Abstract
Uncontrolled growth in a confined space generates mechanical compressive stress within tumors, but little is known about how such stress affects tumor cell behavior. Here we show that compressive stress stimulates migration of mammary carcinoma cells. The enhanced migration is accomplished by a subset of “leader cells” that extend filopodia at the leading edge of the cell sheet. Formation of these leader cells is dependent on cell microorganization and is enhanced by compressive stress. Accompanied by fibronectin deposition and stronger cell–matrix adhesion, the transition to leader-cell phenotype results in stabilization of persistent actomyosin-independent cell extensions and coordinated migration. Our results suggest that compressive stress accumulated during tumor growth can enable coordinated migration of cancer cells by stimulating formation of leader cells and enhancing cell–substrate adhesion. This novel mechanism represents a potential target for the prevention of cancer cell migration and invasion.
Keywords: mechanobiology, solid stress, collective migration, metastasis
The microenvironment plays a crucial role in tumor initiation and progression (1–3). It is known that cells respond to biochemical cues such as secreted growth factors and cytokines (4), as well as metabolic stress resulting from reduced glucose and oxygen availability (5, 6). However, biology is not entirely governed by soluble signals: Cells also respond to mechanical cues in the microenvironment, actively changing shape and cytoskeletal organization (7) and adjusting adhesion affinity (8) when matrix tension or stiffness change. Despite extensive studies on the role of mechanical signals in many aspects of physiology, including endothelial function (9, 10), tissue maintenance (11, 12), and morphogenesis (13, 14), the role of mechano-stimulation in tumor biology is largely unexplored. Emerging data show that cells respond to various mechanical signals including (i) ECM stiffening due to deposition or remodeling of collagen fibers by activated stromal myofibroblasts (15), (ii) elevated interstitial fluid pressure (16), (iii) increased interstitial fluid flow (17, 18), and (iv) compressive stress (solid stress) generated by confined growth (19, 20). Such mechanical stresses may have a profound impact on tumor growth and development (19–23).
Growth-induced mechanical stress accumulates in structural elements of the interstitium and cells and is sufficient to collapse blood and lymphatic vessels (24). Not only can compressive stress affect intraspheroid proliferation and apoptosis (19, 20), but also studies have suggested that compression can induce genotypic and phenotypic changes that are related to malignancy (13, 25, 26). Thus, we hypothesize that compressive stress can select for metastatic cell populations or trigger cancer cell invasion.
Results
Compression Induces Migration of Breast Cancer Cells and Cytoskeletal Remodeling.
To test this hypothesis, we subjected normal and cancer epithelial cells to defined compression by pressing them against a membrane surface with a weighted piston (Fig. S1A). This geometry is similar to that experienced by cells at the periphery of a mammary acinus; in these 3D structures, uncontrolled growth of cancer cells within the acinus lumen effectively presses cells at the periphery of the acinus against the surrounding basement membrane (Fig. S1B). We subjected five established mammary epithelial cell lines (MCF10A, MCF7, 67NR, 4T1, and MDA-MB-231; listed in order of increasing invasion potential) to constant compressive stress and measured migration rates via a scratch-wound assay (throughout this paper, “wound” refers to the denuded area in our 2D cultures where cells were removed or excluded) (Fig. S1A). Stress levels were similar to those estimated in the native breast tumor microenvironment: 5.8 mmHg (21). At this level, compression did not significantly increase cell proliferation (Fig. S2A). However, compressive stress did enhance the motility of highly aggressive 4T1 and MDA-MB-231 cells, as well as 67NR cells, which have undergone partial epithelial–mesenchymal transition (characterized by loss of E-cadherin and vimentin expression) (27). In contrast, compressive stress suppressed the migration of normal mammary epithelial MCF10A and noninvasive, well-differentiated MCF7 cells, which retain certain features of normal mammary epithelium (28) (Fig. 1A). For the MCF10A cells, the slowed migration was associated with a decrease in cell number (Fig. S2A). It should be noted that during the first 16 h of compression, there was slight compaction of the agarose and a corresponding flow of fluid out of the gel. However, the resulting shear forces experienced by the cells were negligible (29, 30) (maximum 3.2 × 10−5 dyn/cm2; see SI Materials and Methods, Analysis of Fluid Dynamics at the Surface During Compression). These results demonstrate that applied compressive stress (ACS) enhances the migration potential of mammary carcinoma cells independent of any changes in cell proliferation.
