] [Google Scholar]
- 13.Holdaway, R. N., Worthy, T. H. & Tennyson, A. J. D. (2001) N. Z. J. Zool. 28, 119–187. [Google Scholar]
- 14.Butler, D. & Merton, D. (1992) The Black Robin: Saving the World's Most Endangered Bird (Oxford Univ. Press, Oxford).
- 15.Clout, M. N. & Craig, J. L. (1995) Ibis 137, S181–S190. [Google Scholar]
- 16.Jamieson, I. G. & Ryan, C. J. (2000) Biol. Conserv. 94, 107–114. [Google Scholar]
- 17.Ardern, S. L. & Lambert, D. M. (1997) Mol. Ecol. 6, 21–28. [Google Scholar]
- 18.Bensch, S., Hasselquist, D. & von Schantz, T. (1994) Evolution 48, 317–326. [DOI] [PubMed] [Google Scholar]
- 19.van Noordwijk, A. J. & Scharloo, W. (1981) Evolution 35, 674–688. [DOI] [PubMed] [Google Scholar]
- 20.Koenig, W. D. (1982) Auk 99, 526–536. [Google Scholar]
- 21.Sokal, R. R. & Rohlf, F. J. (1995) Biometry (Freeman, New York).
- 22.Harvey, P. H. & Pagel, M. (1991) The Comparative Method in Evolutionary Biology (Oxford Univ. Press, Oxford).
- 23.Purvis, A. & Rambaut, A. (1994) caic, Comparative Analysis by Independent Contrasts (Univ. Oxford, Oxford). [DOI] [PubMed]
- 24.Sibley, C. G. & Ahlquist, J. E. (1990) Phylogeny and Classification of Birds: A Study in Molecular Evolution (Yale Univ. Press, New Haven, CT).
- 25.Long, J. L. (1981) Introduced Birds of the World (David & Charles, London).
- 26.Thomson, G. M. (1922) The Naturalisation of Animals and Plants in New Zealand (Cambridge Univ. Press, Cambridge, U.K.).
- 27.Frankham, R. (1997) Heredity 78, 311–327. [DOI] [PubMed] [Google Scholar]
- 28.Bull, P. C., Gaze, P. D. & Robertson, C. J. R. (1985) The Atlas of Bird Distribution in New Zealand (Ornithological Society of New Zealand, Wellington).
- 29.Jones, C. G., Heck, W., Lewis, R. E., Mungroo, Y., Slade, G. & Cade, T. (1994) Ibis 137, S173–S180. [Google Scholar]
- 30.Fox, N. C. & Lock, J. W. (1978) N. Z. J. Ecol. 1, 118–125. [Google Scholar]
- 31.King, C. M. (1984) Immigrant Killers: Introduced Predators and the Conservation of Birds in New Zealand (Oxford Univ. Press, Auckland, New Zealand).
- 32.Craig, J. L. (1994) in Creative Conservation, eds. Olney, P. J. S., Mace, G. M. & Feistner, A. T. C. (Chapman & Hall, London), pp. 50–66.
- 33.Nei, M., Maruyama, T. & Chakraborty, R. (1975) Evolution 29, 1–10. [DOI] [PubMed] [Google Scholar]
- 34.Merilä, J., Björklund, M. & Baker, A. J. (1996) Heredity 77, 410–422. [Google Scholar]
- 35.Armstrong, D. P. & McLean, I. G. (1995) Pacific Conserv. Biol. 2, 39–54. [Google Scholar]
- 36.Griffith, B., Scott, J. M., Carpenter, J. W. & Reed, C. (1989) Science 245, 477–480. [DOI] [PubMed] [Google Scholar]