Abstract
Cullin-3 (Cul3) functions as a scaffolding protein in the Bric-a-brac, Tramtrack, Broad-complex (BTB)–Cul3–Rbx1 ubiquitin E3 ligase complex. Here, we report a previously undescribed role for Cul3 complexes in late endosome (LE) maturation. RNAi-mediated depletion of Cul3 results in a trafficking defect of two cargoes of the endolysosomal pathway, influenza A virus (IAV) and epidermal growth factor receptor (EGFR). IAV is able to reach an acidic endosomal compartment, coinciding with LE/lysosome (LY) markers. However, it remains trapped or the capsid is unable to uncoat after penetration into the cytosol. Similarly, activation and subsequent ubiquitination of EGFR appear normal, whereas downstream EGFR degradation is delayed and its ligand EGF accumulates in LE/LYs. Indeed, Cul3-depleted cells display severe morphological defects in LEs that could account for these trafficking defects; they accumulate acidic LE/LYs, and some cells become highly vacuolated, with enlarged Rab7-positive endosomes. Together, these results suggest a crucial role of Cul3 in regulating late steps in the endolysosomal trafficking pathway.
Keywords: membrane trafficking, multivesicular body, virus entry, endocytosis, influenza virus
By serving as a scaffolding subunit in a large family of E3 ubiquitin ligases, cullins play a central role in the regulation of a multitude of cellular functions (1). The cullin family member, Cul3, is responsible for joining the ligase activity-bearing subunit Rbx1 with a BTB-containing protein. BTB proteins serve as adaptors for the recognition of a wide variety of specific substrate proteins in processes such as cell cycle, apoptosis, antioxidative responses, and cytoskeleton dynamics (2–5).
Recently, we found that Cul3 was present in detergent-resistant membrane fractions (DRMs), by the action of lipid-modified DCNL3, a protein that promotes Cul3 activity through neddylation (6). Moreover, two BTB proteins, Rabankyrin-5 and RhoBTB3, are known regulators of membrane trafficking (7, 8). These observations suggested that Cul3-mediated ubiquitination might have undetected membrane-associated functions. To investigate this possibility, we monitored the internalization and intracellular fate of two endocytosed cargo in Cul3-depleted cells: the EGF/epidermal growth factor receptor (EGFR) complex and influenza A virus (IAV).
EGFR belongs to a cohort of plasma membrane proteins targeted to early endosomes (EE) from where a fraction is brought via late endosomes (LEs) to lysosomes (LYs) for degradation (9). During infectious entry in host cells, IAV particles follow a similar pathway except that when the virus reaches the LEs, the low pH (pH 5.4–4.9) induces an irreversible conformational change in the membrane fusion protein, the hemagglutinin (HA) (10–13). This conformational change triggers the fusion of the viral envelope with the limiting membrane of the endosome. The viral ribonucleoproteins (vRNPs) and the matrix protein (M1) are released into the cytosol where they dissociate from each other. This uncoating allows the vRNPs to be imported into the nucleus where replication takes place (14–16).
Cargo sorting from EEs into LEs and delivery into the LY compartment requires a number of membrane fission and fusion events, as well as the maturation of LEs preparing them for interaction with LYs (17, 18). Maturation occurs through a variety of protein- and lipid-based remodeling events including a switch from Rab5 to Rab7, a PIKfyve-dependent phosphatidylinositide conversion from PtdIns(3)P to PtdIns(3,5)P2, and recruitment of numerous effector proteins from the cytosol (19, 20). There is also a further reduction in luminal pH and formation of intraluminal vesicles (ILVs). For many cargo proteins, such as EGFR, inclusion within ILVs depends on ubiquitination of the cytosolic domain and recognition by components of the endosomal sorting complex required for transport (ESCRT) machinery (21, 22).
Here, we demonstrate that Cul3 depletion resulted in nonproductive accumulation of two endocytic cargoes, IAV and EGF/EGFR in LEs/LYs, accompanied by the accumulation of LEs with abnormal morphology. Together these results suggest a unique role for Cul3 as a critical regulator of LE maturation.
