Abstract
It has generally been assumed that bone mass is controlled by endocrine mechanisms and the local bone environment. Recent findings demonstrate that central pathways are involved in the regulation of bone mass. Estrogen is involved in the regulation of bone homeostasis and the CNS is also a target for estrogen actions. The aim of this study was to investigate in vivo the role of central estrogen receptor-α (ERα) expression for bone mass. Nestin-Cre mice were crossed with ERαflox mice to generate mice lacking ERα expression specifically in nervous tissue (nestin-ERα−/−). Bone mineral density was increased in both the trabecular and cortical bone compartments in nestin-ERα−/− mice compared with controls. Femoral bone strength was increased in nestin-ERα−/− mice, as demonstrated by increased stiffness and maximal load of failure. The high bone mass phenotype in nestin-ERα−/− mice was mainly caused by increased bone formation. Serum leptin levels were elevated as a result of increased leptin expression in white adipose tissue (WAT) and slightly increased amount of WAT in nestin-ERα−/− mice. Leptin receptor mRNA levels were reduced in the hypothalamus but not in bone. In conclusion, inactivation of central ERα signaling results in increased bone mass, demonstrating that the balance between peripheral stimulatory and central inhibitory ERα actions is important for the regulation of bone mass. We propose that the increased bone mass in nestin-ERα−/− mice is mediated via decreased central leptin sensitivity and thereby increased secretion of leptin from WAT, which, in turn, results in increased peripheral leptin-induced bone formation.
Bone mass is maintained by highly regulated processes involving osteoblastic bone formation and osteoclastic bone resorption. Bone is traditionally considered to be regulated by hormones, autocrine/paracrine signals, and mechanical loading. It is now recognized that the regulation of bone involves other systems within the organism, including the CNS. It has been known for long time that bone is an innervated tissue containing both efferent and afferent fibers in bone marrow and the periosteum (1). However, the first clear evidence that central signaling affects bone mass was the finding that leptin-deficient mice, despite their hypogonadism, had high bone mass, and that this phenotype was reversed by intracerebroventricular injections of leptin (2). Furthermore, mice lacking the β2-adrenergic receptor, which binds the main sympathetic neurotransmitter noradrenaline, are resistant to the central bone-reducing effects of leptin, demonstrating that central leptin signaling may affect bone via the sympathetic nervous system (3). Thus, sympathetic signaling appears to be involved in bone regulation and to have a negative impact on bone mass (4). These studies show that neuronal signaling is important for bone regulation and have fortified the link between skeletal and neuronal biology. Furthermore, peripheral leptin treatment increases bone mass both in rodents (2, 5) and in hypoleptinemic women (6). Thus, leptin has opposite peripheral versus central effects on bone mass.
Several neurotransmitters have been shown to be involved in bone regulation, including serotonin. Central serotonin has been suggested to decrease sympathetic signaling and thereby enhance bone mass (7). The notion that peripheral serotonin has a negative influence on bone mass, suggesting that this neurotransmitter may have opposite peripheral versus central effects, is supported by some but not others (8, 9).
Another system involved in bone regulation is the immune system. The immune system may influence bone regulation both by soluble factors, such as cytokines, and by cellular components, including T lymphocytes. Activated T lymphocytes express RANKL (receptor activator of NFκB), a cytokine that is crucial for the differentiation and activation of bone-resorbing osteoclasts, and may therefore affect bone regulation (10).
Estrogens are important endocrine regulators of skeletal growth and maintenance, as demonstrated in both experimental and human studies (11–13). Estrogen deficiency, induced by ovariectomy (ovx) in animal models, or after menopause, reduces trabecular bone mineral density (BMD) as well as cortical bone mass, but estrogen substitution restores both bone compartments (14, 15).
The physiological effects of estrogen are mainly exerted via the two classic nuclear estrogen receptors (ERs), ERα and ERβ, which are ligand-activated transcription factors. We and others have previously demonstrated, using transgenic mouse models, that signaling via ERα protects against ovx-induced trabecular bone loss (14, 16, 17). In contrast, ERβ is not essential for this bone-protective effect of estrogen (14, 17), although the ERα activity can be modulated by ERβ in female bone (18). Bone loss caused by estrogen deficiency is restored by local injections of estrogen, demonstrating that peripheral estrogen action is important for bone regulation (19). Furthermore, using transgenic mouse models, the importance of both osteoblasts (20) and osteoclasts (21) for the peripheral estrogenic effects on bone has been demonstrated. Thus, estrogen has positive peripheral effects on bone.
The CNS is a target for estrogen and the nuclear ERs are widely distributed in the brain (for review of estrogen actions in the brain see ref. 22), but whether central estrogenic signaling affects the skeleton is not known. We have previously tried to address this question by comparing central (intracerebroventricular) estradiol administration to peripheral (subcutaneous) administration (23). We did not detect any differences between administration routes with respect to the influence of estrogen on bone density, but the results might have been confounded by the fact that estradiol can easily pass the blood-brain barrier.
