Abstract
Novelty processing can transform short-term into long-term memory. We propose that this memory-reinforcing effect of novelty could be explained by mechanisms outlined in the “synaptic tagging hypothesis.” Initial short-term memory is sustained by a transient plasticity change at activated synapses and sets synaptic tags. These tags are later able to capture and process the plasticity-related proteins (PRPs), which are required to transform a short-term synaptic change into a long-term one. Novelty is involved in inducing the synthesis of PRPs [Moncada D, et al. (2011) Proc Natl Acad Sci USA 108:12937–12936], which are then captured by the tagged synapses, consolidating memory. In contrast to novelty, stress can impair learning, memory, and synaptic plasticity. Here, we address questions as to whether novelty-induced PRPs are able to prevent the loss of memory caused by stress and if the latter would not interact with the tag-setting process. We used water-maze (WM) training as a spatial learning paradigm to test our hypothesis. Stress was induced by a strong foot shock (FS; 5 × 1 mA, 2 s) applied 5 min after WM training. Our data show that FS reduced long-term but not short-term memory in the WM paradigm. This negative effect on memory consolidation was time- and training-dependent. Interestingly, novelty exposure prevented the stress-induced memory loss of the spatial task and increased BDNF and Arc expression. This rescuing effect was blocked by anisomycin, suggesting that WM-tagged synapses were not reset by FS and were thus able to capture the novelty-induced PRPs, re-establishing FS-impaired long-term memory.
Keywords: memory rescue, memory reinforcement, novelty exploration
Learning and memory are important to survive in a changing environment and use a limited, predetermined neural subset to integrate various stimuli. Memory formation includes widespread brain regions and consists of sequential and parallel events that define memory phases (1–4). Underlying mechanisms are based on the activation of preexisting proteins during an early, labile phase (short-term memory, STM) and the synthesis of new proteins for a late, stable phase (long-term memory, LTM) (1, 5). These cellular processes can be activated by a single experience or by two independent, temporally coupled events. Similar mechanisms seem to be involved in long-term potentiation (LTP), a model of synaptic plasticity and cellular memory. Thus, motivation and emotion can reinforce a normally transient, early LTP (E-LTP) into a prolonged, late-LTP (L-LTP), a phenomenon known as behavioral LTP-reinforcement (3). Novelty exploration in an open field (OF) can also effectively reinforce LTP (6). Behavioral LTP-reinforcement is protein synthesis-dependent (7) and basolateral amygdala-stimulation mimics the reinforcing effects of distinct behavioral stimuli (8, 9). LTP reinforcement by behavioral stimuli has been explained by the synaptic-tagging hypothesis (STH) (3). According to this theory, LTP-induction results not only in transient modifications of synaptic efficacy, but can also set tags at activated synapses, which can capture plasticity-related proteins (PRPs) required to consolidate L-LTP. At the behavioral level, it was shown that STM can be prolonged into LTM if, shortly before or after training, animals explored a novel environment (10, 11). This novelty effect on memory consolidation was protein synthesis-dependent (10, 11). The authors propose that training induces “learning tags” that capture the proteins required for memory consolidation, and novelty exploration induces the required PRP-synthesis.
Recently, we investigated how different combinations of LTP- and long-term depression (LTD)-inducing paradigms interact at the same synapses (12). After LTP-depotentiation, using low-frequency stimulation (which usually results in LTD), synapses became temporarily refractory to novel LTP-induction, obviously competing with LTD-processes. If a behavioral reinforcing stimulus was applied during that time, no LTP-reinforcement was seen, suggesting that depotentiation also resets synaptic LTP-tags (13), although PRPs were made available by the behavioral stimulus. If E-LTP was induced beyond the refractory period, the formerly applied behavioral stimulus was able to reinforce E-LTP into L-LTP. We suggested that LTP and LTD compete at the same synapses, but affective modulation can influence the final outcome (12).
Here we expanded our work to the organism level. We investigated if memory can be recovered after being disrupted by foot shock (FS), using different temporal patterns of a combination of training, FS, and exploration. The cellular mechanisms involved were also studied blocking protein synthesis and evaluating the expression of PRP-associated gene candidates.
Results
Water-Maze Training.
