Abstract
People attend not only to their own experiences, but also to the experiences of those around them. Such social awareness profoundly influences human behavior by enabling observational learning, as well as by motivating cooperation, charity, empathy, and spite. Oxytocin (OT), a neurosecretory hormone synthesized by hypothalamic neurons in the mammalian brain, can enhance affiliation or boost exclusion in different species in distinct contexts, belying any simple mechanistic neural model. Here we show that inhaled OT penetrates the CNS and subsequently enhances the sensitivity of rhesus macaques to rewards occurring to others as well as themselves. Roughly 2 h after inhaling OT, monkeys increased the frequency of prosocial choices associated with reward to another monkey when the alternative was to reward no one. OT also increased attention to the recipient monkey as well as the time it took to render such a decision. In contrast, within the first 2 h following inhalation, OT increased selfish choices associated with delivery of reward to self over a reward to the other monkey, without affecting attention or decision latency. Despite the differences in species typical social behavior, exogenous, inhaled OT causally promotes social donation behavior in rhesus monkeys, as it does in more egalitarian and monogamous ones, like prairie voles and humans, when there is no perceived cost to self. These findings potentially implicate shared neural mechanisms.
Keywords: social decision-making, neuropeptide, other-regarding preference, social gaze
Oxytocin (OT) (1) is a mammalian neurosecretory hormone, synthesized by hypothalamic neurons, which regulates the hypothalamic-pituitary-adrenal axis (2). The most well-understood role of OT in mammals is in female reproduction, with peripheral OT influencing parturition and lactation (3), and central OT affecting mother-offspring bonding and recognition (4, 5). More recently, OT has been found to influence nonparental social behavior in a species-specific manner. For example, OT promotes pair-bonding between males and females in monogamous prairie voles (Microtus ochrogaster) (6, 7) but can also increase aggression (i.e., mate-guarding behavior) and decrease social interaction among females after brief exposure to a male (8). In humans, OT also influences more complex forms of social behavior and cognition (9–14). For example, inhaled OT enhances trusting behavior toward other individuals in economic games, potentially by suppressing aversion to betrayal risk (15), and promotes cooperation within groups (16). However, inhaled OT also provokes cultural and racial biases (17). OT inhalation also enhances sensitivity to the experiences of others by promoting vicarious reward and empathic pain (10, 18, 19). Recently, OT-mediated processes have been implicated in disorders attended by dysfunctional social behavior, including autism, fragile X syndrome, and schizophrenia (19–22). Notably, OT treatment improves social skills in individuals with autism (21, 23, 24), a spectrum of disorders with marked deficits in sensitivity to what happens to others, including impairments in understanding and responding to social cues (22, 25, 26). Variations in a common oxytocin-receptor allele are linked to autism spectrum disorders and are associated with reduced volume in hypothalamus and anterior cingulate cortex (27).
Despite a growing literature, the mechanisms mediating the influence of OT on sensitivity to what happens to others remain only partially understood (9, 14, 19, 21, 28, 29). OT receptors are localized in multiple regions of the brain, with especially high density in areas implicated in affective and social processing. In prairie voles, OT receptors are densely localized in the amygdala, prelimbic cortex (homologous to the cingulate cortex in primates), and nucleus accumbens of the striatum (30). Recently, it has been shown that OT selectively inhibits a dedicated channel from the central nucleus of the amygdala to periaqueductal gray, ultimately reducing fear-induced freezing behavior in rats (31). Similarly, in humans, inhaled OT influences on social behavior are associated with reduced blood oxygen level-dependent (BOLD) signals in the bilateral amygdala and dorsal striatum (28, 29), consistent with the OT-mediated negative affect processing in the amygdala-cingulate circuits (22). These studies provide evidence that OT influences information processing in neural circuits implicated in emotion and social behavior.
