Abstract
The medial (MHb) and lateral (LHb) habenulae are a small group of nuclei that regulate the activity of monoaminergic neurons. Disruptions to these nuclei lead to deficits in a range of cognitive and motor functions from sleep to decision making. Interestingly, the habenular nuclei are present in all vertebrates, suggesting that they provide a common neural mechanism to influence these diverse functions. To unravel conserved habenula circuitry and approach an understanding of their basic function, we investigated the organization of these nuclei in the lamprey, one of the phylogenetically oldest vertebrates. Based on connectivity and molecular expression, we show that the MHb and LHb circuitry is conserved in the lamprey. As in mammals, separate populations of neurons in the LHb homolog project directly or indirectly to dopamine and serotonin neurons through a nucleus homologous to the GABAergic rostromedial mesopontine tegmental nucleus and directly to histamine neurons. The pallidal and hypothalamic inputs to the LHb homolog are also conserved. In contrast to other species, the habenula projecting pallidal nucleus is topographically distinct from the dorsal pallidum, the homolog of the globus pallidus interna. The efferents of the MHb homolog selectively target the interpeduncular nucleus. The MHb afferents arise from sensory (medial olfactory bulb, parapineal, and pretectum) and not limbic areas, as they do in mammals; consequently, the “context” in which this circuitry is recruited may have changed during evolution. Our results indicate that the habenular nuclei provide a common vertebrate circuitry to adapt behavior in response to rewards, stress, and other motivating factors.
Keywords: basal ganglia, neuromodulator
The habenular complex, a small group of nuclei in the epithalamus, links the limbic forebrain to mid- and hind-brain regions involved in adapting the internal cerebral state (1–4). Lesion and genetic inactivation studies have implicated the habenula in a range of functions from control of the circadian rhythm to adapting the behavioral response to fear and reward, as well as to higher cognitive functions, such as attention and value-based decision making (5–8). The habenula is present in all classes of vertebrates, including those that seemingly lack high-level cognitive functions (9). This suggests that it regulates processes crucial to survival through a basic neural mechanism common to all vertebrates. To unravel the neural basis for the habenula's basic and complex functions, it will be important to elucidate the basic circuitry and determine how this has evolved to regulate higher cognitive functions.
In mammals, the habenula is subdivided into two nuclei: the medial habenula (MHb) and lateral habenula (LHb) (10). The LHb receives value-related signals from the basal ganglia [globus pallidus interna/entopeduncular nucleus (GPi/EP)] as well as inputs from the diagonal band of Broca and the lateral hypothalamus (1, 11). The LHb projects to the GABAergic rostromedial mesopontine tegmental nucleus (RMTg), and neurons in both of these nuclei respond to reward in an opposite manner to dopaminergic neurons because they are excited by aversive stimuli or the absence of expected reward (12–14). Stimulation and lesion studies have demonstrated that the mammalian LHb exerts an inhibitory influence on monoaminergic neurons, probably via the RMTg (15–18). Consequently, the LHb-RMTg (GABA)-neuromodulatory pathway is thought to contribute to reinforcement learning and other cognitive functions by suppressing dopamine, the reinforcing signal, and other monoaminergic neurons in response to aversive stimuli or reward prediction errors. Whether this circuitry is conserved in nonmammalian vertebrates is unclear. One recent study has identified a homolog of the LHb in zebrafish based, in part, on habenula projections to the serotonergic raphe nucleus (19). Despite this, no studies of nonmammalian LHb homologs have demonstrated the presence of direct projections to neuromodulatory neurons or an indirect projection to the monoamine system via a GABAergic nucleus. In addition, no studies have addressed the conservation of the LHb afferents in nonmammalian species. Consequently, it is unclear if this LHb circuitry forms part of the common vertebrate circuitry or if it evolved later to regulate higher cognitive functions in mammals.
The MHb receives input mainly from the limbic system and sends cholinergic projections to the interpeduncular nucleus (IPN) (1, 2). Habenula projections to the IPN have been identified in all classes of vertebrates (9), and these projections have been shown to arise from distinct habenula subpopulations in zebrafish and reptiles (20, 21). This suggests that the MHb may be conserved throughout vertebrate phylogeny. In addition, genetic inactivation of the zebrafish homolog of the MHb leads to a disruption in the flight response to fearful stimuli, a basic function that all vertebrates perform (6, 7). Although the MHb may be conserved, the input to this circuitry, as the contextual basis that nonmammalian vertebrates use to regulate their response to fear or aversive stimuli, has not been studied.
Consequently, it is unclear which components of the mammalian habenular circuitry are conserved in lower vertebrates. Our aim was to use the lamprey, one of the phylogenetically oldest vertebrate species whose ancestors diverged from the main vertebrate lineage over 560 million years ago (22), to determine the basic circuitry and function of the habenula. Based on connectivity and molecular expression, we show that homologs of the MHb and LHb and their efferent circuitry are conserved in the lamprey. The homolog of the LHb projects both directly and indirectly, via a GABAergic nucleus, to dopaminergic and serotonergic neurons in the lamprey but also to histaminergic neurons. Our results furthermore demonstrate that a habenula projecting pallidal nucleus (DPh) is present and is distinct from the dorsal pallidum (DP), the lamprey homolog of the GPi (23). During evolution, there appears to have been a change in the other afferent inputs to these circuits, with sensory innervation having been replaced by inputs from the so-called limbic system (i.e., the septum and diagonal band of Broca). Our results show that the habenula circuitry is conserved as part of a common vertebrate mechanism to regulate the neuromodulatory system; consequently, it may form part of the neural basis that all vertebrates use to adapt their behavior in response to rewards, stress, fearful stimuli, and other motivating factors.
Results
The lamprey habenula is asymmetrical, with an enlarged right habenula, and is composed of four distinct nuclei, the left habenula (lfHb), right dorsal habenula (rdHb), right middle habenula (rmHb), and right ventral habenula (rvHb) nuclei (Fig. 1A).
Fig. 1.