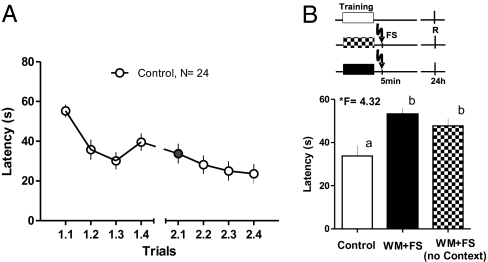
First, we measured the 24-h retention of the spatial memory trace after four training trials in the water maze (WM). Fig. 1A shows the performance during training and retention (control, n = 24). Average latency during the first trial in the retention test was 33.57 ± 4.99 s, a value that appears to be convenient to demonstrate effects of memory-impairing or -facilitating paradigms. The animals also learned during the second session 24 h later, as can be seen in the figure. Therefore, to evaluate retention we used only the latencies in the first trial of the second session to evaluate retention (filled circle in Fig. 1A) under different conditions and manipulations.
Fig. 1.
WM training and the effect of FS. (A) WM training was able to produce appreciable learning, which was maintained up to 24 h after training. Data presented show the averaged escape latency mean and SEM of each trial per day. Trials are identified by two numbers: training day (1 or 2) and trial (1–4). Trial 2.1 (filled circle) was used as a retrieval test in all of the following experiments. (B) WM training and FS. FS application 5 min after the fourth trial interfered with memory at the second day. Two FS groups were tested, one (WM+FS, n = 15) in which the animals remained in the box 30 s before FS application, and another in which the animals received FS immediately after entering the box (WM+FS no context, n = 8) to evaluate the possible influence of contextual learning on the FS effect. Escape latencies increased in both FS groups in the retrieval test 24 h later. (Upper) The experimental design for each group is presented (shaded boxes: training; R: time for retrieval, FS: time for FS). Means and SEM are shown. F values from ANOVA are given, and the post hoc Duncan's test results indicated by lowercase letters (a and b).
FS Impairs Retention in the WM in a Time-Dependent Manner.
Next, we tested the effects of an electrical FS, applied 5 min after training, to interfere with memory retention at 24 h (Fig. 1) (trial 2.1; WM+FS, n = 15).
Fig. 1B shows that FS impaired memory retention at 24 h, as expressed by the higher escape latency. The memory-impairing effect is the same when FS was applied after a 30-s rest period in the punishment box, or when it was applied immediately after entering the box. The ANOVA (*F = 4.32) showed differences among groups. The post hoc Duncan's test showed differences between the control animals and both FS groups, but no differences between the two FS groups.
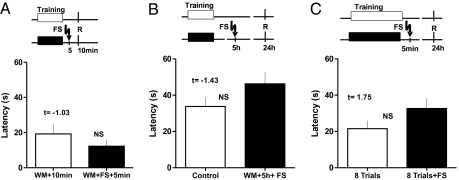
The impairing effects of FS were dependent on the timing between training, FS, and retention test. If the animals were tested for retention 5 min after FS (MWM+FS+5 min, n = 10), their performance was similar to the control group, as shown in Fig. 2A (WM+10 min, n = 10; Student t test, t = −1.03). Application of the FS 5 h after training (Fig. 2B) (WM+5 h+FS, n = 10) did not affect the escape latency (Student t test, t = −1.43), suggesting that the memory trace was not labile at that time.
Fig. 2.
Temporal and training-dependent constrains to FS effects on memory. (A) FS application 5 min after the fourth trial did not interfere with STM because the escape latency was similar to the control group when the retrieval test was performed 5 min later. Data for the group tested at 10 min after finishing the training (WM+10 min) and animals that received FS 5 min after the training and tested 5 min thereafter (WM+FS+5 min) are presented. (B) If application of FS was delayed for 5 h after finishing the fourth trial, no interference with memory was observed because the escape latency was similar to the control group in the retrieval test 24 h later. The control group (Control) and animals with FS 5 h after the training (WM+5 h+FS) are shown. (C) FS after the eighth trial. FS application 5 min after eight training trials did not interfere with memory because the escape latency was similar to the eight-trial group in the retrieval test 24 h later. The control eight-trial group (8 Trials) and animals that received FS 5 min after the training (8WM+FS) are shown. (Upper) The experimental design for each group is presented (shaded boxes: training; R: time for retrieval, FS: time for FS). Means and SEM are shown. F values from ANOVA are given, NS, no significant differences.
Finally, when WM training was prolonged to eight trials (8 Trials+FS, n = 12) (Fig. 2C), no impairing effects of FS on memory were observed. There were no significant differences when latencies were compared with the control group (8 Trials, n = 10; Student t test, t = 1.75).
Novelty Exploration Prevents the Memory Impairment by FS.