Unlike prairie voles or humans (2, 6, 9–11, 13–16, 30, 32, 33), rhesus macaques (Macaca mulatta) live in large, hierarchical social groups with promiscuous mating and uniparental female care of offspring. Precisely how OT might influence social cognition in animals with this type of social structure and mating system, if at all, remains unknown. To answer this question, we capitalized on a recent finding by our group showing that rhesus macaques are sensitive to the rewards experienced by others, and this vicarious reinforcement is sufficient to motivate them to work to reward another monkey when the alternative is delivering reward to no one (34). We found that inhaling OT increased OT levels in cerebral spinal fluid (CSF), demonstrating transnasal penetration into the CNS. Roughly 2 h after OT-inhalation and onward, donor monkeys selectively increased the frequency of choosing an option resulting in reward to an adjacent, visible monkey, when the alternative was rewarding no one. In the same context, OT also increased the frequency that donors looked at the recipient monkey and prolonged choice response times. In contrast, up to about 2 h postinhalation, OT increased selfish decisions when the donors had the option to reward self over the other monkey. These findings invite the hypothesis that OT boosts internal vicarious reinforcement signals in a context-dependent manner in neural circuits homologous to those mediating these processes in humans. Our results demonstrate that OT mediates other-regarding behavior in nonhuman animals, even in those living in despotic societies with uniparental care.
Results
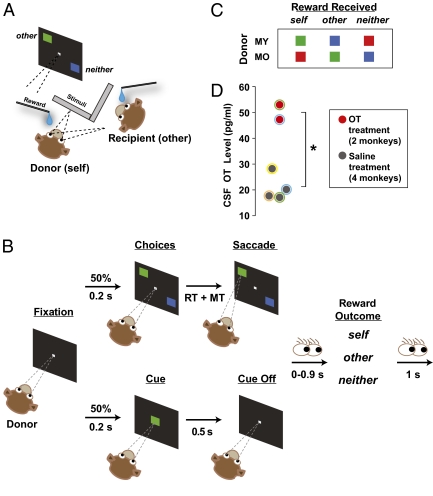
Donor monkeys (hereafter, “self” or “donor”) performed a reward allocation task with an unrelated recipient monkey (“other”) (Fig. 1 A–C) (34). The two monkeys were seated in adjacent primate chairs (Crist), 100-cm apart and at 45° angles to each other. Each monkey viewed his own LCD display, and had a juice-tube positioned in front of his mouth through which reward could be delivered. On each trial, donors chose between two visual shapes, associated with rewarding self, other, or neither. We have previously shown that donors typically prefer the shape delivering reward to other over neither (34). This preference is enhanced by greater familiarity between the two monkeys, and is abolished if the recipient monkey is replaced with a juice collection bottle, thus demonstrating the fundamentally social nature of the task (34).
Fig. 1.
Reward allocation task. (A) Experimental setup. (B) Trial sequence. Choice (Upper) and cued (Lower) trials were randomly interleaved. The eye-gaze cartoons specify the task intervals during which the donors could potentially look at the recipient monkey. MT, movement time; RT, reaction time. (C) Stimuli associated with different reward outcomes to donors and recipient, shown separately for the two donors. (D) OT concentration in the CSF after intranasal OT (in red) or saline (dark gray). *P < 0.05, Welch two-sample t test. Colored outlines on the datapoints represent animal identities.
For each session, we intranasally (35) delivered 25 international units (IU) of OT or saline, on alternating days, to two males using a pediatric nebulizer 30 min before performing the reward allocation task. A session composed of multiple reward allocation trials after either OT or saline administration occurred on each day (Methods). Data from a total of 12 OT and 10 saline control sessions were collected from two donors (MY and MO) while they engaged in the reward allocation task (Fig. 1 A–C) with an unrelated recipient monkey (MD). Five OT and three saline sessions were collected from MY, and seven OT and saline sessions each were collected from MO. For statistical power, we present data collapsed across the two donors, unless otherwise stated.