We have deleted ERα, the main ER for estrogenic bone effects, specifically in nervous tissue, and demonstrated that ERα expression in neuronal cells affects bone mass.
Results
Generation of Mice with a Specific Deletion of ERα in Nervous Tissue.
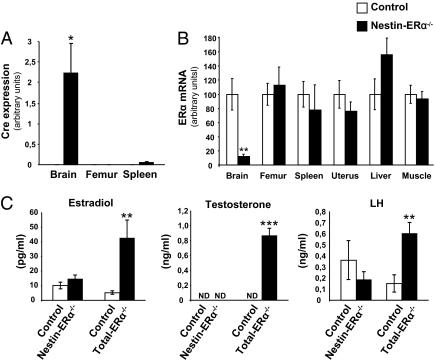
To investigate the role of central ERα signaling for bone regulation, we generated nestin-Cre mice in which ERα is selectively ablated in nervous tissue (Materials and Methods). We analyzed by RT-PCR, the brain-specific Cre expression (Fig. 1A) and selective ERα deletion (Fig. 1B). In brain (hypothalamus), we observed a 88% reduction in ERα expression compared with control mice, but no significant effect on expression was detected in the nonneuronal tissues bone (femur), spleen, uterus, liver, or striated muscle (Fig. 1B). Furthermore, no compensatory alteration in expression of ERβ was detected in any of the tissues examined (Table S1). Body weight was monitored at 3, 6, 9, and 12 wk of age, and no difference between nestin-ERα−/− and control mice was detected at any time point (Table S2).
Fig. 1.
Specificity of ERα deletion in nestin-ERα−/− females. RNA was prepared from the hypothalamic region of the brain and the indicated nonneuronal tissues from 3-mo-old nestin-ERα−/− and control mice. (A) Cre expression, analyzed using real-time PCR, was specifically detected in the brain (n = 4). (B) ERα mRNA levels, shown as percentage of control, were specifically reduced in the brain (n = 6–10). (C) Nestin-ERα−/− females do not exert disturbed sex steroid levels. Serum levels of estradiol, testosterone and LH were measured in nestin-ERα−/− females, ERα−/− females, and their corresponding control females. (n = 4–13). Values are given as mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 vs. control mice, Student's t test. ND, not detectable.
Nestin-ERα−/− Females Have Normal Levels of Sex Steroids, Leutenizing Hormone, and FSH.
To determine whether the negative feedback regulation was disturbed in female nestin-ERα−/− mice serum levels of the sex steroids, estradiol and testosterone, leutenizing hormone (LH) and FSH were analyzed. In ERα-null mutant mice (ERα−/−, mice homozygous for the deletion of ERα exon 3), we confirmed the previously described (16, 24) dramatic increase in serum estradiol levels compared with control mice (Fig. 1C). The same analysis in nestin-ERα−/− mice did not result in significantly increased serum estradiol levels (Fig. 1C). Testosterone and LH levels were unchanged in nestin-ERα−/− females compared with control mice, but the total ERα deletion led to an increase in both testosterone and LH compared with control mice (Fig. 1C). Analysis of serum FSH revealed unchanged levels in nestin-ERα−/− mice compared with WT mice (Table S3).
Nestin-ERα−/− Females Have Increased Trabecular and Cortical Volumetric BMD.
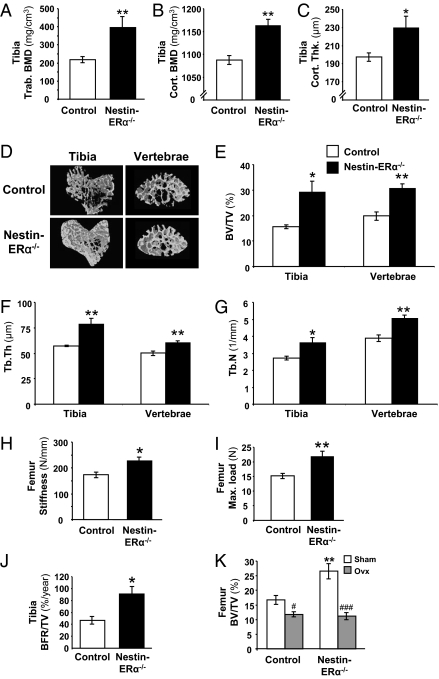
Peripheral quantitative computerized tomography (pQCT) analysis of excised femur and tibia revealed a significant increase in trabecular volumetric BMD in nestin-ERα−/− compared with control mice (tibia: +81%, P < 0.01; femur: +93%, P < 0.001) (Fig. 2A and Fig. S1A). The cortical parameters, volumetric BMD and thickness, were also increased in nestin-ERα−/− mice compared with controls in both tibia and femur (Fig. 2 B and C and Fig. S1 B and C).
