Abstract
Paleorecords of the middle Holocene (MH) from the North American mid-continent can offer insights into ecological responses to pervasive drought that may accompany future climatic warming. We analyzed MH sediments from West Olaf Lake (WOL) and Steel Lake (SL) in Minnesota to examine the effects of warm/dry climatic conditions on prairie–woodland ecosystems. Mineral composition and carbonate δ18O were used to determine climatic variations, whereas pollen assemblages, charcoal δ13C, and charcoal accumulation rates were used to reconstruct vegetation composition, C3 and C4 plant abundance, and fire. The ratio of aragonite/calcite at WOL and δ18O at SL suggest that pronounced droughts occurred during the MH but that drought severity decreased with time. From charcoal δ13C data we estimated that the MH abundance of C4 plants averaged 50% at WOL and 43% at SL. At WOL C4 abundance was negatively correlated with aragonite/calcite, suggesting that severe moisture deficits suppressed C4 plants in favor of weedy C3 plants (e.g., Ambrosia). As climate ameliorated C4 abundance increased (from ≈33 to 66%) at the expense of weedy species, enhancing fuel availability and fire occurrence. In contrast, farther east at SL where climate was cooler and wetter, C4 abundance showed no correlation with δ18O-inferred aridity. Woody C3 plants (e.g., Quercus) were more abundant, biomass flammability was lower, and fires were less important at SL than at WOL. Our results suggest that C4 plants are adapted to warm/dry climatic conditions, but not to extreme droughts, and that the fire regime is controlled by biomass–climate interactions.
Pervasive drought, expected to be associated with climatic change in continental interiors, will have profound ecological, economic, and societal repercussions (1–4). Drought conditions are likely to alter ecosystem function by changing the relative abundance of plant functional groups (e.g., C3 vs. C4) in natural systems (5–7). For example, episodic droughts that occurred throughout the 20th century in the midwestern United States killed woody C3 genera, such as Quercus (8–10), and favored better adapted C3 and C4 herbaceous species (9). General circulation models coupled with dynamic crop-growth models project that, in agricultural systems, such climatic conditions could significantly reduce both C3 (e.g., wheat, Triticum aestivum L.) and C4 (e.g., corn, Zea mays L.) cereal crop yields (11, 12), resulting in billions of dollars of economic loss (13, 14). Empirical evidence of the response of plant functional groups to climatic conditions characteristic of drought is therefore important for evaluating predictions of future change. However, such evidence is mostly limited to historical records (15, 16), which lack the full range of past drought variability (3), and to short-term experimental manipulations (17, 18), which lack a sufficient temporal dimension for understanding future vegetational response.
During the middle Holocene (MH), ≈8.0–4.0 thousand years (ka) B.P. (19), the midwestern United States experienced higher summer temperatures and lower annual precipitation than during the early or late Holocene (20, 21), with episodes of pronounced drought (1). Retrospective studies of this period provide an opportunity to examine vegetational dynamics in response to climatic change. To this end, numerous pollen profiles spanning the Holocene have been published from the midwestern United States, especially Minnesota (22, 23). However, a detailed understanding of the vegetational history of the MH remains limited because of similar pollen morphologies of the taxa within Poaceae (the grass family) and because of the likely underrepresentation of pollen from important taxa that are not wind-dispersed, such as insectpollinated forbs.
On the basis of the distinct carbon-isotopic signatures of C3 and C4 plants (24), charcoal δ13C was recently used by Clark et al. (25) to estimate the relative abundance of C3 and C4 plants across a transect of three sites from North Dakota, Minnesota, and Wisconsin. This study demonstrated for the first time that C4 plant abundance increased during the MH, which is consistent with the generalization that C4 plants are adapted to warm and moisturelimited habitats (26, 27). However, these charcoal δ13C records did not have adequate temporal resolution (≈200 years between samples) to offer insights into the shorter-term dynamics of C3 and C4 response to climatic change within the MH. Furthermore, experimental and field results suggest that, although C4 plants are adapted to warm and moisture-limited habitats, they are not suited to conditions characterized by severe drought (28, 29). To resolve the temporal and spatial details of C3 and C4 plant response, we analyzed climatic and vegetational proxies from the MH portion of well dated sediment cores from two west-central Minnesota lakes, with an average resolution of 58 years between charcoal δ13C samples. Specifically, we tested the hypothesis that C4 plants were more tolerant than C3 plants of warm and dry climatic conditions within the MH.