Fig. 1.
Compression induces migration of mammary carcinoma cells and alters cytoskeletal organization. (A) Cell migration in the scratch-wound assay for five different mammary epithelial cells of increasing invasion potential, either stress-free (control) or subjected to a compressive stress of 5.8 mmHg for 16 h (n = 9; *P < 0.05 compared with corresponding control). (B) MCF10A (Upper) and 67NR cells (Lower) closing the “wound” after 16 h. The compressed 67NR cells at the leading edge exhibited directional alignment and faster migration, whereas compressed MCF10A cells displayed suppressed migration. (Scale bar, 200 μm.) (C) Cytoskeletal staining (phalloidin for actin filaments and tubulin for microtubules) of MCF10A and 67NR cells at the periphery of the cell-denuded area. The 67NR, but not MCF10A, cells demonstrated elongated actin filaments perpendicular to the cell-denuded area and microtubule rearrangement in response to stress. (Scale bar, 10 μm.)
Fig. 1B shows the dramatic difference in cell morphology at the wound edge between MCF10A and 67NR cells—the two cell lines that exhibited the most prominent inhibition or enhancement of migration, respectively. Compression stimulated changes in cell shape and cytoskeletal organization at the wound edge in 67NR, but not MCF10A cells. Specifically, compressed 67NR cells showed actin stress fiber alignment and microtubule rearrangement (Fig. 1C). This cytoskeletal adaptation to mechanical stimulation suggests that (i) compression-induced 67NR cell motility could be mediated by increased tension in the actin cytoskeleton, thereby inducing formation of stress fibers, and (ii) the elevated intracellular tension, in turn, induces formation of a microtubule network to ensure mechanical stability (7, 31).
We next investigated whether higher stresses would further enhance the migration of cancer cells. By titrating the piston weight, we determined that stresses >5.8 mmHg significantly triggered apoptosis, with only 40% of cells viable at 58 mmHg (Fig. S2B); there was a corresponding decrease in migration of viable cells at these higher stress levels (Fig. S2C). Furthermore, continuous compression was necessary for enhanced migration: 67NR cells preconditioned with a compressive stress of 5.8 mmHg migrated more slowly after stress removal than continuously compressed cells (Fig. S2D). These results show that moderate levels of continuously applied compressive stress enhance cell motility, whereas excessive stresses trigger cell death and impede cell migration. For the subsequent studies, the compressive stress was maintained at 5.8 mmHg.
ACS Stimulates Formation of “Leader Cells” with Filopodial Protrusions.
During wound closure, 67NR cells at the leading edge appeared to guide the directional movement of the cell sheet. This subset of cells acted as leader cells, maintaining connections with their trailing neighbors while extending protrusions into the denuded area (Fig. 1C). In the absence of compression (control; Movie S1), these cells extended in random directions and clustered at the wound periphery. In contrast, compressed 67NR cells demonstrated a clear persistence and directionality in their movement at the leading edge of the cell sheet (Movie S2). Quantitative analysis of cell orientation showed that the compressed 67NR cells preferentially aligned perpendicular to the wound periphery with the leading edges extending into the open area (Fig. 2A). Interestingly, there was a striking difference in the formation of leader cells between the control and compressed cultures. The number of leader cells increased by approximately twofold when the 67NR cells were compressed (Fig. 2B). To further characterize these leader cells, we assessed their size, shape, and polarization in compressed and control cultures. Although leader cells were polarized in both control and compressed conditions (Fig. S2E), those under compression had larger cell–substrate contact areas (Fig. 2C) and longer filopodial protrusions (Fig. 2D).
Fig. 2.
Compression promotes formation of leader cells at the scratch-wound periphery. (A) Cell orientation at the wound periphery. For the calculated cell alignment correlation index, a value of 1 indicates that the cells align perpendicular to the cell-denuded areas, and a value of 0 indicates orientation parallel to the wound periphery. Random cell alignment would result in an index of 0.63 according to theory. Uncompressed (control) samples had randomly oriented cells, but compression resulted in directed elongation into the denuded region (n = 7). (B) The fraction of cells around the denuded periphery phenotypically identified as leader cells was dramatically higher in the compressed samples. Leader cells were defined as those cells at the wound margin that extend protrusions into the denuded area (n = 12; *P < 0.005 compared with control). (C) Average projected cell areas of the control and compressed 67NR cells at the leading edge of the “wound” and those in the internal monolayer, far from the edge (n = 8–9; NS, not significant; *P < 0.005 compared with the control in the same group). (D) Comparison of average cell lengths in control and compressed samples. Frontal length (filopodial protrusion length) was measured from the leading tip of the cell to its nucleus (*P < 0.005 compared with the control).