Anatomical evidence for a lamprey homolog of the mammalian MHb. (A) Neurobiotin injection (Inset; green) into IPN, resulting in retrogradely labeled cells in the lfHb and rmHb. (B) Calbindin-immunoreactive cells in the lfHb and rmHb. Calbindin-immunoreactive fibers are observed in the rvHb and rdHb. (C) Anterogradely labeled fibers in the rostral and caudal IPN following injections (Neurobiotin) into the left (purple) and right (red) habenulae. White arrows indicate the mesencephalic Muller cell 3 as a landmark of the rostrocaudal location. (D) Schematic drawing of the habenula-IPN projection in the lamprey. (Scale bars: 200 μM.)
Lamprey lfHb and rmHb Share Molecular and Connectivity Characteristics with the Mammalian MHb.
To determine if a homolog of the mammalian MHb exists in the lamprey, we first explored whether the habenula projects to the IPN. Injections of Neurobiotin (Vector) in the habenula resulted in anterogradely labeled fibers throughout the entire rostrocaudal extent of the IPN (Fig. S1A, n = 3), as has been demonstrated in the larval lamprey (24). Retrograde tracing from the IPN revealed that this habenula-IPN projection specifically arose from two of the four habenular nuclei, the lfHb and rmHb nuclei (Fig. 1A, n = 4), suggesting that these nuclei may be homologs to the mammalian MHb. An additional homology between these nuclei and the mammalian MHb was that neurons in the lfHb and rmHb were shown to be immunoreactive for calbindin (25) (Fig. 1B). Anterograde tracing from the lfHb and right habenula revealed that the lfHb and rmHb project to the rostral and caudal IPN, respectively (Fig. 1 C and D, n = 6). The habenula projection to the IPN is also topographically arranged in other species, although the orientation of the topographic projection varies across species. For example, in zebrafish, the left dorsal habenula and rdHb (MHb homolog) predominantly project to the dorsal and ventral IPN, respectively (21). The projection from the medial and lateral aspects of the MHb to the IPN is also topographically organized in mammals (2). Together, our results suggest that despite the different topography, the lfHb and rmHb may be homologous to the left and right mammalian MHb.
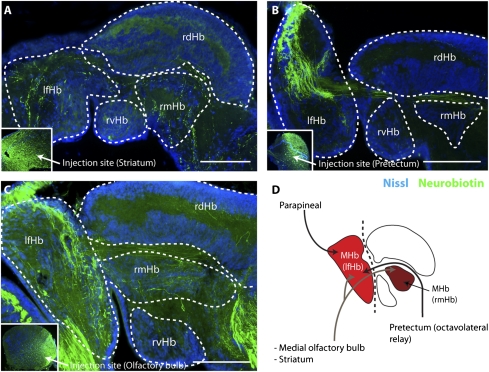
lfHb and rmHb Receive Projections from Sensory Regions and the Striatum.
As mentioned previously, MHb output circuitry is known to regulate the flight response to fearful stimuli, and it receives input from the limbic system and ventral striatum in mammals (1). To determine the “context” in which this output circuitry may be activated in a nonmammalian vertebrate, we analyzed the specific afferent connectivity of these lamprey nuclei. Injections in the lfHb resulted in retrogradely labeled cells in three of the six regions projecting to the habenula: the medial olfactory bulb (mOB), the striatum, and the pretectum (Fig. S2 A–D, n = 3). In addition, it should be noted that the parapineal organ selectively innervates the lfHb (26). This result is not reconfirmed here, because the pineal and parapineal organs were removed during the dissection. These findings suggest that the lamprey homolog of the MHb receives input from two sensory regions in addition to the pretectum and the basal ganglia.
The pretectum may also relay sensory information to the habenula, because previous experiments have shown that cells in the pretectum are activated (shown as an increase in Fos B expression) after weak electrical stimulation (27). Retrograde tracing from the pretectum revealed that the electroceptive and vestibular recipient area of the brain, the octavolateral area, projects directly to the pretectum (Fig. S2 E and F, n = 4). This confirms, as the physiological data predicted, that there is a direct octavolateral-pretectum connection that could relay electroceptive signals from the lateral line receptors to the lamprey homolog of the MHb.
To determine if these sensory areas and the basal ganglia also innervate the rmHb, as the other half of the lamprey MHb homolog, we analyzed the anterograde projections from each of these regions. This revealed that projections from the striatum and the mOB innervate both the lfHb and the rmHb [Fig. 2A, n = 3; Fig. 2C, n = 4; and Fig. 2D). In both cases, fibers were observed innervating the cell-dense areas in the rostral habenula, whereas further caudally, the fibers from these injections passed through the habenula commissure as has previously been reported (28). In contrast, projections from the pretectum selectively innervate the lfHb (Fig. 2B, n = 4; and Fig. 2D) in a pattern almost identical to the parapineal innervation, which has previously been reported (24). These pretectal fibers were not observed passing through the habenula commissure in the caudal habenula. This shows that the lfHb and rmHb, in contrast to the mammalian MHb, receive input from sensory rather than limbic regions. In contrast both the lamprey and mammalian species have a striatal projection to the MHb or its homolog. This suggests that a homolog of the MHb, with projections to the IPN, is present in the lamprey but that the input to this circuitry has been altered during evolution.
Fig. 2.
Afferents of the lamprey homolog of the MHb (lfHb and rmHb) from sensory regions and the striatum. (A–C) Neurobiotin injections (green) into the striatum, pretectum, and mOB, resulting in anterogradely labeled fibers in the lfHb and rmHb. (D) Schematic drawing of the lfHb and rmHb afferents. (Scale bars: 200 μM.)
Separate Populations of Neurons in the rdHb and rvHb Project to Dopaminergic, Serotonergic, and GABAergic Neurons as with the Mammalian LHb.