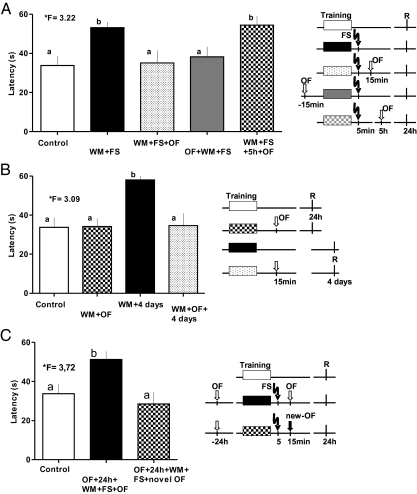
We then assessed if novelty exploration was able to re-establish memory, which was impaired by FS. Exploration of a novel environment (for 3 min) 15 min before or after WM training reduced the escape latency to control levels in the first trial of the retention test, suggesting a recovery of the FS-impaired memory (Fig. 3A). Latencies to escape from water did not significantly differ between control group and the FS groups that explored the OF in temporal vicinity to training (15 min after, WM+FS+OF, n = 15; or 15 min before, OF+WM+FS, n = 13), but they did differ from those that received only the FS. The ability of OF exploration to protect or recover memory was time-dependent. OF exploration was unable to recover memory when applied 5 h after training (WM+FS+5 h+OF, n = 9). The retention of this group was not different from those that received only the FS, but was significantly different from control animals and both OF exploring groups with 15-min intervals (one-way ANOVA, *F = 3.22, followed by a post hoc Duncan's test).
Fig. 3.
Effect of exploration of novel environment on memory affected by FS. (A) Novelty scenery exploration in the OF 10 min after FS or 15 min before spatial training in the WM was able to protect memory from the FS-impairing effects because both groups (WM+FS+OF; OF+WM+FS) were similar to the control group, but different from the WM+FS group. This effect was time-dependent because OF 5 h later (WM+FS+5 h+OF) did not benefit memory recovery. (B) Spatial memory could be stabilized or prolonged for up to 4 d (WM+OF+4 days; statistically different from the WM+4 days group; and similar to control group) by novelty exploration. (C) Previous habituation to OF did not produce a memory recovery effect when the animals were put into the same OF 15 min after the training (OF+24 h+WM+FS+OF). However, if the second OF was changed by a new one, the memory-preserving effect of exploration was re-established (OF+24 h+WM+FS+novel OF). (Right) The experimental design for each group is presented (shaded boxes: training; R: time for retrieval, FS: time of FS). Means and SEM are shown. F-values from ANOVA are given; lowercase letters identify similar and different values after the Duncan's test.
The exploration of a novel OF was able to promote memory retention also in the absence of the FS. Fig. 3B shows that OF exploration reduced the escape latency evaluated 4 d after training (WM+4 d, n = 10 vs. WM+OF+4 d, n = 12). Note, however, that retention at 24 h did not differ in OF-exposed and control animals (Control vs. WM+OF), suggesting that OF exploration prolonged the memory trace (one-way ANOVA, *F = 3.09, post hoc Duncan's test).
To confirm that the effect was caused by novelty exploration in the OF scenery, we habituated a group of animals in the OF apparatus 24 h before the training. Animals were exposed four times to 3-min OF exploration with 5-min intertrial intervals (OF+24 h+WM+FS+OF, n = 10). Our data show (Fig. 3C) that the escape latency was significantly higher compared with the control group, indicating that the protective effect of OF exploration on memory disruption by FS was lost when the novelty value of the arena was reduced by previous habituation (one-way ANOVA, *F = 3.72, post hoc Duncan's test). Moreover, if the animals were habituated to one OF arena before training and placed to explore a new OF arena after training and FS (OF+24 h+WM+FS+novel OF, n = 10), the animals showed an escape latency comparable to the control group (Fig. 3C).
Novelty Exploration Effect on Memory Was Protein Synthesis-Dependent.
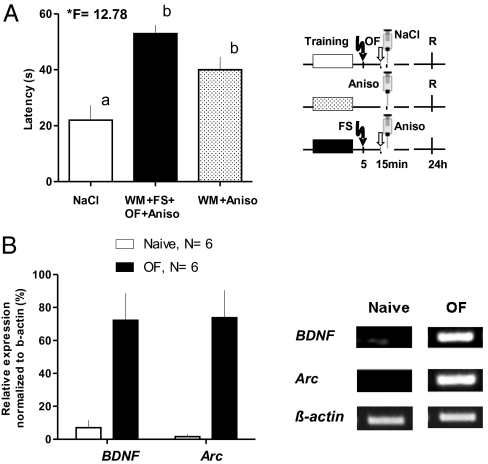
We also addressed the question if the effect of novelty exploration in the OF on memory was protein synthesis-dependent. Fig. 4A shows that anisomycin, a reversible blocker of protein synthesis, injected 15 min after WM training (WM+Aniso, n = 8) impairs retention at 24 h. In the same way, treatment after training, FS, and novelty exploration (WM+FS+OF+Aniso, n = 13) abolishes the recovery of memory by OF, supporting the theory that the effect of novelty exploration on memory was mediated by protein synthesis. Both groups differed significantly from the saline control (NaCl, n = 10; ANOVA *F = 12.78).