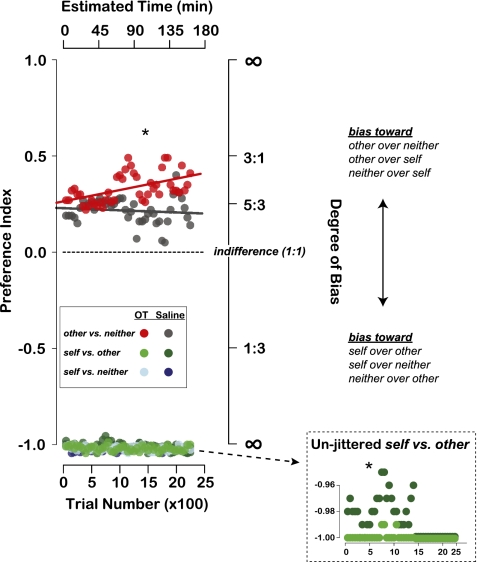
OT inhalation, compared with saline, significantly increased OT concentration in CSF as measured by cervical draws (P < 0.05, Welch two-sample t test) (Fig. 1D), confirming transnasal penetration into the CNS. Thirty minutes after OT administration, donors began the reward allocation task. For choices between delivering reward to other and neither, OT selectively amplified reward donations to other (Fig. 2). Preference for other increased linearly over time after OT but not after saline (OT: different from 0, r2 = 0.26, P < 0.0005; saline: r2 = 0.01, P = 0.47, linear regression) (Fig. 2). OT-induced enhancement of prosocial choices was largest in the later half of a given session (i.e., ∼110 min after OT administration and ∼80 min after task initiation; preference index mean difference between OT vs. saline: 0.17, P < 0.00001, Welch two-sample t test) (Fig. 2). Individual donors showed a similar pattern (MY: 0.18, P < 0.00001; MO: 0.19, P < 0.01). We found a significant difference between the two treatment conditions even when we averaged across the entire duration of the task (mean difference of 0.12, P < 0.00001; MY: 0.15, P < 0.00001; MO: 0.06, P < 0.05, Welch two-sample t test).
Fig. 2.
Intranasal OT promotes both vicarious and self reinforcement. Choice preference index (moving averages of 200 trials per session, 50-trial step) for OT (red) and saline (gray) across all reward options (other vs. neither, self vs. other, and self vs. neither). Datapoints from self vs. other and self vs. neither are jittered along the ordinate for visibility. (Inset) Unjittered and magnified data from self vs. other trials. Data from self vs. neither trials were effectively overlapping between the OT and saline conditions, and therefore not shown in an unjittered format. OT, 12 sessions; saline, 10 sessions. Lines show linear regression on other vs. neither trials.
In contrast, in the early half of a given session (i.e., up to ∼80 min into the task), OT slightly but significantly increased selfish choices on self vs. other trials compared with saline control (mean difference between OT and saline of −0.02, P < 0.00001, Welch two-sample t test; Inset in Fig. 2 shows unjittered self vs. other trials), but had no effect on self vs. neither trials (mean difference of −0.002, P = 0.36). Individual donors showed a similar selfish bias (MY: −0.003, P < 0.06; MO: −0.04, P < 0.00001). The absence of OT effect on self vs. neither trials might be due to the fact that this context does not involve a potential reward to another monkey, although we cannot rule out the possibility that donors were maximally self-regarding in this context in the absence of OT. Thus, OT robustly enhanced prosocial choices when there was no potential cost to self, but slightly increased selfish choices when there was potential for direct self reward.
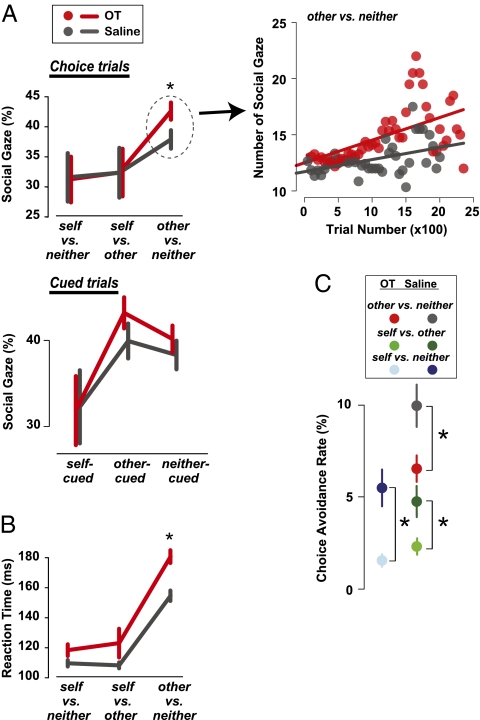
Donor monkeys often shift gaze to the recipient monkey after making a choice, and this attention to the recipient is enhanced after prosocial choices compared with selfish choices (34). OT further enhanced this overt other-oriented attention to the recipient after donors made a decision on other vs. neither trials (Fig. 3A) (OT vs. saline: mean difference of 4.70%, P < 0.05, Welch two-sample t test). In contrast, we did not observe any effects of OT on donor's attention to the recipient when direct self reward was involved (self vs. neither: mean difference of −0.36%, P = 0.95; self vs. other: 0.03%, P = 0.99) (Fig. 3A). We also found that donors looked more frequently to the recipient when rewards were delivered to him compared with when rewards were delivered to self, even on cued trials in which rewards were delivered by computer without any action by donors (gaze frequency on self-cued vs. other-cued trials: OT, P < 0.005; saline: P = 0.05) (Fig. 3A). However, OT did not modulate this difference in social attention on cued trials (all comparisons P > 0.23, Welch two-sample t test) (Fig. 3A), suggesting that OT enhances other-oriented attention selectively following prosocial decisions rather than in response to anything happening to the other monkey (i.e., after active choices on other vs. neither trials). As in the other-oriented choice preference, attention to the recipient monkey also increased linearly over time after OT (slope significantly different from 0: r2 = 0.31, P < 0.00001, linear regression) (Fig. 3A, Right). The frequency of looking at the recipient monkey in the saline control also increased over the course of the session (r2 = 0.19, P < 0.005), but with a significantly lower rate of rise than the OT condition (differences in OT and saline slopes greater than zero: P < 0.005, permutation test) (Fig. 3A). This finding suggests that OT enhances the intensity of vicarious reinforcement in part by modulating attentional mechanisms.
