Abstract
Chronic obstructive pulmonary disease (COPD) will soon be the third most common cause of death globally. Despite smoking cessation, neutrophilic mucosal inflammation persistently damages the airways and fails to protect from recurrent infections. This maladaptive and excess inflammation is also refractory to glucocorticosteroids (GC). Here, we identify serum amyloid A (SAA) as a candidate mediator of GC refractory inflammation in COPD. Extrahepatic SAA was detected locally in COPD bronchoalveolar lavage fluid, which correlated with IL-8 and neutrophil elastase, consistent with neutrophil recruitment and activation. Immunohistochemistry detected SAA was in close proximity to airway epithelium, and in vitro SAA triggered release of IL-8 and other proinflammatory mediators by airway epithelial cells in an ALX/FPR2 (formyl peptide receptor 2) receptor-dependent manner. Lipoxin A4 (LXA4) can also interact with ALX/FPR2 receptors and lead to allosteric inhibition of SAA-initiated epithelial responses (pA2 13 nM). During acute exacerbation, peripheral blood SAA levels increased dramatically and were disproportionately increased relative to LXA4. Human lung macrophages (CD68+) colocalized with SAA and GCs markedly increased SAA in vitro (THP-1, pEC50 43 nM). To determine its direct actions, SAA was administered into murine lung, leading to induction of CXC chemokine ligand 1/2 and a neutrophilic response that was inhibited by 15-epi-LXA4 but not dexamethasone. Taken together, these findings identify SAA as a therapeutic target for inhibition and implicate SAA as a mediator of GC-resistant lung inflammation that can overwhelm organ protective signaling by lipoxins at ALX/FPR2 receptors.
Keywords: resolution, leukocyte activation, G protein-coupled receptor, innate immunity
Airway mucosal inflammation is an essential effector arm of innate host defense, but the principal determinants of its intensity and persistence remain poorly understood. In particular, molecular aberrations in diseases where inflammation is intractable and resolution is impaired have not been well delineated. Understanding these control mechanisms may help to redress disease states, such as chronic obstructive pulmonary disease (COPD), an incurable fatal lung disease where glucocorticoid (GC)-insensitive inflammation (reviewed in ref. 1) persists, despite smoking cessation (2). COPD is already the fifth leading cause of death worldwide and, given its long disease latency, its impact will continue to rise in coming decades (3). The primary cause of COPD is chronic exposure to cigarette smoke, which is known to permanently alter the lung transcriptome (4). Because the underlying cause of persistent innate immune activation remains poorly understood in COPD, there is an urgent need to identify new insights into the pathobiology of COPD that will lead to novel therapeutic strategies.
The lead member of a new class of endogenous proresolving mediators is lipoxin A4 (LXA4) (5), which is generated in the lung during cell–cell interactions including in COPD (6), when infiltrating leukocytes interact with airway epithelial cells (reviewed in ref. 7). LXA4 interacts with ALX/FPR2 (formyl peptide receptor 2) receptors to transduce anti-inflammatory signals that block neutrophil activation and recruitment as part of a resolution program for airway inflammation. For example, LXA4 opposes the actions of the 5-lipoxygenase–derived eicosanoid leukotriene B4 (LTB4), which is a potent agonist for neutrophil transmigration and activation (reviewed in ref. 5). Of interest, serum amyloid A (SAA) can also interact with ALX/FPR2 receptors (8) and, in sharp contrast to LXA4, stimulates neutrophil chemotaxis and functional responses (9, 10). SAA is a hepatic acute-phase protein, the serum levels of which strongly predict the intensity of acute exacerbations of COPD (AECOPD) (11) that are triggered by airway infection and associated with further increases in inflammation (12). Thus, as SAA is increased in COPD, we reasoned that if it were present in the lung it might account for persistent lung inflammation.
Here, we have shown that SAA is present in inflamed lungs, elevated in AECOPD, and is closely related to neutrophilic activation in COPD airways. During AECOPD, levels of circulating SAA were substantially greater than either of the anti-inflammatory mediators LXA4 or annexin A1 (ANXA1), a steroid-inducible anti-inflammatory protein. In vitro, SAA potently activated proinflammatory mediator release from airway epithelial cells in an ALX/FPR2-dependent manner. In vivo, SAA elicited a robust neutrophilic response that was counteracted by administration of the ALX/FPR2 anti-inflammatory ligand 15-epi-LXA4. Importantly, SAA's proinflammatory actions appeared to underlie the GC refractory nature of neutrophil-mediated inflammation in COPD (and potentially other diseases of chronic neutrophilic inflammation), as GCs, the most common anti-inflammatory drug in current disease management, induced extrahepatic SAA expression and failed to suppress extrahepatic SAA production. These data identify SAA as a candidate pathogenic mediator of lung inflammation and mechanism for defective resolution, raising the possibility of SAA and ALX/FPR2 receptors as previously unexplored therapeutic targets in COPD.