Study Sites
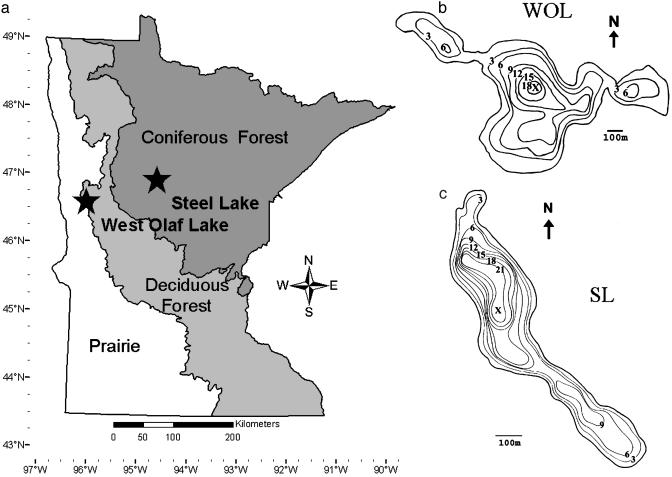
Our two sites, ≈120 km apart, straddle the pre-European-settlement prairie–forest border (Fig. 1a), which was controlled by interactions among climate, fire, soils, and topography (30). West Olaf Lake (WOL; 46° 37′ N, 96° 11′ W) has an area of 58 hectares, a maximum water depth of ≈18 m (Fig. 1b), and anoxic conditions below ≈9 m during the midsummer of 2002. At Detroit Lakes, MN, ≈40 km northeast of WOL, the mean annual temperature is 4.2°C, and the mean annual precipitation is 62 cm. Before European settlement, the vegetation near WOL was oak woodland and brushland dominated by Quercus macrocarpa Michx. (bur oak), Quercus ellipsoidalis E.J. Hill (pin oak), Populus tremuloides Michx. (quaking aspen), and Corylus cornuta Marsh. (beaked hazelnut), with upland tall-grass prairie openings (31, 32).
Fig. 1.
(a) Location of study sites within the major vegetational zones of Minnesota. (b and c) Bathymetric maps (depth in meters) of WOL (b) and SL (c) with coring locations indicated by X.
Steel Lake (SL; 46° 58′ N, 94° 41′ W) covers 23 hectares and has a maximum water depth of ≈21 m (Fig. 1c), with midsummer anoxia below ≈8 m in 2002. At Park Rapids, MN, ≈30 km west of SL, mean annual temperature and precipitation are 4.6°C and 67 cm, respectively. Before European settlement, the vegetation near SL was coniferous–hardwood forest consisting of Pinus strobus L. (white pine), P. resinosa Aiton (red pine), P. banksiana Lamb. (jack pine), Betula papyrifera Marsh. (paper birch), and Populus tremuloides Michx. (31, 32).
Materials and Methods
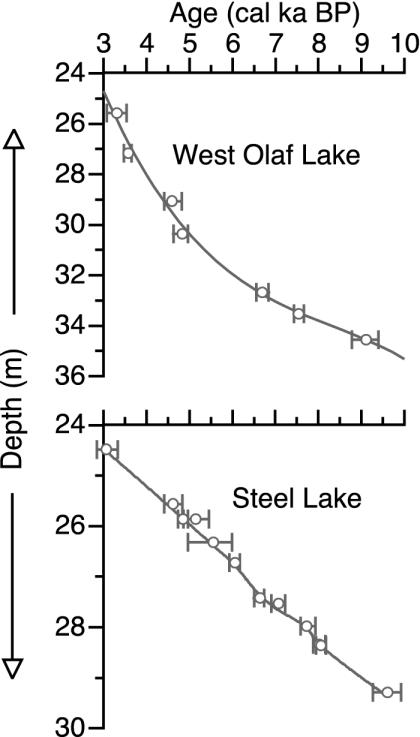
Stratigraphically overlapping sediment cores were obtained from the deepest part of each lake with a modified Livingstone piston sampler (33). Sediment subsamples were sieved with distilled water to isolate terrestrial plant macrofossils for 14C dating. Macrofossils were treated with an acid–base–acid protocol and submitted to Lawrence Livermore National Laboratory for accelerator-mass-spectrometry 14C dating. Age–depth models were developed based on seven 14C dates from WOL and 12 14C dates from SL (Table 1 and Fig. 2) that were converted to calibrated years with calib 4.3 (34) using the atmospheric decadal calibration data set (35) (http://depts.washington.edu/qil/calib/calib.html).
Table 1. 14C dates from WOL and SL.