Fig. 3.
Intranasal OT enhances attention to the recipient monkey and increases the deliberation time for making donation decisions. (A) Gaze to the face of the other monkey after reward delivery. (Left) Percentages of gaze shifts to the recipient monkey on choice trials (Upper) and cued trials (Lower). (Right) Number of gaze shifts over the course of each day session for other vs. neither choice trials (moving averages of 200 trials per session, 50-trial step). Lines through the datapoints show linear regressions. (B) Response times, measured as saccade onset times following target onset (ms). (C) OT reduced choice avoidance [i.e., declining to choose by breaking fixation upon target onset (such as, reward options), which, in the task resulted in a time out for 5 s]. *P < 0.05, Welch two-sample t test.
We also examined the time required by monkeys to render a decision. Response times in the reward allocation task are generally slower when donor monkeys choose between delivering reward to other vs. neither, compared with when self reward is involved (34). OT selectively prolonged response times on other vs. neither trials (mean difference between OT and saline of 26.0 ms, P < 0.00001, Welch two-sample t test) (Fig. 3B), possibly reflecting internal processes, such as deliberation and control. On self vs. neither and self vs. other trials, however, OT only showed a trend on response times (self vs. other: mean difference of 14.78 ms; self vs. neither: 8.72 ms; both P < 0.13) (Fig. 3B). Finally, on some trials, donors avoided making a decision, opting to wait until the next trial (although they could not predict the subsequent reward options). OT reduced this choice avoidance behavior across all trial types (all P < 0.05, Welch two-sample t test) (Fig. 3C), perhaps because of overall enhancement in subjective reinforcement.
Inhaled OT thus influenced reward donation decisions by rhesus macaques when there was an option to reward another monkey (other vs. neither and self vs. other, but not self vs. neither). OT enhanced reward donations on other vs. neither trials, but increased selfish behavior on self vs. other trials (Fig. 2). OT-induced changes in attention to the recipient monkey (Fig. 3A) and decision time (Fig. 3B) were both specific to the donation context (other vs. neither), whereas OT-induced reductions in choice avoidance behavior (Fig. 3C) were global.
Discussion
Compared with some other nonhuman primates, social behavior of rhesus monkeys is primarily characterized by competition and aggression, and shows very weak, if any, inclination toward cooperation (36, 37). In a prior study, different levels of endogenous OT were reported in more socially affiliative mother-reared compared with more socially agnostic nursery-reared macaques (38). Here we show that exogenous OT promotes social donation behavior in rhesus macaques, as it does in more egalitarian and monogamous species, like prairie voles and humans. OT-induced prosocial donations were accompanied by enhanced other-oriented attention and decision times. In contrast, in a context in which there was a potential for rewarding self or another monkey, OT slightly increased the tendency for donors to choose selfishly without influencing overt attention and, at most, minimally affecting decision times. The absence of OT-induced enhancement of overt attention on these trials suggests that OT modulates other-oriented preferences through vicarious reinforcement (34). These findings are consistent with context-dependent effects of OT on human social behavior (16, 17, 39) (for a review of human social processing, see ref. 40), implying similar neural mechanisms.