To explore whether the other nuclei in the lamprey habenula (rdHb and rvHb) may be homologs to the mammalian LHb, we analyzed the remaining habenula efferents. Anterogradely labeled fibers from the habenula were observed in four areas of the brain, in addition to the IPN. These fibers were observed in three areas with neuromodulatory neurons: the histaminergic periventricular hypothalamus, the serotonergic ventral mammillary region (vMAM), and the dopaminergic nucleus tuberculi posterior (ntp) (29–31) (Fig. S1, n = 3). In addition, fibers were observed in the dorsal mammillary region (dMAM), which contains GABAergic neurons (32) (Fig. S1 A and F). To determine if habenula fibers directly contact neuromodulatory and GABAergic neurons in these regions, as they do in mammals, we combined anterograde labeling with immunohistochemistry. Anterogradely labeled fibers from the right habenula were observed in close apposition to dopaminergic processes and cell bodies in the ntp (Fig. 3A, n = 3). The ntp in the lamprey is homologous to the mammalian substantia nigra pars compacta (SNc)/ventral tegmental area (VTA) (29); thus, Hb-SNc/VTA projections are present in the lamprey as they are from the mammalian LHb (2). The cell bodies and processes of GABAergic neurons in the dMAM were peppered with fibers from the right habenula (Fig. 3B, n = 3). Anterogradely labeled fibers were also observed in close apposition to serotonergic neurons in the vMAM (Fig. 3C, n = 3). Finally, anterogradely labeled habenula fibers that projected to the periventricular hypothalamus selectively innervated the cell bodies of histaminergic neurons (Fig. 3D, n = 3). Together, these results indicate that projections from the habenula make direct “putative” contacts with dopaminergic, serotonergic, and GABAergic neurons as with the mammalian LHb. In addition, the results indicate that a projection to histaminergic neurons exists in the lamprey and may be present in other species, although this remains to be examined.
Fig. 3.
Direct habenula projections to dopaminergic, serotonergic, histaminergic, and GABAergic neurons are homologous to the projections of the mammalian LHb. Confocal images of anterogradely labeled fibers from injections (Neurobiotin) in the right habenula in close apposition to neurons immunoreactive for tyrosine hydroxylase (TH) in the ntp (A), GABA in the dMAM (B), 5-HT in the vMAM (C), and histamine in the periventricular hypothalamus (Hyp; D). (E) Schematic drawing showing the locations of the different immunoreactive populations in transverse sections of the diencephalon. (Scale bars: 50 μM.)
To test if separate habenula populations independently control each of these regions, we explored whether the habenula populations projecting to each of these areas arose from distinct subpopulations. Retrograde tracing revealed that separate populations of neurons in the rdHb and rvHb give rise to the projections to the ntp (dopamine), hypothalamus (histamine), dMAM (GABA), and vMAM [5-hydroxytryptophan (5-HT)] (Fig. 4 A–E). Projections to the ntp arose from a cluster of neurons in the rostrolateral portion of the rdHb (Fig. 4A, n = 4). Labeled cells in the rvHb and rostromedial regions of the rdHb send projections to the vMAM (Fig. 4B, n = 3). Sparsely labeled neurons in all areas of the rvHb and rdHb projected to the dMAM (Fig. 4C, n = 3). Finally, neurons in the caudal region of the rdHb project to the periventricular hypothalamus (Fig. 4D, n = 2). This suggests that efferent projections of the rdHb and rvHb are homologous to the mammalian LHb and that separate habenula subpopulations can potentially regulate each population of neuromodulatory neurons independently. In addition, these separate populations are topographically distinct; with the medial regions of these nuclei projecting to the serotonergic vMAM and the lateral regions projecting to the dopaminergic ntp, this topographic arrangement resembles the projections of the medial and lateral subdivision of the mammalian LHb (2). Consequently, the efferent circuitry of these nuclei appears homologous to that of the mammalian LHb.
Fig. 4.
Separate populations of neurons in the rvHb and rdHb innervate neuromodulatory and GABAergic neurons as with the LHb in mammals. Retrogradely labeled cells in the rvHb and rdHb following injections (Neurobiotin; green) in the ntp (A), vMAM (B), dMAM (C), and periventricular hypothalamus (Hyp; D). Insets in A–D show the injection sites. (E) Schematic drawing showing the areas of rvHb and rdHb that project to the different neuromodulatory and GABAergic regions. DA, dopamine; Hist, histamine. (Scale bars: 200 μM.)
GABAergic Neurons in the dMAM Receive Input from the Homolog of the LHb and Project to Dopamine and 5-HT Neurons as with the Mammalian RMTg.
In mammals, the LHb can exert an inhibitory influence on monoaminergic neurons by projecting disynaptically via GABAergic neurons, including those in the RMTg (15, 17, 18). If the GABAergic dMAM is homologous to the mammalian RMTg, GABAergic neurons in this region should project to the dopaminergic ntp and the serotonergic vMAM. In agreement with a possible homology, GABAergic neurons in the dMAM were retrogradely labeled following injections in either the ntp (dopamine) or the vMAM (5-HT) (yellow arrows in Fig. 5). As in mammals, not all retrogradely labeled neurons in this nucleus were GABAergic (white arrows in Fig. 5B, n = 3; and Fig. 5D, n = 4). This suggests the dMAM may be homologous to the mammalian RMTg. An additional similarity between the dMAM and the RMTg is that they are both located adjacent to dopaminergic nuclei. In mammals, the RMTg is also referred to as the tail of the VTA because of its close proximity to the dopaminergic neurons in the VTA, and in the lamprey, this nucleus is adjacent to the dopaminergic ntp. This suggests that both direct habenula monoaminergic and disynaptic habenula-GABA-monoaminergic projections are present in the lamprey as they are in mammals (33, 34).
Fig. 5.
GABAergic neurons in the dMAM project to dopamine and 5-HT neurons, as with the mammalian RMTg. (A–D) Retrogradely labeled GABAergic (yellow arrows) and non-GABAergic (white arrows) cells in the dMAM following injections (Neurobiotin; green) in the vMAM (A) and ntp (C). (Scale bars: A and C, 200 μM; B and D, 50 μM).
Lateral Hypothalamic and Pallidal Projections to the Lamprey rdHb and rvHb Resemble the Afferent Input to the Mammalian LHb.
In mammals, the LHb receives major input from the lateral hypothalamus, value-related signals from the GPi/EP, and inputs from the limbic system. Retrograde tracing revealed that the lateral hypothalamus and an area referred to as the subhippocampal lobe (35) project to the lamprey rdHb (Fig. S3, n = 3). No labeling was observed in any of the regions that project to the lamprey homolog of the MHb (Fig. S3).