Compressive Stress Induces Leader-Cell Phenotype in Border Cells, Regardless of Cell Microorganization.
Because (i) only cells at the wound edge adopt leader cell phenotype and (ii) leader cell morphology was not observed in sparsely seeded cultures, even when compressed (control, Movie S3; compressed, Movie S4), we hypothesized that the microorganization of cells within the sheet influences leader-cell formation. Specifically, it appeared that the extent of free-cell perimeter (the fraction of cell perimeter not in contact with other cells) was important.
To investigate this possibility, we controlled free-cell perimeter using microcontact printing. Groups of 67NR cells were forced into various defined geometries and their morphological changes were tracked over time. In circle patterns, all cells around the denuded periphery have roughly the same extent of free perimeter. On the other hand, cells at vertices (such as the points of the rosette) have, on average, more free perimeter (Fig. 3 and Fig. S3). We first confirmed that compression-induced leader-cell formation and enhanced wound closure occurred in the circle pattern formed by microcontact printing, similar to that seen in the scratch-wound assays. As expected, leader cells rarely formed in the uncompressed circle patterns, indicated by a relatively smooth periphery of cell-denuded areas, but were frequent in compressed cultures (Fig. 3A); furthermore, wound closure rates were increased with compression (Fig. 3B), consistent with the scratch-wound cultures. This result also verified that leader-cell formation is not influenced by cell damage in the in vitro scratch assay.
Fig. 3.
Free-cell perimeter determines leader-cell formation in control, but not compressed cultures. Shown are morphological changes and cell migration rates when 67NR cell monolayers (yellow and gray) were patterned into circles (A), rosettes (C), and squares (F) and cultured under stress-free (control) or compressed (5.8 mmHg) conditions. Solid and open triangles represent edge cells and point/corner cells, respectively (n = 6–8). (Scale bar, 100 μm.) (B) Average migration speed of control and compressed cells in circle patterns (n = 6–7; *P < 0.005). (D) Average migration speed of edge cells and point cells in the uncompressed cultures (n = 17; **P < 0.05 compared with edge cells). (E) Average migration speed of control and compressed cells in rosette patterns (n = 13–17; *P < 0.005). (G) Square patterns (500 × 500 μm) distort due to cell migration, and this pattern distortion can be quantified using a shape change index. For compressed cells, the index is ∼1, suggesting that the square pattern expands uniformly around the boundary; in contrast, control samples had much higher indexes, indicating that the shape expanded preferentially along the diagonals (n = 6; *P < 0.005).
Next, we patterned 67NR cells in a rosette geometry with leader cells predesignated at the tips (Fig. 3C). Interestingly, in the absence of compression, the preformed leader cells (point cells) extended more frequently from the rosette vertices and migrated faster than the other boundary cells (Fig. 3D). In contrast, with compression, there was no preferential location for leader-cell formation—cells extended and migrated from random locations around the rosette, not just from the pattern vertices (Fig. 3C). As a result, there was faster overall migration in these compressed cultures (Fig. 3E).
To quantify the effect of free-cell perimeter on leader-cell formation, we confined colonies of 67NR cells to a square geometry using micro contact printing (Fig. 3F). In these patterns, cells at the corners have more free perimeter than those on the edges, and quantitative comparison is accomplished via the change in shape of the square. Consistent with the rosette data, with no compression, cells at the four corners were more likely to become leader cells; with compression, almost all boundary cells adopted the leader-cell phenotype (Fig. 3F). Quantitative analysis of the patterns confirmed that there was preferential extension from the corners in the control, but not the compressed, cultures (Fig. 3G). Thus, leader-cell formation in uncompressed cultures is sensitive to local cell–cell spatial organization, which determines free-cell perimeter and can influence the dynamics of spreading. The positional advantage disappears with compression, which distends all border cells, likely inducing changes in shape or cytoskeletal tension that mimic those in uncompressed corner cells.
Actomyosin Contractility Is Required for Migration of Leader Cells, but Not Their Formation.
