Abstract
The type III histone deacetylase sirtuin 1 (Sirt1) is a suppressor of both innate and adoptive immune responses. We have recently found that Sirt1 expression is highly induced in anergic T cells. However, the transcriptional program to regulate Sirt1 expression in T cells remains uncharacterized. Here we report that the early responsive genes 2 and 3, which can be up-regulated by T-cell receptor-mediated activation of nuclear factor of activated T-cell transcription factors and are involved in peripheral T-cell tolerance, bind to the sirt1 promoter to transcript sirt1 mRNA. In addition, the forkhead transcription factor, FoxO3a, interacts with early responsive genes 2/3 on the sirt1 promoter to synergistically regulate Sirt1 expression. Interestingly, IL-2, a cytokine that can reverse T-cell anergy, suppresses sirt1 transcription by sequestering FoxO3a to the cytoplasm through activating the PI3K-AKT pathway. Expression of the constitutively active form of FoxO3a blocks IL-2–mediated reversal of T-cell tolerance by retaining sirt1 expression. Our findings here provide a molecular explanation of IL-2–mediated reversion of T-cell anergy.
Keywords: T-cell anergic gene, FoxO3a sequestration
When self-reactive T cells recognize MHC/antigen complexes through their receptors (T-cell receptor, TCR) at peripheral lymphoid organs, they will either be deleted by apoptosis or silenced through a mechanism known as anergy (1, 2). T-cell anergy is a critical mechanism to prevent autoimmunity in mammals and failure in this anergic induction process causes autoimmune diseases, such as type 1 diabetes, multiple sclerosis, and rheumatoid arthritis (3–6). It is well accepted that T-cell anergy is induced and maintained by a group of suppressive proteins, such as Itch, Cbl-b, early growth-response genes (EGR) 2 and 3, Grail, and Sirt1, up-regulated by self-antigen/TCR ligation in the absence of costimulated signaling mediated by the CD28 receptor (7–10). The up-regulation of these suppressive genes requires the nuclear factor of activated T cells (NFAT), which can be activated by the TCR-mediated calcium/calcineurin pathway (11–13).
We and others have recently demonstrated that the class III histone deacetylase Sirt1, which plays important roles in many aspects of biological functions, such as aging, metabolism, and apoptosis, inhibits T-cell immune response (9, 14–16). Sirt1 expression in T cells is regulated by TCR-mediated signaling. The up-regulated Sirt1 protein antagonizes T-cell immune response by suppressing the activations of NF-κB and activated protein 1 (AP-1) transcription factors, both of which are required for production of IL-2, a cytokine that promotes T-cell proliferation (9, 16, 17). Genetic depletion of the sirt1 gene in mice leads to elevated T-cell immune response and the development of a lupus-like autoimmune syndrome (9, 15). In addition, Sirt1 is also found to suppress innate-immune responses through opposing NF-κB–mediated inflammatory cytokine production by macrophages (18).
In the present study we report that TCR-mediated sirt1 transcription is promoted through EGR2 and EGR3, as well as the forkhead O family transcription factor 3a (FoxO3a). FoxO3a binds to the sirt1 promoter through its interaction with EGR2/3 to synergistically promote sirt1 mRNA transcription. IL-2 sequesters FoxO3a to suppress sirt1 mRNA expression during T-cell activation, presumably allowing accelerated T-cell proliferation and differentiation. Because Sirt1 is required for maintaining and IL-2 reverses T-cell peripheral tolerance, our findings here provide a possible explanation about the IL-2–mediated switch from anergy to activation.
Results
Differential Sirt1 Expression in Mouse Primary T Cells.
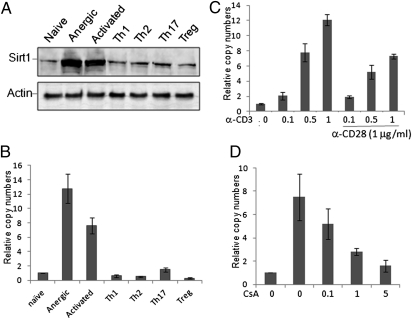
We have recently reported that sirt1 gene transcription is induced by TCR-mediated anergic signaling, and this increased Sirt1 expression is required to maintain peripheral T-cell tolerance (9). To further analyze the transcriptional regulation of Sirt1 expression in T cells, we compared Sirt1 protein and mRNA expression levels in naïve, activated, anergic, and differentiated effector T cells. The highest levels of Sirt1 protein expression were detected in anergic T cells generated in OT-II TCR transgenic mice treated with ovalbumen (OVA)323–339 peptide, confirming the results of our previous study (9). Stimulation of T cells with anti-CD3 plus anti-CD28 also dramatically induced Sirt1 expression. In contrast, Sirt1 protein expression levels in Th1, Th2, Th17, and regulatory T cells (Tregs) were significantly reduced compared with its expression in anergic and activated T cells (Fig. 1A). A similar pattern of sirt1 mRNA expression in CD4+ T cells was observed by real-time RT-PCR analysis (Fig. 1B). Therefore, Sirt1 expression is differentially regulated during T-cell immune responses.