Results
SAA in COPD Bronchoalveolar Lavage Fluid Is Associated with IL-8 and Neutrophil Activation.
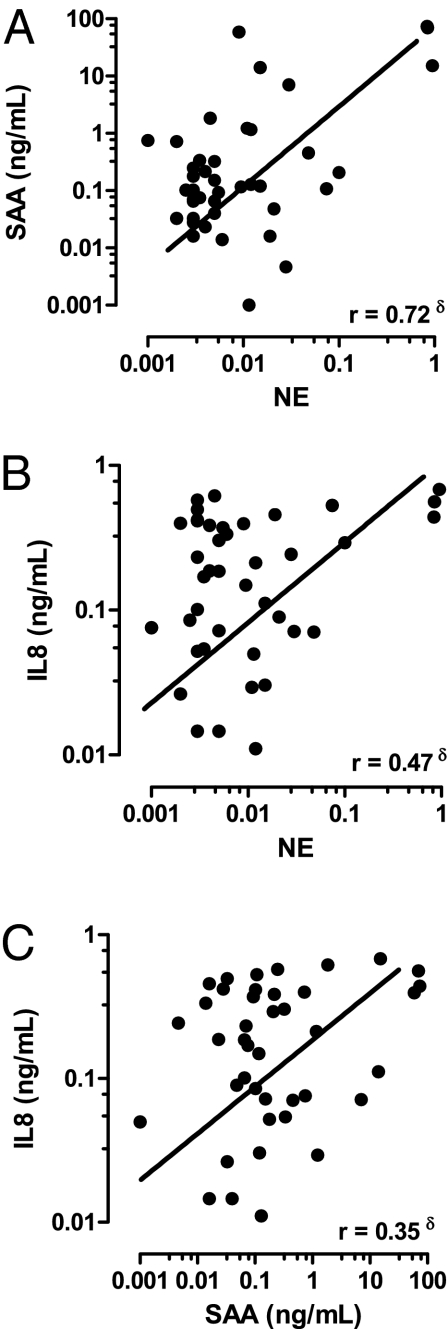
The relationship between airway SAA and neutrophilic inflammation in COPD was explored by measuring SAA and neutrophil elastase (NE) activity, a marker of neutrophil activation, in bronchoalveolar lavage fluid (BALF) archived from subjects with stable (nonexacerbating) COPD. BALF SAA levels positively correlated with NE (P = 0.001, r = 0.72,) (Fig. 1A and Table S1). In the same cohort, NE also associated with IL-8, an independent and major neutrophil chemotactic factor (P = 0.002, r = 0.47) (Fig. 1B) and a significant association was observed between SAA and IL-8 levels (Fig. 1C) (P = 0.03, r = 0.35). SAA levels in BALF collected during stable disease were not significantly different across the Global Initiative for Obstructive Lung Disease disease severity stages.
Fig. 1.
Relationship between lung SAA, IL-8 and neutrophil activity. Correlation between secreted (A) SAA and NE activity; (B) IL-8 and NE, and SAA and IL-8 were determined in BALF from COPD subject (n = 41). (δP < 0.05, Pearson correlation).
SAA Promotes Mucosal Epithelial Inflammatory Responses via ALX/FPR2.
In control lung, modest ALX/ FPR2 staining was observed in airway epithelium (Fig. 2A, Upper). In COPD lung, ALX/FPR2 expression displayed more prominent apical and basolateral decoration of the airway mucosal lining (Fig. 2A, Lower), implicating the epithelium as a potentially major target for SAA. Staining for SAA in the same specimens demonstrated widespread and intense SAA staining that was prominent within the submucosa in close proximity to the basolateral epithelium.
Fig. 2.
ALX/FPR2 is expressed in COPD lungs and is a functional receptor for SAA in human airway epithelial cells. (A) Representative Isotype (ISO), ALX/FPR2 (ALX) and SAA staining on serial Control (Upper) and COPD sections (Insets: 100× magnification). ALX/FPR2 inset demonstrates prominent apical and basolateral (BL) epithelial ALX/FPR2 expression in COPD. MCP-1 (B) and GM-CSF (C) levels in cell-free supernatants were measured in null- A549 cells (open bar) and rhALX- A549 cells (black bar) treated with Vehicle (VEH) or recombinant SAA (1 mg/mL) for 24 h. rhALX- A549 cells were preincubated with LXA4 (10−8 M or 10−7 M) (D) or 15-epi-LXA4 (10−7 M) (E) for 30 min followed by recombinant SAA (10−10 M to 10−7 M, 24 h). Release of IL-8 was measured in cell-free supernatants by ELISA (see SI Methods). The data are expressed as a percentage of the maximal SAA-stimulated IL-8 release and the mean ± SEM for n = 5–7 measured in duplicate, #P < 0.05.