Previous studies have shown that cell microorganization within a monolayer defines patterns of intracellular tension generated by the actomyosin cytoskeleton (32). Such intracellular tension is important for determining sites of cell proliferation (32) and mammary branching morphogenesis (33) in vitro. To investigate whether similar myosin-dependent intracellular tension is involved in ACS-induced coordinated migration, we altered cellular mechanics using molecular and pharmacological approaches.
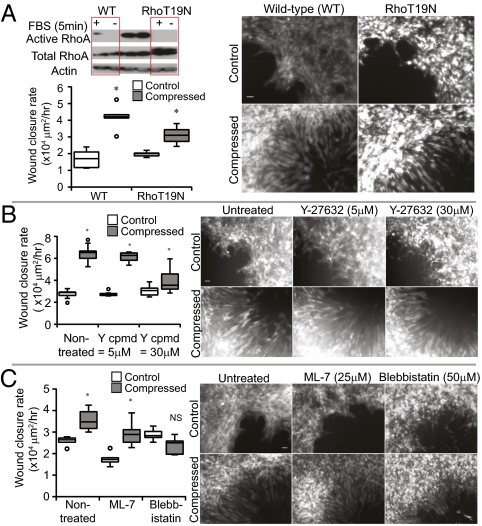
Intracellular tension generated by the actomyosin cytoskeleton is regulated in part by signaling through the small GTPase RhoA, its downstream effector Rho kinase (ROCK), and myosin light chain kinase (MLCK). To decrease the actomyosin-mediated contractile tension, we therefore used dominant-negative RhoA (RhoA-T19N) retrovirus, ROCK inhibitor Y-27632, or MLCK inhibitor ML-7. Compression still enhanced wound closure rates of RhoT19N cells compared with uncompressed controls, but the effect was slightly reduced compared with the induction seen in wild-type cells. In addition, inhibition of RhoA activity did not suppress leader-cell formation in the compressed cultures (Fig. 4A). Similar results were seen with Y-27632 and ML-7 treatment (Fig. 4 B and C). Our finding that RhoA is not involved in compression-induced leader-cell formation was surprising, considering previous work demonstrating its role in the formation of leader cells during in vitro wound healing (34). It is possible that external mechanical stimulation enables other RhoA-independent signaling mechanisms. Interestingly, when actomyosin contractility was completely blocked by the myosin II ATPase inhibitor Blebbistatin (35), compressive stress was still able to induce leader-cell formation despite compromised cell motility (Fig. 4C). These results indicate that compression can initiate directional protrusions for leader-cell formation independent of actomyosin contractility; however, the contractile machinery is still necessary for sheet migration.
Fig. 4.
Actomyosin contractility is not necessary for compression-induced leader-cell formation. (A–C) Migration rates in the scratch-wound assay for 67NR cells transduced with dominant-negative RhoT19N retrovirus (A; n = 6) or treated with Y-27632 (B; n = 6–13), ML-7 (C; n = 6), or Blebbistatin (C; n = 6) under stress-free or compressed (5.8 mmHg) conditions for 16 h and corresponding representative images of 67NR leader cells at the wound edge (*P < 0.005 compared with the respective control; NS, not significant compared with the respective control). (Scale bar, 50 μm.) The inhibitors of actomyosin contractility did not abolish leader-cell formation despite reduced wound closure rate. (A) Western blot showing RhoA activation pull-down to analyze the transduction efficiency. Error bars represent SEM.
Cell Adhesion Is Modulated During Compression-Induced Migration.
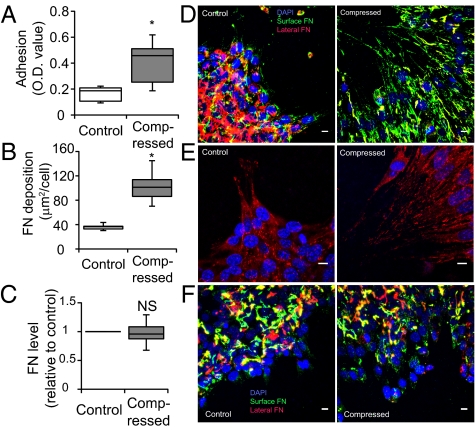
Cell migration is a coordinated interaction between cells and their surroundings. In our 67NR cells, homotypic E-cadherin adhesion was not necessary for—and did not interfere with—compression-induced migration (Fig. S4). Therefore, we next tested whether compression affects cell–substrate adhesion. We quantified the ability of cells to resist detachment caused by fluid shear forces. Compressed 67NR cells exhibited 2.5-fold higher cell-substrate adhesion than uncompressed cells (Fig. 5A). Analyzing the distribution of fibronectin in the culture, we found that more fibronectin was localized at the cell–substrate interface in the compressed samples than in controls (Fig. 5 B and D), which was consistent with the immunostaining pattern of vinculin, a protein marker of focal adhesions (Fig. 5E). There was also fibronectin between cells, which likely allowed for cohesion of the cell sheet independent of E-cadherin expression. However, the change in fibronectin distribution was not related to altered fibronectin transcription (quantitative PCR analysis; Fig. 5C).
