Abstract
Microglial priming predisposes the brain to neurodegeneration and affects disease progression. The signal to switch from the quiescent to the primed state is unknown. We show that deleting the C3 convertase regulator complement receptor 1-related protein y (Crry) induces microglial priming. Mice that were double-knockout for Crry and either C3 or factor B did not show priming, demonstrating dependence on alternative pathway activation. Colocalization of C3b/iC3b and CR3 implicated the CR3/iC3b interaction in priming. Systemic lipopolysaccharide challenge overactivated primed microglia with florid expression of proinflammatory molecules, which were blocked by complement inhibition. Relevance for neurodegenerative disease is exemplified by human multiple sclerosis (MS) and by experimental autoimmune encephalomyelitis (EAE), a model of MS. In human MS, microglial priming was evident in perilesional white matter, in close proximity to C3b/iC3b deposits. EAE was accelerated and exacerbated in Crry-deficient mice, and was dependent on C activation. In summary, C3-dependent microglial priming confers susceptibility to other challenges. Our observations are relevant to progression in MS and other neurological diseases exacerbated by acute insults.
In the context of aging or neurodegeneration, microglial priming appears to exacerbate disease. Stimuli that lead to microglial priming, such as systemic infections and elevated plasma IL-1β/TNF-α, are correlated with accelerated cognitive decline in Alzheimer's disease patients (1, 2). In Alzheimer's disease models, repeated LPS challenges exacerbate tau pathology (3), inflammation (4), and amyloid deposition (5). In prion disease models, microglial priming is evident even in the preclinical stage, and LPS challenge exacerbates neuronal death, induces acute cognitive impairment, and accelerates disease progression (6–8). These studies all suggest that microglial priming places subjects at risk for exacerbation from an early stage of disease (7). Despite the likelihood that microglial priming is an important event in neurodegenerative diseases, its triggers are not well understood. Identification of pathways that lead to microglial priming could support the design of therapies that either reverse priming or block the pathways that activate primed microglia after peripheral infection, surgery, or other insults.
We hypothesize that the complement system—one of the most important humoral signaling systems, contributing substantially to immune surveillance and homeostasis, and highly expressed in the CNS—is involved in microglial priming (9). We show here that complement dysregulation in the CNS triggers microglial priming for subsequent activation in mice and humans. The findings presented here suggest that targeted inhibition of complement to reduce microglial priming and/or block subsequent activation can provide a unique therapeutic approach to neurodegenerative disease.
Results
Microglial Changes in Complement Receptor 1-Related Protein y (Crry)−/− CNS Depend on an Intact Alternative Pathway.
To study the role of the complement system in microglial priming, we used a mouse strain that lacks a major regulator of the complement cascade, Crry, strongly expressed by microglia (10). Crry regulates the C3 convertase enzymes in rodents (11). It regulates C3 activation on self tissues by decaying the C3 convertase and catalyzing factor I (fI)-mediated degradation of C3b. We generated Crry−/− mice by treating female Crry+/− mice with neutralizing anti-C5 mAb through pregnancy (12). Crry−/− offspring, although healthy and fertile, had markedly reduced plasma C activity and C3 levels despite increased hepatic C3 synthesis, demonstrating chronic C3 activation and consumption (12, 13).
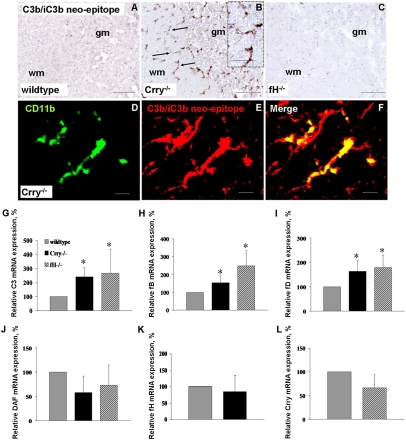
Histological examination revealed hypercellularity in the CNS of Crry−/− mice relative to WT at both 10 and 32 wk but no associated neuropathology. Immunoreactivity for CD11b (CR3) identified the supernumerary cells as microglia (Fig. 1 A–E) that were significantly increased in CNS white and gray matter of Crry−/− mice relative to WT (brain and spinal cord, white matter and gray matter, P < 0.001, one-way ANOVA) (Fig. 1F). Microglia showed a highly ramified activated morphology (Fig. 1B) at 10 and 32 wk, but a quiescent morphology was observed in controls.
Fig. 1.
Alternative pathway-dependent microglial phenotype in Crry−/− spinal cord. (A–E) CD11b immunostaining in spinal cord of WT (A and Inset), Crry−/− (B and Inset), Crry−/−C3−/− (C), Crry−/−fB−/− (D), and fH−/− mice (E). (F) Quantification of CD11b immunostaining in brain and spinal cord of WT (n = 7), Crry−/− (n = 7), Crry−/−C3−/− (n = 3), Crry−/−fB−/− (n = 3), and fH−/− mice (n = 4). Values are given as means ± SD. Error bars are for n, number of animals. *P < 0.001 determined by one-way ANOVA. wm, white matter; gm, gray matter. (Scale bar: A–E, 100 μm; Insets, 50 μm.)