Lung epithelial A549 (null-A549; deficient for ALX/FPR2) and hALX-A549 cells genetically engineered to express the receptor were exposed to recombinant SAA. In the absence of ALX/FPR2, SAA did not induce significant amounts of monocyte chemoattractant protein-1 (MCP-1) or GM-CSF (Fig. 2 B and C). In the presence of ALX/FPR2, SAA markedly increased both MCP-1 (Fig. 2B) and GM-CSF levels (Fig. 2C). In hALX-A549 cells, SAA also induced a concentration dependent increase in IL-8 (Fig. 2D). As little as 10−9 M SAA, led to 3.79 ± 1.97 ng IL-8/mL, and 50 ng SAA/mL (an SAA concentration found in COPD BALF) (Fig. 1A) resulted in a marked increase to 37.47 ± 7.92 ng IL-8/mL (Fig. 2D). The EC50 of SAA for hALX-A549 generated IL-8 was 8.5 nM. In addition to A549 cells, SAA induced production of the inflammatory mediators MCP-1, GM-CSF, and IL-8 in the bronchial epithelial BEAS-2B cell line and primary human COPD epithelial cells (Figs. S1 and S2).
Because ALX/FPR2 engagement by LXs blocks proinflammatory mediator release by injured airway epithelial cells (13), the impact of LXA4 and 15-epi LXA4 on SAA-initiated IL-8 release was determined. LXA4-exposed cells demonstrated a rightward shift in the SAA concentration response with depressed maxima (Fig. 2D). Both LXA4 and 15-epi-LXA4 were equipotent at 10−7 M, with similar IC50 values (25.74 nM for LXA4 and 24.83 nM for 15-epi-LXA4) for SAA-mediated IL-8 release (Fig. 2 D and E). However, the rightward shift and depressed SAA maxima caused by both LXs (Fig. 2 D and E), precluded simple competitive antagonism at a single binding site as a mechanism for inhibition of IL-8 release. We therefore applied classic Schild regression analysis, which yielded a regression slope less than unity (0.34) with an estimated pA2 of 13 nM; indicating negative cooperative binding secondary to allosteric modulation as the basis for antagonism.
SAA Is Disproportionately Expressed Relative to LXs During an AECOPD in Humans.
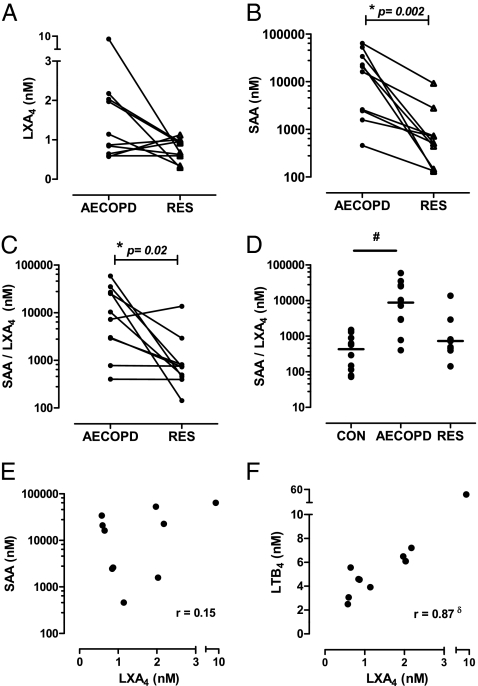
Because both SAA and LXA4 can serve as endogenous ALX/FPR2 ligands, the relative time course of their systemic formation was determined prospectively from the same individual during AECOPD and Resolution. There was a trend for increased plasma LXA4 concentrations during AECOPD (2.0 ± 0.79 nM) vs. Resolution (0.80 ± 0.09 nM) (Fig. 3A), with 7 of 10 matching samples displaying increased LXA4 levels at AECOPD. SAA concentrations rose sharply and were significantly elevated during AECOPD relative to Resolution (Fig. 3B) [18,595 ± 7,112 nM (AECOPD) vs. 510 ± 800 nM (Resolution), P < 0.05 for n = 10]. As a consequence, the amount of SAA relative to LXA4 was significantly increased during the acute phase of an exacerbation (Figs. 3C) [8,791 ± 6,092 SAA/LXA4 (AECOPD) vs. 735 ± 1,305 SAA/LXA4 (Resolution), P < 0.05 for n = 10]. In addition, SAA/LXA4 ratios were determined from a control cohort with normal lung function (Fig. 3D). The SAA/LXA4 ratio for Control (432 ± 166 SAA/LXA4) was even lower than AECOPD resolution (735 ± 1,305 SAA/LXA4), although this difference did not reach statistical significance at this sample size (P = 0.25). No association between LXA4 and SAA levels (Fig. 3E) (r = 0.152, P = 0.68) was observed during AECOPD, whereas LXA4 and the proinflammatory 5-lipoxygenase-derived eicosanoid LTB4 were significantly correlated (Fig. 3F) (r = 0.87, P = 0.002). Because ANXA1 can also oppose neutrophilic inflammation via its interaction with ALX/FPR2 receptors (14), plasma ANXA1 levels were determined in matched samples. Levels for ANXA1 increased during an AECOPD (Fig. S3A). As observed for LXA4, the levels of SAA relative to ANXA1 were also significantly elevated during an AECOPD (Fig. S3B). In addition, an inverse relationship between SAA and ANXA1 was observed during AECOPD (Fig. S3C).