Fig. 1.
Analysis of Sirt1 expression in CD4+ T cells. (A and B) Mouse naïve CD4+ T cells were left unstimulated (naïve) or stimulated with anti-CD3 plus anti-CD28 for 24 h (activated). Th1, Th2, and Th17 cells were generated under each specific polarization condition for 5 d. CD4+CD25+ cells from 6- to 8-wk-old mice were isolated as Tregs. For induction of anergic T cells, OT-II TCR transgenic mice were treated with OVA323–339 peptide. Seven days after treatment, CD4+ T cells in the lymph nodes from the treated mice were used as anergic T cells. (A) Cells were lysed with Nonidet P-40 lysis buffer and Sirt1 protein expression was analyzed by Western blotting (Upper) using β-Actin as a loading control (Lower). (B) Total RNA was purified and the mRNA level of sirt1 was analyzed by real-time RT-PCR. (C and D) Naïve T cells were stimulated with different amounts of anti-CD3 in the absence or presence of 1 μg/mL of anti-CD28 for 24 h (C) or stimulated with anti-CD3 plus anti-CD28 (1 μg/mL each) with different doses of CsA for 24 h (D). Total RNA was extracted and sirt1 mRNA levels were determined by real-time RT-PCR. Error bars represent data from three independent experiments (mean ± SE).
TCR recognition of autoreactive antigen in the absence of costimulation induces T-cell tolerance through activation of NFAT (12, 19). In fact TCR-stimulation alone with anti-CD3 antibody induced sirt1 transcription in a dose-dependent manner. Addition of anti-CD28 antibody did not further enhance, and in contrast slightly inhibited sirt1 gene transcription (Fig. 1C). Treatment of T cells with the calcineurin inhibitor cyclosporine A (CsA), which suppresses its downstream transcription factor NFAT, inhibited sirt1 gene transcription in a dose-dependent manner (Fig. 1D), suggesting that the TCR-NFAT pathway is involved in sirt1 transcription and that CD28-mediated signaling may suppress sirt1 gene transcription in T cells.
Transcription Factors EGR2 and EGR3 Are Involved in sirt1 Gene Expression in T Cells.
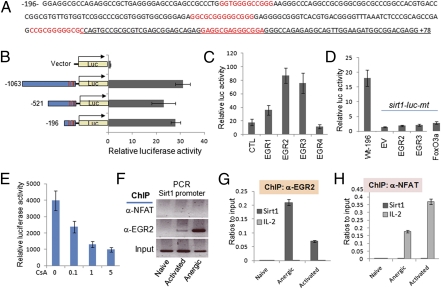
Analyzing the sirt1 promoter sequence did not identify any conserved NFAT-binding sites within the −30 kp region of sirt1 promoter in mammals. Instead, four conserved binding elements for the EGR family transcription factors were identified, three of which locate within the −196 bp region, and the fourth one is in the first exon (Fig. 2A), implying that EGR family transcription factors may regulate sirt1 transcription. To test this theory, we subcloned the 1-kb region (−1,063 to +79), which is often the optimal region carrying transcription factor-binding activity, into a pGL3 luciferase vector. In contrast to a basal-level pGL3-luciferase activity, this 1-kb fragment mediated significant luciferase activity (Fig. 2B). A serial deletion of this 1-kb region identified that the −196 to +79-bp region, which contains all four EGR binding sites, carries the transcriptional activity. These results suggest that EGR family transcription factors may be involved in sirt1 mRNA transcription. There are four members of EGR family transcription factors; we then tested which of them mediates sirt1 transcription. As shown in Fig. 2C, cotransfection of EGR2 and EGR3 transcription factors dramatically enhanced sirt1-luciferase activity. In contrast, EGR1 and EGR4 coexpression showed a modest luciferase activity of sirt1 promoter (Fig. 2C). Mutation of the EGR-binding sites completely abolished the sirt1 reporter activity, even when either EGR2 or EGR3 was coexpressed (Fig. 2D). Therefore, our data indicate that EGR family transcription factors EGR2 and EGR3 regulate sirt1 gene transcription. Interestingly, both egr2 and egr3 genes are downstream of the NFAT transcription factor in T cells (20, 21). It is likely that TCR-mediated NFAT activation promotes egr2 and egr3 mRNA transcription for sirt1 gene expression in T cells. To support this hypothesis, treatment of cells with the calcineurin inhibitor CsA dose-dependently suppressed sirt1 luciferase activity (Fig. 2E).
Fig. 2.