To determine whether, in the absence of Crry, activation of the alternative pathway was driving the activated microglial morphology, spinal cord tissue from Crry−/−C3−/− (Fig. 1C) and Crry−/−fB−/− (Fig. 1D) mice was analyzed. In both cases, the morphology (Fig. 1A) and number (Fig. 1F) of CD11b-positive microglia were the same as in WT, demonstrating that the microglial changes in C-sufficient Crry−/− mice required a functional alternative pathway.
To test whether the activated microglial morphology also occurred in other situations of complement dysregulation, spinal cord from fH−/− mice, also characterized by chronic C3 activation (14), was examined. These mice displayed a quiescent microglial morphology (Fig. 1 A and E) and no increase in number of CD11b-positive microglia, resembling WT (Fig. 1F), showing that the increased microglial number and activated morphology are specific to Crry deficiency because they are absent in another situation of chronic C3 activation. A scheme of the alternative pathway activation and regulation of C3 is shown in Fig. S1.
Microglia Are Primed in Crry−/− Mice.
Morphologically activated microglia can be either functionally activated or primed, distinguishable because primed cells do not express inflammatory cytokines (7, 15). Expression of IL-1β and TNF-α in Crry−/− CNS was assessed by quantitative PCR (qPCR). There was no difference in expression levels of these proinflammatory cytokines between naïve Crry−/− and WT mice, supporting the contention that microglia in Crry−/− mice are primed but not activated.
C3 Cleavage Products Are Present on Microglia of Crry−/− Mice.
Deposition of C3 fragments was tested by immunohistochemistry using a mAb (clone 3/26) that detects only fragments C3b, iC3b, and C3c, here referred to as C3b/iC3b because the soluble C3c fragment is not retained in tissue (Fig. 2 A–F) (16). This mAb gave no staining in WT spinal cords (Fig. 2A) but yielded strong staining on microglia in gray and white matter in Crry−/− spinal cords (Fig. 2B). Double-immunolabeling for C3b/iC3b and the microglial marker CD11b showed partial colocalization on microglia (Fig. 2 D–F). C3b/iC3b staining was absent on microglia in fH−/− mice (Fig. 2C). Altogether, these data show that C3b and/or iC3b specifically accumulate on microglia of Crry−/− mice. To determine why microglial cells were susceptible to C3b/iC3b deposition, we tested whether neurons express other regulators of the C3 convertase. In situ hybridization for decay-accelerating factor (DAF) expression on mouse and human CNS tissue showed that DAF mRNA is highly expressed by neurons but is undetectable in microglia in both mouse spinal cord and human brain tissue (Fig. S2). These data suggest that, in the absence of Crry, microglial cell susceptibility to C3b/iC3b deposition is caused by lack of other regulators of C3 activation, such as DAF. In addition, all components of the alternative pathway amplification loop were up-regulated in Crry−/− mice spinal cords (Fig. 2 G–L), supporting involvement of the alternative pathway (SI Results).
Fig. 2.
Alternative pathway activation in Crry−/− spinal cord. (A–C) Immunostaining for C3b/iC3b neoepitope, showing deposition in Crry−/− (B) but not in WT (A) or fH−/− (C) spinal cords. (D–F) Colocalization of C3b/iC3b with the microglial marker CD11b in Crry−/− mice. (Scale bar: A–C, 100 μm; D–F, 10 μm.) (G–L) Relative C3 (G), fB (H), fD (I), DAF (J), fH (K), and Crry (L) mRNA expression in spinal cords of WT (n = 7), Crry−/− (n = 7), and fH−/− (n = 4) mice. Values are normalized to the expression of β-actin and given as percentage (means ± SD) of WT control levels. Error bars are for n, number of animals. *P < 0.05 determined by one-way ANOVA.
Proinflammatory Molecules Are Up-Regulated in Crry−/− CNS upon LPS Challenge.
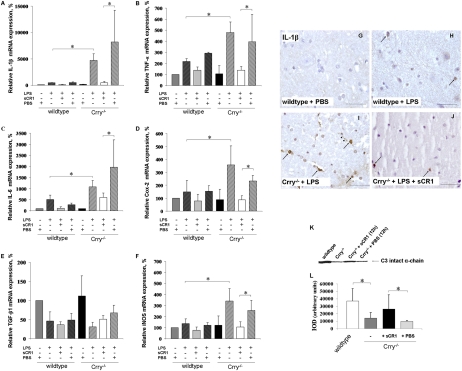
In view of the known effects of systemic infection on the innate immune system in the CNS, we studied the consequences of systemic LPS challenge. In WT mice, Crry mRNA decreased fourfold after LPS (P < 0.001; Fig. S3A). In both WT and Crry−/− animals, LPS challenge resulted in increased C3 mRNA expression levels (Fig. S3B). The LPS challenge affected expression of proinflammatory cytokines IL-1β, TNF-α, and IL-6 and proinflammatory mediator inducible nitric oxide synthase (iNOS) in CNS of WT. Notably, in the Crry−/− mice, expression of these proinflammatory molecules was markedly increased to levels much higher than those detected for the challenged WT mice. Cox-2 expression was significantly increased in spinal cord and hippocampus of Crry−/− mice after LPS challenge but was unchanged in WT CNS. Expression of the anti-inflammatory cytokine TGF-β was reduced in both Crry−/− and WT spinal cords and hippocampi (Fig. 3 A–F and Fig. S4).