Fig. 3.
Relationship between SAA and LXA4 during AECOPD. (A) LXA4 and (B) SAA concentration (nM) in matching blood samples from COPD subjects during (i) the acute phase of an exacerbation (AECOPD) and at (ii) clinical recovery (Resolution) (n = 10, *P < 0.05 by Wilcoxon-matched paired t test). (C) The relative ratio of SAA/LXA4 was determined for the matching AECOPD and recovery samples. (D) The SAA/LXA4 ratio was also determined for a control cohort with normal lung function (#P < 0.05 by ANOVA vs. Control). The association of LXA4 with (E) SAA and (F) LTB4 was compared during the AECOPD phase, demonstrating a significant correlation with LTB4 only (δP = 0.002, r = 0.87 Pearson correlation).
SAA Elicits Airway Neutrophilic Inflammation That Is Inhibitable by 15-epi-LXA4.
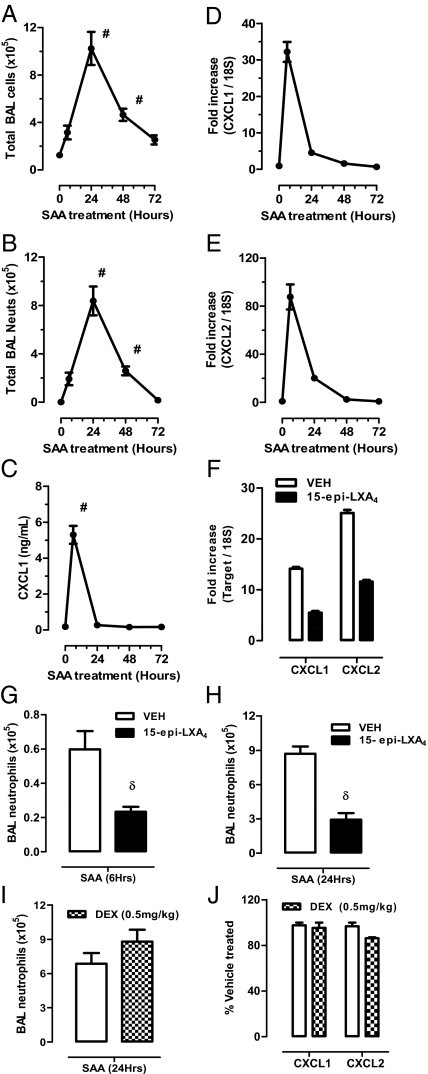
To determine if the actions of SAA were opposed by LXs in vivo, a murine airway challenge model was established. Intranasal SAA (2 μg) challenge elicited an acute inflammatory response peaking at 24 h that was neutrophilic in nature (8.4 ± 1.2 × 105 BALF neutrophils at 24 h) (Fig. 4 A and B). A significant increase in secreted CXC chemokine ligand (CXCL) 1 in BALF was seen during this early phase (6 h) (Fig. 4C). An increase in transcript expression of the neutrophil chemokines, CXCL1 and CXCL2 (the murine homologs of KC and MIP-2), as assessed by TaqMan quantitative PCR (qPCR) (Fig. 4 D and E), was also observed. Of note, secreted CXCL2 in BALF was not increased above control levels, which may indicate rapid consumption of this neutrophil chemokine at the site of SAA-mediated inflammation. The murine ALX/FPR2 receptor pathway was targeted in vivo by locally delivering 15-epi-LXA4 concurrently with SAA, which reduced CXCL1 and CXCL2 expression by 61% and 54%, respectively (Fig. 4F). Recruitment of BALF neutrophils was similarly reduced by 15-epi-LXA4 at early (6 h) and peak (24 h) time points, respectively (Fig. 4 G and H). In contrast to 15-epi-LXA4, when dexamethasone (DEX) (0.5 mg/kg, i.p.) was given 2 h before SAA challenge, there was no significant change in airway neutrophils (Fig. 4I). In addition, DEX did not inhibit SAA induction of CXCL1/2 expression (Fig. 4J). As a control for acute inflammation, the same dose of DEX displayed a 49% reduction in LPS (1 μg, i.n.) triggered BALF neutrophilia (LPS; 7.0 ± 0.7 × 105 vs. DEX-LPS 3.5 ± 0.5 × 105, P < 0.05). To evaluate whether local SAA effects were also mediated by LTB4, levels of LTB4 in BALF were measured. SAA did not induce measureable local LTB4 production (EIA, Cayman Chemicals; detection limit = 13 pg/mL), and treatment with 15-epi-LXA4 did not significantly change LTB4 levels.