Fig. 4.
Protein synthesis-dependency and gene expression by OF exploration. (A) Application of anisomycin immediately after exploration the OF (WM+FS+OF+Aniso) prevented the memory-preserving effect of OF exploration compared with controls (NaCl). When anisomycin was applied 15 min after training in the absence of FS or OF a similar result was obtained, confirming the amnesic effect of protein synthesis inhibition. (Right) The experimental design for each group is presented (abbreviations as in Fig. 3). Means and SEM are shown. F-values from ANOVA are given; lowercase letters identify similar and different values after the Duncan's test. (B) Semiquantitative RT-PCR allowed the identification and verification of an increase in gene expression for Arc and BDNF in those animals that were exposed to novelty scenery for 3-min spontaneous exploration. (Right) Representative examples for bands of naive rats (Naive) and for explorer animals (OF) for three genes are shown: Arc and BDNF as immediate-early genes, and β-actin as control.
Novelty Exploration Induces Gene Expression.
Finally, we studied the possible participation of Arc and BDNF gene expression, as potential molecular players in the mechanisms of memory rescue by OF exploration. The findings are shown in Fig. 4B. Arc and BDNF gene expression was increased in the hippocampus of all of the animals that performed a spontaneous exploration in the OF for 3 min (OF, n = 6) compared with naive animals not exposed to OF (Naive, n = 6).
Discussion
Exploration of a novel environment in OF is able to convert a STM into a LTM trace (11). We have now studied if it could also recover the spatial-memory impairment caused by FS in a spatial-memory model. We show that four trials in the WM were sufficient for rats to learn to find the platform during training. This LTM trace was preserved for 24 h. FS impaired the consolidation of spatial LTM in the WM but not STM. This impairment was dependent on the timing between WM training and FS, as well as the training intensity. When memory was measured 5 min after FS, the animals performed similar to the control group; however, LTM was prevented at 24 h. FS application 5 h after training did not affect LTM measured 24 after training. Similarly, FS application after a more intense training (eight trials) was not able to block memory at 24 h. FS is a stressful event, and stress impairs spatial memory and related cellular processes, such as LTP (14, 15). An alternative explanation would be that impairment was produced by a concurrent memory process of contextual fear-conditioning initiated by exposure and punishment in the FS box. Rosen et al. (16) have shown that applying FS immediately after placing the animals in the box prevents contextual fear conditioning. Using this approach, we found that our results showed no significant change in the impairing effect of FS, reducing the likelihood of the memory-competition hypothesis and favoring the stressful effects of FS as the causal factor for the spatial-memory impairment.
Our results agree with the consolidation theory (17). FS shortly after training interfered with consolidation of LTM, although it did not affect short-term storage mechanisms. When FS was applied hours later, LTM was already established and not affected by FS anymore (18, 19). Prolonged training (eight trials) created a stronger memory trace. The longer training probably allowed some consolidation during the prolonged time of training, finally being refractory to FS. It does not seem likely that FS affected retrieval 24 h later by mechanisms different from impairing memory consolidation. FS can directly affect retrieval of a consolidated memory trace only in a short period (less than 30 min) but not after 4 h (20).
Our central question was whether exposing the animal to a novel environment, before or after training and FS, was able to prevent the memory-impairing effect of FS. Novelty exploration in an OF is effective to enhance memory and hippocampal LTP (6, 10, 11). We now show that novelty exploration was also able to protect memory from FS-induced impairments. Exploration of a novel environment 15 min before or after training reduced the escape latency to control levels in the first trial of the retention test, despite the application of FS after training. Furthermore, the main factor modulating memory consolidation seemed to be novelty and not simply exploration or movement, because exploration of a familiar environment did not produce the same memory-protective effect. If the familiar OF box was exchanged by a different one (color, ground, cues), the protective effect reappeared. Interestingly, novelty exploration did not improve memory performance at 24 h in the retention test (in animals without FS), suggesting that it modulates the mechanisms involved in the stabilization of the memory trace, but not those related to the acquisition or encoding. Novelty can increase dopamine activity in the hippocampus and the medial prefrontal cortex (21). It has been proposed that the ventral tegmental area-hippocampus projection acts as a novelty-detection mechanism to reinforce the storage of relevant spatial information within the hippocampus (22, 23). We have confirmed that memory consolidation in the WM is protein synthesis-dependent, and provided evidence that the effect of novelty exploration on memory consolidation was protein synthesis-dependent because application of anisomycin disrupted the protective effect of novelty.