Anterograde tracing revealed that fibers from the subhippocampal lobe predominantly innervate the rostrolateral regions of the rdHb; this region includes the area that projects to the dopaminergic neurons (Fig. 6A, n = 4; and Fig. 6C). Labeling was also observed in the medial and caudal regions of the rdHb. In some cases, few fibers were observed in the rvHb. In contrast, fibers labeled from injections in the lateral hypothalamus predominantly projected to the rvHb and medial regions of the rdHb (Fig. 6B, n = 4; and Fig. 6C). Sparsely labeled fibers were observed in the lateral and caudal regions of the rdHb, and a few fibers were observed in the lfHb, although these latter fibers were not observed with each injection (2 of 4 injections), suggesting they may have been labeled through fibers of passage (Fig. 6 B and C).
Fig. 6.
Afferents of the lamprey homolog of the LHb (rvHb and rdHb). Neurobiotin injections (green) into the DPh (subhippocampal lobe) (A) and lateral hypothalamus (B), resulting in anterogradely labeled fibers in the rvHb and rdHb. (C) Schematic drawing of the rvHb and rdHb afferents. Injection (Neurobiotin) in the DPh (subhippocampal lobe) (D) resulting in retrogradely labeled cells in the striatum (E). (F) Schematic drawing of a sagittal section of the lamprey brain showing the location and projections of the DP and DPh. DP, dorsal pallidum; DPh, habenula projecting dorsal pallidum; Hb, habenula; Hyp, hypothalamus; Th, thalamus. (Scale bars: A and B, 200 μM; D and E, 100 μM.)
To determine if the subhippocampal lobe might represent a basal ganglia output nucleus, we injected into this nucleus to determine if it received striatal input. These injections resulted in retrogradely labeled neurons throughout the striatum, both within and surrounding the neuronally dense striatal band (Fig. 6D, n = 4; and Fig. 6E). In addition, complementary experiments showed dense anterograde labeling throughout the subhippocampal lobe after striatal injections (Fig. S3, n = 3). This suggests that the subhippocampal lobe might represent an output nucleus of the lamprey basal ganglia and not a pallial nucleus as has been suggested (36). Interestingly, this nucleus is topographically distinct from the recently characterized DP, the lamprey homolog of the GPi/globus pallidus externa (GPe) (Fig. 6F) (23). To avoid confusion, we would propose a different nomenclature for this nucleus to reflect its proposed pallidal nature and rename it as the habenula projecting dorsal pallidum (DPh).
Together, these results suggest that the afferent circuitry, with the lateral hypothalamus projecting to the medial part of the LHb and the pallidum (DPh) projecting to the lateral part of the LHb, are conserved both in the lamprey and in mammals. In contrast, the limbic innervation from the diagonal band of Broca that is seen in the mammalian LHb appears to be lacking in the lamprey.
Discussion
The habenula has been implicated in a range of basic and complex functions ranging from sleep to cognition (reviewed in 4, 37, 38). Our results show that the efferent “effector” circuitry of the habenula, including homologs of the mammalian MHb and LHb, is present in the phylogenetically oldest group of vertebrates (Fig. 7). This suggests that these circuits, despite their role in cognitive functions, form a fundamental component of all vertebrate brains and have not been significantly altered during evolution. This raises two fundamental questions. First, what are the basic functions of this circuitry? Second, how did a circuit present in the oldest vertebrates come to be used to control cognitive functions, including value-based decision making? These questions will be considered below with respect to both the MHb and LHb circuitry.
Fig. 7.
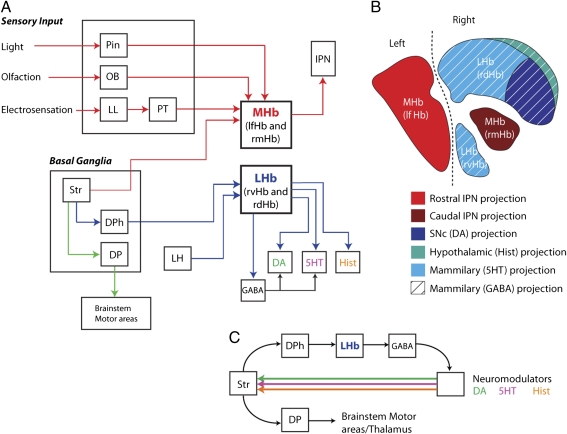
Schematic drawings showing the connectivity and topography of the lamprey homologs of the LHb (rvHb and rdHb) and MHb (lfHb and rmHb). (A) Connectivity diagram of the homologs of the MHb (lfHb and rmHb) and LHbl (rvHb and rdHb). (B) Schematic drawing of the habenula showing the locations of cells that project to the IPN, neuromodulatory systems, and the GABAergic dMAM. (C) Proposed scheme for two separate output nuclei of the basal ganglia, with one targeting the LHb (DPh) and the other targeting brainstem motor areas and the thalamus (DP). DA, dopamine; DP, dorsal pallidum; DPh, habenula projecting dorsal pallidum; Hist, histamine; LH, lateral hypothalamus; LL, lateral line receptors; OB, olfactory bulb; Pin, parapineal organ; PT, pretectum; Str, striatum.
Note on Topography.
The asymmetry of the habenula in the lamprey is similar to that of fish and amphibians but differs from mammalian species, where it is symmetrical (9). One clear difference is that the homologs of the LHb in the lamprey (rvHb and rdHb) are completely lateralized, whereas they are located bilaterally in mammals and zebrafish (2, 37). One explanation may be that the lamprey homologs of the LHb expand dorsally during development. Such a dorsal expansion of the LHb can be observed in primates (11) and appears to have occurred with the rdHb (LHb) in the lamprey. This dorsal expansion may have forced the rvHb (LHb) from the left-hand side, ventromedially, to the position where it appears on the right-hand side. Developmental studies will be needed to test this hypothesis, and we cannot rule out the possibility that the lateralization is attributable to other developmental differences.
Conservation, Function, and Evolution of the MHb Circuitry.