Fig. 4.
Local SAA delivery promotes lung inflammation and CXCL1/2 that is suppressed by 15-epi-LXA4. Mice were challenged with SAA (2 μg) via intranasal administration and at the indicated timepoints (h) were killed. (A) Total BAL cells and differential analysis was used to determine (B) neutrophil BAL numbers. (C) Secreted CXCL1 concentration in BALF was determined by ELISA. (D and E) CXCL1 and CXCL2 mRNA in response to SAA and the effect of concurrent 15-epi-LXA4 (4 μg) on CXCL1/2 expression (F) and neutrophil recruitment at 6 h (G) and 24 h (H) were determined by TaqMan QPCR. (I) In a separate experiment, mice were pretreated with DEX (0.5 mg/kg i.p.) for 2 h before intranasal SAA challenge (1 μg, 24 h). (J) The effect of DEX on SAA-mediated CXCL1/2 expression in lung tissue was determined by qPCR. Data are presented at a percentage of the SAA+VEH-treated group. Results are expressed as mean ± SEM (n = 4–8) where #P < 0.05 by ANOVA and δP < 0.05 by unpaired t test.
SAA Localized with Macrophages in COPD Lungs.
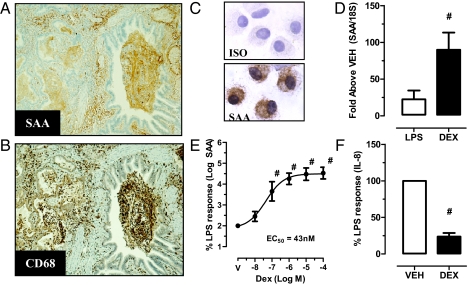
The presence and cellular distribution of SAA was assessed immuno-histochemically in resected human lung tissue (Table S2). Control subjects were negative for SAA in seven of eight lung specimens, with one specimen displaying moderate staining. In contrast, a moderate staining pattern within the parenchyma was observed in 7 of 22 COPD lung specimens. No reactivity was observed in 9 of 22 lung specimens with clinically stable comorbid COPD. Intense SAA immunoreactivity was observed in 6 of 22 COPD specimens that were diffuse in nature and localized to the submucosa, vascular, and bronchoalveolar compartments (Fig. 5A). CD68+ macrophages were present in BALF and lung parenchyma (Fig. 5B) and colocalized with SAA-positive regions. Because SAA can serve as a precursor for amyloid protein AA serial sections were stained with Congo red. No sample showed observable birefringence, indicating the absence of insoluble AA amyloid fibril deposition. Smoking history was not significantly different across the three SAA staining profiles [No SAA: mean 47 pack years (10–140), moderate SAA: 59 pack years (30–120), and intense SAA: 36 pack years (25–48)]. Additionally FEV1% predicted was not significantly different across the staining profiles [No SAA: mean 79% (54–89), moderate SAA 77% (52–91), and intense SAA 78% (66–85)].
Fig. 5.
SAA is elevated in COPD airways and colocalizes with BAL and tissue macrophages. (A) A representative specimen of Intense SAA staining in COPD. (B) Serial sections were immunostained for CD68+ macrophages (50× magnification). (C) SAA expression in bronchoalveolar COPD macrophages was detected by immunocytochemistry. (D) SAA expression was measured by qPCR in THP-1 cells treated with LPS (1 μg/mL) or DEX (10−6 M) for 24 h. (E) The combination of DEX and LPS was next assessed and normalized to LPS alone, which was designated as 100%. Note that because of the large synergistic effect of DEX and LPS (35,000% at 10−6 M DEX), the y axis was log-transformed. The DEX concentration dose–response curve for LPS treated THP-1 cells generated an EC50 of 43 nM. (F) IL-8 levels were also measured at the DEX dose (10−6 M), causing maximal induction of SAA, demonstrating effective suppression of IL-8. The data are expressed as a percentage of the LPS response and the mean ± SEM for n = 4–5, #P < 0.05.
A positive correlation between SAA mRNA and protein was found in BALF (P = 0.01, r = 0.83) (Table S1), implicating alveolar macrophages as a major candidate for production of BALF SAA. BALF cytospot immunostaining confirmed that alveolar macrophages from COPD subjects were positive for SAA, with prominent staining observed in the cytoplasmic region (Fig. 5C). The regulation of SAA expression was further investigated in human macrophages (THP-1 cell line) exposed to bacterial LPS (1 μg/mL), which is abundant in the airways during AECOPD (15). LPS induced THP-1 SAA mRNA levels by 25 ± 12-fold (Fig. 5D). DEX also induced SAA mRNA by 90 ± 23-fold, and synergistically increased LPS-induced SAA expression in a dose-dependent manner with 10−6 M DEX plus LPS, resulting in a 2,564 ± 754-fold increase. The calculated half maximal concentration (EC50) was 43 nM (Fig. 5E). As a control, DEX (10−6 M) effectively decreased LPS-induced IL-8 release by 76% in the same incubations (Fig. 5F). To further investigate whether innate stimuli relevant to COPD and infectious AECOPD can directly promote de novo local synthesis of SAA in airway tissue, SAA mRNA in whole lung from mice treated under three separate conditions were determined (Fig. S4). BALB/c mice subchronically exposed to cigarette smoke for 4 d displayed a 34-fold increase in SAA expression (Fig. S4A). Intranasal administration of LPS (10 μg per mouse) induced a 350-fold peak increase in SAA 24 h following challenge (Fig. S4B). In addition, intranasal administration of influenza (HKx31) transiently induced SAA expression, peaking at day 5 postinfection (17-fold) (Fig. S4C).