Hippocampal Arc and BDNF gene expression was also increased in animals that spontaneously explored a novel OF for 3 min, which is supported by previous data of increased Arc expression in CA1 (24). Furthermore, Arc-knockout mice failed to develop long-term plasticity and LTM for implicit and explicit learning tasks, despite intact STM, suggesting that Arc is critical for the consolidation of an enduring plasticity and memory storage (25). Additionally, spatial learning increased Arc-mRNA levels in the hippocampus and entorhinal cortex (26), but its inhibition by intrahippocampal infusions of antisense Arc-mRNA impaired L-LTP without affecting its induction, and impaired consolidation of spatial LTM without affecting acquisition or STM (27). Thus, Arc seems to play a basic function in the stabilization of activity-dependent hippocampal plasticity (26, 28).
This increase in Arc-expression could be a consequence of an increased BDNF production. It has been demonstrated that BDNF can trigger Arc-expression via the Ras-Raf-MAPK signaling pathway through ERK (29, 30). In the dorsal hippocampus, BDNF-mRNA expression can be induced by novelty exploration triggered by NMDA-receptor-dependent mechanisms (31), likely in the same neuronal population involved in spatial memory acquisition. Similarly, some of the known effects of BDNF on LTP (32, 33) and memory stabilization (34–36) could require Arc activation. It was also reported that BDNF infusion can prevent LTP and spatial memory impairments in the WM provoked by chronic immobilization stress (37). We propose that the protective effect of novelty exploration on memory could be mediated by an increased BDNF expression and the subsequent expression of Arc and other PRPs, which are captured by tagged synapses. Alternatively, the negative effects of FS on retention can be the result of a reduction in the BDNF expression induced by WM training (38, 39).
Our data can be explained in terms of the STH (3, 40, 41). At the cellular level, it was shown that electrical or behavioral activation of neuromodulatory structures, such as the basolateral amygdala (8, 42) or the medial septum (43), within an effective associative time window can reinforce E-LTP into L-LTP. It has been demonstrated that all of these forms of synaptic memory consolidation require PRP-synthesis (7, 8, 43). We have suggested that LTP reinforcement can also be explained by the STH (3); that is, E-LTP induction sets synaptic tags at glutamatergic inputs, which can capture PRPs synthesized by activation of neuromodulatory inputs within a specific time window, resulting in L-LTP. WM training induces plasticity changes in neural circuits (involving the hippocampus), which become consolidated when PRPs are captured at tagged synapses. Novelty by itself can activate cellular processes similar to the ones involved in WM learning, donating PRPs synthesized under novelty exploration (44). This coactivation of neuromodulatory systems is only effective if it occurs in a short temporal frame on the same neuronal population bearing active synaptic tags. FS disrupted WM-LTM consolidation, which could be rescued by subsequent OF, suggesting that FS interfered with molecular cascades responsible for the activation of gene transduction and translation, but not with tag-setting.
Memory formation, like LTP, is a time-dependent, multiphasic process (17, 45) sustained by sequential and parallel mechanisms (1, 2, 46). Thus, one can block STM without affecting LTM (2). To shed light into these processes, we have recently investigated whether LTP can be rescued from depotentiation by amygdala stimulation or by behavioral reinforcement (12). We hypothesized, that if tags survive depotentiation, and PRPs can be donated by amygdala activation or behavioral stimuli, the potentiated state could be recovered. However, we could not restore LTP at depotentiated synapses, supporting our hypothesis that depotentiation resets synaptic tags (13). In the present study however, we have shown that FS does not reset tags. WM training induced a limited burst of PRP synthesis, enough to maintain memory for up to 24 h. However, the result of the whole process was fragile or suboptimal and was disrupted by FS. WM-induced synaptic tags, however, survived and remained able to capture PRPs induced by novelty. When animals explore a novel environment, an increased dopamine release in the hippocampus has been observed (47), with several potential effects [e.g., affecting Arc expression (48) and activating protein synthesis via PKA-dependent mechanisms (49–51)]. PRPs, triggered by dopamine, could then be captured by “surviving” tags. In addition, one has to keep in mind that memory consolidation involves not only the hippocampus, but also other brain structures, such as the prefrontal cortex. Interestingly, novelty also raises dopamine in the prefrontal cortex (21).
Our results show that memory depends on complex interactions between cellular events modulated by inputs of different origin and neurochemical signature. Understanding these interactions can be an important contribution to develop methods of reinforcing and improving memory in persons affected by dementia and other memory-impairing conditions.