| Lab number | Depth, cm* | 14C date, year B.P. | Calibrated age† | Material |
|---|---|---|---|---|
| WOL | ||||
| CAMS-85892 | 2,554-2,562 | 3,110 ± 80 | 3,315 (3,076-3,540) | Charcoal |
| CAMS-85893 | 2,712-2,720 | 3,345 ± 40 | 3,577 (3,472-3,662) | Charcoal |
| CAMS-85225 | 2,902-2,910 | 4,080 ± 70 | 4,595 (4,419-4,822) | Charcoal |
| CAMS-85894 | 3,032-3,040 | 4,260 ± 45 | 4,832 (4,626-4,964) | Charcoal |
| CAMS-85895 | 3,265-3,271 | 5,875 ± 45 | 6,692 (6,554-6,845) | Charcoal |
| CAMS-87119 | 3,350-3,356 | 6,680 ± 50 | 7,539 (7,422-7,656) | Charcoal |
| CAMS-29670 | 3,455 | 8,140 ± 70 | 9,107 (8,782-9,398) | Wood |
| SL | ||||
| CAMS-71312 | 2,448 | 2,920 ± 90 | 3,071 (2,849-3,335) | One Alnus seed |
| CAMS-70220 | 2,556 | 4,100 ± 90 | 4,620 (4,411-4,836) | Leaf fragments |
| CAMS-70221 | 2,586 | 4,290 ± 40 | 4,853 (4,731-4,968) | Wood |
| CAMS-69532 | 2,586 | 4,510 ± 100 | 5,150 (4,862-5,454) | One Betula seed |
| CAMS-70222 | 2,632 | 4,840 ± 210 | 5,559 (4,970-5,994) | Wood |
| CAMS-66722 | 2,672.5 | 5,280 ± 40 | 6,063 (5,935-6,173) | Leaf fragments |
| CAMS-68539 | 2,742 | 5,830 ± 40 | 6,642 (6,501-6,732) | One bud |
| CAMS-68540 | 2,753 | 6,170 ± 50 | 7,076 (6,909-7,227) | Wood |
| CAMS-70223 | 2,798 | 6,890 ± 90 | 7,729 (7,580-7,926) | Charcoal, leaf fragment |
| CAMS-70224 | 2,858 | 7,880 ± 70 | 8,719 (8,539-8,991)‡ | One seed bract |
| CAMS-71313 | 2,866 | 8,160 ± 50 | 9,115 (9,008-9,271)‡ | Two Cyperaceae seeds |
| CAMS-71314 | 2,888 | 7,210 ± 60 | 8,016 (7,876-8,164) | One Cyperaceae seed |
| CAMS-70225 | 2,890 | 7,240 ± 60 | 8,060 (7,949-8,169) | One seed bract |
| CAMS-70226 | 2,983 | 8,580 ± 130 | 9,607 (9,272-9,924) | Three Betula seeds |
Depth from lake water surface.
Calibration was performed by using calib 4.3 with the median value chosen as the calibrated age. The 2σ range is in parentheses.
Dates from a turbidite layer are excluded from the age—depth model following Wright et al. (51).
Fig. 2.
Age–depth models for sediment cores from WOL and SL with depth from the water surface. Error bars represent the 2σ probability of calibrated age ranges from calib4.3. The WOL dates are fit with a third-order polynomial, and the SL dates are fit with a locally weighted polynomial regression (59, 60).
Sediment mineral composition was determined by x-ray diffraction with a Scintag θ–θ diffractometer following conventional procedures (36). Minerals were quantified as the dry weight percentage of total major identified minerals, which included aragonite, calcite, dolomite, feldspars, and quartz (37). We used the ratio of aragonite/calcite to infer climatic change at WOL; this proxy was not applicable at SL because of the absence of aragonite in its sediment. We did not conduct δ18O analysis at WOL because of the presence of aragonite, which has a fractionation factor different from calcite and therefore makes it difficult to derive climatic inferences from δ18O.
Carbonate δ18O was analyzed at SL because the mineralogical profile was dominated by calcite with no significant stratigraphic change in the composition of carbonate mineral types. δ18O was determined by reacting sediment with ultra-pure phosphoric acid at 70°C in an automated Kiel device interfaced with an isotope–ratio mass spectrometer (IRMS; Finnigan MAT 252). The instrumental standard error for δ18O analysis was 0.1‰.
Macroscopic charcoal particles were concentrated from sub-samples of 3-cm3 sediment from WOL and 8-cm3 sediment from SL by disaggregating the sediment with 10% KOH and 10% HCl and washing it through a 180-μm sieve. The sediment subsamples from SL were of a greater volume than those from WOL to obtain an adequate number of charcoal particles for δ13C analysis. Charcoal particles were identified and counted at ×30 magnification and converted to charcoal accumulation rates (CHAR) following Long et al. (38). For charcoal δ13C analysis, a minimum of 35 pieces of randomly selected charcoal particles of generally similar size were analyzed per sample. Samples were combusted in an elemental analyzer (Carlo Erba NC2500) interfaced with an IRMS (Finnigan MAT 252). The instrumental standard error for charcoal δ13C analysis was ±0.1‰. Replicate samples were analyzed throughout each core for ≈10% of the samples to determine the reproducibility of charcoal δ13C signatures, which revealed an average error of ±2‰.
As the average isotopic composition of C3 and C4 plants (–27‰ and –13‰, respectively) (39) is unaffected by charring (40), we used these values as end members in a mixing model described by Clark et al. (25) to estimate the relative abundance of C3 and C4 plants on the landscape from charcoal δ13C values. Our estimates of C3 and C4 proportions are not restricted to grasses because potential charcoal sources include other C3 plants (trees, shrubs, and other herbaceous species). We assume that the charcoal was produced from terrestrial vegetation. Littoral and wetland vegetation in this region rarely burn, and today they cover only a small fraction of each lake's watershed.
Subsamples of 1-cm3 sediment were prepared for pollen analysis following standard methods (41), with Lycopodium spore tablets added to determine pollen concentrations. At least 300 pollen grains were counted per sample at ×400 magnification. Pollen percentages were based on the sum of arboreal and nonarboreal pollen types, excluding spores and aquatics. Pollen accumulation rates show similar patterns as pollen percentages and are therefore not presented.