Fig. 5.
Compression up-regulates 67NR cell–matrix adhesion via localized fibronectin secretion. (A) Compression enhances cell-substrate adhesion. Uncompressed and compressed samples were exposed to detractive fluid shear and the remaining adherent cells were quantified using a colorimetric assay in which crystal violet stain was quantified via optical density (OD) at 540 nm (n = 8; *P < 0.005). (B) Quantification of fibronectin accumulation at the cell–substrate interface. Results are expressed as surface fibronectin-positive pixel area relative to the total number of DAPI-stained nuclei (n = 11–12; *P < 0.005 compared with the control). (C) Quantitative PCR of control and compressed 67NR cells showed no significant difference in fibronectin messenger level between the two groups (NS, not significant). (D) Fibronectin staining of 67NR cells at the periphery of the cell-denuded area. Fibronectin at the cell–substrate interface in the compressed, but not control, samples was fibrillar and oriented in the direction of migration (n = 17). (Scale bar, 10 μm.) (E) Vinculin-stained cells at the periphery of the cell-denuded area. 67NR cells were either uncompressed (control) or exposed to a compressive stress of 5.8 mmHg for 16 h. Vinculin-positive (red) focal adhesions were detected underneath compression-induced filopodia of elongated cells (n = 16). (Scale bar, 10 μm.) (F) Fibronectin staining of 67NR cells treated with 1 μM cycloheximide at the periphery of the cell-denuded area. The formation of oriented and fibril-like patterns of fibronectin observed earlier in the nontreated compressed cultures (D) was abolished after inhibition of protein synthesis, suggesting that the cells secrete fibronectin during their movement for enhanced cell–matrix adhesion (n = 8). (Scale bar, 10 μm.)
The distinct fibronectin patterns could have been formed by rearrangement of existing extracellular fibronectin or by directed secretion of newly synthesized fibronectin by the cells. To investigate this further, we inhibited all protein translation by treating the 67NR cells with cycloheximide before compression and then monitored migration and fibronectin patterns. We confirmed that cycloheximide-treated 67NR cells still adhered to and migrated on fibronectin substrates, but, in general, speeds were slower compared with the untreated cells (Fig. S5). However, inhibition of protein synthesis abolished the oriented, fibril-like pattern of fibronectin seen in the untreated, compressed cultures (Fig. 5F). These results suggest that mechanical compression induces localized secretion of fibronectin by the migrating 67NR cells, and this localized secretion enhances leader-cell formation.
Discussion
Uncontrolled proliferation of cancer cells generates mechanical, compressive stresses (19, 20). We hypothesized that such stresses can facilitate tumor progression and found that cells at the periphery of a discontinuous sheet can undergo a phenotypic transformation when compressed. These cells at the sheet boundary became leader cells and participated in coordinated cell migration (collective migration) (36, 37), extending protrusions in the direction of migration and guiding “followers” at their rear. Leader cells have been observed in collective migration during cancer cell invasion, vascular sprouting, wound closure, and embryogenesis (36, 37). Furthermore, there is some evidence that mechanical forces can modulate coordinated migration in vascular morphogenesis: It has been shown that forces exerted by flowing fluids can induce changes in endothelial junction structure (38), differentiation of vascular wall cells (39), and tip cell formation (40). Interestingly, our leader cells are morphologically and functionally similar to endothelial tip cells. Therefore, it is possible that similar mechanisms are involved in the coordinated migration of epithelium and endothelium.