Materials and Methods
Two-month-old (250–300 g) male Wistar rats were used. The animals were obtained from professional breeders (CENPALAB) and housed in translucent plastic cages (five animals per cage) under controlled environmental conditions (23 °C, 50% relative humidity, 12-h light-dark cycle), with free access to water and food (Rat Chow; CENPALAB) throughout the experiment. All efforts were made to minimize the number of animals used and their suffering. The experimental protocols for this study followed the National Guidelines for the Care and Use of Laboratory Animals, Cuba, and were approved by the institutional Bioethics Committee of Centro Internacional de Restauración Neurológica (CIREN), Cuba.
Behavioral Training.
The animals were trained in the WM for four consecutive trials, during which they could search (60 s maximum) for a hidden platform to escape from water. Retention tests were carried out at different times after training (10 min, 24 h, or 4 d). This weak version of a WM protocol was used with the intention of creating a labile memory trace (52), which could be interfered by FS. The apparatus consisted of a circular swimming pool (diameter of 1.50 m, filled with water at 22 °C) marked by four virtual equispaced points named N, S, E, and W, respectively. Animals were released from one of these positions each time in a previously selected random sequence (S, W, N, and E). The start point for retrieval was W. In some initial experiments, we used an eight-trial protocol with a different sequence of starting points; at the retrieval test the release position was E. The behavior of the animals was captured by a television camera connected to a personal computer. The data were collected and processed using the SMART program (Panlab, v 2.0). Several behavioral variables were measured (velocity, path length, and time at walls) but escape latency (in seconds) was finally selected as a reliable measure of learning and memory.
Blocking Memory by FS.
To interfere with the memory process we applied an electrical FS, which consisted of five shocks at 10-s intervals (1 mA for 2 s). The animals were placed in a 25 × 30-cm training cage (Coulbourn Instruments) for 30 s and then the FS was applied. After the FS, the animals were placed back into their home cages.
Preserving Memory by Spontaneous Exploration in Novelty Scenery.
Ten minutes after FS, the animals were placed in an OF box for 3 min (OF, 50 × 50 cm, blue walls and floor), which was a completely novel environment for them. To evaluate the effect of novelty, some animals were previously habituated to the OF by placing them into the arena for four times (5 min between trials) or placed in an alternative OF of the same dimensions and shape but different in the color of the walls and floor (brown and white, respectively) and printed Times New Roman capitals (M, O, X, and Z, size 650 points in black) attached to each of the walls.
Surgery.
A group of animals was implanted with bilateral intrahippocampal cannulas to allow the injection of substances. The animals were anesthetized with chloral hydrate (400 mg/kg, i.p.) and fixed in a stereotaxic frame (David Kopf). Bilaterally, guide cannulas were placed into the hippocampus (anteroposterior = −4.0; mediolateral = ±4.0, and dorsoventral = −3.0 mm from Bregma) (10). Additionally, three miniscrews were secured on the skull and the implant was covered with dental acrylic. Water and food remained ad libitum through all of the experiments. At the end of this experiment, animals were killed and the brains processed for conventional histology to confirm the location of the cannulas. Only data from animals with a correct cannula position were analyzed. Two animals were rejected for this reason.
Protein Synthesis Inhibition.
To study the requirement of new protein synthesis for memory rescue by novelty exploration, anisomycin was applied shortly after OF (Sigma) to a group of cannulated rats. Eighty micrograms of anisomycin were bilaterally injected into the CA1 region (0.8 μL each site) dissolved in HCl, diluted in saline solution, and adjusted to a pH of 7.4 with NaOH (10).
Arc and BDNF Expression by Novelty Scenery Exploration.
Gene-expression assays were performed for Arc and BDNF using β-actin as control. Following decapitation (30 min after OF in the case of exploring animals), hippocampi were quickly dissected and total RNA from the dorsal hippocampus was separately purified with TRIzol (Roche Diagnostics). To synthesize cDNA, 5 μg of total RNA and 0.5 μg oligo(dT) (Invitrogen) were mixed. The mixture was heated to 70 °C for 10 min. First-strand buffer, 0.1 M DTT, and 25 mM dNTPs were added and mixed contents were incubated at 42 °C for 2 min. One microliter of M-MLV reverse-transcriptase was added and the mixture was incubated at 42 °C for 50 min. The reaction was inactivated by heating at 75 °C for 15 min. PCR reactions were carried out using 2 μL of cDNA mixed with 25 mM dNTPs, 50 pMol of each specific primer, 5 μL of DMSO, and 1 U Taq DNA polymerase (Invitrogen). Cycling conditions were: 94 °C for 3 min; 40 cycles for 1 min at 94 °C, annealing temperature for 1 min, 72 °C for 1 min and 72 °C for 5 min (for β-actin and BDNF), and cycle conditions: 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C, and 1 min at 60 °C for Arc.