Fig. 3.
Spinal cord expression of inflammatory mediators is up-regulated in Crry−/− mice after systemic LPS challenge. (A–F) Relative mRNA expression of IL-1β (A), TNF-α (B), IL-6 (C), Cox-2 (D), TGF-β1 (E), and iNOS (F) in spinal cords of WT and Crry−/− mice before (−LPS; WT n = 3; Crry−/− n = 4) and after (+LPS; WT n = 5; Crry−/− n = 8) systemic challenge with LPS. Some groups were pretreated at 12 h earlier with sCR1 (+sCR1; WT n = 4; Crry−/− n = 4) or PBS (+PBS; WT n = 3; Crry−/− n = 3). Values are normalized to expression of β-actin and given as percentage (means ± SD) of WT controls. Asterisks indicate statistically significant differences determined by one-way ANOVA. Error bars are for n, number of animals. (G–J) Immunohistochemical staining for IL-1β. IL-1β microglial immunoreactivity is indicated by arrows. (Scale bar: G–J, 50 μm.) (K) Western blot analysis of plasma from Crry−/− mice before and after systemic treatment with one dose (20 mg/kg) of sCR1. Plasma from WT and untreated Crry−/− mice is included as additional controls. C3 intact α-chain is shown (arrow). (L) Densitometry of Western blot analysis. Values are expressed as mean ± SD of the integrated optical density (IOD) for the immunoreactive band. Error bars are for n, number of animals. Asterisks indicate statistically significant differences determined by one-way ANOVA.
To determine which cells express the cytokines, CNS tissue was stained for IL-1β (7, 15). LPS treatment induced IL-1β expression in microglia in spinal cords of all Crry−/− mice, whereas only trace staining of microglia in WT was detected, which agrees with the mRNA expression data (Fig. 3 G–J). Soluble complement receptor 1 (sCR1) treatment inhibited LPS-induced changes in gene expression in the CNS of Crry−/− mice (Fig. 3 A–F, Fig. S4, and SI Results).
To determine whether LPS increased plasma levels of inflammatory cytokines in Crry−/− mice compared with WT, plasma IL-6 and TNF-α were measured at 2 h after the systemic LPS challenge. Plasma levels of IL-6 and TNF-α increased twofold but were similar in Crry−/− and WT mice.
Primed Microglia Are Present in Normal-Appearing Multiple Sclerosis (MS) Tissue.
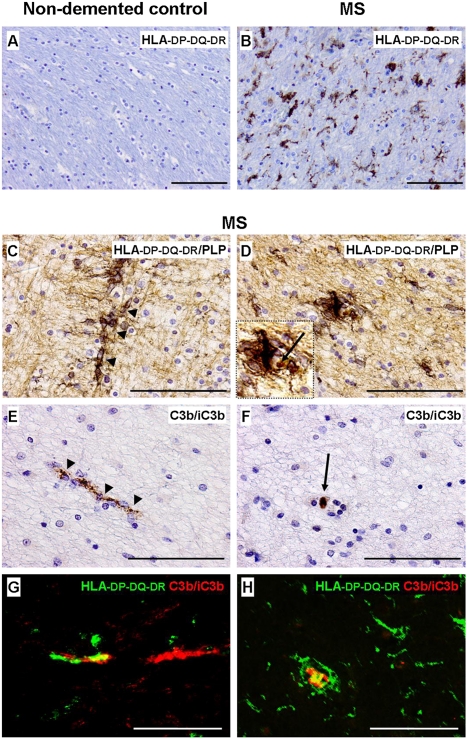
Microglial morphology and activity vary in distinct MS lesion areas (17) and may range from quiescent to highly activated morphology. Microglial expression of IL-1β (18) and deposition of activated C3 (C3d) (19, 20) have been independently reported but no account has been published of an investigation in the same lesion/patient or analysis in the context of microglial priming. MS brain tissue and white matter from nondemented controls (Table S1) were examined for evidence of microglial priming by morphology, staining for all three subunits (HLA-DP, HLA-DQ, and HLA-DR) of the HLA (HLA-DP-DQ-DR), IL-1β, and C3 fragment (C3b/iC3b) deposition (Table S2 and Fig. 4 A–F). Microglia in normal-appearing white matter from MS brains were abundant, enlarged, thickly ramified, and stained strongly for HLA-DP-DQ-DR (Fig. 4B). Clusters of microglia were localized along segments of proteolipid protein (PLP)-positive myelinated nerve fibers in normal-appearing white matter close to and distant from active and inactive lesions, but they were absent from demyelinated regions (Fig. 4 C and D). Microglial clusters were not observed and HLA-DP-DQ-DR staining was virtually undetectable in control white matter (Fig. 4A). C3b/iC3b staining was observed in normal-appearing MS brains, located in nerve fiber segments in close proximity to microglial clusters (Fig. 4 E–H). Double-staining for HLA-DP-DQ-DR and C3b/iC3b showed partial colocalization, suggesting that microglia contact C3b/iC3b-positive myelinated nerve fibers at discrete locations (Fig. 4 G and H). No C3b/iC3b staining was found in control white matter. IL-1β staining was detected in microglia in the rim of active lesions but was absent in normal-appearing white matter in MS and in control white matter (Table S2). In summary, these data show that perilesional microglia in MS show an enlarged, ramified morphology and stain for C3 fragments but do not express proinflammatory cytokines (IL-1β–negative), compatible with a primed phenotype.