Our results show that nuclei homologous to the mammalian MHb are present in the lamprey. It is therefore likely that these nuclei are present in all vertebrates, because the habenula-IPN projections have been observed in all classes of vertebrates (9) and have been shown to arise specifically from the MHb and its homologs in zebrafish, lizards, and mammalian species (2, 20, 21).
The functions of the MHb are still being elucidated, but two recent studies show that genetic inactivation of the zebrafish homolog of the MHb, or its septal inputs, reduces the flight response to fearful stimuli (6, 7). This deficit was interpreted as an inability to use contextual information to switch the behavioral response to fearful stimuli. In line with this proposed role, lesions of the MHb in rodents lead to a deficit in avoidance learning and prevent animals from using contextual cues to escape a stressful situation (ref. 38 and references therein). This suggests that selecting and adapting the strategy to cope with aversive stimuli may be a conserved function of the MHb. In the lamprey, activation of the sensory regions projecting to the homolog of the MHb can also initiate behavioral responses to aversive stimuli. Specifically, activation of the lateral line (electroreceptors), which are known to be used for predator detection, results in an increase in habenula activity and initiates responses characteristic of flight and freezing behavior (27). In addition, locomotion can be initiated by application of olfactory stimuli to the mOB (28). This shows that sensory stimuli can trigger an innate behavioral response to aversive or appetitive stimuli in the lamprey, possibly via the MHb circuitry. Such innate responses to olfactory stimuli are also observed in zebrafish, where exposure to a substance from zebrafish skin extract can induce freezing behavior (39). In this species, as with the lamprey, there are direct olfactory projections to the homolog of the MHb that may control this behavior (40). In contrast, the MHb in mammalian species does not appear to receive input directly from sensory areas; rather, the major input arises from the septum (fimbrialis septi and triangularis septi) (1). These septal nuclei receive the majority of their input from the hippocampus (41, 42), and lesions of the habenula lead to deficits in hippocampal-dependent tasks, such as spatial learning (5). This may suggest that the mammalian MHb receives contextual information from the hippocampus, which could be used to elicit a behavioral response to a learned aversive condition as opposed to an innate response to particular sensory stimuli.
The topographic organization of the MHb-IPN circuitry appears to be an additional feature that is conserved in vertebrates, although the topographic arrangement differs across species (2, 21). This is clearly observed in zebrafish, where the left and right MHb homologs project to the dorsal and ventral IPN, respectively (21), in contrast to the lamprey, where the lfHb and rmHb innervate the rostral and caudal IPN, respectively. In zebrafish, the ventral and dorsal parts of the IPN are known to project to the median raphe and griseum centrale, respectively (7). The griseum centrale is thought to contain areas homologous to the periaqueductal gray (37), which, in mammalian species, contains discrete populations of neurons that can initiate different responses to stress, such as fight, flight, or freezing behaviors and vocalizations (43, 44). This suggests that topographic relationships between the MHb-IPN and the brainstem regions is conserved and may provide a mechanism by which different populations in the MHb can initiate different behavioral reactions to aversive stimuli through parallel pathways.
In summary, our results suggest that the MHb circuitry is conserved in all vertebrates, likely providing a common mechanism that adapts the behavioral strategy to aversive stimuli. Our results suggest that there has been a change in the afferent regulation of this circuitry during evolution, with contextual information from the hippocampus taking on an important role and probably replacing the direct sensory innervation that is seen in the lamprey and in fish (40). Such a switch may allow mammals to adapt their behavior flexibly in response to the contextual situation instead of responding innately to a given stimuli.
Conservation, Function, and Evolution of the LHb Circuitry.
It has been suggested that a homolog of the LHb was lacking in the larval lamprey (24), because projections to an area homologous to the mammalian SNc/VTA were not found. However, projections to the region containing the ntp were observed, and subsequent studies have shown that this nucleus is homologous to the SNc/VTA (29). This suggests that the circuitry we observe may also be present in the larval lamprey. Studies of the efferent connections of nonmammalian homologs of the LHb have failed to demonstrate the presence of direct habenula projections to dopamine, serotonin, or other neuromodulatory neurons in the midbrain and hypothalamus; in addition, indirect habenula-GABA-neuromodulatory projections have not been observed. Specifically, no projections from the homolog of the LHb to dopaminergic, histaminergic, or GABAergic nuclei have been observed in zebrafish (38). In lizards, however, habenula projections to the dopaminergic ventral tegmentum, raphe nuclei, and hypothalamus are observed, suggesting that the circuitry observed in the lamprey and in mammals may also be present in reptiles (20). Consequently, the lack of these projections in zebrafish may be unique to this species, or even to teleosts, or these projections may exist but remain to be demonstrated. This suggests that as with the MHb circuitry, the LHb and its efferent circuitry constitute a fundamental component of the vertebrate brain.
The mammalian LHb is thought to play a key role in cognitive functions, such as value-based decision making, because of its regulation of monoaminergic neurons in response to reward (4). Our results show that the complete LHb efferent circuitry is present in the lamprey. This suggests that this circuitry is fundamental even for vertebrates that presumably lack higher cognitive functions and could contribute to value-based decision making during simple behaviors that are common to all vertebrates, such as foraging. In mammals, neurons in both the LHb and the RMTg respond to reward in an opposite manner to dopaminergic neurons because they are excited by aversive stimuli or the absence of expected reward (12, 14). Stimulation and lesion studies have demonstrated that the mammalian LHb exerts an inhibitory influence on monoaminergic neurons via a disynaptic pathway thought to involve the GABAergic RMTg (13, 17, 18, 45, 46). Consequently, the LHb-RMTg (GABA)-neuromodulatory pathway is thought to contribute to reinforcement learning by suppressing dopamine, the reinforcing signal (47), in response to aversive stimuli or reward prediction errors. The presence of this pathway in the lamprey suggests that this pathway may be conserved as a common mechanism for reward-based learning that allows vertebrates to suppress actions that lead to unwanted consequences.