Results
Cul3 Depletion Causes a Defect in IAV Entry.
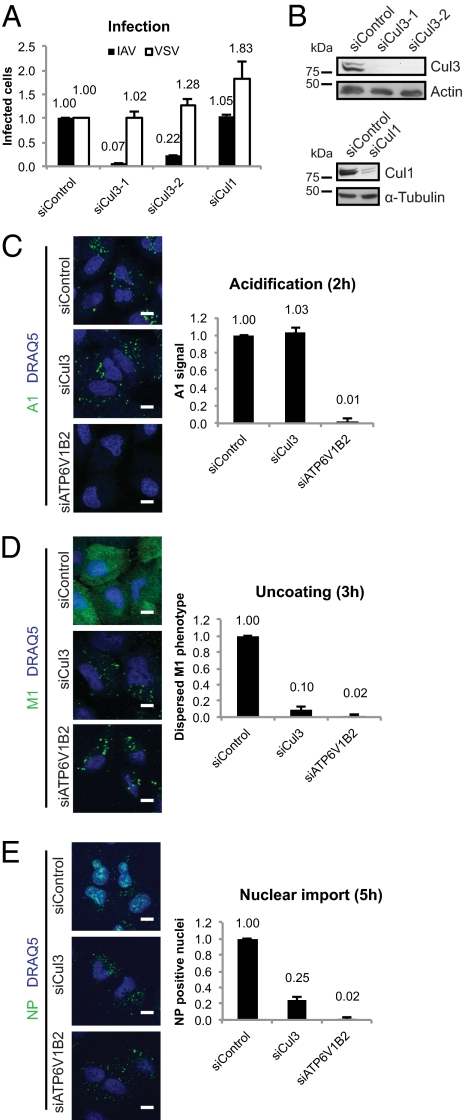
To identify host cell factors of IAV, our collaboration partner, 3-V Biosciences, Inc., performed an siRNA screen in A549 cells and found Cul3 as a possible regulator of infection. We validated these results with two siRNAs against Cul3 that efficiently decreased Cul3 expression (Fig. 1 A and B). As measured by viral nuclear protein (NP) expression, IAV infection in Cul3-depleted A549 cells was decreased up to 93%, compared with control. Vesicular stomatitis virus (VSV), which unlike IAV fuses in EEs (23, 24), was not affected. Depletion of another cullin family member, Cul1, did not affect IAV infection, although it did elevate VSV infection (Fig. 1 A and B). These results indicated that IAV, a virus that penetrates from acidic LEs, depends on Cul3 for infection.
Fig. 1.
Depletion of Cul3 allows delivery of IAV to acidified compartments but blocks IAV uncoating. (A) Virus infection in A549 cells measured by IAV NP and VSV-G protein production. Shown is mean infection, normalized to control siRNA with SEM from three independent experiments. (B) Verification of knockdown efficiency in A549 cells. (C) IAV HA acidification at 2 h after infection (p.i.), as measured by staining with A1 antibody. Nuclei were stained with DNA dye, DRAQ5. Quantification shows A1 positive area per cell, normalized to siControl. (D) Uncoating assay of IAV, with anti-M1 staining, 3 h p.i. Quantification shows cell classification, based on M1 dispersal phenotype, classified as uncoated, and normalized to siControl. (E) Nuclear import assay of IAV NP, 5 h p.i. Quantification shows percentage of NP positive nuclei, normalized to siControl. For C–E, single confocal sections from the midpoint of cells are shown. Bars for all graphs represent means ± SEM from at least three independent experiments. siRNAs against a subunit of V-ATPase (ATP6V1B2) were used as a negative control for all three assays. (Scale bars: 10 μm.)