The acute phase SAA isoforms (SAA1 and SAA2) are 95% homologous and indistinguishable by qPCR, whereas mouse SAA3 (71% sequence identify with SAA1) is a pseudogene in humans (16). The in vivo expression of SAA isoforms was assessed in murine lung tissue following LPS challenge in the presence of a clinically used inhaled GC, budesonide (Fig. S5). Budesonide (intranasally) induced SAA1/2 expression ∼fivefold and LPS induced SAA1/2 by greater than 20-fold (Fig. S5B). Airway administration of budesonide (10 μg) 6 h after LPS (3 μg) did not inhibit peak neutrophilic airway inflammation (Fig. S5A) or SAA1/2 lung transcript expression (Fig. S5B), whereas SAA3 was reduced by 50% (Fig. S5C). Secreted SAA in BALF was increased by budesonide, in particular after concomitant LPS challenge for 24 h (Fig. S5D). In contrast, the neutrophil agonists CXCL1, CXCL2, IL-17A) and G-CSF were inhibited by at least 50% by budesonide (Fig. S5 G–J). LPS-initiated increases in ALX/FPR2 expression were also decreased by budesonide (Fig. S5E). ANXA1 expression was not significantly changed by either LPS or budesonide (Fig. S5F).
Discussion
In this study, we have demonstrated that SAA is a potent and highly efficacious proinflammatory mediator in the lung that is markedly up-regulated in exacerbations and produced locally, in response to diverse innate stimuli. SAA was also intimately associated with neutrophil accumulation and activation in clinical samples and experimental models. Whereas LXA4 and 15-epi-LXA4 can suppress SAA actions via a noncompetitive interaction of an allosteric type, the very high levels of SAA in COPD may overwhelm this defense mechanism. Induced sputum from subjects with COPD contain both LXA4 and 15-epi-LXA4 (6), and we demonstrate SAA levels in excess of 50 ng/mL in COPD BALF. Furthermore, GCs, the most effective current agents to suppress mucosal inflammation, increased rather than suppressed SAA production. Although SAA has been shown to target other receptor complexes, including Scavenger receptor class B type I (SR-BI) (17), receptor for advanced glycation end-products (RAGE) (18), Toll-like receptor (TLR) 4 (19), and TLR2 (20), here we provide evidence that 15-epi-LXA4 can ameliorate the adverse effects of SAA in vivo. Because bioactive stable analogs of 15-epi-LXA4 have been prepared and proven efficacious in murine models of lung inflammation (21), the present translational findings are unique in their demonstration of a clinical indication for LX stable analogs in COPD.
In addition to COPD, severe asthma is also characterized by GC refractory and persistent inflammation. Although the airway neutrophila of severe asthma appears to stem, in part, from increased proinflammatory leukotriene production and a defect in anti-inflammatory LX generation (22), AECOPD was associated with increased LXA4 without significant change in the relative metabolic fate of arachidonic acid to LTB4 and LXA4. The majority (7 of 10) of acute episodes were associated with an increase in LXA4 levels during an AECOPD. This heterogeneous response may reflect alternative resolution phenotypes previously described, where early and late resolvers were discriminated on the basis of differences in temporal LX generation (23). During AECOPD, high amounts of circulating SAA were also significantly greater than ANXA1, which can interact with ALX/FPR2 receptors to block neutrophil diapadesis (14). Delivery of SAA to the lungs in vivo proved sufficient to facilitate airway neutrophilia. This response was inhibited by concurrent instillation of equal amounts of 15-epi-LXA4 with SAA. LXA4 is an autacoid that is rapidly generated and rapidly inactivated at local sites of inflammation, so only a small fraction of the intranasal exogenous lipoxin would be available to the lower respiratory tract. The natural isomer 15-epi-LXA4 displays a modestly prolonged half-life (∼twofold) (reviewed in ref. 24), but it is also rapidly inactivated in the lung. Thus, intranasal administration of SAA and 15-epi-LXA4 was performed to demonstrate proof-of-principle that SAA-initiated acute lung inflammation can be decreased by LX signaling, and proof of therapeutic utility was demonstrated. The findings presented here demonstrate that blocking SAA in vivo is achievable with ALX/FPR2-targeted compounds in 10- to 100-times excess doses. Given that there are numerous precedents in G protein-coupled receptor pharmacology where it has been possible to make drugs with up to 1,000-fold higher affinity than the endogenous agonist, it is very likely that clinically useful drugs exploiting this mechanism can be created. Taken together, these findings suggest that the relative amounts of SAA and LXs can modulate the neutrophil-rich airway inflammation in COPD and point to a pivotal role for ALX/FPR2 receptor signaling in airway epithelial cells for integrating the host functional response to these mediators.