Our results also indicate that the afferent control of this circuitry is conserved, because the two major inputs to the mammalian LHb, the lateral hypothalamus and a pallidal nucleus, are also present in the lamprey. It is likely that this afferent circuitry is present in all vertebrates, because the lateral hypothalamus and pallidum project to the habenula in fish, amphibians, and mammals (1, 48–50), but experiments are needed to confirm that these nuclei project selectively to the homologs of the LHb in fish and amphibians. Interestingly, our results suggest that the DPh is topographically distinct from the DP, the lamprey homolog of the GPi/EP (23). Consequently, this nucleus may represent an additional output nucleus of the basal ganglia that is present in parallel with the homologs of the GPi, GPe, and substantia nigra pars reticulata. In contrast to the DP, where neurons are GABAergic, the majority of neurons in the DPh are glutamatergic (34), although a few GABAergic (31) and cholinergic (51) neurons are also located in this area. Consequently, these nuclei appear to be distinct both in their projections and molecular expression. In primates, single-cell tracing studies have also demonstrated that there are two classes of pallidal neurons in the GPi, one that projects selectively to the habenula and another that projects to thalamic and brainstem motor areas (52). The habenula projecting pallidal neurons are located around the edge and through the middle of the GPi, whereas the thalamic projecting neurons form the core of the nucleus. This suggests that as in the lamprey, these neurons comprise two distinct populations. Recent electrophysiological recordings in primates have shown that these two classes of pallidal neurons also encode different types of information, because the habenula projecting pallidal neurons respond to reward-related information; for example, one type is excited by aversive stimuli or to cues that predict an adverse outcome (11). In contrast, the thalamic projecting GPi neurons respond to aspects of movement kinematics (53). Although these populations have been examined to a lesser extent in other rodents, it also appears that they may be separate, because the habenula projecting neurons are located in the rostral two-thirds of the EP and at least some of these projection neurons are cholinergic (54, 55). In contrast to this, a study that examined the axonal projections of thalamic projecting EP neurons demonstrated that a few thalamic projecting EP neurons also project to the LHb (56). However, whether there are also pallidal neurons that selectively innervate the habenula was not examined in this study. Taken together, these results suggest that at least in the lamprey and in primates, the habenula and thalamic projecting pallidal neurons represent distinct populations that differ in their topographic location; their molecular expression; the information they encode; and, of course, their projections. The presence of a pallidal projection to the habenula in the lamprey and all other vertebrates studied suggests that this pathway is conserved as a potential source of value-related signals to the LHb. Because of the differences in the DPhs and thalamic projecting pallidal nuclei in the lamprey and in primates, we propose that the vertebrate pallidum may be composed of two kinds of output nuclei: one targeting the habenula (DPh) with value-related signals and another targeting the brainstem and thalamic motor areas (DP/GPi/EP) with action signals.
In addition to the indirect habenula-GABA-neuromodulator projections, our results indicate that direct habenula-neuromodulator projections are part of the conserved habenula circuitry. In mammals, these direct habenula connections to monoaminergic neurons have been confirmed by ultrastructural analysis; as with the lamprey, they have been shown to arise from separate populations in the LHb (33, 57). Our results indicate that a separate population of LHb neurons also projects to histaminergic neurons in the hypothalamus. A projection from the LHb to the histaminergic tuberomammillary nucleus has also been demonstrated in rodents, but no direct projections to histaminergic neurons have been reported (58). In addition, anterogradely labeled fibers from the LHb have been shown to project to the hypothalamus in amphibians and mammals (58, 59). This suggests that the LHb may have an as yet unappreciated role in regulating the histaminergic system, along with the dopaminergic and serotonergic systems. Direct projections to each of these neuromodulatory neurons may account for the sparse evidence that the habenula can excite monoaminergic neurons, because these projections arise from glutamatergic habenula neurons (60–62). This leads to the interesting possibility that separate populations of LHb neurons may differentially regulate the firing of neuromodulatory neurons, exciting or inhibiting them in response to different stimuli.
In summary, our results show that the complete mammalian LHb circuitry is conserved in a nonmammalian vertebrate, likely as a common mechanism by which all vertebrates regulate their different neuromodulatory systems. The alterations in dopaminergic firing, caused by the LHb, are thought to contribute to reward learning by altering the efficacy of corticopalliostriatal synapses (63). Because the complete basal ganglia circuitry has also recently been shown to be present in the lamprey (23), this suggests that the habenula, neuromodulatory system, and basal ganglia, together, may have formed evolution's blueprint for a vertebrate action selection and reinforcement learning mechanism.
Conclusion
To survive, all animals need to adapt their behavior in response to rewards, stress, fearful stimuli, and other motivating factors. Our results suggest that the habenula circuitry, including homologs of the mammalian MHb and LHb, are conserved throughout vertebrate phylogeny and may provide a common mechanism for these functions, with the LHb and its homologs providing a circuitry that can affect the neuromodulatory system to adapt behavior in response to rewards, disappointment, and other motivating stimuli. Parallel circuitry through the MHb and its homologs provides a pathway, through the IPN, by which contextual or sensory information could influence brainstem regions involved in initiating the behavioral response to aversive stimuli. We suggest that together with the neuromodulatory system and the basal ganglia, this habenula circuitry provides part of the fundamental neural basis that all vertebrates use to select actions and adapt them in response to motivating stimuli and the contextual situation.
Materials and Methods
Experiments were performed on a total of 84 adult river lampreys (Lampetra fluviatilis). Information on ethical committee approval and guidelines adhered to is available in SI Materials and Methods.
Anatomy.
The dissection, injections, fixation, and sectioning of the lamprey brains for anatomical experiments were performed as previously described (64) (SI Materials and Methods). Neurobiotin [20% (wt/vol) in distilled water; Vector] was pressure-injected unilaterally into the habenula, lfHb, right habenula, rdHb, ntp, lateral hypothalamus, periventricular hypothalamus, dMAM and vMAM, IPN, mOB, striatum, subhippocampal lobe, and pretectum.
Neurobiotin was demonstrated by incubation with streptavidin conjugated to different fluorophores (1:1,000; Jackson ImmunoResearch).
Immunohistochemistry.