Fig. 2.
Deletion of ERα in nervous tissue results in increased bone mass. pQCT analysis of the tibia demonstrated increased trabecular volumetric bone mineral density (Tibia Trab. BMD) (A), increased cortical volumetric BMD (Tibia Cort. BMD) (B), and increased cortical thickness (Tibia Cort. Thk.) (C) in 3-mo-old nestin-ERα−/− mice compared with controls (n = 6). (D) Representative images of μCT analyses of trabecular bone in the proximal metaphysis of the tibia and in vertebrae L5. Bone volume per total volume (BV/TV) (E), trabecular thickness (Tb.Th) (F), and trabecular number (Tb.N) (G) of trabecular bone in the proximal metaphysis of the tibia and in vertebrae L5 (n = 5–8). Three-point bending of femur demonstrated increased (H) stiffness and (I) maximal load (Max. load) at failure in nestin-ERα−/− mice (n = 5). (J) Dynamic histomorphometric analysis of tibia giving bone formation rate per tissue volume (BFR/TV; n = 4–5). (K) Effect of ovx on bone mass in nestin-ERα−/− and control females. Three-month-old female mice were ovariectomized or sham-operated and terminated after 4 wk (n = 5–8). Values are given as mean ± SEM; *P < 0.05, **P < 0.01 vs. control; #P < 0.05, ###P < 0.001 vs. sham, Student's t test.
To further examine the trabecular bone effects in nestin-ERα−/− females, μCT analyses of proximal tibia and vertebrae were performed. Trabecular bone volume per tissue volume (BV/TV) was increased in nestin-ERα−/− mice compared with control mice (+87%, P < 0.05 and 55%, P < 0.01 in tibia and vertebra, respectively) (Fig. 2 D and E). This increase in bone volume was both because of increased trabecular thickness (+37% and 21%, P < 0.01 in tibia and vertebrae, respectively) (Fig. 2F) and because of trabecular number (+33%, P < 0.05 and 30%, P < 0.01 in tibia and vertebrae, respectively) (Fig. 2G) in nestin-ERα−/− females compared with controls. Similar results were seen after static histomorphometric analysis of distal femur (Table S4).
Bone Strength Is Increased in Nestin-ERα−/− Females.
To evaluate the biomechanical properties of the bone, three-point bending was performed. Deletion of central ERα signaling resulted in increased femur stiffness compared with controls (+31%, P < 0.05) (Fig. 2H). Furthermore, the maximal load at failure (Fmax) (Fig. 2I) as well as the force applied at the start of the plastic deformation (Fyield, +40%, P < 0.01) were increased in nestin-ERα−/− mice compared with control mice.
Effect on Bone Remodeling in Nestin-ERα−/− Females.
To determine the importance of central ERα signaling for bone formation, a dynamic histomorphometric analysis of tibia was performed. The tissue-referent bone formation rate (BFR/TV), which relates the amount of newly formed bone to the total tissue volume (bone and bone marrow), was significantly increased in nestin-ERα−/− females compared with controls (Fig. 2J), and there was a tendency to an increase (P = 0.07) in the bone-referent bone formation rate (BFR/BS), which relates the newly formed bone to the bone surface (Table S4). Expression of the osteoblast-specific protein bone sialoprotein (BSP) in femur revealed a significant increase in nestin-ERα−/− mice compared with control mice (+91%, P < 0.05) (Table S3). Furthermore, we found a tendency (+86%, P = 0.06) to increased expression of dentin matrix acidic phosphoprotein 1, a marker for osteocyte differentiation, in the bone of nestin-ERα−/− mice compared with control mice (Table S3). The serum level of osteocalcin, a marker for bone formation, was unchanged when comparing nestin-ERα−/− females with control mice (Table S3). The number of osteoclasts, as determined by histomorphometric analysis, transcription of osteoclast-related genes (cathepsin K, RANKL, tartrate resistant acid phosphatase isoform 5b, osteoprotegerin) and the level of collagen type I c-telopeptide (RatLaps), a serum marker for bone resorption, were not significantly altered in nestin-ERα−/− mice (Tables S3 and S4).
Effect of Ovariectomy on Bone in Nestin-ERα−/− Females.
A high bone-mass phenotype might depend on developmental effects causing early high bone-mass accrual. Therefore, we ovariectomized the mice to determine whether the high bone mass was because of a dynamic event. Ovx of control mice resulted in decreased BV/TV, as measured by μCT (Fig. 2K). Ovx of nestin-ERα−/− mice resulted in a significantly more pronounced loss of BV/TV in both femur and vertebra compared with the ovx-induced bone loss in the corresponding bone compartment in control mice (P < 0.01) (Fig. 2K and Fig. S2A). BV/TV in femur and vertebrae were not significantly distinguishable between ovariectomized control and nestin-ERα−/−. Similar results for trabecular BMD were seen after pQCT analysis of femur and tibia (Fig. S2B).