EGR2/3 regulates sirt1 transcription in T cells. (A) The −196 bp to +79 bp region of Sirt1 promoter sequence is shown. EGR-binding sites are indicated in red and the partial sequence of the first exon of sirt1 gene is underlined. (B) HEK293 cells were transfected with control pGL4-luc or with each of the pGL4-sirt1-luc plasmids that carry −1 kb, −0.5 kb, or −196 bp region of sirt1 promoter region, or with mutations of all four EGR-binding sites. Luciferase activity in the lysates of transfected cells were determined by a dual luciferase assay. Error bars represent data from three independent experiments. (C and D) pGL4-sirt1-luc that carries −196 bp region of the sirt1 promoter (C) or its mutant on all four EGR-binding sites (D) were cotransfected without or with EGR1, EGR2, EGR3, and EGR4, or FoxO3a expression plasmids, as indicated. The luciferase activity in transfected cells was determined. (E) Cells were transfected with pGL4-sirt1-luc that carries −196 bp region of the sirt1 promoter and treatment with different doses of CsA for 24 h and sirt1-luciferase activities were analyzed. (F–H) Naïve, activated, and anergic T cells were generated as described in Fig. 1. The binding of EGR2 (F, Middle, and G) and NFAT (F, Top, and H) to the sirt1 promoter was determined with ChIP assay. Their binding to the il-2 promoter was analyzed as a control (F and H, light-gray bars).
To further confirm the involvement of EGR family transcription factors in sirt1 gene transcription, we used a ChIP assay and analyzed whether EGR transcription factors bind to the sirt1 promoter in T cells. The binding of EGR2 to the sirt1 promoter was detected in activated T cells, and this binding was significantly increased in tolerized T cells isolated from OT-II TCR transgenic mice that were treated with OVA323–339 peptide. In contrast, only background levels of EGR2 binding to the sirt1 promoter was detected in naïve CD4 T cells (Fig. 2 F and G). In contrast, NFAT binding to the il-2 but not sirt1 promoter was detected in activated and anergic T cells (Fig. 2H). Unfortunately, we could not analyze sirt1 promoter-binding activity of EGR3 because none of the anti-EGR3 antibodies in our hand worked for ChIP analysis. It has been reported that EGR2, EGR3, and Sirt1 are up-regulated to maintain T-cell tolerance, and that EGR2 and EGR3 are downstream of NFAT (10, 21). Our results here provide a possible link of EGR family transcription factors to Sirt1 in T-cell anergy induction.
FoxO3a Promotes Sirt1 Transcription Through Its Binding with EGR2/3 in T Cells.
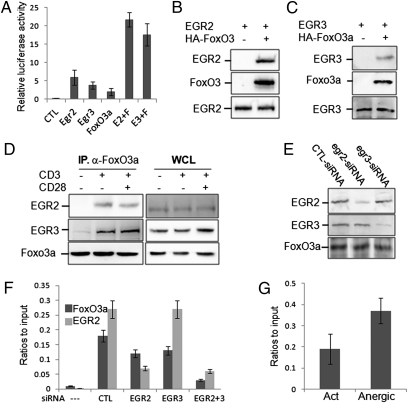
It has been reported that FoxO3a is a critical transcription factor for sirt1 expression in human cells through its binding with p53 (22). Therefore, we attempted to test whether FoxO3a is also involved in sirt1 transcription in mouse T cells. Expression of FoxO3a induced a modest sirt1-luciferase activity. Coexpression with EGR2/3 synergistically promoted sirt1 luciferase activity (Fig. 3A). Mutation of the EGR-binding sites in the sirt1 promoter completely abolished FoxO3a-mediated sirt1-luciferase activity (Fig. 2D). In human cells, FoxO3a binds to the sirt1 promoter via interaction with p53 as two p53 binding sites were identified in the promoter region of human sirt1 gene (22). However, the conserved p53 binding sites do not exist at the mouse sirt1 promoter (Fig. 2A). Therefore, we asked whether FoxO3a promotes sirt1 gene transcription in T cells by binding to EGR2 and EGR3 at the sirt1 promoter. Indeed, EGR2 and EGR3 proteins were detected in the anti-HA immunoprecipitates from the lysates of HEK293 cells when HA-FoxO3a expression plasmid DNA was cotransfected, but not in the nontransfected controls, suggesting FoxO3a interacts with both EGR2 and EGR3 in transiently transfected cells (Fig. 3 B and C). Their interaction was also detected in mouse primary T cells upon anti-CD3 stimulation, but anti-CD28 did not further enhance FoxO3a-interaction with EGR2 and EGR3, suggesting that FoxO3a may promote Sirt1 transcription through binding to EGR2/3 (Fig. 3D).
Fig. 3.