Given the context-specific increase in attention to the other monkey and more deliberative decision latency, it is conceivable that these behaviors are related. Several hypotheses are plausible. On the one hand, OT may increase attention to the other monkey via neural circuits mediating orienting behavior, including amygdala, parietal cortex, and superior colliculus. Increased attention to the recipient may enhance vicarious reinforcement experienced from delivering juice to him. Alternatively, OT may influence neural circuits involved in decision-making, including the striatum and anterior cingulate cortex (see introductory paragraphs). Slowed response times may reflect more deliberate processing of the potential outcomes available (41). A future study designed to probe the temporal evolution of OT-induced effects on attention and decision-making will be needed to resolve these hypotheses.
The direction of OT-induced social enhancement also appears to vary as a function of time. OT initially enhanced self reinforcement but later amplified vicarious reinforcement, although the largest OT-induced effects were prosocial. Although this interaction between time-dependent and context-dependent effects of OT may be specific to our reward allocation task and thus can only be extrapolated with caution, these results suggest that OT may influence self- and other-regarding behaviors via distinct underlying neural mechanisms.
Why might OT promote self reinforcement bias on self vs. other but not on self vs. neither trials? The key difference between the two contexts is the alternative option. In one context, the alternative option has a social consequence (i.e., rewarding the recipient), whereas in the other context, the alternative option does not (i.e., nothing happens to either donor or recipient). OT-induced self reinforcement may depend on the contrast between rewarding self and another individual. We hypothesize that when a decision context presents this contrast, OT can promote selfish behavior. OT influences on self and vicarious reinforcement (16, 17, 39) thus appear to depend on the social state of the underlying neural circuits.
Previous studies in monogamous prairie voles and promiscuous montane voles (Microtus montanus) have suggested that mating system may be a key predictor of OT influences on social behavior through the topology of OT receptor localization in neural circuits, mediating reinforcement and motivation (33). A more general difference between prairie voles and montane voles is the frequency and intensity of social interaction (33). Compared with montane voles, prairie voles are biparental, show more selective aggression, and spend more time in close physical proximity (33). Humans and rhesus macaques, too, are highly social mammals; intranasal OT induces prosocial tendencies in humans (15, 16) and, as we now report, in rhesus macaques. These findings suggest that OT may play a critical role in modulating social behavior in highly gregarious mammals, regardless of mating system or parental care strategy.
Intranasal administration of OT in humans has also been shown to increase gaze to the eyes of others (19). We found that OT enhanced gaze directed at the face of the other monkey following active social decision-making but not following passive reward delivery. This finding invites the possibility that OT gates the activity of attention circuits in the brain specifically during active interaction with others. Evidence from human functional neuroimaging studies is consistent with this idea. For example, OT selectively modulates BOLD signal in the anterior cingulate cortex, amygdala, midbrain, and dorsal striatum during a trust game involving other human players, but not during a nonsocial decision-making task (29). Functional connectivity between the amygdala and midbrain structures is also reduced by OT when human participants view emotional faces (28). Finally, OT reduces the subjective evaluation of aversively conditioned faces, and this reduction is accompanied by suppressed BOLD responses in the amygdala and the fusiform gyrus (42).
Consistent with our results, OT modulates deliberation times during social decision-making in humans. For example, OT slows overall evaluation time for rating faces in a nonspecific manner, regardless of whether the images were aversively conditioned or not (42). OT can also speed up decision times; for example, OT decreased overall key press reaction times for evaluating in-group favoritism and out-group derogation in an implicit association test (17).
OT enhanced the frequency of prosocial decisions in the absence of opportunity for direct self reward, but provoked an increase in selfish decisions when choosing between self and other. Such a dual function has also been reported in humans. OT can both promote cooperation and increase out-group bias depending on behavioral context (16, 17, 39). Thus, OT does not appear to have a universal prosocial influence on behavior, but rather amplifies ongoing social information processing (21), perhaps by influencing already existing preferences. It is plausible that OT mediates prosociality and generosity only in an indirect manner. Alternatively, OT may play a more direct and causal role in modulating context-dependent social information processing (e.g., refs. 27–29 for neural evidence), specifically by enhancing the gain of neural circuits mediating vicarious reinforcement and attention.