Nestin-ERα−/− Females Have Increased Circulating Levels of Leptin.
To assess the possibility that central estrogen signaling affects bone via leptin or the serotonergic system, both peripheral and central serotonin levels and serum leptin levels were analyzed. Furthermore, because central leptin and serotonin have been suggested to affect bone via an influence on sympathetic nerve signaling, parameters reflecting sympathetic signaling were also analyzed.
Peripheral serotonin signaling.
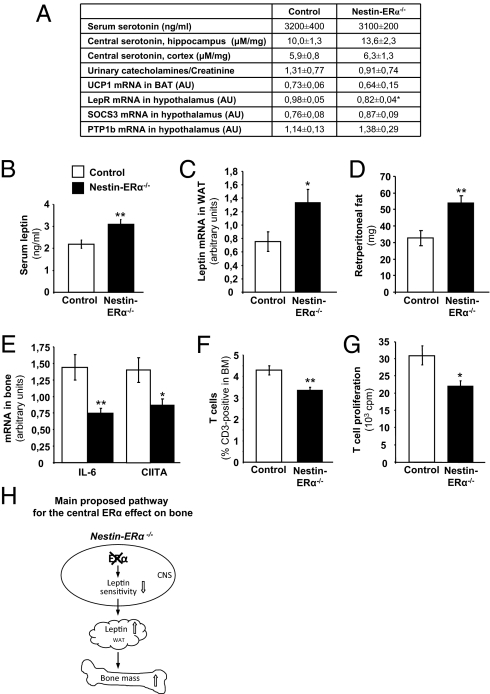
Serum levels of serotonin were not affected in nestin-ERα−/− mice compared with control females (Fig. 3A). Furthermore, as the effects of serotonin on bone have been suggested to be mediated by actions via the serotonin receptor 1b (8), the expression of this receptor was investigated in tibia. However, no expression differences between nestin-ERα−/− and control mice were detected (Table S3).
Fig. 3.
Possible pathways for the central ERα effect on bone. (A) Peripheral (serum) and central serotonin levels, measured using ELISA and HPLC, respectively (n = 8–10). Urinary catecholamine secretion (ng/mL) related to urinary creatinine (Cr) (mg/dL) content (n = 7). UCP1 mRNA expression in BAT (n = 5–9). Leptin receptor (LepR), SOCS3, and PTP1b mRNA expression in hypothalamus (n = 5–7). (B) Serum levels of leptin (n = 9–10). (C) Leptin mRNA expression in WAT (n = 6–7). (D) Retroperitoneal fat mass (mg) (n = 6–7). (E) mRNA expression in bone (tibia) of IL-6 and CIITA (n = 9). (F) T-cell frequency (CD3+ cells) in bone marrow (BM) (n = 9–10) and (G) T-cell proliferation in spleen (n = 6–10). (H) Schematic presentation of the main proposed mechanism for the central ERα effect on bone (see Discussion). Values are given as mean ± SEM; *P < 0.05, **P < 0.01 vs. control, Student's t test.
Central serotonin signaling.
No difference in central serotonin levels were detected between nestin-ERα−/− and control mice (Fig. 3A).
Sympathetic nerve signaling.
To determine the possible role of central ERα signaling for sympathetic nerve signaling, catecholamine excretion in urine was measured. No difference was detected between nestin-ERα−/− and control mice (Fig. 3A). Furthermore, Ucp1 expression in brown adipose tissue, a marker for sympathetic tone (7), was also unchanged in nestin-ERα−/− females (Fig. 3A).
Leptin signaling.
Serum leptin levels were significantly increased in nestin-ERα−/− mice compared with control mice (+41%, P < 0.001) (Fig. 3B). Importantly, the mRNA levels of leptin in white adipose tissue (WAT) were clearly increased in nestin-ERα−/− mice compared with control mice (+77%, P < 0.05) (Fig. 3C). No significant body weight difference between nestin-ERα−/− and control mice was detected but retroperitoneal fat mass was increased in nestin-ERα−/− mice (+55%, P < 0.05) (Fig. 3D and Table S2). Hypothalamic, but not bone, mRNA expression of the leptin receptor was decreased in nestin-ERα−/− mice compared with control mice (Fig. 3A and Table S3). The expression of suppressor of cytokine signaling-3 (SOCS3) and protein tyrosine phosphatase-1b (PTP1b), two negative downstream regulators of leptin signaling (25), was unaffected in the hypothalamus of nestin-ERα−/− mice (Fig. 3A).
Nestin-ERα−/− Females Have Altered Regulation of T Lymphocytes.