Results and Interpretations
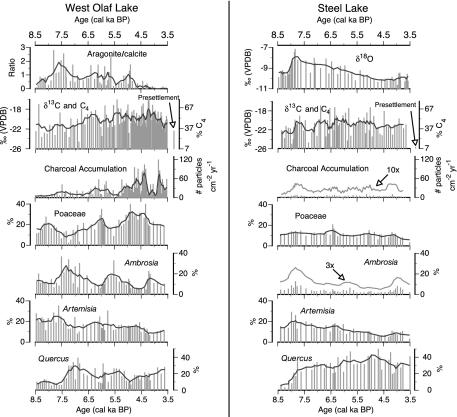
Climatic Variation Within the MH. We combine the records of aragonite/calcite at WOL and δ18O at SL to infer the temporal and spatial patterns of the regional climate during the MH (Fig. 3). Aragonite and calcite in the MH sediments of WOL are most likely endogenic because their stratigraphic patterns differ greatly from those of detrital minerals, such as quartz and feldspars (J.T., unpublished data). Aragonite is a polymorph of calcite that precipitates preferentially over calcite as lake-water Mg/Ca molar ratios increase to >7 (42). Today, aquifers in the WOL region have Mg/Ca ratios <1 (www.pca.state.mn.us/water/groundwater/gwmap/gwbaseline.html), suggesting that the presence of aragonite during the MH was likely due to concentration within the lake, controlled by water balance related to temperature and/or aridity, rather than by the input of high Mg/Ca ground water.
Fig. 3.
Data used for climatic interpretation (aragonite/calcite ratios and δ18O), charcoal δ13C and %C4, charcoal accumulation rates, and Poaceae, Ambrosia, Artemisia, and Quercus pollen abundance at WOL and SL. Bars represent raw data, and black curves are five-sample moving averages of the raw data. Gray curves are exaggerations of the five-sample moving averages.
Calcite δ18O in lake sediment is determined by moisture source, atmospheric and water temperatures, evaporation, and precipitation seasonality. A change in moisture source is unlikely (43), and the fractionation effect of atmospheric temperature prevails over that of water temperature (44). We do not have sufficient information to tease apart the relative importance of the other factors. However, the similarity in the general stratigraphic trends of δ18O and aragonite/calcite implies common climatic controls. Distinguishing between the effects of temperature and aridity is difficult, but both factors were likely important during the MH. This inference is supported by quantitative climatic reconstructions in the region, including pollen evidence of warm and dry conditions (21) and diatom evidence of saline water associated with low effective moisture (45).
Within the MH, aragonite/calcite at WOL reaches peak values around 7.6 ka BP, suggesting maximum warmth and aridity at that time. As the MH progressed, variable and decreasing aragonite/calcite ratios suggest fluctuating but generally lower temperatures and greater effective moisture (Fig. 3). Similar to aragonite/calcite, δ18O at SL reaches its peak value (–7.9‰) at the beginning of the MH and then displays a decreasing trend, which also suggests that conditions were warmest and driest at the beginning of the MH (Fig. 3).
In contrast to the similarity of general temporal trends, the spatial difference in effective moisture was likely large between the two sites. The presence of aragonite at WOL and its absence at SL indicate that lake-water Mg/Ca ratios were greater in WOL than in SL, suggesting greater moisture deficits at WOL throughout the MH. Alternatively, the presence of aragonite at WOL and not at SL could be caused by individual lake interactions with ground water (46). However, because the basin of WOL is substantially larger than that of SL, SL should be more susceptible to changes in moisture balance, with a greater tendency for aragonite precipitation. Greater moisture deficits at WOL than at SL are not unexpected, because annual precipitation minus evaporation decreases westward across Minnesota today (47) and during the Holocene (48). The inferred large difference in moisture between our two sites implies steep climatic gradients during the MH.
Plant Communities, C3/C4 Abundance, and Fire. Ambrosia, Artemisia, Poaceae, and Quercus were the most abundant pollen types at both WOL and SL during the MH. However, as with climate, distinct differences existed in community composition and structure between these sites. The combination of high Ambrosia, a weedy C3 taxon that invades disturbed areas, and Artemisia, a noninvasive C3 taxon that includes herbs and shrubs, along with moderately low Poaceae pollen at WOL during the early MH (Fig. 3) suggests a highly disturbed and patchy mid- to short-grass prairie (49, 50). Quercus was likely restricted to small groves near streams and lakes. As the MH progressed, the pollen assemblages at WOL became more typical of tall-grass prairie; grass pollen abundance gradually increased from 8% to 33% at the expense of Ambrosia, which decreased from 28% to 5%, and Artemisia, which decreased from 25% to 9%. In comparison with the pollen assemblages at WOL, at SL Ambrosia and Poaceae were less abundant, Quercus was more widespread, and Artemisia was slightly less abundant (Fig. 3), as is characteristic of an oak–prairie savanna or parkland (51). As the MH progressed, Quercus pollen increased in abundance from 14% to 43%, Ambrosia decreased from 9% to 2%, Artemisia decreased from 20% to 6%, and Poaceae showed little change, ranging between 15% and 9%.