As shown in Fig. 1C, we found that the virus was endocytosed in Cul3-depleted cells and entered cytoplasmic vacuoles where HA underwent the acid-induced conformational change, detected with the conformation-specific antibody, A1 (25). No acidified HA was observed in cells depleted of a subunit of the vacuolar-type H+-ATPase (V-ATPase), ATP6V1B2. After penetration into the cytosol, uncoating of the viral capsid can be detected by the diffuse staining of M1 in the cytoplasm (15). In contrast to control cells, Cul3-depleted cells showed an endosome-like distribution of M1 staining (Fig. 1D). Likewise, NP accumulated in endosome-like structures in the cytoplasm, and not in the nucleus as in control cells (Fig. 1E) (15). Except for the conversion of HA to the acid conformation, the overall picture resembled that of V-ATPase inactivation. We found most of the virus to reach LEs, identified by the colocalization of acidified A1-positive virus particles with vesicles containing the LE/LY marker LAMP1 and the LE/TGN marker cation-independent mannose-6-phosphate receptor (CI-M6PR). In contrast, virus particles were not found in vesicles bearing the EE marker EEA1 (Fig. 2A). Similarly, after 3 h when most viruses had escaped from endosomes in control cells (Fig. 1D), the M1 in Cul3-depleted cells was still associated with LAMP1 and CI-M6PR-, but not EEA1-positive, vesicles (Fig. 2B). Because CI-M6PR is absent from lysosomes (26), we conclude that virus particles were trapped in LEs upon Cul3 depletion.
Fig. 2.
IAV is blocked in LAMP1-positive LEs/LYs in Cul3-depleted cells. (A and B) Colocalization of IAV with endosomal markers. siRNA-transfected A549 cells were infected for 1 (A) or 3 (B) h with IAV, and stained with A1 (A) or M1 (red) (B) and either EEA1, CI-M6PR or LAMP1 (green) antibodies. Shown are single confocal sections. (Scale bars: 2 μm.) Quantification of colocalization shows the mean fraction of A1 or M1-positive virus particles that colocalized with the endosomal markers EEA1, CI-M6PR, or LAMP1 with SEM from at least three independent experiments. (C) Endocytosis bypass assay. Shown is the mean infection for corresponding type of experiment; acid bypass (filled bars) or normal infection (open bars), normalized to siControl with SEM from three independent experiments.
Finally, we tested whether the block in IAV infection upon Cul3 depletion could be bypassed by low pH-induced viral penetration at the plasma membrane (27, 28), which allows vRNP delivery directly into the cytoplasm without endocytosis and vesicular trafficking. When the bypass was performed in Cul3-depleted cells, infection was rescued to a level comparable to cells treated with control siRNAs or siATP6V1B2 (Fig. 2C). We concluded that Cul3-depletion caused specific defects in the cellular internalization and trafficking machinery, blocking virus infection at a prepenetration step.
The results suggested that Cul3 is not required for viral trafficking to LEs or for the acidification of HA in late endocytic compartments. However, it is needed for the productive penetration of the vRNPs and M1 from these compartments or for uncoating of the viral capsids in the cytoplasm.
Cul3 Depletion Leads to Accumulation of EGF and EGFR.
To test whether Cul3 depletion affected endocytosis and processing of physiological cargo destined to LEs and lysosomal degradation, we analyzed the fate of EGFR and its ligand EGF in HeLa cells. Fluorescence microscopy showed that the amount of EGF-TexasRed (EGF-TxRed) accumulating in cytoplasmic vesicles almost doubled upon Cul3 depletion (Fig. 3A). The difference was most pronounced 30–60 min after internalization (Fig. 3A, Right).
Fig. 3.
Labeled EGF and EGFR accumulate in Cul3-depleted cells. (A) EGF intracellular accumulation over time. siRNA-transfected HeLa cells were starved overnight and treated with 100 ng/mL EGF-TxRed for 5 min (pulse) followed by replacement to full medium (chase). The minutes on images and on the x axis of graph represent total time from addition of EGF-TxRed (5 min pulse plus chase). Quantification shows mean EGF-TxRed signal per cell, normalized to siControl 5 min with SEM from at least three independent experiments. (Scale bars: 10 μm.) Immunoblotting for EGFR of HeLa cells transfected with (B) siRNAs (star in Cul3 blot marks neddylated form of Cul3) or (C) HA-Cul3 or HA-control plasmid.