Regulation of sirt1 transcription by FoxO3a. (A) pGL4-sirt1-luc plasmid DNA was cotransfected with different combinations of EGR2 (E2), EGR3 (E3), and FoxO3a (F) expression plasmids, as indicated (50 ng each). The luciferase activities in transfected cells were determined. (B and C) HA-tagged FoxO3a expression plasmid DNA was cotransfected with EGR2 (B) or with EGR3 (C) into HEK293 cells. FoxO3a protein in the lysates of transfected cells was immunoprecipitated with anti-HA antibody. The binding of EGR2 or EGR3 with FoxO3a was determined by coimmunoprecipitation and Western blotting. (D) Mouse primary T cells were stimulated with anti-CD3 plus anti-CD28 for 2 h. The interaction of FoxO3a with EGR2 and EGR3 was determined by coimmunoprecipitation with anti-FoxO3a antibody and Western blotting with anti-EGR2 (Top, Left) and EGR3 (Middle, Left) antibodies. The same membrane was reblotted with anti-FoxO3a (Bottom, Left). The protein expression levels of EGR2 (Top, Right), EGR3 (Middle, Right), and FoxO3a (Bottom, Right) in the whole-cell lysates (WCL) were determined by Western blotting as loading controls. (E and F) Naïve T cells were transfected with siRNA against EGR2 or EGR3 by nuclear infection. The cells were then stimulated with anti-CD3 plus anti-CD28 for 24 h. (E) The protein expression levels of EGR2 (Top), EGR3 (Middle), and FoxO3a (Bottom) were determined by Western blotting. (F) The binding of FoxO3a and EGR2 to sirt1 promoter was determined by ChIP assay. The error bars represent data from three independent experiments (mean ± SE). Student t test was used for statistic analysis. (G) The sirt1 promoter-binding activities of FoxO3a in the activated and anergic T cells were analyzed by ChIP assay.
If FoxO3a binds to the sirt1 promoter through EGR2/3, suppression of EGR2 and EGR3 expression should inhibit FoxO3a-binding to the sirt1 promoter. We then used a siRNA-mediated knockdown approach to test this hypothesis. As shown in Fig. 3E, EGR2-specific siRNA inhibited 70–80% of EGR2 expression without affecting EGR3 protein levels. Similarly, EGR3-specific siRNA knocked-down EGR3 but not EGR2 expression in T cells. When T cells only received EGR2 or EGR3 siRNA, a modest reduction of FoxO3a-binding to sirt1 promoter was detected. In contrast, the sirt1 promoter binding activity of FoxO3a was significantly inhibited in T cells when both EGR2 and EGR3 were knocked-down (Fig. 3F). As expected, suppression of EGR2 expression inhibited its binding to the sirt1 promoter, but EGR3 binding activity was unaffected (Fig. 3F). In addition, a significant increase in FoxO3a binding to sirt1 promoter DNA in anergic T cells was detected compared with that in the activated T cells (Fig. 3G). Collectively, our data suggest that FoxO3a interacts with EGR2/3 to promote sirt1 mRNA transcription in T cells.
IL-2 Suppresses sirt1 Transcription in T Cells.
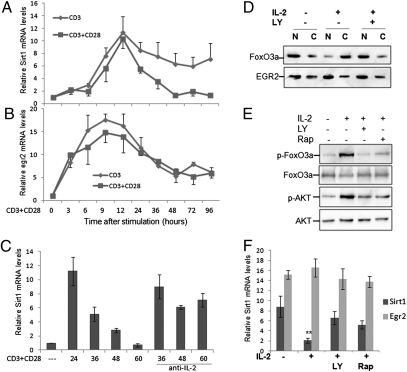
Sirt1 is highly expressed in anergic T cells and during the early stage of T-cell activation, but it is down-regulated in activated and differentiated effector T cells, suggesting that cytokine-mediated signaling might be involved in regulation of Sirt1 expression. To test this theory we analyzed the dynamics of sirt1 mRNA transcription in mouse primary T cells. As shown in Fig. 4A, a significant increase in sirt1 mRNA levels was detected at 12 h after TCR alone or TCR plus CD28 stimulation, which reached its peak at 24 h. A rapid decrease in sirt1 mRNA transcription was observed from 36 h in T cells when stimulated with both anti-CD3 and anti-CD28, and the sirt1 mRNA levels dropped to a similar level to that in naïve T cells at 2 d poststimulation. However, when T cells were stimulated with anti-CD3 alone, only a modest decline in sirt1 mRNA levels was observed, and this high level of sirt1 gene transcription was maintained at day 4 after TCR stimulation. These results indicate that TCR-mediated signaling alone is sufficient in sirt1 gene transcription and that CD28 costimulation induces a negative factor to suppress sirt1 gene transcription. In contrast, a dramatic increase in egr2 mRNA was detected within 3 h of TCR/CD28 stimulation, which peaked at 6–9 h. The expression of egr2 mRNA was maintained at relatively high levels during a 4-d period cultivation, although with a significant decline at about a day after stimulation, and CD28 costimulation appears not to affect egr2 gene transcription (Fig. 4B). Because both egr2 and egr3 are downstream genes of NFAT, this relative delay in sirt1 mRNA transcription during the early stages of T-cell activation suggests that sirt1 gene transcription depends on EGR2 and EGR3 protein expression in T cells.