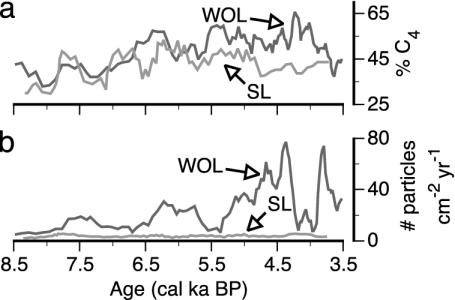
The MH plant communities inferred from pollen agree with previous studies from the regions near WOL (50) and SL (25). However, our charcoal δ13C results provide information on C4 dynamics during the MH that is unavailable from pollen assemblages alone. MH charcoal δ13C ranges from –16 to –25‰ at WOL and from –17 to –26‰ at SL. The δ13C-based estimates of C4 plant abundance for the entire MH averaged 50% and 43% at WOL and SL, respectively. The largest differences in C4 abundance between our sites occurred after ≈5.7 ka BP, when charcoal δ13C averages –19‰ at WOL and –21‰ at SL, equivalent to 57 and 43% contribution of C4 plants to sediment charcoal, respectively (Fig. 4a). In comparison, the δ13C of single charcoal samples from WOL and SL representing presettlement vegetation were –22‰ and –26‰, respectively, which is equivalent to 36% and 7% C4 (Fig. 3). These values of C4 abundance are much lower than their respective MH C4 abundance estimates of 50% and 43%, potentially reflecting regional forest development, which occurred under the mesic conditions of the late Holocene (51).
Fig. 4.
Comparison of C4 abundance (a) and charcoal accumulation rates (b)at WOL (black curves) and SL (gray curves).
Century-scale fluctuations in charcoal δ13Cofupto7‰ at WOL and 8‰ at SL during the MH suggest large variations in the relative abundance of C3 and C4 plants (Fig. 3). High sample-to-sample variation may also result partially from the ±2‰ error associated with our charcoal-δ13C analysis; thus, we focus on the long-term trends. The δ13C-estimated average abundance of C4 plants at WOL ranges from a low of 33% at 8.0 ka BP to a high of 66% at 4.2 ka BP, with an overall increasing trend from the early to late MH. In conjunction with the overall positive relationship between charcoal δ13C and Poaceae pollen interpolated to a common sampling resolution (r = 0.49, P < 0.0001, n = 60), this trend suggests that C4 grasses became more abundant as the MH progressed. Before European settlement near WOL, Andropogon, Bouteloua, Panicum, and Sorghastrum were the dominant C4 grasses, and these genera were likely among the C4 grass genera present during the late MH. Unlike WOL, no strong trend exists in C4 abundance from the early to late MH at SL, and the average C4 plant abundance there ranges from 30% to 53%. However, at SL, larger fluctuations in C4 abundance occurred during the early MH than during the late MH.
CHAR also exhibit distinct differences between our two sites (Fig. 4b). At WOL, CHAR range from 6 to 77 particles of charcoal cm–2·yr–1 and generally increase from the early to late MH, with the greatest increase after ≈5.2 ka BP, followed by a sharp decrease at ≈4.3 ka BP (Fig. 3). The increase in CHAR, particularly between ≈5.2 and 4.3 ka BP, suggests an increase in fire importance and/or the amount of biomass on the landscape during the later part of the MH at WOL. At SL, CHAR vary between 2 and 5 particles of charcoal cm–2·yr–1 with no strong temporal trend, suggesting little change in the fire regime during the MH (Fig. 3). Furthermore, CHAR are much lower than at WOL, suggesting that fire was relatively unimportant and/or that fire did not consume as much biomass at SL.
Discussion
Two lines of evidence support the hypothesis that C4 plants were more tolerant of warm and dry MH climatic conditions than C3 plants. First, at WOL, where moisture deficits were greater than at SL throughout the MH, C4 plants were more abundant than at SL after ≈5.7 ka BP. Second, consistent with Clark et al. (25), C4 plants were more abundant during the warm and dry MH than during the mesic period before European settlement, although our data from the latter period are limited.