The immune system is known to be involved in the regulation of bone mass, mostly via secretion of cytokines, but also via altered activity of various cells, including T lymphocytes. Analysis of the proinflammatory cytokine IL-6, which has a role in T-cell survival, proliferation, and activation (26) in tibia, revealed decreased expression in nestin-ERα−/− females compared with controls (−48%, P < 0.01) (Fig. 3E). The expression of class II MHC transactivator (CIITA), a regulator of MHC II, which is involved in antigen presentation by macrophages and T-cell activation, was also decreased in nestin-ERα−/− tibia compared with controls (−38%, P < 0.05) (Fig. 3E). The frequency of T lymphocytes (CD3+ cells) in bone marrow was significantly decreased in nestin-ERα−/− females (−21%, P < 0.01) (Fig. 3F). Furthermore, T-cell proliferation in spleen was decreased in nestin-ERα−/− mice compared with control mice (−28%, P < 0.05) (Fig. 3G).
Discussion
We have deleted ERα signaling in nervous tissue, using the Cre-loxP system, to determine the importance of central ERα signaling for the regulation of bone mass. Deletion of ERα in neuronal cells resulted in a substantial increase in bone mass. We propose that the main mechanism for the increased bone mass in nestin-ERα−/− mice is mediated via decreased central leptin sensitivity in the hypothalamus, and thereby increased secretion of leptin from WAT, which, in turn, results in increased peripheral leptin-induced bone formation.
The Cre-mouse used in this study is nestin-Cre, which is a widely used and well-described mouse model for deleting gene expression in the CNS (27). The specificity of the mouse model was validated using RT-PCR and Cre expression showing impaired ERα expression in brain but not in bone.
Nestin-ERα−/− mice had increased BMD in both the trabecular and cortical bone compartments and in both the appendicular and axial skeleton. The increase in trabecular BMD was because of both an increase in trabecular number and trabecular thickness. Furthermore, cortical thickness and bone strength were increased in nestin-ERα−/− mice. Thus, prevention of ERα signaling in the nervous system leads to increased bone density and increased bone strength.
Previous studies have shown that total deletion of ERα results in a preserved bone mass phenotype caused by disturbed negative feedback of sex steroids acting via the androgen receptor (17, 24). To determine whether a disturbed negative feedback might cause the increased bone mass seen in nestin-ERα−/− mice, serum levels of sex steroids and LH were measured. As demonstrated previously, total ERα deletion resulted in dramatically increased serum sex steroid levels (16, 24). However, no significant increase was detected in nestin-ERα−/− mice, demonstrating that a disturbed negative feedback regulation of sex steroids is not the primary cause of the increased bone mass. These data support previous findings of normal LH levels in neuron-specific ERα knockout female mice (28).
Dynamic histomorphometric analyses demonstrated a significantly increased bone formation rate in nestin-ERα−/− females, indicating that the bone formation rate is increased in mice lacking central ERα expression. Furthermore, expression analyses revealed increased expression of osteoblast- and osteocyte-specific transcripts after central ERα deletion, supporting the conclusion that bone formation is affected in these mice.
Nestin-ERα−/− mice might have a higher bone mass because of increased accrual of bone during early growth. To test this theory, control and nestin-ERα−/− mice were subjected to ovx and bone mass was measured after 4 wk. The bone mass in ovx nestin-ERα−/− mice was equal to the bone mass in ovx control littermates. Thus, there was a significantly higher bone loss in nestin-ERα−/− compared with controls. These data demonstrate that the increased bone mass seen in nestin-ERα−/− mice is not because of a higher bone accrual during early development, but is a dynamic event.
ERs are present both peripherally and centrally. Several studies have shown that estrogen clearly has positive peripheral effects on bone (19–21, 29). In contrast, deletion of central ERα signaling in the present study resulted in increased bone mass, suggesting that central estrogen signaling has a negative impact on bone and that the peripheral and central actions of estrogen on bone are opposite.
Another substance with the same suggested properties regarding opposite central versus peripheral effects on bone is the neurotransmitter serotonin (7, 8). Both central and peripheral serotonin levels were normal in nestin-ERα−/−. Central serotonin signaling affects bone via altered sympathetic signaling (7), and sympathetic tone, as determined by catecholamine secretion in urine and UCP1 (uncoupling protein 1) expression in brown adipose tissue (BAT), was not affected in nestin-ERα−/− mice. Therefore, no support was obtained for the possibility that the bone phenotype seen in nestin-ERα−/− mice is caused by altered serotonin regulation or sympathetic tone. These data are in accordance with previous results suggesting a more prominent role for ERβ in the regulation of the central serotonergic system (30).