The formation of leader cells in coordinated migration is likely related to the balance of intracellular stresses generated in the cytoskeleton (7, 41). A shift in stress balance due to interactions with other cells (32, 36, 42) or cell distortion as a cell actively adapts to its local matrix environment (7, 43) can initiate cytoskeletal rearrangement, proliferation, and morphogenesis (32, 33). Our experiments without compression demonstrate the ability of the cell microorganization to control the coordinated migration: In the absence of exogenous compression, the stress balance—determined by the cell's position within the monolayer and mediated through actomyosin (32)—affected leader-cell formation. Cells at rosette tips or at the corners of a square pattern have more free-cell perimeter available for extension and formation of new adhesions, resulting in a change in the cell stress balance. We propose that this shift in intracellular forces initiates changes in cytoskeleton and focal adhesions that quickly translate into leader-cell formation.
In contrast, when cells were subjected to applied compressive stress, there was no preferential location for leader-cell formation around the sheet boundary. Even cells that were not in “preferred” positions for self-induced leader-cell formation (i.e., cells in the “edge” positions) became leaders. As the leader cells spread, they secreted fibronectin, facilitating cell-substrate interactions (Fig. 5 B and D). This result is consistent with reports that the persistent movement of leader cells requires cell adhesion to fibronectin (44). Our results are also consistent with the study by Chen et al., who confined individual cells to patterned surfaces and found that cell spreading controls the number of focal adhesions (43). Evidently, the increased free-cell perimeter and surface adhesion caused by compression-induced cell distension (Fig. 2 C and D) affect cytoskeletal tension and trigger leader-cell formation (Fig. 1C), independent of any preexisting force balance established by the cells themselves. Our observations that (i) ACS-induced coordinated migration is abolished upon removal of the applied stress (Fig. S2D) and (ii) inhibition of actomyosin contractility has no effect on ACS-induced leader-cell formation (Fig. 4) support the concept that the applied stress substitutes for intracellular tension generated by actomyosin contractility to enable and sustain the transformation (Fig. 6). Indeed, it has been reported that external force application can induce intracellular tension during maturation of focal contacts, independent of Rho/ROCK-dependent actomyosin-driven cell contractility (45). Upon removal of the applied stress, the tension balance could return to normal and the cells revert to nonleader phenotype.
Fig. 6.
Proposed model of compression-modulated leader-cell formation and coordinated migration. (A) Cells seeded at the corners and edges of square islands have different extents of free perimeter, which affects actomyosin-driven intracellular stress. (B) In uncompressed cultures, free perimeter affects leader-cell formation. On average, the corner cells in the square islands have more free-cell perimeter than the edge cells and are therefore able to extend more protrusions than the edge cells, resulting in higher intracellular stress. (C) The resulting change in force balance within the cell likely causes their phenotypic change into “leader” cells. In our system, cell–cell adhesion is maintained, so cells adjacent to the leader cells (either behind or on the sides) appear to be pulled in the coordinated migration. As a result, the sheet preferentially extends from the corners of the square pattern. (D) In contrast, when the culture is compressed, all cells around the periphery of the island are deformed, or extruded, against the substrate, into the empty space. Similar to the case of the active extension of the uncompressed corner cells, cell extrusion has the effect of increasing cell-substrate contact (and also intracellular stress). (E) Hence, all cells around the periphery of the square pattern can become leader cells. The leader cells then continue to secrete and deposit fibronectin during cell spreading and movement, thereby forming new adhesion contacts with the substrate and resulting in enhanced coordinated migration.
The fact that aggressive carcinoma cells, but not MCF10A cells, respond to compression raises the interesting possibility that cancer cells are somehow “primed” to respond to changes in the mechanical environment. Previous reports have shown that MCF10A cells have a higher apparent elastic modulus than cancer cells (31, 46). This result is consistent with our finding that uncompressed (control) MCF10A cells have a denser network of cytoskeletal structures (particularly microtubules) compared with uncompressed (control) 67NR cells (Fig. 1C). The relatively higher level of cell stiffness may make nontumorigenic MCF10A cells less mechano-sensitive. Our results are consistent with results from 3D cultures, where increased matrix density or stiffness has been shown to trigger malignant transformation (23, 47, 48). It is possible that in these systems, cell growth produces compressive stress that initiates the invasion in a way analogous to our applied compression.
Our results establish a direct link between mechanical stress and enhancement of coordinated cancer cell motility via formation of leader cells. The concept of mechanical induction of tumor invasiveness could open the door to a unique class of targets for blocking mechanical stress pathways and guide the development of approaches for drug screening that take into account mechanical as well as genetic and biological factors. In addition, this work provides unique insight into how physical determinants can influence coordinated migration, a process relevant to other physiological events such as vascular sprouting and wound healing (36, 37).