The sequence of each primer, the annealing temperatures, and the length of the amplified products were as follows: BDNF, forward and reverse primer sequences (5′ to 3′): forward: TTGGCCTACCCAGCTG TGCGGAC/reverse: CTCTTCGATCACGTGCTCAAAAGTG (annealing temperature 60 °C, product length 130 bp); Arc, TGC TCC AGG GTC TTG GGG AGT/AGG AGA GCT GCC TGA GCA GG (60 °C, 472 bp); β-actin, ATTTGGCACCACACTTTCTACA/TCACGCACGATTTCCCTCTCAG (51 °C, 379 bp). β-Actin was used as an endogenous control. DNA products were electrophoresed on 1.2% agarose gels at 100 mV and visualized with ethidium bromide. PCR products were separated and documented by digital imaging (29).
A base pair-long product for Arc and BDNF were amplified by semiquantitative PCR with specific primers.
For the semiquantitative analysis, we used the free online program ImageJ (Version 1.44; Wayne Rasband, National Institute of Health; http://imagej.nih.gov/ij). For this analysis we first subtracted the background activity to the target band, and then normalized using β-actin as reference.
Experimental Groups.
Statistics.
A one-way ANOVA was used to compare the escape latencies among groups. As post hoc test we used the Duncan's multiple ranges test. A Student t test was also used when only two groups were compared. Significant differences were considered when P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Alain Garcia Varona for animal care, Irenia Horruitinier for skillful help in the animal training, and Dr. Diego Moncada for his useful advice on how to administer anisomycin.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114198109/-/DCSupplemental.
References
- 1.Matthies H. In search of cellular mechanisms of memory. Prog Neurobiol. 1989;32:277–349. doi: 10.1016/0301-0082(89)90024-5. [DOI] [PubMed] [Google Scholar]
- 2.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Frey S, Frey JU. ‘Synaptic tagging’ and ‘cross-tagging’ and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 5.Flexner JB, Flexner LB, Stellar E. Memory in mice as affected by intracerebral puromycin. Science. 1963;141:57–59. doi: 10.1126/science.141.3575.57. [DOI] [PubMed] [Google Scholar]
- 6.Straube T, Korz V, Frey JU. Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett. 2003;344:5–8. doi: 10.1016/s0304-3940(03)00349-5. [DOI] [PubMed] [Google Scholar]
- 7.Bergado JA, Almaguer-Melian W, Kostenko S, Frey S, Frey JU. Behavioral reinforcement of long-term potentiation in rat dentate gyrus in vivo is protein synthesis-dependent. Neurosci Lett. 2003;351:56–58. doi: 10.1016/s0304-3940(03)00943-1. [DOI] [PubMed] [Google Scholar]
- 8.Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. Reinforcement of early long-term potentiation (early-LTP) in dentate gyrus by stimulation of the basolateral amygdala: Heterosynaptic induction mechanisms of late-LTP. J Neurosci. 2001;21:3697–3703. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almaguer-Melian W, Martínez-Martí L, Frey JU, Bergado JA. The amygdala is part of the behavioural reinforcement system modulating long-term potentiation in rat hippocampus. Neuroscience. 2003;119:319–322. doi: 10.1016/s0306-4522(02)00867-9. [DOI] [PubMed] [Google Scholar]
- 10.Ballarini F, Moncada D, Martinez MC, Alen N, Viola H. Behavioral tagging is a general mechanism of long-term memory formation. Proc Natl Acad Sci USA. 2009;106:14599–14604. doi: 10.1073/pnas.0907078106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moncada D, Viola H. Induction of long-term memory by exposure to novelty requires protein synthesis: Evidence for a behavioral tagging. J Neurosci. 2007;27:7476–7481. doi: 10.1523/JNEUROSCI.1083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almaguer-Melian W, Bergado JA, López-Rojas J, Frey S, Frey JU. Differential effects of electrical stimulation patterns, motivational-behavioral stimuli and their order of application on functional plasticity processes within one input in the dentate gyrus of freely moving rats in vivo. Neuroscience. 2010;165:1546–1558. doi: 10.1016/j.neuroscience.2009.11.068. [DOI] [PubMed] [Google Scholar]
- 13.Sajikumar S, Frey JU. Resetting of ‘synaptic tags’ is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Bodnoff SR, et al. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15:61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong W, et al. The stress experience dependent long-term depression disassociated with stress effect on spatial memory task. Neurosci Res. 2003;46:415–421. doi: 10.1016/s0168-0102(03)00120-2. [DOI] [PubMed] [Google Scholar]
- 16.Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S. Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning. Brain Res. 1998;796:132–142. doi: 10.1016/s0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- 17.Muller GE, Pilzecker A. Experimental Contribution to the Theory of Memory. Z Psychol. 1900;1:1–300. [Google Scholar]