We also observed an increase in the amount of the receptor, EGFR, in cells transfected with siCul3 (Fig. 3B and Fig. S1A). An increase in protein level after Cul3 depletion was also observed for another receptor-tyrosine kinase, insulin-like growth factor receptor (IGF1R), whereas no increase was detected for transferrin receptor (TfR), which unlike EGFR and IGF1R is not destined for degradation in LYs (Fig. S1B). In addition, overexpression of Cul3 did not affect the level of EGFR (Fig. 3C), suggesting that Cul3 is required but not limiting for efficient EGF and EGFR trafficking and degradation.
Cul3 Regulates Lysosomal Degradation of EGFR, but Not Its Activation and Ubiquitination.
The ninefold increase in EGFR protein levels (Fig. 3B) was accompanied by a mere twofold elevation in mRNA levels (Fig. 4A), suggesting that the receptor may not be efficiently down-regulated by degradation, i.e., that there could be a problem for example with trafficking to LYs. To directly assess degradation of EGFR, we used cycloheximide (CHX) to block protein translation (Fig. 4B). Whereas in control cells the relative amount of EGFR was decreased 2 h and 4 h after CHX addition, EGFR degradation was significantly delayed in Cul3-depleted cells. Together these results suggested that accumulation of EGFR and its ligand EGF in Cul3-depleted cells resulted from delayed degradation of endocytic cargo destined to the LYs.
Fig. 4.
EGFR lysosomal degradation is delayed, whereas phosphorylation and ubiquitination occur upon Cul3 depletion. (A) EGFR mRNA levels from siRNA transfected HeLa cells, quantified by quantitative RT-PCR, normalized to GAPDH, relative to siControl with corresponding SEM from at least three independent experiments. (B) Degradation of EGFR. siRNA-transfected HeLa cells were untreated (0 h) or treated with 10 μM CHX for 2 or 4 h and analyzed for EGFR protein levels. Graph shows mean EGFR protein amounts, normalized to GAPDH, relative to 0 h of corresponding siRNA with SEM from five independent experiments. **P < 0.01. (C) Activation of EGFR. siRNA-transfected HeLa cells were serum starved, stimulated with 100 ng/mL EGF, and analyzed for EGFR and p-EGFR (EGFR-pY1173) levels. (D) Ubiquitination of EGFR. Cell lysates (input), treated as in C, were immunoprecipitated with EGFR antibodies (under limiting conditions to pull down equal amounts of EGFR) and analyzed for EGFR ubiquitination by immunoblotting.
For EGFR to be sorted into the degradative pathway, it needs to be activated by its ligand EGF, which induces receptor dimerization, autophosphorylation, and ubiquitination (9, 21). EGFR was phosphorylated after EGF addition with similar kinetics in Cul3-depleted cells as in control cells (Fig. 4C). We also observed EGFR to be ubiquitinated upon EGF stimulation in Cul3-depleted cells, in contrast to c-Cbl depletion (Fig. 4D and Fig. S2A). Whereas ubiquitination appeared similar to control conditions, deubiquitination of EGFR may have been slightly delayed in the absence of Cul3. However, although Cul3 has recently been proposed to regulate several deubiquitinating enzymes (DUBs) (29), we found that levels of c-Cbl and the DUBs, AMSH and UBPY, known to be involved in lysosomal degradation of EGFR (30), were unaffected by Cul3 depletion (Fig. S2 B and C).
We conclude that Cul3 is required for efficient EGFR degradation, but does not regulate the activation and ubiquitination of EGFR after ligand stimulation. These findings are consistent with a role of Cul3 in endosome maturation or trafficking, as described above for IAV.
EGF Accumulates in LE/LYs upon Cul3 Depletion.