Fig. 4.
IL-2 suppresses sirt1 transcription in T cells. (A and B) Naïve T cells were stimulated with anti-CD3 (A) or anti-CD3 plus anti-CD28 (B) for different amounts of time as indicated (hours). The mRNA levels of sirt1 and egr2 were determined by real-time PCR. (C) Naïve CD4+ T cells were stimulated with anti-CD3 plus anti-CD28 for 24 h. The cells were then cultured with IL-2 (10 ng/mL) or with anti–IL-2 neutralization antibody (10 μg/mL) for each indicated amount of time (hours). (D) Naïve T cells were stimulated with anti-CD3 plus anti-CD28 for 12 h followed by cultivation with 10 ng/mL of IL-2 or with PI3K inhibitor LY294002 or with rapamycin for an additional 6 h. Cytoplasmic (C) and nuclear (N) fractionations were performed and the protein levels of FoxO3a and EGR2 were analyzed by Western blotting (A). The levels of phosphorylated FoxO3a (p-FoxO3a) and AKT (p-AKT), as well as their total protein levels in the whole-cell lysates, were analyzed by Western blotting. (E and F) Anergic CD4+ T cells were isolated from OT-II TCR transgenic mice treated with OVA323–339 peptide. Purified cells were cultured with anti-CD3 plus anti-CD28 or with IL-2 or PI3K inhibitor LY294002 or rapamycin for 12 h. The levels of phosphorylated and total FoxO3a and AKT were analyzed by Western blotting (E). The mRNA levels of sirt1 and egr2 were determined by real-time PCR. Error bars represent data from three independent experiments (F).
Because sirt1 transcription is inhibited in all lineages of effector cells tested, including Th1, Th2, Th17, and Treg (Fig. 1 A and B), and IL-2 is the common cytokine during their differentiation, we asked whether IL-2 is involved in suppression of sirt1 mRNA transcription. To test this theory, we stimulated CD4+ naïve T cells with anti-CD3 plus anti-CD28 for 24 h, followed by addition of recombinant mouse IL-2 or anti-mouse IL-2 neutralization antibody. The exogenous IL-2, which induced T-cell proliferation, significantly reduced sirt1 mRNA and protein expression (Fig. 4C). In contrast, neutralization of IL-2 that was produced by activated T cells upon TCR/CD28 stimuli maintained the high levels of sirt1 mRNA expression (Fig. 4C). These data indicate that IL-2 is a negative factor in regulating sirt1 gene expression in T cells.
IL-2 Inhibits sirt1 Gene Transcription Through Phosphorylation of FoxO3a in T Cells.
It has been reported that IL-2 sequesters FoxO3a from the nucleus to the cytoplasm to promote T-cell proliferation and survival (23). It is possible that IL-2 suppresses sirt1 transcription by sequestering FoxO3a to cytoplasm in T cells. Subcellular fractionation experiments confirmed that IL-2 sequestered FoxO3a to the cytoplasm. This IL-2–mediated sequestration of FoxO3a is inhibited by pretreatment of T cells with the PI3K inhibitor LY294002. In contrast, the subcellular distribution of EGR2 was affected neither by IL-2 nor by PI3K inhibitor LY294002 treatment (Fig. 4D). Therefore, IL-2 appears to sequester FoxO3a through the PI3K-AKT pathway. To support this finding, we further detected an elevated phosphorylation of FoxO3a and AKT by IL-2 treatment of anergic T cells, and this IL-2–mediated AKT phosphorylation was inhibited by LY294002 (Fig. 4E). Exogenous IL-2 reverses T-cell tolerance, and our recent findings show that TCR-mediated Sirt1 expression contributes significantly to maintain T-cell anergy (9). Combined with our present data, these findings suggest that IL-2 may reverse T-cell anergy by suppressing sirt1 transcription. To test this hypothesis, we isolated CD4+ T cells from OT-II mice pretreated with OVA323–339 peptide and cultured them in the absence or presence of IL-2 for 12 h. As shown in Fig. 4F, IL-2 dramatically inhibited sirt1 mRNA expression without affecting egr2 mRNA in tolerized T cells. Treatment with PI3K inhibitor LY partially blocked IL-2-mediated down-regulation of sirt1 mRNA expression. Therefore, suppression of sirt1 transcription appears to be an explanation for IL-2–mediated reversal of T-cell anergy.