Recently, OT has been evaluated for potential therapeutic use in clinical conditions attended by dysfunctional social behavior, such as autism spectrum disorders, antisocial personality disorder, and schizophrenia (20–24, 43, 44). Notably, the intranasal nebulization method (35) we developed here is well-tolerated by children for delivery of other therapeutics (i.e., albuterol), thus opening up avenues for early OT intervention in neuropsychiatric conditions with social deficits. Furthermore, choice-specific effect of OT on increasing other-oriented attention suggests a potential need for active decision-making during OT interventions.
The current finding opens up new opportunities for uncovering the mechanisms underlying the influences of OT on social behavior in a species much more closely related to humans than rodents. Rhesus monkeys have long served as the primary model species for probing the neural mechanisms mediating high-level cognition. Given the strong similarities in social behavior and cognition, and the apparent homologies in underlying neural circuitry, the rhesus macaque provides a powerful model for probing the mechanisms mediating some of the basic behaviors that make complex human social interactions possible.
Methods
General Procedures and Behavioral Task.
All procedures were approved by the Duke University Institutional Animal Care and Use Committee. Two donor monkeys (MY and MO) and a recipient monkey (MD) participated in the study. All animals underwent standard surgical procedures for implanting a head-restraint prosthesis at least 6 mo before the present study. The head-restraint prosthesis allowed us to monitor eye position, sampled at 1,000 Hz (SR Research; Eyelink), as well as conduct single-unit recordings in other experiments, not reported here. Both the donor and recipient were head-restrained throughout the experiment. Donors and recipient were unrelated, middle-ranked, and not cage mates. Face of recipient (other; corresponding horizontal and vertical eye positions) was empirically mapped. Rewards were 0.5–1.0 mL of cherry-flavored juice. Within each block, reward size was constant for all three outcomes. A separate solenoid was designated for rewarding neither that only produced clicks but delivered no fluid. To prevent monkeys from forming secondary associations between solenoid clicks and different reward types, all solenoid valves (including the one used to deliver “neither” reward) used to deliver juice rewards were placed in another room. Masking white noise was also played in the experimental room.
Donors began the trial by shifting gaze (± 2.5°) to a central stimulus (0.5° × 0.5°), and maintained fixation (for 200 ms). Choice and cue trials were presented at equal frequencies and randomly interleaved. On choice trials (Fig. 1B), two visual targets (4° × 4°) appeared at two random locations of 7° eccentricity and reflected about the vertical meridian. Donors shifted their gaze to one target (± 2.5°) to indicate their choice. On cued trials (Fig. 1B), donors maintained fixation while a cue appeared centrally (for 500 ms). On both trial types, the reward onset was followed by a 0 to 0.9 s delay. Donors could freely look around for 0–0.9 s following making a choice and for another 1 s after the reward onset. Data from error trials are not included in analyses.
Data from 12 OT (MY: 5, MO: 7) and 10 saline (MY: 3, MO: 7) sessions were collected on strictly alternating days. Each day session was, on average, 1,274 ± 141 (mean ± SEM) trials. Within each day session, several blocks of the task (a median of 6 and 6.5 blocks for OT and saline, respectively) were completed by the donors. Each of these blocks typically consisted of 192 ± 10 (mean ± SEM) and 205 ± 15 trials for OT and saline, respectively.
Intranasal OT Protocol.