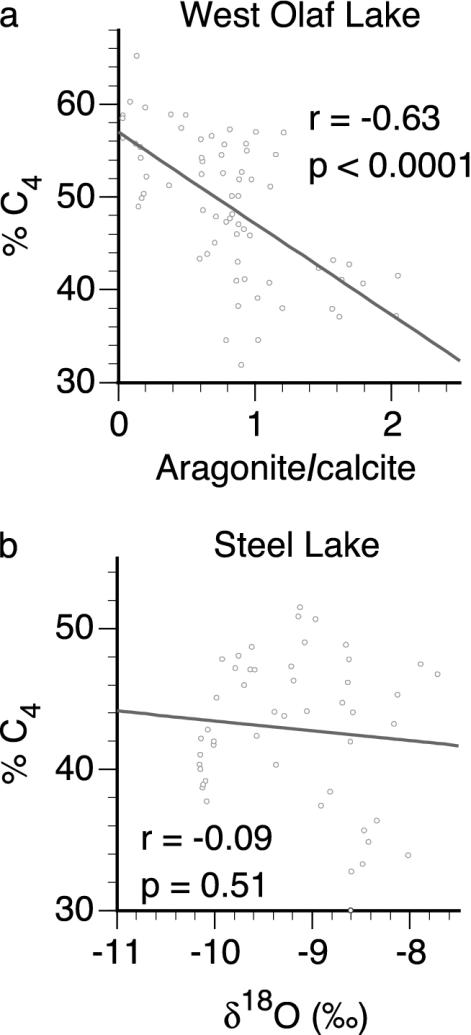
However, patterns of C3 and C4 plant response at our sites also differ from the predictions of the hypothesis that C4 plants were more tolerant of MH climatic conditions than C3 plants. For example, at WOL, the aragonite/calcite ratio and C4 abundance display generally opposite stratigraphic trends during the MH (Fig. 3). When interpolated to a common sampling resolution, these two time series have a significant negative relationship (r = –0.63, P < 0.0001, n = 67) (Fig. 5a). Thus, C4 plants did not generally dominate in the warmest and driest early part of the MH at WOL. Rather, such climatic conditions appeared to have created bare ground, which favored the establishment of weedy C3 species, such as Ambrosia, over C4 grasses, as suggested by the overall positive relationship between average Ambrosia pollen and aragonite/calcite data interpolated to a common sampling resolution (r = 0.45, P < 0.0001, n = 52). As MH temperatures became cooler and aridity decreased, C4 grasses increased in abundance.
Fig. 5.
Correlation between aragonite/calcite and C4 abundance at WOL (a) and δ18O and C4 abundance at SL (b). The five-sample moving averages of each time series were resampled to obtain the same resolution for correlation.
This counterintuitive C4 plant response to warm and dry climatic conditions is not restricted to the early MH. During the severe drought of 1930s Dust Bowl period, weedy C3 species colonized bare ground formerly dominated by C4 grasses in prairies of the midwestern United States (52). Furthermore, today C4 productivity declines relative to C3 productivity westward beyond the prairie–forest ecotone (53) with the decline of mean annual precipitation, most of which is summer precipitation (5–7). Thus, although C4 grasses are adapted to warm and semiarid regions and have various mechanisms to cope with drought (54, 55), they require adequate warm-season moisture (6, 7, 56). Drought-like conditions characterized by high interannual moisture variability at WOL during the early MH could have greatly reduced the proportion of C4 plants relative to C3 weedy species (49). We cannot tease apart the specific climatic factors that resulted in the severe early MH droughts using our climatic proxies. Regardless, an increase in overall moisture availability during the later MH appears to have favored C4 plants relative to C3 plants.
Farther east, at SL, greater effective moisture throughout the MH relative to WOL caused woody C3 species to be more abundant at SL compared to WOL. C4 plants were likely present in the uplands near SL, as they were at Deming Lake ≈40 km northwest of SL (25). At SL, C4 abundance and δ18O are not correlated during the MH (r = –0.09, P = 0.51, n = 48) (Fig. 5b), suggesting that C4 abundance did not respond to the trend toward cooler and moister conditions. Thus, as the result of overall cooler and moister conditions at SL, climatic variation within the MH exerted a weaker control on the abundance of C4 plants relative to C3 plants at SL.
Our data also suggest that fuel dynamics and climate interacted to control the fire regimes at our sites. At WOL, the extremely warm and dry conditions of the early MH likely limited productivity and the accumulation of flammable fuels, as can occur in semiarid grasslands today (57). During the late MH, the cooler and wetter climatic conditions resulted in increased productivity and C4 grasses, which enhanced biomass accumulation and changed the vegetation structure at WOL, as inferred from our pollen and charcoal δ13C data. Thus, fire was a direct consequence of changes in fuel conditions in response to climatic variation; the warmest and driest conditions of the early MH did not result in maximum burning because of biomass limitation (1, 25). Unlike those at WOL, MH fires at SL were likely never biomass-limited, and the fire regime did not show a large response to increased effective moisture from the early to late MH. A plausible explanation for the much lower CHAR at SL than at WOL is that woody species with relatively low flammability, such as Quercus, were more abundant at SL throughout the MH. The lower biomass flammability along with cooler and moister conditions at SL caused fires to be less frequent and prevented a discernible change in burning at this site throughout the MH. Thus, fires responded to fuel and climatic conditions, and they did not appear to have been the primary driver of vegetation change at either of our sites, which is in agreement with other studies from the region (25, 58).
This study provides a detailed temporal and spatial record of C3 and C4 dynamics during the MH in the mid-continent of North America. Our data, together with other recent results (1, 25), demonstrate the complex responses of vegetation to climatic change in this region. Our results confirm that C4 plants appear to be adapted to warm and arid environments. However, this relationship may be reversed under climatic conditions characterized by severe moisture deficits, which compromise the advantage of C4 grasses over C3 weedy species (28, 29). Furthermore, these results illustrate that the fire regimes of grassland–woodland ecosystems are determined by the interactions of biomass, fuel flammability, and climatic change. Documenting these complex relationships in paleorecords is important for anticipating how these ecosystems may respond to future climatic changes in the continental interiors of North America.