Fig. 4.
Microglial morphology and C3b/iC3b immunoreactivity in normal-appearing MS tissue. (A and B) Immunostaining for the HLA-DP-DQ-DR microglial marker showing increased immunoreactivity and thick, ramified microglial morphology in normal-appearing MS tissue (B) compared with control white matter (A). (C) A cluster of thickly ramified microglial cells (in black) on a proteolipid protein (PLP)-positive nerve fiber (in light brown) in normal-appearing tissue surrounding an MS lesion. (D) Transverse view of a microglial cluster (in black) showing thick microglial ramifications around a central core. (E) A C3b/iC3b-positive segment of nerve fiber located in intact MS tissue. (F) Transverse view of E. (G and H) Double-immunostaining for HLA-DP-DQ-DR (green) and C3b/iC3b (red) in intact MS tissue showing partial colocalization (in yellow), suggesting discrete areas of contact between the C3b/iC3b localized on the nerve fiber and the HLA-DP-DQ-DR microglial marker.
Experimental Autoimmune Encephalomyelitis (EAE) Is Accelerated and Exacerbated in Crry−/− Mice.
To explore whether microglial priming might influence the course of MS, Crry−/− mice were tested in the EAE model of MS, in which both C regulation and microglial activation are implicated (21, 22). EAE was induced in WT and Crry−/− mice, the course was monitored, and the endpoint pathology was assessed. In WT animals, disease developed predictably at 16 d after initial immunization and was mild with minimal loss of myelin and axons as anticipated (23). In contrast, Crry−/− mice fell ill between days 10 and 14, and disease progressed much faster. Disease severity was greatly increased in Crry−/− mice (Fig. 5A), with all animals paralyzed and requiring killing on or before day 21. The induction of disease in the Crry−/− mice was specific for the myelin antigen used because treatment with adjuvant alone did not induce any clinical disease or CNS inflammation. At the endpoint, Crry−/− mice showed marked inflammation (Fig. 5 C, H&E; F, Iba-1; and I, IL-1β) and myelin loss [Fig. 5L, Luxol fast blue (LFB) staining] in subpial perivascular areas. The inflammatory cells were predominantly microglia/macrophage, strongly ionized calcium-binding adaptor molecule 1 (Iba-1)–positive, and localized in the hypercellular subpial perivascular area. Quantification of equivalent subpial perivascular areas revealed fourfold increased H&E staining (Fig. 5D), 10-fold increased Iba-1 staining (Fig. 5G), 25-fold increased IL-1β staining (Fig. 5J), and twofold decreased LFB staining (Fig. 5M) in Crry−/− spinal cords compared with WT.
Fig. 5.
Induction and characterization of EAE in Crry−/− mice. (A) Clinical disease score for EAE, showing earlier onset and increased disease severity in Crry−/− mice (n = 6) compared with WT (n = 4). All animals were killed by day 21. Values are expressed as mean ± SD. Error bars are for n, number of animals. *P < 0.05 determined by Mann–Whitney U nonparametric test. (B–M) Inflammation and myelin loss were assessed by H&E staining (B–D), Iba-1 immunostaining of macrophages (E–G), and IL-1β immunostaining (H–J) and LFB staining (K–M) of myelin in WT (B, E, H, and K) and Crry−/− (C, F, I, and L) EAE spinal cords. Perivascular infiltration, Iba-1–positive microglia/macrophages, IL-1β deposition, and demyelination were evident in Crry−/− spinal cords (arrows in C). Arrowheads in K and L indicate a blood vessel. (Scale bars: 100 μm.) Quantification of perivascular infiltration (D), Iba-1 staining (G), IL-1β deposition (J), and perivascular LFB staining (M) in Crry−/− mice compared with WT. Values are expressed as mean ± SD. Error bars are for n, number of animals. *P < 0.05 in D and M; *P < 0.01 in G and J; determined by two-tailed Student's t test.