Donor monkeys were transported in the primate chair from the colony room to the experimental room. After stabilizing their heads, OT (25 IU/mL; Agrilabs) was delivered via nebulization (Pari Baby Nebulizer) into the nose and mouth continuously for 5 min (5 IU/min) when the donor monkeys were fully awake. On alternating days, nebulized saline served as a control. Before experimental sessions, donor monkeys were first habituated to the nebulizer and then accustomed to saline delivery using the nebulizer in an incremental fashion until they were completely relaxed during the procedure, which typically took about a week. In fact, donor monkeys showed no distress during this procedure. Testing began exactly 30 min after each treatment, at which time a recipient monkey was brought to the experimental setup. In the guinea pig CNS, radioactively labeled OT lasts up to 4 h (45). In humans, intranasal delivery of a similar peptide, vasopressin (differing by only two amino acids), increases its concentration in the CSF after 10 min, and elevated vasopressin levels are maintained for more than 80 min after administration (35). In that study (35), vasopressin levels increased significantly after 30 min. Previous studies in humans have not measured inhaled OT uptake into the CNS. Fig. 1D plots CSF OT levels in monkeys 35 min after inhalation, demonstrating efficacy of the intranasal nebulization method (see below). Note that the mask was always pressed very tightly to minimize potential leakage, but nonetheless leakage could have occurred. It is worth noting that CSF OT levels may have continued to increase after the time of CSF measurement, warranting caution in linking absolute CSF OT levels with changes in behavior. Despite these uncertainties, our nebulization technique resulted in a ∼2.5-fold increase in CSF OT levels roughly 0.5 h after inhalation.
CSF OT Protocol.
To determine whether inhaled OT penetrates the CNS after nebulization, OT concentration in CSF was measured via cervical punctures (on average 35 min after the beginning of inhalation). Cervical punctures were performed by a licensed veterinarian, and targeted the cisterna magna through the juncture between the occipital base and atlas (C1) through the atlanto-occipital membrane. Monkeys were first anesthetized with ketamine (3 mg/kg, i.m.) and dexdomitor (0.075 mg/kg, i.m.). To reverse anesthesia, we administered antisedan (0.075 mg/kg, i.m.) once the animal was returned to its cage after the draw. Approximately 0.5 mL of CSF was drawn using a 24 to 27 gauge needle. At the performing veterinarian's discretion, bupivacaine was administered subcutaneously at the insertion site following needle removal. CSF was immediately frozen on dry ice and sent off-site to be assayed for OT (Biomarkers Core Labs, Yerkes National Primate Research Center, Atlanta, GA) using a commercially prepared kit [Assay Designs (now Enzo Life Sciences); cat. # 900–153: Oxytocin ELISA kit, with very low reactivity with vasopressin]. Samples were assayed “neat” with a range of 15.6–1,000 μL assay volume. This assay has near-zero reactivity with vasopressin, which is chemically similar to OT, thus providing specific quantitation of OT.
Data Analysis.
Preference index was a contrast ratio of frequency of choosing an option, nA or nB:
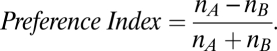 |
For choices between self vs. other, nA and nB were number of choices to reward other and self, respectively. For choices between other vs. neither, nA and nB were number of choices to reward other and neither, respectively. Finally, for choices between self vs. neither, nA and nB were number of choices to reward neither and self, respectively. Indices ranged from –1 to 1, with 1 corresponding to always choosing the “prosocial” option to reward the recipient monkey (when that was an option) or to withhold reward from self (self vs. neither). An index of –1 indicated that donors always chose an “antisocial” option to reward self (when that was an option) or to withhold reward from the other monkey (other vs. neither). Preference index of 0 indicated indifference. Frequency of donors looking at recipients was computed from number of gaze shifts to the recipient's facial region (within ± 8.5° spanning from the center of the recipient's face). Reaction times (time from target onset to movement onset) were computed using a 20°/s velocity threshold (46).