Primary antibodies against calbindin (Sigma–Aldrich), tyrosine hydroxylase (Millipore), 5-HT (Immuno Star, Inc.), histamine (kindly donated by Pertti Panula, University of Helsinki, Helsinki, Finland), and GABA (kindly donated by Peter Streit, Brain Research Institute, Zurich, Switzerland) were used in this study. Sections were subsequently incubated with different secondary antibodies (1:500; Jackson ImmunoResearch Laboratories) and Neurotrace (1:500; Molecular Probes) (details on primary and secondary antibodies used are available in SI Materials and Methods).
Analysis.
Photomicrographs of key results were taken with a Zeiss Axiocam (Carl Zeiss AB) or an Olympus XM10 (Olympus Sverige AB) digital camera. Illustrations were prepared using Adobe Illustrator and Adobe Photoshop CS2 (both from Adobe Systems, Inc.). Images were only adjusted for brightness and contrast.
Confocal Z-stacks of the sections were obtained using a Zeiss Laser scanning microscope 510 (Carl Zeiss AB), and the projection images were processed using Zeiss LSM software (Carl Zeiss AB) and Adobe Photoshop CS2.
Supplementary Material
Acknowledgments
This work was funded by Grant EU FP7 “Lampetra” 21610 (to S.G.), Swedish Research Council Grants VR-M 3026 and VR-NT 621-2007-6049 (to S.G), Karolinska Institute research funds (to B.R.), FP7 Select-and-Act (S.G.), and the European Union Cortex Training Program (M.S.-J.).
Footnotes
The authors declare no conflict of interest.
See Author Summary on page 667.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119348109/-/DCSupplemental.
References
- 1.Herkenham M, Nauta WJ. Afferent connections of the habenular nuclei in the rat. A horseradish peroxidase study, with a note on the fiber-of-passage problem. J Comp Neurol. 1977;173:123–146. doi: 10.1002/cne.901730107. [DOI] [PubMed] [Google Scholar]
- 2.Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland RJ. The dorsal diencephalic conduction system: A review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982;6:1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 4.Hikosaka O. The habenula: From stress evasion to value-based decision-making. Nat Rev Neurosci. 2010;11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lecourtier L, Neijt HC, Kelly PH. Habenula lesions cause impaired cognitive performance in rats: Implications for schizophrenia. Eur J Neurosci. 2004;19:2551–2560. doi: 10.1111/j.0953-816X.2004.03356.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee A, et al. The habenula prevents helpless behavior in larval zebrafish. Curr Biol. 2010;20:2211–2216. doi: 10.1016/j.cub.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 7.Agetsuma M, et al. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- 8.Valjakka A, et al. The fasciculus retroflexus controls the integrity of REM sleep by supporting the generation of hippocampal theta rhythm and rapid eye movements in rats. Brain Res Bull. 1998;47:171–184. doi: 10.1016/s0361-9230(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 9.Concha ML, Wilson SW. Asymmetry in the epithalamus of vertebrates. J Anat. 2001;199(Pt 1-2):63–84. doi: 10.1046/j.1469-7580.2001.19910063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianco IH, Wilson SW. The habenular nuclei: A conserved asymmetric relay station in the vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jhou TC, Geisler S, Marinelli M, Degarmo BA, Zahm DS. The mesopontine rostromedial tegmental nucleus: A structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J Comp Neurol. 2009;513:566–596. doi: 10.1002/cne.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang RY, Aghajanian GK. Physiological evidence for habenula as major link between forebrain and midbrain raphe. Science. 1977;197(4298):89–91. doi: 10.1126/science.194312. [DOI] [PubMed] [Google Scholar]
- 17.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amo R, et al. Identification of the zebrafish ventral habenula as a homolog of the mammalian lateral habenula. J Neurosci. 2010;30:1566–1574. doi: 10.1523/JNEUROSCI.3690-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Distel H, Ebbesson SO. Habenular projections in the monitor lizard (Varanus benegalensis) Exp Brain Res. 1981;43:324–329. doi: 10.1007/BF00238374. [DOI] [PubMed] [Google Scholar]
- 21.Aizawa H, et al. Laterotopic representation of left-right information onto the dorso-ventral axis of a zebrafish midbrain target nucleus. Curr Biol. 2005;15:238–243. doi: 10.1016/j.cub.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Hedges SB. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson-Jones M, Samuelsson E, Ericsson J, Robertson B, Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Yañez J, Anadon R. Afferent and efferent connections of the habenula in the larval sea lamprey (Petromyzon marinus L.): An experimental study. J Comp Neurol. 1994;345:148–160. doi: 10.1002/cne.903450112. [DOI] [PubMed] [Google Scholar]
- 25.Séquier JM, Hunziker W, Andressen C, Celio MR. Calbindin D-28k Protein and mRNA Localization in the Rat Brain. Eur J Neurosci. 1990;2:1118–1126. doi: 10.1111/j.1460-9568.1990.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 26.Yáñez J, Pombal MA, Anadón R. Afferent and efferent connections of the parapineal organ in lampreys: A tract tracing and immunocytochemical study. J Comp Neurol. 1999;403:171–189. doi: 10.1002/(sici)1096-9861(19990111)403:2<171::aid-cne3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Chung-Davidson YW, Yun SS, Teeter J, Li W. Brain pathways and behavioral responses to weak electric fields in parasitic sea lampreys (Petromyzon marinus) Behav Neurosci. 2004;118:611–619. doi: 10.1037/0735-7044.118.3.611. [DOI] [PubMed] [Google Scholar]
- 28.Derjean D, et al. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010;8:e1000567. doi: 10.1371/journal.pbio.1000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pombal MA, El Manira A, Grillner S. Afferents of the lamprey striatum with special reference to the dopaminergic system: A combined tracing and immunohistochemical study. J Comp Neurol. 1997;386:71–91. [PubMed] [Google Scholar]