Leptin, a hormone involved in the regulation of food intake and energy expenditure, has also been shown to be involved in the regulation of bone mass, with opposing central versus peripheral effects (2, 5). Central leptin signaling has negative effects on bone mass, tentatively via increased sympathetic output (2, 3). However, sympathetic signaling in nestin-ERα−/− mice was unaffected, indicating that the bone phenotype is not mediated via altered central leptin signaling. A direct peripheral effect of leptin in bone is supported by the fact that the signaling form of the leptin receptor is expressed by several skeletal cell types, including osteoblasts, chondrocytes, and bone marrow stromal progenitor cells (31–33). Peripheral leptin signaling has been demonstrated to increase osteoblast differentiation and bone formation, leading to increased bone mass (6, 31–33). Serum levels of leptin and bone formation were both increased in nestin-ERα−/− mice, suggesting that increased peripheral leptin signaling is involved in the high bone-mass phenotype (Fig. 3H). Circulating leptin is mainly adipocyte-derived and is regulated by the leptin expression within WAT and the amount of WAT. Importantly, we observed a substantial increase in leptin mRNA levels in WAT and the amount of WAT (retroperitoneal) was also increased. Thus, the elevated serum leptin levels in the nestin-ERα−/− mice were the result of both a clearly increased leptin expression within the WAT and an increase of the amount of WAT. It is well established that decreased central leptin sensitivity (increased resistance) results in elevated serum leptin levels (34) and can result from impaired central leptin receptor signaling (25). Importantly, we found the expression of the signaling form of the leptin receptor to be decreased in the hypothalamus in nestin-ERα−/− mice. The expression of SOCS3 and PTP1b, two negative regulators of leptin signaling (25), was unaffected in the hypothalamus of nestin-ERα−/− mice, suggesting that the effect is on the receptor level and not on downstream signaling mechanisms. These data are in concert with previous studies demonstrating coexpression of ERs and leptin receptors in the hypothalamus (35, 36) and estrogenic effects on central leptin sensitivity (37–39). In contrast, expression of leptin receptors in bone was unaffected in nestin-ERα−/− mice, supporting the notion that the impaired leptin sensitivity is specific to the nervous system. Taking these data together, we propose that the main mechanism for the increased bone mass in the nestin-ERα−/− mice is mediated via peripheral leptin action: deletion of ERα in nervous tissue results in decreased expression of leptin receptors in hypothalamus, leading to reduced central leptin sensitivity and thereby increased secretion of leptin from WAT, resulting in the observed elevated serum leptin levels in nestin-ERα−/− mice. The elevated serum leptin levels in turn increase bone mass by augmenting bone formation (Fig. 3H).
An alternative mechanism that might contribute to the high bone-mass phenotype in the nestin-ERα−/− mice is altered T-cell regulation. Activation of T cells results in decreased bone mass (40). Interestingly, expression in bone of both CIITA and IL-6, two factors known to enhance T-cell activation (26, 41, 42), as well as T-cell frequency and proliferation, were decreased in nestin-ERα−/− mice, supporting a contributing role for altered T-cell regulation in the nestin-ERα−/− bone phenotype. However, the reduction of the number of T cells in nestin-ERα−/− mice was not leading to a significantly reduced number of osteoclasts or bone resorption, arguing against a major impact of altered T-cell regulation for the increased bone mass.
The present study suggests that the balance between peripheral stimulatory and central inhibitory ERα actions determines the bone mass. Because estrogen treatment is known to be associated with side effects, such as breast cancer and thromboembolism (43, 44), it would be beneficial to develop a bone-specific estrogen treatment. One may, therefore, speculate that it would be useful to separate the peripheral effects of estrogen from its central effects. For an optimal bone health, it is probably favorable to treat with an ERα-specific agonist with low penetrance to the CNS, resulting in stimulatory peripheral bone effects without having inhibitory central bone effects. However, further studies are required to characterize the relative importance of central- versus peripheral ERα-mediated actions for other nonbone related estrogen effects. These studies might result in the development of a treatment preserving bone mass with minimal side effects in other tissues.
In summary, although peripheral ERα activation increases bone mass, we here demonstrate that central ERα activation decreases bone mass, indicating that the balance between peripheral stimulatory and central inhibitory ERα actions is important in the regulation of bone mass. We propose that the main mechanism for the increased bone mass in the nestin-ERα−/− mice is mediated via decreased central leptin sensitivity in the hypothalamus, and thereby increased secretion of leptin from WAT, which, in turn, results in increased peripheral leptin-induced bone formation.
Materials and Methods
Animals.
All animal experiments have been approved by the local Ethical Committee for Animal Research at the University of Gothenburg.
Nestin-Cre mice, which express the Cre recombinase specifically in neuronal and glial precursor cells (27), were mated with ERαflox/flox mice bearing floxed ER L2 alleles (in which loxP sites flank exon 3 encoding the DNA binding domain), to generate mice specifically lacking ERα in nervous tissue. Double-heterozygous Cre+/−ERαflox/+ mice were mated with ERαflox/flox mice to generate Cre+/−ERαflox/flox (Nestin-ERα−/−) mice and Cre−/−ERαflox/flox (control) mice (n = 6–10 per genotype). The generation of mice with a total ERα inactivation (ERα−/−) has previously been described (45). These mice have a deletion in exon 3 of the ERα gene and they do not express any of the isoforms of the ERα protein (45). The total ERα−/− mice and WT control (ERα+/+) littermates were generated by breeding male ERα+/− with female ERα+/− mice.