Acknowledgments
We thank Ernst Fehr, Markus Heinrichs, and Steven P. Wise for helpful discussions and valuable suggestions; Benjamin Y. Hayden, David B. Barack, and Jean-Francois Gariépy for comments; Monica L. Carlson for technical assistance; and Francis Sun DVM at the Duke Division of Laboratory Animal Resource for cerebrospinal fluid collection. This work was supported by a Ruth K. Broad Biomedical Foundation Postdoctoral grant (to S.W.C.C.); a Predoctoral grant (to R.B.E.); the Davis Foundation (K.K.W.), a National Institutes of Health T32 Postdoctoral Grant (to S.W.C.C.); and National Institutes of Health Grant MH086712 (to S.W.C.C., K.K.W., and M.L.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Du Vigneaud V, et al. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J Am Chem Soc. 1953;75:4879–4880. [Google Scholar]
- 2.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 3.Soloff MS, Alexandrova M, Fernstrom MJ. Oxytocin receptors: Triggers for parturition and lactation? Science. 1979;204:1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen CA. In: Oxytocin Control of Maternal Behavior: Regulation of Sex Steroids and Offspring Stimuli. The Integrative Neurobiology of Affiliation. Carter CS, Lederhendler I, Kirkpatrick B, editors. Cambridge, MA: MIT Press; 1999. pp. 301–320. [Google Scholar]
- 5.Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Horm Behav. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Bales KL, et al. Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm Behav. 2007;52:274–279. doi: 10.1016/j.yhbeh.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bales KL, Carter CS. Sex differences and developmental effects of oxytocin on aggression and social behavior in prairie voles (Microtus ochrogaster) Horm Behav. 2003;44:178–184. doi: 10.1016/s0018-506x(03)00154-5. [DOI] [PubMed] [Google Scholar]
- 9.Fehr E, Camerer CF. Social neuroeconomics: The neural circuitry of social preferences. Trends Cogn Sci. 2007;11:419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Singer T, et al. Effects of oxytocin and prosocial behavior on brain responses to direct and vicariously experienced pain. Emotion. 2008;8:781–791. doi: 10.1037/a0014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein DJ. Oxytocin and vasopressin: Social neuropeptides. CNS Spectr. 2009;14:602–606. doi: 10.1017/s1092852900023841. [DOI] [PubMed] [Google Scholar]
- 12.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Zak PJ, Kurzban R, Matzner WT. Oxytocin is associated with human trustworthiness. Horm Behav. 2005;48:522–527. doi: 10.1016/j.yhbeh.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Lindenberg A. Impact of prosocial neuropeptides on human brain function. Prog Brain Res. 2008;170:463–470. doi: 10.1016/S0079-6123(08)00436-6. [DOI] [PubMed] [Google Scholar]
- 15.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 16.De Dreu CK, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 17.De Dreu CK, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJ. Oxytocin promotes human ethnocentrism. Proc Natl Acad Sci USA. 2011;108:1262–1266. doi: 10.1073/pnas.1015316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Bartz JA, Hollander E. The neuroscience of affiliation: Forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. 2006;50:518–528. doi: 10.1016/j.yhbeh.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 23.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollander E, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 25.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 26.Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- 27.Tost H, et al. A common allele in the oxytocin receptor gene (OXTR) impacts prosocial temperament and human hypothalamic-limbic structure and function. Proc Natl Acad Sci USA. 2010;107:13936–13941. doi: 10.1073/pnas.1003296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirsch P, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333:104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 32.Bales KL, Pfeifer LA, Carter CS. Sex differences and developmental effects of manipulations of oxytocin on alloparenting and anxiety in prairie voles. Dev Psychobiol. 2004;44:123–131. doi: 10.1002/dev.10165. [DOI] [PubMed] [Google Scholar]
- 33.Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- 34.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Born J, et al. Sniffing neuropeptides: A transnasal approach to the human brain. Nat Neurosci. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- 36.Maestripieri D. Macachiavellian Intelligence: How Rhesus Macaques and Humans Have Conquered the World. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 37.Thierry B, Mewa S, Kaumanns W. Macaque Societies: A Model for the Study of Social Organization. Cambridge; New York, NY: Cambridge University Press; 2004. xv, 418 pp. [Google Scholar]
- 38.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 39.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 41.Gold JI, Shadlen MN. Neural computations that underlie decisions about sensory stimuli. Trends Cogn Sci. 2001;5:10–16. doi: 10.1016/s1364-6613(00)01567-9. [DOI] [PubMed] [Google Scholar]
- 42.Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J Neurosci. 2008;28:6607–6615. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 44.Bartz JA, Hollander E. Oxytocin and experimental therapeutics in autism spectrum disorders. Prog Brain Res. 2008;170:451–462. doi: 10.1016/S0079-6123(08)00435-4. [DOI] [PubMed] [Google Scholar]
- 45.Robinson IC, Jones PM. Oxytocin and neurophysin in plasma and CSF during suckling in the guinea-pig. Neuroendocrinology. 1982;34:59–63. doi: 10.1159/000123278. [DOI] [PubMed] [Google Scholar]
- 46.Paré M, Munoz DP. Saccadic reaction time in the monkey: Advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]