It has been shown that the mammalian target of rapamycin (mTOR) kinase inhibitor rapamycin, which inhibits both mTORC1 and mTORC2 in T cells, blocks the ability of IL-2 to reverse anergy (24, 25). As FoxO3a phosphorylation and inhibition is also mediated by mTORC2, we then asked whether rapamycin is able to block FoxO3a phosphorylation and the down-regulation of sirt1. In fact, treatment of T cells with rapamycin inhibited FoxO3a phosphorylation and sirt1 down-regulation that is induced by IL-2 in anergic T cells (Fig. 4E). As a consequence, rapamycin treatment also partially blocked the ability of IL-2 to suppress sirt1 gene expression (Fig. 4F).
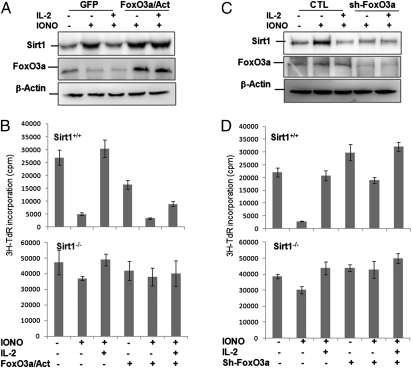
Because IL-2–mediated suppression of FoxO3a transcriptional activity is responsible for sirt1 gene down-regulation and reversal of T-cell tolerance, we asked whether the constitutively active form of FoxO3a can maintain sirt1 expression in anergic T cells when stimulated with IL-2. To this end, the constitutively active form of FoxO3a with a triple mutation of the AKT phosphorylation sites (TM-FoxO3a), which is not susceptible to inactivation by Akt phosphorylation (26), was delivered into CD4+ during expansion phase and rested. Anergy was induced by ionomycin treatment. Data in Fig. 5A show that IL-2 treatment failed to down-regulate Sirt1 protein expression in T cells that express the constitutively active form of FoxO3a. As a consequence, this constitutively active FoxO3a expression partially blocked the ability of IL-2 in reversal of T-cell tolerance. The constitutively active FoxO3a suppresses IL-2–mediated T-cell anergy reversal is through Sirt1, because its expression in Sirt1−/− T cells failed to maintain T-cell anergy (Fig. 5B). However, knockdown of FoxO3a expression in T cells results in reduced Sirt1 expression, even after ionomycin treatment (Fig. 5C). As a consequence, FoxO3a knockdown leads to a partial breakdown of tolerance of wild-type T cells (Fig. 5D). Collectively, our findings indicate that IL-2–mediated suppression of FoxO3a transcriptional activation inhibits sirt1 gene transcription to reverse T-cell tolerance.
Fig. 5.
Expression of the constitutively active form of FoxO3a blocks IL-2 ability to reverse T-cell anergy. Naïve CD4 T cells from wild-type and Sirt1−/− mice were purified and cultivated with anti-CD3 plus anti-CD28 for 24 h followed by infection with retrovirus that carry (A and B) GFP only (GFP) or GFP with the constitutively active form FoxO3a (FoxO3a/Act), or (C and D) with control shRNA or with the shRNA against FoxO3a. Three days after infection, GFP+ cells were sorted out, rested, and then treated with or without ionomycin for 16 h to induce tolerance. Cells were restimulated with anti-CD3 and anti-CD28 in the absence or presence of IL-2. (A and C) Sirt1 (Top) and FoxO3a (Middle) expression levels were determined by Western blotting using β-Actin as a control (Bottom). (B and D) The proliferation was determined by 3H-thymidine (3H-TdR) incorporation. Error bars represent data from three independent experiments.
Discussion
Peripheral tolerance of self-reactive T cells is induced by the reorganization of MHC/antigen complexes through TCR in the absence of costimulatory signaling (1, 2). Tolerized or anergic T cells express the high-affinity IL-2Ra (CD25), but do not produce IL-2, even after reobtaining a combined TCR/CD28 stimulation. Adding exogenous IL-2 leads to the breakdown of this tolerance, a phenomenon that has been known for decades. The present study provides a possible molecular explanation of IL-2-mediated reversal of T-cell tolerance by suppressing Sirt1 expression (Fig. 6). This conclusion is supported by the following observations: (i) the TCR-NFAT-EGR2/3 pathway induces Sirt1 expression to maintain T-cell tolerance; (ii) FoxO3a interacts with EGR2/3 to synergistically promote sirt1 gene expression during T-cell anergy induction; (iii) IL-2 sequesters FoxO3a to suppress sirt1 gene expression leading to reversal of T-cell tolerance; (iv) the constitutively active form of FoxO3a partially blocks IL-2–mediated reversal of T-cell anergy of wild-type but not Sirt1−/− T cells; and (v) knockdown of FoxO3a inhibits sirt1 expression, enhances T-cell activation and partially breaks down T-cell tolerance.