To explore whether complement inhibition ameliorated CNS disease associated with microglial priming/activation, Crry−/− mice were treated with sCR1 systemically, daily from day 8 after induction of EAE. To focus on the effect of sCR1 treatment on microglial priming rather than on clinical disease severity, all mice were killed on day 14, before they developed significant clinical disease. Crry−/− mice treated with sCR1 showed quiescent microglial morphology and no signs of subpial and perivascular inflammatory infiltrates at killing, whereas untreated Crry−/− mice at this time point in the course of EAE induction displayed highly activated microglia and abundant infiltrating inflammatory cells (Fig. S5 A–D). In a separate experiment, naïve Crry−/− mice were treated with sCR1 for 1 wk. Immunohistology showed no change in the microglial primed phenotype (Fig. S5E). Altogether, these data demonstrate that, in the context of a leaky blood–brain barrier (BBB), systemic C inhibition reverses the primed microglial morphology and ameliorates the exacerbated EAE associated with microglial priming. In contrast, in the presence of an intact BBB, systemic C inhibition had no effect, suggesting that local C production drives the phenotype.
Discussion
Microglial priming is a preconditioning event found in several chronic neurodegenerative diseases, including prion disease (7) and Alzheimer's disease (24), and it even occurs as a feature of normal brain aging (25, 26). In vitro exposure of rat microglia to amyloid β protein primed cells for an exacerbated inflammatory response to phorbol ester (27), whereas IFN-γ primed monocytes/macrophages for an enhanced TNF-α response to LPS (28). In these contexts, priming prepares the cell for an enhanced activation response to a second noxious stimulus, for example, a peripheral infection (6–8, 15), surgery (26), or stroke (29), resulting in increased inflammatory response and pathology.
Crry−/− mice, although subject to spontaneous and chronic activation of complement, are healthy, fertile, and display no spontaneous pathology when maintained in pathogen-free conditions (12). Although they exhibited no clinical evidence of neurological disease, histological examination of brain and spinal cord showed increased numbers of microglia. The microglia appeared activated, having thick ramifications and strong CR3 immunoreactivity. However, the lack of expression of proinflammatory cytokines (7) and absence of overt neuropathology suggested that the microglia were not traditionally activated. This different functional phenotype is defined as primed. Priming readies the cell for an enhanced response to further stimuli: Indeed, Crry−/− mice respond much more vigorously than WT mice do to both local and peripheral challenges. Low-dose systemic administration of LPS greatly increased CNS expression of proinflammatory molecules, including neurotoxic cytokines, and of iNOS, which catalyses production of the neurotoxic radical NO.
Although the primary role of Crry is to regulate complement activation, it has also been implicated as a regulator of T-cell activity (30). We showed here that microglial priming in Crry−/− mice depends on and requires both C3 and fB; thus, priming is a consequence of uncontrolled activation of complement via the alternative pathway. Because the active products of complement are short-lived and exert most of their effects locally, it is likely that local complement activation in the CNS is the driver. In this context, it is important to note that local expression of C3 and the alternative pathway components, fB and fD, are increased in Crry−/− mice. Staining with a C3b/iC3b fragment-specific monoclonal antibody showed deposition of C3b/iC3b specifically on microglia, the major site of Crry expression in the CNS. C cleavage products activate microglia, either via receptors (C3a, C5a, or iC3b) or directly (membrane attack complex). C5a, membrane attack complex, and iC3b have all been implicated as microglial activators in various contexts (21, 31). The colocalization of C3b/iC3b and CR3 on microglia suggests that priming is triggered by iC3b/CR3 interaction.
Further support that Crry plays an important role comes from fH−/− mice, which showed a similar degree of C dysregulation but did not have primed microglia. In fH−/− mice, Crry will catalyze the rapid and complete breakdown of C3b to C3d, whereas, in Crry−/− mice, fH catalyses only the first cleavage of C3b, leading to the accumulation of iC3b (32) (Fig. S1). We propose a mechanism whereby local accumulation of iC3b engages CR3 and triggers microglial priming in the Crry−/− CNS.
Pretreatment of Crry−/− mice with sCR1, a strong inhibitor of complement activation, prevented the LPS-triggered switch from primed to activated microglia in Crry−/− mice and the consequent inflammatory mediator production. Thus, complement-derived mediators are also responsible for triggering this switch within the CNS. Further generation of iC3b might play a role, but downstream complement mediators, including C5a and membrane attack complex, previously implicated in LPS-triggered CNS injury, may also contribute (33).