Acknowledgments
We thank B. Clegg, D. Gavin, and J. Hollis for field assistance; S. Greenberg for isotopic analyses; and R. Hughes and D. Moore for assistance with x-ray diffraction analyses. Comments from J. Clark, B. Clegg, D. Gavin, E. Grimm, P. Henne, J. Lynch, K. Robertson, H. E. Wright, and an anonymous reviewer improved the manuscript. This research was supported by a Packard Fellowship in Science and Engineering and by National Science Foundation Grant EAR 99-05327 (to F.S.H.). The 14C dating was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under Contract W-7405-Eng-48.
Abbreviations: MH, middle Holocene; WOL, West Olaf Lake; SL, Steel Lake; ka, thousand years; CHAR, charcoal accumulation rates.
References
- 1.Clark, J. S., Grimm, E. C., Donovan, J. J., Fritz, S. C., Engstrom, D. R. & Almendinger, J. E. (2002) Ecology 83, 595–601. [Google Scholar]
- 2.Intergovernmental Panel on Climate Change (2001) Climate Change 2001: The Scientific Basis (Cambridge Univ. Press, Cambridge, U.K.).
- 3.Woodhouse, C. A. & Overpeck, J. T. (1998) Bull. Am. Meteorol. Soc. 79, 2693–2714. [Google Scholar]
- 4.de Menocal, P. B. (2001) Science 292, 667–673.11303088 [Google Scholar]
- 5.Epstein, H. E., Lauenroth, W. K., Burke, I. C. & Coffin, D. P. (1997) Ecology 78, 722–731. [Google Scholar]
- 6.Paruelo, J. M. & Lauenroth, W. K. (1996) Ecol. Appl. 6, 1212–1224. [Google Scholar]
- 7.Epstein, H. E., Lauenroth, W. K., Burke, I. C. & Coffin, D. P. (1998) Plant Ecol. 134, 173–195. [Google Scholar]
- 8.Transeau, E. N. (1935) Ecology 16, 423–437. [Google Scholar]
- 9.Weaver, J. E. (1968) Prairie Plants and Their Environment (Univ. of Nebraska Press, Lincoln).
- 10.Faber-Langendoen, D. & Tester, J. R. (1993) Bull. Torrey Bot. Club 120, 248–256. [Google Scholar]
- 11.Rosenzweig, C. & Hillel, D. (1993) J. Environ. Qual. 22, 9–22. [Google Scholar]
- 12.Tubiello, F. N., Rosenzweig, C., Goldberg, R. A., Jagtap, S. & Jones, J. W. (2002) Climate Res. 20, 259–270. [Google Scholar]
- 13.White, R. & Etkin, D. (1997) Nat. Hazards 16, 135–163. [Google Scholar]
- 14.Riebsame, W. E., Changnon, S. A. & Karl, T. R. (1991) Drought and Natural Resources Management in the United States: Impacts and Implications of the 1987–89 Drought (Westview, Boulder, CO).
- 15.Weaver, J. E., Stoddart, L. E. & Noll, W. M. (1935) Ecology 16, 612–629. [Google Scholar]
- 16.Albertson, F. W. & Tomaneck, G. W. (1965) Ecology 46, 714–720. [Google Scholar]
- 17.Knapp, A. K., Fay, P. A., Blair, J. M., Collins, S. L., Smith, M. D., Carlisle, J. D., Harper, C. W., Danner, B. T., Lett, M. S. & McCarron, J. K. (2002) Science 298, 2202–2205. [DOI] [PubMed] [Google Scholar]
- 18.Danner, B. T. & Knapp, A. K. (2003) Global Change Biol. 9, 266–275. [Google Scholar]
- 19.Wright, H. E. (1976) Q. Res. 6, 581–596. [Google Scholar]
- 20.Cooperative Holocene Mapping Project Members (1988) Science 241, 1043–1052. [Google Scholar]
- 21.Bartlein, P. J. & Whitlock, C. (1993) in Elk Lake, Minnesota: Evidence for Rapid Climate Change in the North-Central United States, eds. Bradbury, J. P. & Dean, W. E. (Geol. Soc. Am., Boulder, CO), pp. 275–293.
- 22.Webb, T., III, Cushing, E. J. & Wright, H. E. (1983) in Late-Quaternary Environments of the United States, ed. Wright, H. E. (Univ. of Minnesota Press, Minneapolis), Vol. 2, pp. 142–165. [Google Scholar]
- 23.Wright, H. E. (1992) Q. Res. 38, 129–134. [Google Scholar]
- 24.Bender, M. M. (1971) Phytochemistry 10, 1239–1245. [Google Scholar]
- 25.Clark, J. S., Grimm, E. C., Lynch, J. & Mueller, P. G. (2001) Ecology 82, 620–636. [Google Scholar]
- 26.Raven, P. H., Evert, R. F. & Eichhorn, S. E. (1992) Biology of Plants (Worth, New York).
- 27.Hopkins, W. G. (1999) Introduction to Plant Physiology (Wiley, New York).
- 28.Long, S. P. (1999) in C4 Plant Biology, eds. Sage, R. F. & Monson, R. K. (Academic, San Diego), pp. 215–249.