- 18.Davis S, et al. The formation and stability of recognition memory: What happens upon recall? Front Beha Neurosci. 2010;4:177. doi: 10.3389/fnbeh.2010.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DJ. Psychobiology of active and inactive memory. Psychol Bull. 1979;86:1054–1083. [PubMed] [Google Scholar]
- 20.de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 21.Ihalainen JA, Riekkinen P, Jr, Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- 22.Lisman JE, Grace AA. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang SH, Redondo RL, Morris RG. Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proc Natl Acad Sci USA. 2010;107:19537–19542. doi: 10.1073/pnas.1008638107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 25.Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 26.Guzowski JF, et al. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: A comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Link W, et al. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng F, Luo Y, Wang H. Regulation of brain-derived neurotrophic factor-mediated transcription of the immediate early gene Arc by intracellular calcium and calmodulin. J Neurosci Res. 2009;87:380–392. doi: 10.1002/jnr.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ying SW, et al. Brain-derived neurotrophic factor induces long-term potentiation in intact adult hippocampus: Requirement for ERK activation coupled to CREB and upregulation of Arc synthesis. J Neurosci. 2002;22:1532–1540. doi: 10.1523/JNEUROSCI.22-05-01532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gwag BJ, Springer JE. Activation of NMDA receptors increases brain-derived neurotrophic factor (BDNF) mRNA expression in the hippocampal formation. Neuroreport. 1993;5:125–128. doi: 10.1097/00001756-199311180-00007. [DOI] [PubMed] [Google Scholar]
- 32.Dragunow M, et al. Brain-derived neurotrophic factor expression after long-term potentiation. Neurosci Lett. 1993;160:232–236. doi: 10.1016/0304-3940(93)90420-p. [DOI] [PubMed] [Google Scholar]
- 33.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Adasme T, et al. Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci USA. 2011;108:3029–3034. doi: 10.1073/pnas.1013580108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slipczuk L, et al. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS ONE. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- 38.Brun VH, Ytterbo K, Morris RGM, Moser MB, Moser EI. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J Neurosci. 2001;21:356–362. doi: 10.1523/JNEUROSCI.21-01-00356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 40.Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 41.Frey U, Morris RGM. Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- 42.Bergado JA, Frey S, López J, Almaguer-Melian W, Frey JU. Cholinergic afferents to the locus coeruleus and noradrenergic afferents to the medial septum mediate LTP-reinforcement in the dentate gyrus by stimulation of the amygdala. Neurobiol Learn Mem. 2007;88:331–341. doi: 10.1016/j.nlm.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 43.Frey S, Bergado JA, Frey JU. Modulation of late phases of long-term potentiation in rat dentate gyrus by stimulation of the medial septum. Neuroscience. 2003;118:1055–1062. doi: 10.1016/s0306-4522(03)00029-0. [DOI] [PubMed] [Google Scholar]
- 44.Moncada D, Ballarini F, Martinez MC, Frey JU, Viola H. Identification of transmitter systems and learning tag molecules involved in behavioral tagging during memory formation. Proc Natl Acad Sci USA. 2011;108:12931–12936. doi: 10.1073/pnas.1104495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 46.Matthies H. [Cellular mechanisms of learning processes and the shaping of memory] (in German) Z Psychol Z Angew Psychol. 1976;184:308–328. [PubMed] [Google Scholar]
- 47.Hamilton TJ, et al. Dopamine modulates synaptic plasticity in dendrites of rat and human dentate granule cells. Proc Natl Acad Sci USA. 2010;107:18185–18190. doi: 10.1073/pnas.1011558107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vazdarjanova A, et al. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J Comp Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- 49.Frey U, Huang Y-Y, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 50.Frey U, Schroeder H, Matthies H. Dopaminergic antagonists prevent long-term maintenance of posttetanic LTP in the CA1 region of rat hippocampal slices. Brain Res. 1990;522:69–75. doi: 10.1016/0006-8993(90)91578-5. [DOI] [PubMed] [Google Scholar]
- 51.Huang Y-Y, Kandel ER. D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proc Natl Acad Sci USA. 1995;92:2446–2450. doi: 10.1073/pnas.92.7.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cruz-Aguado R, Almaguer-Melian W, Díaz CM, Lorigados L, Bergado J. Behavioral and biochemical effects of glutathione depletion in the rat brain. Brain Res Bull. 2001;55:327–333. doi: 10.1016/s0361-9230(01)00484-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.