The demonstration of complement-triggered microglial priming in Crry−/− mice led us to speculate that similar events may occur in human neurological disease. Indeed, in MS, microglia displaying features—enlarged, thickly ramified, IL-1β–negative, compatible with priming—were present in regions with normal-appearing myelin and intact axons around plaques. Primed microglia were clustered in close proximity to segments of nerve fibers positive for C3b/iC3b. Double-immunostaining for microglial markers and C3b/iC3b showed colocalization, similar to our findings in Crry−/− mice, suggesting an interaction between axonal C3b/iC3b and its receptor on microglia. Activated C3 was independently identified in association with ramified microglia in perilesional white matter bordering plaques (19, 20), IL-1β was up-regulated in the rim but not the perilesional white matter, whereas C3 was up-regulated in the rim and the perilesional white matter of chronic active lesions (18). Complement activation in MS suggests local dysregulation; in support of this, we have recently shown that plasma fH levels predict the clinical course of MS (34).
Microglial priming in MS might sensitize to disease exacerbations and progression triggered by diverse stimuli. To test this possibility in the Crry−/− model, EAE was induced. Disease was markedly accelerated and exacerbated in Crry−/− mice, despite the fact that C3 levels were very low (12), and C3 deficiency was reportedly protective in EAE in some but not all studies (35–37). Microglial activation is well recognized as an early pathogenic feature of EAE, and, indeed, inhibitors of microglial activation suppress disease (38). We showed that treatment of mice with sCR1 before onset of overt disease in EAE reversed the primed microglia and suppressed CNS inflammation; in contrast, treatment of naïve Crry−/− mice with sCR1 did not reverse microglial priming, implicating intrathecal complement activation as the driver. Primed microglia in Crry−/− likely respond more quickly and more vigorously to the immunization challenge; indeed, levels of the inflammatory cytokine IL-1β in Crry−/− spinal cord at death were markedly elevated compared with WT levels. We have previously shown that axonal injury, an early event in EAE, activates complement (39–41), providing the additional drive to microglial activation, production of neurotoxic mediators, and destruction of axons and myelin.
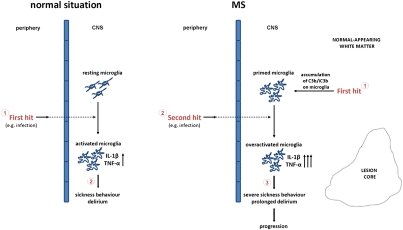
We propose a model of microglial priming based on the findings presented in this paper (Fig. 6). It follows that, if either microglial priming or the switch from the primed to the activated state can be inhibited, disease progression and exacerbation would be reduced. The choice of complement inhibitor is critical because complement plays vital roles in the fight against infections (9). Inhibitors that specifically target pathways of microglial priming by reducing accumulation of iC3b or interfering with the iC3b/CR3 interaction are excellent candidates. Because the BBB is likely to be intact in the primed CNS, and we show here that systemic C inhibition alone is not sufficient, such agents must be able to cross the barrier. Targeting the switch from primed to activated might be easier as the barrier is breached early in CNS inflammation (42, 43). Therapies could be timed and targeted to reduce the impact of systemic insults on primed microglia in those at risk, for example, by giving them as prophylactic cover for surgery or as prompt interventions in systemic infection.
Fig. 6.
Model of microglial priming. In the normal situation, a peripheral event, such as an infection, is the first hit to the brain (via mechanisms yet to be defined and discussed in the text), activating resting microglia to produce inflammatory mediators affecting neuronal function and producing sickness behavior or delirium. In pathological situations such as MS, a first hit (disease) primes microglia for overactivation in response to a second hit. In normal-appearing brain tissue, accumulation of C3b/iC3b on microglia, either secondary to preexisting neurodegeneration or caused by a primary (possibly genetic) dysregulation of C3 activation, is the first hit that primes microglial cells. Under these circumstances, a peripheral injurious event, such as an infection, is the second hit to the brain that overactivates primed microglia to produce an elevated amount of inflammatory mediators, determining severe sickness behavior and prolonged delirium leading to disease progression.
Materials and Methods
Animals.
C57BL/6 Crry−/− mice were generated as described (12). C57BL/6 fH−/− spinal cords were a gift from Matthew Pickering (Imperial College London, London). Spinal cords from Crry−/− mice backcrossed onto either C3 or fB deficiency (Crry−/−C3−/− and Crry−/−fB−/−, respectively) were a gift from John Atkinson (Washington University School of Medicine). For details on genotyping and housing of mice, see SI Materials and Methods.
Western Blot Analysis.
See SI Materials and Methods for details.
Characterization of CNS Changes.
See SI Materials and Methods for details.
Peripheral LPS Challenge.
See SI Materials and Methods for details.
Induction of EAE.
See SI Materials and Methods for details.
qPCR Analysis.
Total RNA was extracted from spinal cords and hippocampi and amplified with specific primer pairs (Table S3) as previously described (44). At least two independent experiments were performed in triplicate for each cDNA. Values are expressed as mean ± SD per group of mice.