- 29.Sage, R. F., Wedin, D. A. & Li, M. (1999) in C4 Plant Biology, eds. Sage, R. F. & Monson, R. K. (Academic, New York), pp. 313–373.
- 30.Grimm, E. C. (1984) Ecol. Monogr. 54, 291–311. [Google Scholar]
- 31.Swink, F. & Wilhelm, G. (1994) Plants of the Chicago Region (Indiana Acad. Sci., Indianapolis).
- 32.Marschner, J. H. (1974) The Original Vegetation of Minnesota (U.S. Forest Service, St. Paul).
- 33.Wright, H. E. (1991) J. Paleolimnol. 6, 37–49. [Google Scholar]
- 34.Stuiver, M. & Reimer, P. J. (1993) Radiocarbon 35, 215–230. [Google Scholar]
- 35.Stuiver, M., Reimer, P. J., Bard, E., Beck, J. W., Burr, G. S., Hughen, K. A., Kromer, B., McCormac, G., Van der Plicht, J. & Spurk, M. (1998) Radiocarbon 40, 1041–1083. [Google Scholar]
- 36.Moore, D. M. & Reynolds, R. C., Jr. (1997) X-Ray Diffraction and the Identification and Analysis of Clay Minerals (Oxford Univ. Press, New York).
- 37.Hughes, R. E., Moore, D. M. & Glass, H. D. (1994) in Quantitative Methods in Soil Mineralogy, eds. Amonette, J. E. & Zelazny, L. W. (Soil Sci. Soc. Am., Madison, WI), pp. 330–359.
- 38.Long, C. J., Whitlock, C., Bartlein, P. J. & Millspaugh, S. H. (1998) Can. J. Forest Res. 28, 774–787. [Google Scholar]
- 39.Smith, B. N. & Epstein, S. (1971) Plant Physiol. 47, 380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beuning, K. R. M. & Scott, J. E. (2002) Palaeogeogr. Palaeoclimatol. Palaeoecol. 177, 169–181. [Google Scholar]
- 41.Faegri, K., Iverson, J., Kaland, P. E. & Krzywinski, K. (1989) Textbook of Pollen Analysis (Wiley, New York).
- 42.Kelts, K. & Talbot, M. R. (1990) in Large Lakes: Ecological Structure and Function, eds. Tilzer, M. M. & Surruya, C. (Springer, Berlin), pp. 288–315.
- 43.Simplins, W. W. (1995) J. Hydrol. 172, 185–207. [Google Scholar]
- 44.Stuiver, M. (1970) J. Geophys. Res. Atmos. 75, 5247–5257. [Google Scholar]
- 45.Laird, K. R., Fritz, S. C., Grimm, E. C. & Mueller, P. G. (1996) Limnol. Oceanogr. 41, 890–902. [Google Scholar]
- 46.Digerfeldt, G., Almendinger, J. E. & Bjorck, S. (1992) Palaeogeogr. Palaeoclimatol. Palaeoecol. 94, 99–118. [Google Scholar]
- 47.Winter, T. C. & Woo, M. K. (1990) in Surface Water Hydrology, eds. Wolman, M. G. & Riggs, H. C. (Geol. Soc. Am. Press, Boulder, CO).
- 48.McAndrews, J. H. (1966) Torrey Bot. Club Mem. 22, 1–72. [Google Scholar]
- 49.Grimm, E. C. (2001) Proc. R. Irish Acad. B 101, 47–64. [Google Scholar]
- 50.Almquist-Jacobson, H., Almendinger, J. E. & Hobbie, S. (1992) Q. Res. 38, 103–116. [Google Scholar]
- 51.Wright, H. E., Stefanova, I., Tian, J., Brown, T. A. & Hu, F. S. (2004) Q. Sci. Rev., in press.
- 52.Weaver, J. E. & Albertson, F. W. (1943) Ecol. Monogr. 13, 63–117. [Google Scholar]
- 53.Tieszen, L. L., Reed, B. C., Bliss, N. B., Wylie, B. K. & DeJong, D. D. (1997) Ecol. Appl. 7, 59–78. [Google Scholar]
- 54.Heckathorn, S. A. & Delucia, E. H. (1991) Bot. Gazette 152, 263–268. [Google Scholar]
- 55.Knapp, A. K. (1985) Ecology 66, 1309–1320. [Google Scholar]
- 56.Knapp, A. K. & Medina, E. (1999) in C4 Plant Biology, eds. Sage, R. F. & Monson, R. K. (Academic, San Diego), pp. 251–283.
- 57.Oesterheld, M., Loreti, J., Semmartin, M. & Paruelo, J. M. (1999) in Ecosystems of the World: Ecosystems of Disturbed Ground, ed. Walker, L. R. (Elsevier, New York), Vol. 16, pp. 287–306. [Google Scholar]
- 58.Olson, D. E. L. (1993) M.S. thesis (Univ. of Minnesota, Minneapolis).
- 59.Cleveland, W. S. (1979) J. Am. Stat. Assoc. 74, 829–836. [Google Scholar]
- 60.Cleveland, W. S. & Devlin, S. J. (1988) J. Am. Stat. Assoc. 83, 596–610. [Google Scholar]