Fig. 6.
A proposed model for IL-2–mediated reversal of T-cell tolerance through modulating Sirt1 expression. (A) Schematic representation of the transcriptional regulation of sirt1 during T-cell anergy induction. TCR signaling activates NFAT through calcium/calmodulin/calcineurin pathway to induce egr2/3 expression. EGR2 and EGR3 bind to FoxO3a at sirt1 promoter to synergistically induce sirt1 gene transcription for inducing and maintaining T-cell tolerance. (B) Schematic representation of IL-2–mediated reversal of T-cell anergy. When anergic T cells receive IL-2 (may be also CD28), its binding to IL-2R activates PI3K/AKT. The activated AKT phosphorylates FoxO3a leading to sequestering FoxO3a to cytoplasm. Therefore, sirt1 gene expression is inhibited leading to breakdown of T-cell tolerance.
Anergic T cells express CD25, the high-affinity IL-2 receptor, but not IL-2 cytokines, as the transcription factors mediating IL-2 expression, such as NFAT, NF-κB, and AP-1, are inhibited (27–29). The Sirt1-mediated deacetylation of both AP-1 and NF-κB transcription factors is responsible, at least partially, for suppressing IL-2 expression in anergic T cells (9, 18, 30, 31). It is well established that exogenous IL-2, together with TCR and CD28 stimuli, can induce the proliferation of anergic T cells (32), but the underlying molecular mechanism remains unknown. IL-2 suppresses FoxO3a transcriptional activity by sequestering FoxO3a from nuclear to cytoplasm in a PI3K-AKT–dependent manner (23, 33). As FoxO3a is a transcription factor of sirt1, and sirt1-deficency leads to the breakdown of T-cell tolerance, this sequestration of FoxO3a is likely a molecular explanation of IL-2–mediated reversal of T-cell anergy.
The forkhead transcription factor FoxO3a has been found as a transcription factor for sirt1 gene expression in human cells (22). Our present study also demonstrates that FoxO3a promotes sirt1 gene transcription in mouse T cells. In human cells, FoxO3a binds to the sirt1 promoter through an interaction with p53, which has been found to regulate sirt1 gene transcription upon DNA damage (34). However, p53 binding sites do not exist in mouse sirt1 promoter region, implying that FoxO3a regulates sirt1 gene transcription in mouse T cells differently from that in human cells. Interestingly, we analyzed the sirt1 promoter sequence and found four conserved binding sites for EGR-family transcription factors. Further analysis as reported in this study indicates that FoxO3a interacts with EGR2 and EGR3 to synergistically regulate sirt1 mRNA transcription in T cells.
Materials and Methods
Isolating Mouse Naïve T Cells and in Vivo CD4+ T-Cell Anergy Induction.
Primary T cells were isolated from the lymph nodes and spleens of 6- to 8-wk-old mice. CD4+CD25−CD44lowCD62hi naïve T cells were purified using a naïve CD4+ T-cell isolation kit (Miltenyi Biotec). These cells were maintained in RPMI media supplemented with 10% FBS, 100 U/mL penicillin, 200 μg/mL streptomycin, and 0.25 μg/mL amphotericin, and stimulated with anti-CD3 plus anti-CD28 (5 μg/mL each). For in vivo T-cell anergy induction, OT-II mice were treated with 200 μg OVA323–339 peptide by tail intravenous injection. Mice were killed 7 d after treatment, CD4+ T cells were isolated, and the anergy induction was confirmed as previously described (6).
Transfection, Immunoprecipitation, and Western Blotting.
Transfection, immunoprecipitation, and Western blotting to analyze protein–protein interactions and expressions were performed as previously reported (14). Detailed methods are described in SI Materials and Methods.
Chromatin Immunoprecipitation.
ChIP assays were performed as previously reported (14, 35) and a detailed method is described in SI Materials and Methods.
Luciferase Assay.
Experiments were performed as previously described (36).
Supplementary Material
Acknowledgments
We thank Dr. Fang Feng (Northwestern University) for early responsive-gene expression plasmids. This study was supported by National Institutes of Health Grant R01AI079056 and “The Type I Diabetes Pathfinder Award” (DK083050) from the National Institute of Health (to D.F.); and National Institutes of Health Training Grant 5K12GM088020-02 (to K.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118462109/-/DCSupplemental.