Histological Analysis and Immunohistochemistry.
Fixed brains and spinal cords were sectioned (7 μm) and stained for inflammation (H&E), demyelination (LFB), and axonal loss (Palmgren's silver stain). Frozen O.C.T.-embedded spinal cord was transverse-sectioned (7 μm), acetone-fixed, blocked in 0.03% H2O2 followed by 10% normal goat serum, then incubated sequentially with primary antibody (Table S4) in PBS/BSA, appropriate secondary biotinylated antibody (Table S4) diluted in PBS/BSA, and peroxidase-labeled polystreptavidin (1:400 in PBS/BSA; Sigma-Aldrich). For fluorescence double-labeling, slides were incubated with the relevant primary antibodies (Table S4) diluted in PBS/BSA and sequentially with TRITC- or FITC-conjugated relevant Ig. Further details are in SI Materials and Methods.
Treatment of EAE and Naïve Crry−/− Mice with sCR1.
See SI Materials and Methods for details.
Quantitative Analysis of Immunohistochemistry.
See SI Materials and Methods for details.
MS Tissue Analyses.
See SI Materials and Methods for details.
Statistical Analysis.
See SI Materials and Methods for details.
Supplementary Material
Acknowledgments
We thank Dr. Phil Taylor for assistance with the LPS experiments, Dr. Kees Fluiter for assistance with the in situ hybridization experiment, and Prof. Matthew Pickering for kindly providing the fH−/− mouse tissue. This work was supported by Wellcome Trust Value in People Award 084542 (to V.R.), Wellcome Trust Programme Grant 068590 (to B.P.M.), Medical Research Council New Investigator Grant G0700102 (to R.M.D.), and National Institutes of Health Grant 2 R01 AI041592-13A1 (to X.W.).
Footnotes
Conflict of interest statement: V.R. and F.B. are shareholders of Regenesance BV, a biopharmaceutical spin-off company that develops therapeutics that affect complement activation.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1111924109/-/DCSupplemental.
References
- 1.Holmes C, et al. Systemic infection, interleukin 1β, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmes C, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology. 2009;73:768–774. doi: 10.1212/WNL.0b013e3181b6bb95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sly LM, et al. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer's disease. Brain Res Bull. 2001;56:581–588. doi: 10.1016/s0361-9230(01)00730-4. [DOI] [PubMed] [Google Scholar]
- 5.Brugg B, et al. Inflammatory processes induce β-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci USA. 1995;92:3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham C, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Combrinck MI, Perry VH, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112(1):7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 9.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davoust N, Nataf S, Holers VM, Barnum SR. Expression of the murine complement regulatory protein Crry by glial cells and neurons. Glia. 1999;27(2):162–170. doi: 10.1002/(sici)1098-1136(199908)27:2<162::aid-glia6>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Molina H, et al. Distinct receptor and regulatory properties of recombinant mouse complement receptor 1 (CR1) and Crry, the two genetic homologues of human CR1. J Exp Med. 1992;175(1):121–129. doi: 10.1084/jem.175.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruseva MM, et al. Crry deficiency in complement sufficient mice: C3 consumption occurs without associated renal injury. Mol Immunol. 2009;46:803–811. doi: 10.1016/j.molimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, et al. Membrane protein Crry maintains homeostasis of the complement system. J Immunol. 2008;181:2732–2740. doi: 10.4049/jimmunol.181.4.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering MC, et al. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 15.Ransohoff RM, Perry VH. Microglial physiology: Unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 16.Mastellos D, et al. Novel monoclonal antibodies against mouse C3 interfering with complement activation: Description of fine specificity and applications to various immunoassays. Mol Immunol. 2004;40:1213–1221. doi: 10.1016/j.molimm.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Wekerle H, Lassmann H. The immunology of inflammatory demyelinating disease. In: Compston A, et al., editors. McAlpine's Multiple Sclerosis. 4th Ed. Philadelphia: Churchill Livingstone; 2006. pp. 491–555. [Google Scholar]
- 18.Koning N, Bö L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- 19.Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65(1):32–46. doi: 10.1002/ana.21524. [DOI] [PubMed] [Google Scholar]
- 20.Prineas JW, et al. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001;50:646–657. doi: 10.1002/ana.1255. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths MR, Neal JW, Fontaine M, Das T, Gasque P. Complement factor H, a marker of self protects against experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4368–4377. doi: 10.4049/jimmunol.0800205. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, et al. Inhibition of glial cell activation ameliorates the severity of experimental autoimmune encephalomyelitis. Neurosci Res. 2007;59:457–466. doi: 10.1016/j.neures.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Mead RJ, et al. Deficiency of the complement regulator CD59a enhances disease severity, demyelination and axonal injury in murine acute experimental allergic encephalomyelitis. Lab Invest. 2004;84(1):21–28. doi: 10.1038/labinvest.3700015. [DOI] [PubMed] [Google Scholar]