Female mice were terminated at 3–4 mo of age and phenotyped. T-cell proliferation was analyzed in 7-mo-old female mice. Leptin, leptin receptor, PTP1b, and suppressor of cytokine signaling-3 mRNA expression and fat content were analyzed in 9-wk-old mice. Ovx or sham-operation was performed in 3-mo-old mice, which were terminated 4-wk later.
Further details are provided in SI Materials and Methods.
Serum Measurements.
Commercially available RIA kits were used to determine serum levels of testosterone (MP Biomedicals), estradiol (Siemens Medical Solutions Diagnostics), and LH (IDS Nordic). Urinary elimination of catecholamines, serum serotonin levels, and serum leptin levels were measured using ELISA (Labor Diagnostika Nord, Fitzgerald Industries International, Crystal Chem). Creatinine content was measured in urine using a colorimetric assay (Cayman Chemical). Serum levels of FSH were measured by immunofluorometric assays (Delfia; Wallac), as previously described (46).
Measurement of Central Serotonin Levels.
Levels of serotonin in frozen (−80 °C) brain tissue (cortex and hippocampus) were analyzed using HPLC. Further details are provided in SI Materials and Methods.
Gene Expression Analyses.
Total RNA from tibia, femur, hypothalamus, WAT, BAT, spleen, uterus, liver, and striated muscle was prepared for real-time PCR analysis. Further details are provided in SI Materials and Methods.
pQCT.
pQCT was performed with the Stratec pQCT XCT Research M (Norland; v5.4B9), operating at a resolution of 70 μm, as described previously (47).
Trabecular BMD was determined ex vivo, with a metaphyseal scan of the proximal tibia and the distal femur at a distance from the growth plate corresponding to 3% of the total length of the bone. The trabecular bone region was defined as the inner 45% of the cross-sectional area. Cortical BMD was analyzed with a middiaphyseal scan of femur and tibia.
MicroCT.
μCT analyses were performed on the distal femur, proximal tibia, and lumbar vertebra (L5) by using Skyscan 1072 scanner (Skyscan), as previously described (48). Further details are provided in SI Materials and Methods.
Histomorphometry.
Trabecular bone from femur and tibia was evaluated using static and dynamic histomorphometric analyses (49, 50). Further details are provided in SI Materials and Methods.
Three-Point Bending.
The femurs were fixed in Burkhardt's formaldehyde for 2 d and after that stored at +4 C° in 70% ethanol for μCT and pQCT scanning. Before the mechanical testing, the bones were rinsed from ethanol and kept in PBS for 24 h. The biomechanics was analyzed with the three-point bending test (span length 55 mm, loading speed 0.155 mm/min) for the mid femur using the Instron universal testing machine (Instron 3366; Instron Corp.). The biomechanical parameters were calculated based on the recorded load deformation curves.
Flow Cytometry.
For flow cytometry analyses, bone marrow cells were stained with FITC-conjugated antibodies to CD3 for detection of T lymphocytes. The cells were then subjected to FACS on a FACSCalibur (BD Pharmingen). Further details are provided in SI Materials and Methods.
T-Cell Proliferation.
Con A-induced T-cell proliferation was performed on isolated spleen cells from 7-mo-old female mice, as previously described (51) and presented as mean of counts per minute from triplicate stimulated cultures minus mean of counts per minute from unstimulated cultures.
Supplementary Material
Acknowledgments
This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, the Swedish Combine project, an Avtal om Läkarutbildning och Forskning/Läkarutbildningsavtalet research grant in Gothenburg, the Lundberg Foundation, the Torsten and Ragnar Söderberg's Foundation, the Novo Nordisk Foundation, Reumatikerförbundet, the Gustav V 80-års fond, Åke Wiberg, and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant DK071122.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111436109/-/DCSupplemental.