References
- 1.Saibil SD, Deenick EK, Ohashi PS. The sound of silence: Modulating anergy in T lymphocytes. Curr Opin Immunol. 2007;19:658–664. doi: 10.1016/j.coi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Fathman CG, Lineberry NB. Molecular mechanisms of CD4+ T-cell anergy. Nat Rev Immunol. 2007;7:599–609. doi: 10.1038/nri2131. [DOI] [PubMed] [Google Scholar]
- 3.Brumeanu TD, Bona CA, Casares S. T-cell tolerance and autoimmune diabetes. Int Rev Immunol. 2001;20:301–331. doi: 10.3109/08830180109043041. [DOI] [PubMed] [Google Scholar]
- 4.Khan S, Greenberg JD, Bhardwaj N. Dendritic cells as targets for therapy in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:566–571. doi: 10.1038/nrrheum.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waldner H, Collins M, Kuchroo VK. Activation of antigen-presenting cells by microbial products breaks self tolerance and induces autoimmune disease. J Clin Invest. 2004;113:990–997. doi: 10.1172/JCI19388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SM, et al. Prevention and treatment of diabetes with resveratrol in a non-obese mouse model of type 1 diabetes. Diabetologia. 2011;54:1136–1146. doi: 10.1007/s00125-011-2064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 8.Anandasabapathy N, et al. GRAIL: An E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–547. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, et al. The type III histone deacetylase Sirt1 is essential for maintenance of T cell tolerance in mice. J Clin Invest. 2009;119:3048–3058. doi: 10.1172/JCI38902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Safford M, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 11.Baine I, Abe BT, Macian F. Regulation of T-cell tolerance by calcium/NFAT signaling. Immunol Rev. 2009;231:225–240. doi: 10.1111/j.1600-065X.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 12.Soto-Nieves N, et al. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. J Exp Med. 2009;206:867–876. doi: 10.1084/jem.20082731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu G, et al. Phospholipase Cgamma1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kong S, et al. The type III histone deacetylase Sirt1 protein suppresses p300-mediated histone H3 lysine 56 acetylation at Bclaf1 promoter to inhibit T cell activation. J Biol Chem. 2011;286:16967–16975. doi: 10.1074/jbc.M111.218206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequeira J, et al. sirt1-null mice develop an autoimmune-like condition. Exp Cell Res. 2008;314:3069–3074. doi: 10.1016/j.yexcr.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Kwon HS, et al. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe. 2008;3:158–167. doi: 10.1016/j.chom.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 18.Schug TT, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30:4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macián F, et al. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 20.Rengarajan J, et al. Sequential involvement of NFAT and Egr transcription factors in FasL regulation. Immunity. 2000;12:293–300. doi: 10.1016/s1074-7613(00)80182-x. [DOI] [PubMed] [Google Scholar]
- 21.Xiao S, et al. FasL promoter activation by IL-2 through SP1 and NFAT but not Egr-2 and Egr-3. Eur J Immunol. 1999;29:3456–3465. doi: 10.1002/(SICI)1521-4141(199911)29:11<3456::AID-IMMU3456>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 22.Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- 23.Stahl M, et al. The forkhead transcription factor FoxO regulates transcription of p27Kip1 and Bim in response to IL-2. J Immunol. 2002;168:5024–5031. doi: 10.4049/jimmunol.168.10.5024. [DOI] [PubMed] [Google Scholar]
- 24.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell JD, Delgoffe GM. The mammalian target of rapamycin: Linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skurk C, et al. The Akt-regulated forkhead transcription factor FOXO3a controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–1525. doi: 10.1074/jbc.M304736200. [DOI] [PubMed] [Google Scholar]
- 27.Mondino A, et al. Defective transcription of the IL-2 gene is associated with impaired expression of c-Fos, FosB, and JunB in anergic T helper 1 cells. J Immunol. 1996;157:2048–2057. [PubMed] [Google Scholar]
- 28.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 29.Sundstedt A, et al. In vivo anergized CD4+ T cells express perturbed AP-1 and NF-kappa B transcription factors. Proc Natl Acad Sci USA. 1996;93:979–984. doi: 10.1073/pnas.93.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salminen A, Kaarniranta K. NF-kappaB signaling in the aging process. J Clin Immunol. 2009;29:397–405. doi: 10.1007/s10875-009-9296-6. [DOI] [PubMed] [Google Scholar]
- 31.Yeung F, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nossal GJ. Tolerance and ways to break it. Ann N Y Acad Sci. 1993;690:34–41. doi: 10.1111/j.1749-6632.1993.tb43993.x. [DOI] [PubMed] [Google Scholar]
- 33.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo J, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- 35.Lee SM, Gao B, Fang D. FoxP3 maintains Treg unresponsiveness by selectively inhibiting the promoter DNA-binding activity of AP-1. Blood. 2008;111:3599–3606. doi: 10.1182/blood-2007-09-115014. [DOI] [PubMed] [Google Scholar]
- 36.Chen A, et al. The HECT-type E3 ubiquitin ligase AIP2 inhibits activation-induced T-cell death by catalyzing EGR2 ubiquitination. Mol Cell Biol. 2009;29:5348–5356. doi: 10.1128/MCB.00407-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.