- 24.Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer's disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol. 1997;94(1):1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- 25.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosczyk HA, Sparkman NL, Johnson RW. Neuroinflammation and cognitive function in aged mice following minor surgery. Exp Gerontol. 2008;43:840–846. doi: 10.1016/j.exger.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Muiswinkel FL, Veerhuis R, Eikelenboom P. Amyloid β protein primes cultured rat microglial cells for an enhanced phorbol 12-myristate 13-acetate-induced respiratory burst activity. J Neurochem. 1996;66:2468–2476. doi: 10.1046/j.1471-4159.1996.66062468.x. [DOI] [PubMed] [Google Scholar]
- 28.Hayes MP, Freeman SL, Donnelly RP. IFN-γ priming of monocytes enhances LPS-induced TNF production by augmenting both transcription and MRNA stability. Cytokine. 1995;7:427–435. doi: 10.1006/cyto.1995.0058. [DOI] [PubMed] [Google Scholar]
- 29.Moisse K, Welch I, Hill T, Volkening K, Strong MJ. Transient middle cerebral artery occlusion induces microglial priming in the lumbar spinal cord: A novel model of neuroinflammation. J Neuroinflammation. 2008;5:29. doi: 10.1186/1742-2094-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiménez-Periañez A, et al. Complement regulatory protein Crry/p65-mediated signaling in T lymphocytes: Role of its cytoplasmic domain and partitioning into lipid rafts. J Leukoc Biol. 2005;78:1386–1396. doi: 10.1189/jlb.1104642. [DOI] [PubMed] [Google Scholar]
- 31.Woodruff TM, Ager RR, Tenner AJ, Noakes PG, Taylor SM. The role of the complement system and the activation fragment C5a in the central nervous system. Neuromolecular Med. 2010;12(2):179–192. doi: 10.1007/s12017-009-8085-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim YU, et al. Mouse complement regulatory protein Crry/p65 uses the specific mechanisms of both human decay-accelerating factor and membrane cofactor protein. J Exp Med. 1995;181(1):151–159. doi: 10.1084/jem.181.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crane JW, Buller KM. Systemic blockade of complement C5a receptors reduces lipopolysacharride-induced responses in the paraventricular nucleus and the central amygdala. Neurosci Lett. 2007;424(1):10–15. doi: 10.1016/j.neulet.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Ingram G, et al. Complement regulator factor H as a serum biomarker of multiple sclerosis disease state. Brain. 2010;133:1602–1611. doi: 10.1093/brain/awq085. [DOI] [PubMed] [Google Scholar]
- 35.Szalai AJ, Hu X, Adams JE, Barnum SR. Complement in experimental autoimmune encephalomyelitis revisited: C3 is required for development of maximal disease. Mol Immunol. 2007;44:3132–3136. doi: 10.1016/j.molimm.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calida DM, et al. Cutting edge: C3, a key component of complement activation, is not required for the development of myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis in mice. J Immunol. 2001;166:723–726. doi: 10.4049/jimmunol.166.2.723. [DOI] [PubMed] [Google Scholar]
- 37.Nataf S, Carroll SL, Wetsel RA, Szalai AJ, Barnum SR. Attenuation of experimental autoimmune demyelination in complement-deficient mice. J Immunol. 2000;165:5867–5873. doi: 10.4049/jimmunol.165.10.5867. [DOI] [PubMed] [Google Scholar]
- 38.Wang XS, et al. Idazoxan attenuates spinal cord injury by enhanced astrocytic activation and reduced microglial activation in rat experimental autoimmune encephalomyelitis. Brain Res. 2009;1253:198–209. doi: 10.1016/j.brainres.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 39.Ramaglia V, King RH, Morgan BP, Baas F. Deficiency of the complement regulator CD59a exacerbates Wallerian degeneration. Mol Immunol. 2009;46:1892–1896. doi: 10.1016/j.molimm.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Ramaglia V, et al. Soluble complement receptor 1 protects the peripheral nerve from early axon loss after injury. Am J Pathol. 2008;172:1043–1052. doi: 10.2353/ajpath.2008.070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramaglia V, et al. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J Neurosci. 2007;27:7663–7672. doi: 10.1523/JNEUROSCI.5623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clawson CC, Hartmann JF, Vernier RL. Electron microscopy of the effect of gram-negative endotoxin on the blood-brain barrier. J Comp Neurol. 1966;127:183–198. doi: 10.1002/cne.901270204. [DOI] [PubMed] [Google Scholar]
- 43.Nishioku T, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood–brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol. 2009;29:309–316. doi: 10.1007/s10571-008-9322-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donev RM, Cole DS, Sivasankar B, Hughes TR, Morgan BP. p53 regulates cellular resistance to complement lysis through enhanced expression of CD59. Cancer Res. 2006;66:2451–2458. doi: 10.1158/0008-5472.CAN-05-3191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.