The distribution of tissue SAA was widely dispersed in most specimens, and also concordantly localized with ALX/FPR2 receptors that were preferentially distributed along the epithelium, including the basolateral margin; placing SAA and ALX/FPR2 in close proximity. Here, ALX/FPR2 expression markedly enhanced SAA-mediated mucosal inflammatory responses by human airway epithelial cells. Of note, genetic deletion of ALX/FPR2 in mice identified a critical role for this receptor in SAA-mediated leukocyte chemotaxis (25). ALX/FPR2 expression was prominent at the apical and basolateral epithelial surfaces of COPD airways. Bronchial cell injury induces ALX/FPR2 epithelial expression, in part, via prostaglandin E2 (PGE2) (13) and the staining pattern in COPD airways is consistent with increased PGE2 levels observed in COPD airways (26). Of interest, GCs can up-regulate ALX/FPR2 expression in leukocytes (27), suggesting potential amplification of proinflammatory signaling in conjunction with GC-induced increases in SAA.
Although extrahepatic SAA production by tissue macrophages has recently been recognized in other chronic inflammatory conditions, notably rheumatoid arthritis (28, 29) and atherosclerosis (30), the findings herein point to SAA as a major disease locus in lung disease, with CD68+ macrophages as a potentially important source for airway SAA (but does not exclude epithelium, fibroblasts or other cells as extrahepatic contributors). Because SAA expression was markedly induced by DEX, SAA may prove to be a major candidate for GC-induced neutrophil survival. Annexin A1 (Lipocortin 1) is another prominent GC-inducible protein, but Annexin A1 and related degradative peptides serve as ALX/FPR2 ligands that, like LXs, transduce anti-inflammatory signals for neutrophils (14). The marked induction of SAA by GCs and in AECOPD would likely also block ALX/FPR2 signaling by Annexin A1 and related anti-inflammatory peptides. Our results are unique in their characterization of de novo SAA isoform expression in inflamed lungs and demonstrate that budesonide, a clinically used GC, increased SAA1/2 lung expression. These findings are consistent with SAA1 containing a functional glucocortoid response element (GRE) in its promoter region that is induced by GCs through a GC receptor (GR)-dependent manner (31, 32). Taken together, these findings may help to explain the clinical observations that inflammation in COPD is refractory to inhaled GCs (33).
In summary, our results demonstrate that SAA and LXs can regulate airway inflammatory responses in vitro and in vivo via interactions with ALX/FPR2 receptors, and that proinflammatory SAA is disproportionately expressed relative to proresolving LXs in AECOPD. Because SAA is associated with AECOPD severity (11), the imbalance during severe events may represent a fundamental mechanism for prolonging and intensifying inflammation that is associated with AECOPD-related hospitalization, respiratory failure, and death. Findings presented here suggest that harnessing natural LX counter regulatory pathways with stable LXA4 analogs holds promise as a unique GC-independent therapeutic approach to reducing SAA-mediated inflammation in COPD and dampening the severity of episodes of AECOPD.
Methods
Full details are provided in SI Methods. Briefly, there were three separate patient cohorts involving a total of 91 patients, as summarized in Table S3. See Table S4 for AECOPD characteristics.
Supplementary Material
Acknowledgments
We thank Yilin Zhang, Maria Bishara, and Huei Jiunn Soew (University of Melbourne) for their technical assistance, and Philip Antippa (Royal Melbourne Hospital, Melbourne, Australia) for providing lung resection specimens. The work was funded in part by National Health and Medical Research Council of Australia (S.B.) and by Grants HL68669 and HL090927 (to B.D.L.).
Footnotes
Conflict of interest statement: B.D.L. is a co-inventor on patents on lipoxins in airway disease that are assigned to Brigham and Women's Hospital and licensed for clinical development.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109382109/-/DCSupplemental.