Materials and Methods
A detailed description of the materials and methods is included in SI Materials and Methods. Briefly, mammary carcinoma (MCF7, 67NR, 4T1, and MDA-MB-231) and normal (MCF10A) cell lines were subjected to 16-h compressive stress at a level of 5.8 mmHg using the in vitro compression device (Figs. S1A and Fig. S6) and their motility was measured with the scratch-wound assay. Images of cells at the periphery of the wound were captured with an inverted microscope (Olympus) for analyses of cell orientation and migration. To control free-cell perimeter, 67NR cells were patterned by seeding them on surfaces “stamped” with fibronectin to form circles, squares, and rosettes using microcontact printing, as previously described (49, 50) with minor modifications. For circles and rosettes, the fibronectin (and cells) was excluded from the shapes; for squares, the fibronectin (and cells) was confined to the shape. The 67NR cells were also stained with Alexa Fluor-546 phalloidin (Molecular Probes-Invitrogen), anti-vinculin antibody (Sigma), and antiserum against fibronectin (Sigma) for F-actin, focal adhesions, and fibronectin, respectively. Immunofluorescence images were collected with a confocal microscope (Olympus) and analyzed with ImageJ or Matlab for leader cell frequency and filopodial protrusions as well as fibronectin deposition. The transcriptional expression level of fibronectin was measured by quantitative real-time PCR, using total RNA extracted from the 67NR cells. The effect of compression on the cell-surface adhesion strength was determined by the number of compressed or control cells remaining on the surface after exposure to shear forces. Finally, to determine the role of actomyosin contractility in compression-induced coordinated migration, various chemical inhibitors or molecular modification were used to down-regulate RhoA/ROCK or myosin-associated pathways. Data are presented as mean ± SEM, and P ≤ 0.05 was considered significant in unpaired Wilcoxon–Mann–Whitney tests.
Supplementary Material
Acknowledgments
We thank A. Jain and Dr. T. R. Sodunke for their help with microfabrication, Dr. S.-S. Chae for his help with molecular manipulations, and Dr. F. R. Miller for providing the 67NR and 4T1 cells. Funding for this work was provided by National Institutes of Health Grants P01CA080124 (to R.K.J., L.L.M., and Y.B.) and HL64240 (to L.L.M.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118910109/-/DCSupplemental.
References
- 1.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 2.Wong SY, Hynes RO. Tumor-lymphatic interactions in an activated stromal microenvironment. J Cell Biochem. 2007;101:840–850. doi: 10.1002/jcb.21146. [DOI] [PubMed] [Google Scholar]
- 3.Fukumura D, Duda DG, Munn LL, Jain RK. Tumor microvasculature and microenvironment: Novel insights through intravital imaging in pre-clinical models. Microcirculation. 2010;17:206–225. doi: 10.1111/j.1549-8719.2010.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bierie B, Moses HL. Tumour microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolková K, et al. Mitochondrial bioenergetic adaptations of breast cancer cells to aglycemia and hypoxia. J Bioenerg Biomembr. 2010;42:55–67. doi: 10.1007/s10863-009-9267-x. [DOI] [PubMed] [Google Scholar]
- 7.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- 9.Chien S. Mechanical and chemical regulation of endothelial cell polarity. Circ Res. 2006;98:863–865. doi: 10.1161/01.RES.0000219686.29872.e2. [DOI] [PubMed] [Google Scholar]
- 10.Yao Y, Rabodzey A, Dewey CF., Jr Glycocalyx modulates the motility and proliferative response of vascular endothelium to fluid shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H1023–H1030. doi: 10.1152/ajpheart.00162.2007. [DOI] [PubMed] [Google Scholar]
- 11.Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–786. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- 12.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 13.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 14.Nerurkar NL, Ramasubramanian A, Taber LA. Morphogenetic adaptation of the looping embryonic heart to altered mechanical loads. Dev Dyn. 2006;235:1822–1829. doi: 10.1002/dvdy.20813. [DOI] [PubMed] [Google Scholar]
- 15.Shao ZM, Nguyen M, Barsky SH. Human breast carcinoma desmoplasia is PDGF initiated. Oncogene. 2000;19:4337–4345. doi: 10.1038/sj.onc.1203785. [DOI] [PubMed] [Google Scholar]
- 16.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: Implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- 17.Ng CP, Hinz B, Swartz MA. Interstitial fluid flow induces myofibroblast differentiation and collagen alignment in vitro. J Cell Sci. 2005;118:4731–4739. doi: 10.1242/jcs.02605. [DOI] [PubMed] [Google Scholar]