- 30.Abalo XM, et al. Development of the serotonergic system in the central nervous system of the sea lamprey. J Chem Neuroanat. 2007;34(1-2):29–46. doi: 10.1016/j.jchemneu.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Brodin L, Hökfelt T, Grillner S, Panula P. Distribution of histaminergic neurons in the brain of the lamprey Lampetra fluviatilis as revealed by histamine-immunohistochemistry. J Comp Neurol. 1990;292:435–442. doi: 10.1002/cne.902920309. [DOI] [PubMed] [Google Scholar]
- 32.Robertson B, Auclair F, Ménard A, Grillner S, Dubuc R. GABA distribution in lamprey is phylogenetically conserved. J Comp Neurol. 2007;503:47–63. doi: 10.1002/cne.21348. [DOI] [PubMed] [Google Scholar]
- 33.Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J Comp Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villar-Cerviño V, Barreiro-Iglesias A, Mazan S, Rodicio MC, Anadón R. Glutamatergic neuronal populations in the forebrain of the sea lamprey, Petromyzon marinus: An in situ hybridization and immunocytochemical study. J Comp Neurol. 2011;519:1712–1735. doi: 10.1002/cne.22597. [DOI] [PubMed] [Google Scholar]
- 36.Northcutt RG, Puzdrowski RL. Projections of the olfactory bulb and nervus terminalis in the silver lamprey. Brain Behav Evol. 1988;32(2):96–107. doi: 10.1159/000116537. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto H, Agetsuma M, Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev Neurobiol. 2011 doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- 38.Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Jesuthasan SJ, Mathuru AS. The alarm response in zebrafish: Innate fear in a vertebrate genetic model. J Neurogenet. 2008;22:211–228. doi: 10.1080/01677060802298475. [DOI] [PubMed] [Google Scholar]
- 40.Miyasaka N, et al. From the olfactory bulb to higher brain centers: Genetic visualization of secondary olfactory pathways in zebrafish. J Neurosci. 2009;29:4756–4767. doi: 10.1523/JNEUROSCI.0118-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nauta WJ. Hippocampal projections and related neural pathways to the midbrain in the cat. Brain. 1958;81:319–340. doi: 10.1093/brain/81.3.319. [DOI] [PubMed] [Google Scholar]
- 42.Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian HH, Balnave RJ, Holstege G. The midbrain periaqueductal gray control of respiration. J Neurosci. 2008;28:12274–12283. doi: 10.1523/JNEUROSCI.4168-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 45.Nishikawa T, Fage D, Scatton B. Evidence for, and nature of, the tonic inhibitory influence of habenulointerpeduncular pathways upon cerebral dopaminergic transmission in the rat. Brain Res. 1986;373:324–336. doi: 10.1016/0006-8993(86)90347-1. [DOI] [PubMed] [Google Scholar]
- 46.Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188(1):84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 47.Schultz W. Dopamine signals for reward value and risk: Basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yañez J, Anadón R. Afferent and efferent connections of the habenula in the rainbow trout (Oncorhynchus mykiss): An indocarbocyanine dye (DiI) study. J Comp Neurol. 1996;372:529–543. doi: 10.1002/(SICI)1096-9861(19960902)372:4<529::AID-CNE3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 49.Marín O, González A, Smeets WJ. Basal ganglia organization in amphibians: Efferent connections of the striatum and the nucleus accumbens. J Comp Neurol. 1997;380:23–50. [PubMed] [Google Scholar]
- 50.Bruce LL, Neary TJ. Afferent projections to the lateral and dorsomedial hypothalamus in a lizard, Gekko gecko. Brain Behav Evol. 1995;46(1):30–42. doi: 10.1159/000113256. [DOI] [PubMed] [Google Scholar]
- 51.Pombal MA, Marín O, González A. Distribution of choline acetyltransferase-immunoreactive structures in the lamprey brain. J Comp Neurol. 2001;431:105–126. doi: 10.1002/1096-9861(20010226)431:1<105::aid-cne1058>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 52.Parent M, Lévesque M, Parent A. Two types of projection neurons in the internal pallidum of primates: Single-axon tracing and three-dimensional reconstruction. J Comp Neurol. 2001;439:162–175. doi: 10.1002/cne.1340. [DOI] [PubMed] [Google Scholar]
- 53.Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- 54.Rajakumar N, Elisevich K, Flumerfelt BA. Compartmental origin of the striato-entopeduncular projection in the rat. J Comp Neurol. 1993;331:286–296. doi: 10.1002/cne.903310210. [DOI] [PubMed] [Google Scholar]
- 55.Moriizumi T, Hattori T. Choline acetyltransferase-immunoreactive neurons in the rat entopeduncular nucleus. Neuroscience. 1992;46:721–728. doi: 10.1016/0306-4522(92)90158-x. [DOI] [PubMed] [Google Scholar]
- 56.Kha HT, Finkelstein DI, Pow DV, Lawrence AJ, Horne MK. Study of projections from the entopeduncular nucleus to the thalamus of the rat. J Comp Neurol. 2000;426:366–377. [PubMed] [Google Scholar]
- 57.Li B, et al. Synaptic potentiation onto habenula neurons in the learned helplessness model of depression. Nature. 2011;470:535–539. doi: 10.1038/nature09742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kiss J, Csáki A, Bokor H, Kocsis K, Kocsis B. Possible glutamatergic/aspartatergic projections to the supramammillary nucleus and their origins in the rat studied by selective [(3)H]D-aspartate labelling and immunocytochemistry. Neuroscience. 2002;111:671–691. doi: 10.1016/s0306-4522(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 59.Neary TJ. Afferent projections to the hypothalamus in ranid frogs. Brain Behav Evol. 1995;46(1):1–13. doi: 10.1159/000113254. [DOI] [PubMed] [Google Scholar]
- 60.Matsuda Y, Fujimura K. Action of habenular efferents on ventral tegmental area neurons studied in vitro. Brain Res Bull. 1992;28:743–749. doi: 10.1016/0361-9230(92)90254-u. [DOI] [PubMed] [Google Scholar]
- 61.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geisler S, Wise RA. Functional implications of glutamatergic projections to the ventral tegmental area. Rev Neurosci. 2008;19:227–244. doi: 10.1515/revneuro.2008.19.4-5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Jones MR, Grillner S, Robertson B. Selective projection patterns from subtypes of retinal ganglion cells to tectum and pretectum: Distribution and relation to behavior. J Comp Neurol. 2009;517:257–275. doi: 10.1002/cne.22203. [DOI] [PubMed] [Google Scholar]