References
- 1.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 2.Willemse BW, et al. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 3.Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD) Lancet. 2004;364:613–620. doi: 10.1016/S0140-6736(04)16855-4. [DOI] [PubMed] [Google Scholar]
- 4.Spira A, et al. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc Natl Acad Sci USA. 2004;101:10143–10148. doi: 10.1073/pnas.0401422101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 6.Vachier I, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115:55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 7.Haworth O, Levy BD. Endogenous lipid mediators in the resolution of airway inflammation. Eur Respir J. 2007;30:980–992. doi: 10.1183/09031936.00005807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Su SB, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189:395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Kebir D, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179:616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 10.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101:1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 11.Bozinovski S, et al. Serum amyloid a is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:269–278. doi: 10.1164/rccm.200705-678OC. [DOI] [PubMed] [Google Scholar]
- 12.Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 13.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168:1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perretti M, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sethi S. Infectious etiology of acute exacerbations of chronic bronchitis. Chest. 2000;117(5) Suppl 2:380S–385S. doi: 10.1378/chest.117.5_suppl_2.380s. [DOI] [PubMed] [Google Scholar]
- 16.Kluve-Beckerman B, Drumm ML, Benson MD. Nonexpression of the human serum amyloid A three (SAA3) gene. DNA Cell Biol. 1991;10:651–661. doi: 10.1089/dna.1991.10.651. [DOI] [PubMed] [Google Scholar]
- 17.Marsche G, et al. The lipidation status of acute-phase protein serum amyloid A determines cholesterol mobilization via scavenger receptor class B, type I. Biochem J. 2007;402:117–124. doi: 10.1042/BJ20061406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto H, Katagiri Y, Kiire A, Momohara S, Kamatani N. Serum amyloid A activates nuclear factor-kappaB in rheumatoid synovial fibroblasts through binding to receptor of advanced glycation end-products. J Rheumatol. 2008;35:752–756. [PubMed] [Google Scholar]
- 19.Sandri S, et al. Is serum amyloid A an endogenous TLR4 agonist? J Leukoc Biol. 2008;83:1174–1180. doi: 10.1189/jlb.0407203. [DOI] [PubMed] [Google Scholar]
- 20.Cheng N, He R, Tian J, Ye PP, Ye RD. Cutting edge: TLR2 is a functional receptor for acute-phase serum amyloid A. J Immunol. 2008;181:22–26. doi: 10.4049/jimmunol.181.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy BD, et al. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BD, et al. Severe Asthma Research Program, National Heart, Lung, and Blood Institute Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172:824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris T, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci USA. 2010;107:8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiang N, et al. The lipoxin receptor ALX: Potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 25.Dufton N, et al. Anti-inflammatory role of the murine formyl-peptide receptor 2: Ligand-specific effects on leukocyte responses and experimental inflammation. J Immunol. 2010;184:2611–2619. doi: 10.4049/jimmunol.0903526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Chen P, Hanaoka M, Droma Y, Kubo K. Enhanced levels of prostaglandin E2 and matrix metalloproteinase-2 correlate with the severity of airflow limitation in stable COPD. Respirology. 2008;13:1014–1021. doi: 10.1111/j.1440-1843.2008.01365.x. [DOI] [PubMed] [Google Scholar]
- 27.Sawmynaden P, Perretti M. Glucocorticoid upregulation of the annexin-A1 receptor in leukocytes. Biochem Biophys Res Commun. 2006;349:1351–1355. doi: 10.1016/j.bbrc.2006.08.179. [DOI] [PubMed] [Google Scholar]
- 28.Mullan RH, et al. Acute-phase serum amyloid A stimulation of angiogenesis, leukocyte recruitment, and matrix degradation in rheumatoid arthritis through an NF-kappaB-dependent signal transduction pathway. Arthritis Rheum. 2006;54:105–114. doi: 10.1002/art.21518. [DOI] [PubMed] [Google Scholar]
- 29.O'Hara R, Murphy EP, Whitehead AS, FitzGerald O, Bresnihan B. Local expression of the serum amyloid A and formyl peptide receptor-like 1 genes in synovial tissue is associated with matrix metalloproteinase production in patients with inflammatory arthritis. Arthritis Rheum. 2004;50:1788–1799. doi: 10.1002/art.20301. [DOI] [PubMed] [Google Scholar]
- 30.Fyfe AI, et al. Association between serum amyloid A proteins and coronary artery disease: Evidence from two distinct arteriosclerotic processes. Circulation. 1997;96:2914–2919. doi: 10.1161/01.cir.96.9.2914. [DOI] [PubMed] [Google Scholar]
- 31.Thorn CF, Lu ZY, Whitehead AS. Tissue-specific regulation of the human acute-phase serum amyloid A genes, SAA1 and SAA2, by glucocorticoids in hepatic and epithelial cells. Eur J Immunol. 2003;33:2630–2639. doi: 10.1002/eji.200323985. [DOI] [PubMed] [Google Scholar]
- 32.Thorn CF, Whitehead AS. Differential glucocorticoid enhancement of the cytokine-driven transcriptional activation of the human acute phase serum amyloid A genes, SAA1 and SAA2. J Immunol. 2002;169:399–406. doi: 10.4049/jimmunol.169.1.399. [DOI] [PubMed] [Google Scholar]
- 33.Culpitt SV, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:24–31. doi: 10.1164/rccm.200204-298OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.