- 18.Ng CP, Swartz MA. Fibroblast alignment under interstitial fluid flow using a novel 3-D tissue culture model. Am J Physiol Heart Circ Physiol. 2003;284:H1771–H1777. doi: 10.1152/ajpheart.01008.2002. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G, Tse J, Jain RK, Munn LL. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE. 2009;4:e4632. doi: 10.1371/journal.pone.0004632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 21.Butcher DT, Alliston T, Weaver VM. A tense situation: Forcing tumour progression. Nat Rev Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann M, et al. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89–95. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Padera TP, et al. Pathology: Cancer cells compress intratumour vessels. Nature. 2004;427:695. doi: 10.1038/427695a. [DOI] [PubMed] [Google Scholar]
- 25.Koike C, et al. Solid stress facilitates spheroid formation: Potential involvement of hyaluronan. Br J Cancer. 2002;86:947–953. doi: 10.1038/sj.bjc.6600158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Lou Y, et al. Epithelial-mesenchymal transition (EMT) is not sufficient for spontaneous murine breast cancer metastasis. Dev Dyn. 2008;237:2755–2768. doi: 10.1002/dvdy.21658. [DOI] [PubMed] [Google Scholar]
- 28.van Deurs B, Zou ZZ, Briand P, Balslev Y, Petersen OW. Epithelial membrane polarity: A stable, differentiated feature of an established human breast carcinoma cell line MCF-7. J Histochem Cytochem. 1987;35:461–469. doi: 10.1177/35.4.3546491. [DOI] [PubMed] [Google Scholar]
- 29.Chen KD, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 30.Wang DM, Tarbell JM. Modeling interstitial flow in an artery wall allows estimation of wall shear stress on smooth muscle cells. J Biomech Eng. 1995;117:358–363. doi: 10.1115/1.2794192. [DOI] [PubMed] [Google Scholar]
- 31.Li R, Gundersen GG. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9:860–873. doi: 10.1038/nrm2522. [DOI] [PubMed] [Google Scholar]
- 32.Nelson CM, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci USA. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nelson CM, Vanduijn MM, Inman JL, Fletcher DA, Bissell MJ. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 2006;314:298–300. doi: 10.1126/science.1131000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 36.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 37.Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122:3203–3208. doi: 10.1242/jcs.036525. [DOI] [PubMed] [Google Scholar]
- 38.Berardi DE, Tarbell JM. Stretch and shear interactions affect intercellular junction protein expression and turnover in endothelial cells. Cell Mol Bioeng. 2009;2:320–331. doi: 10.1007/s12195-009-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi ZD, Abraham G, Tarbell JM. Shear stress modulation of smooth muscle cell marker genes in 2-D and 3-D depends on mechanotransduction by heparan sulfate proteoglycans and ERK1/2. PLoS ONE. 2010;5:e12196. doi: 10.1371/journal.pone.0012196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song JW, Munn LL. Fluid forces control endothelial sprouting. Proc Natl Acad Sci USA. 2011;108:15342–15347. doi: 10.1073/pnas.1105316108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingber DE. Mechanical control of tissue growth: Function follows form. Proc Natl Acad Sci USA. 2005;102:11571–11572. doi: 10.1073/pnas.0505939102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 43.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochem Biophys Res Commun. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 44.Binamé F, Lassus P, Hibner U. Transforming growth factor beta controls the directional migration of hepatocyte cohorts by modulating their adhesion to fibronectin. Mol Biol Cell. 2008;19:945–956. doi: 10.1091/mbc.E07-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riveline D, et al. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153:1175–1186. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suresh S. Biomechanics and biophysics of cancer cells. Acta Biomater. 2007;3:413–438. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Provenzano PP, Inman DR, Eliceiri KW, Keely PJ. Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK-ERK linkage. Oncogene. 2009;28:4326–4343. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alom Ruiz S, Chen CS. Microcontact printing: A tool to pattern. Soft Matter. 2007;3:168–177. doi: 10.1039/b613349e. [DOI] [PubMed] [Google Scholar]
- 50.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–2376. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.