References
- 1.Gajda M, Litwin JA, Cichocki T, Timmermans JP, Adriaensen D. Development of sensory innervation in rat tibia: Co-localization of CGRP and substance P with growth-associated protein 43 (GAP-43) J Anat. 2005;207:135–144. doi: 10.1111/j.1469-7580.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ducy P, et al. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 3.Elefteriou F, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 4.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Cirmanová V, Bayer M, Stárka L, Zajícková K. The effect of leptin on bone: An evolving concept of action. Physiol Res. 2008;57(Suppl 1):S143–S151. doi: 10.33549/physiolres.931499. [DOI] [PubMed] [Google Scholar]
- 6.Sienkiewicz E, et al. Long-term metreleptin treatment increases bone mineral density and content at the lumbar spine of lean hypoleptinemic women. Metabolism. 2011;60:1211–1221. doi: 10.1016/j.metabol.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lacey DL, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 11.Vanderschueren D, et al. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 13.Vandenput L, Ohlsson C. Estrogens as regulators of bone health in men. Nat Rev Endocrinol. 2009;5:437–443. doi: 10.1038/nrendo.2009.112. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg MK, et al. Estrogen receptor specificity for the effects of estrogen in ovariectomized mice. J Endocrinol. 2002;174:167–178. doi: 10.1677/joe.0.1740167. [DOI] [PubMed] [Google Scholar]
- 15.Turner RT. Mice, estrogen, and postmenopausal osteoporosis. J Bone Miner Res. 1999;14:187–191. doi: 10.1359/jbmr.1999.14.2.187. [DOI] [PubMed] [Google Scholar]
- 16.Börjesson AE, et al. Roles of transactivating functions 1 and 2 of estrogen receptor-alpha in bone. Proc Natl Acad Sci USA. 2011;108:6288–6293. doi: 10.1073/pnas.1100454108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims NA, et al. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J Clin Invest. 2003;111:1319–1327. doi: 10.1172/JCI17246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg MK, et al. Estrogen receptor (ER)-beta reduces ERalpha-regulated gene transcription, supporting a “ying yang” relationship between ERalpha and ERbeta in mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 19.Takano-Yamamoto T, Rodan GA. Direct effects of 17 beta-estradiol on trabecular bone in ovariectomized rats. Proc Natl Acad Sci USA. 1990;87:2172–2176. doi: 10.1073/pnas.87.6.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sjögren K, et al. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J Bone Miner Res. 2009;24:1263–1270. doi: 10.1359/jbmr.090208. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura T, et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: A case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson N, et al. Investigation of central versus peripheral effects of estradiol in ovariectomized mice. J Endocrinol. 2005;187:303–309. doi: 10.1677/joe.1.06181. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg MK, et al. Estrogen receptor specificity in the regulation of the skeleton in female mice. J Endocrinol. 2001;171:229–236. doi: 10.1677/joe.0.1710229. [DOI] [PubMed] [Google Scholar]
- 25.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130:27–33. doi: 10.1016/j.clim.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tronche F, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 28.Wintermantel TM, et al. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin-Millan M, et al. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura M, et al. Differential distribution of estrogen receptor (ER)-alpha and ER-beta in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-beta in midbrain serotonergic systems. Neuroscience. 2005;130:445–456. doi: 10.1016/j.neuroscience.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Cornish J, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 32.Thomas T, et al. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 33.Williams GA, et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. 2011;26:1698–1709. doi: 10.1002/jbmr.367. [DOI] [PubMed] [Google Scholar]
- 34.Frederich RC, et al. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat Med. 1995;1:1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 35.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Del Bianco-Borges B, Cabral FJ, Franci CR. Co-expression of leptin and oestrogen receptors in the preoptic-hypothalamic area. J Neuroendocrinol. 2010;22:996–1003. doi: 10.1111/j.1365-2826.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 37.Bennett PA, et al. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69:417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- 38.Ainslie DA, et al. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- 39.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong YY, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 41.Cenci S, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci USA. 2003;100:10405–10410. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, leukemia inhibitory factor, and oncostatin M stimulate bone resorption and regulate the expression of receptor activator of NF-kappa B ligand, osteoprotegerin, and receptor activator of NF-kappa B in mouse calvariae. J Immunol. 2002;169:3353–3362. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 43.Daly E, et al. Risk of venous thromboembolism in users of hormone replacement therapy. Lancet. 1996;348:977–980. doi: 10.1016/S0140-6736(96)07113-9. [DOI] [PubMed] [Google Scholar]
- 44.Collaborative Group on Hormonal Factors in Breast Cancer Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet. 1997;350:1047–1059. [PubMed] [Google Scholar]
- 45.Dupont S, et al. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 46.van Casteren JI, Schoonen WG, Kloosterboer HJ. Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod. 2000;62:886–894. doi: 10.1095/biolreprod62.4.886. [DOI] [PubMed] [Google Scholar]
- 47.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Movérare S, et al. Differential effects on bone of estrogen receptor alpha and androgen receptor activation in orchidectomized adult male mice. Proc Natl Acad Sci USA. 2003;100:13573–13578. doi: 10.1073/pnas.2233084100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eriksen T, Koch R, Nautrup CP. Microradiography of the feline marginal periodontium with a microfocal high-resolution X-ray system. Scand J Dent Res. 1994;102:284–289. doi: 10.1111/j.1600-0722.1994.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 50.Parfitt AM, et al. Report of the ASBMR Histomorphometry Nomenclature Committee Bone histomorphometry: Standardization of nomenclature, symbols, and units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 51.Islander U, et al. Influence of oestrogen receptor alpha and beta on the immune system in aged female mice. Immunology. 2003;110:149–157. doi: 10.1046/j.1365-2567.2003.01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.