To better define the possible defect in EGF/EGFR endosomal trafficking in Cul3-depleted cells, we assayed the kinetics and localization of EGF-TxRed by immunofluorescence with different endosomal markers. Trafficking of EGF-TxRed out of EEA1-positive EEs was mostly unaffected (Fig. 5A), except for a slight delay at 30 min after uptake. Moreover, we did not detect a major difference in the kinetics of EGF-TxRed colocalization with HRS, a component of the ESCRT-0 complex present on EEs (Fig. 5B), implying that Cul3 depletion does not significantly affect trafficking of EGF at the level of EEs. The general increase of EGF-TxRed colocalization with EEA1 and HRS likely reflected increased amounts of internalized EGF-TxRed (Fig. 3A). In contrast, EGF-TxRed colocalization with LAMP1-positive LE/LY compartments was significantly increased in Cul3-depleted cells 60 and 90 min after internalization compared with maximum at 30 min (Fig. 5C and Fig. S3A), consistent with the delay in EGF/EGFR degradation. Finally, it is unlikely that Cul3 regulated transport of acid hydrolases from the TGN to endosomes as cleavage of the cathepsin D precursor to its active form was unaffected in Cul3-depleted cells (Fig. S3B). Cathepsin D cleavage requires transport of the enzyme to LE/LYs and acidification (31).
Fig. 5.
EGF-TxRed colocalization with LAMP1-positive LEs/LYs is prolonged upon Cul3 depletion. (A–C) siRNA-transfected HeLa cells were serum starved, pulsed for 5 min with EGF-TxRed (100 ng/mL), and chased with full medium. Cells were fixed and stained with antibodies against EEA1 (A), HRS (B), or LAMP1 (C). n > 200 cells for each timepoint and condition. Bars represent means with SEM from at least three independent experiments. P values were calculated between siControl and siCul3 colocalization and compared with maximum values (Fig. S3A). The P values from EGF colocalization with EEA1 and HRS, or other timepoints of LAMP1 colocalization are not statistically significant. (Scale bars: 10 μm.) *P < 0.05, **P < 0.01.
These results suggested that degradation of EGFR/EGF complexes was delayed. Like IAV, they were trapped in LE/LYs.
Cul3 Depletion Distorts LE Morphology.
The late endocytic organelles were clearly expanded with respect to size and number in Cul3-depleted HeLa and A549 cells, as visualized by staining with the LE/LY markers LAMP1, CD63, and the lipid lyso(bis)phosphatidic acid (LBPA)/ bis(monoacylglyceryl)phosphate (BMP) (Fig. 6A and Fig. S4A). A comparable expansion of LE/LY compartments was detected when cells were depleted of known regulators of ILV formation and endosome maturation such as the ESCRT-0 component HRS, Rab7, and the ATP6V1B2 subunit of V-ATPase (Fig. S4B), suggesting that Cul3 regulates an aspect of endosome maturation.
Fig. 6.
LE/LY morphology is distorted in Cul3-depleted cells. (A) Cul3 depletion results in accumulation of LE/LYs. siRNA-transfected HeLa and A549 cells were stained with antibody against CD63 (green) and nuclei with DRAQ5 (blue). Images are z-projections of confocal slices. Graph shows FACS measurement of total CD63 immunofluorescence associated to cells. Bars represent means with SEM, normalized to control siRNA from three independent experiments. **P < 0.01, *P < 0.05. (B) LysoTracker Green DND-26 staining of live siRNA transfected HeLa cells. Some Cul3-depleted cells showed a highly vacuolated phenotype, as seen in the phase contrast image. (C) mRFP-Rab5, EGFP-Rab7, and siRNA cotransfected HeLa cells, imaged live. (D) Quantification of vacuolated phenotype from stably transfected HeLa cells, with inducible EGFP-Rab7 expression. Bars represent means with SEM where n > 300 cells, from three independent experiments. **P < 0.01. (E) Live imaging of fluid phase marker Dextran-Alexa Fluor 488 loaded cells. (F) Thin section EM of siRNA-transfected HeLa cells. (Scale bars: A, 20 μm; B, C, and E, 10 μm; F, 1 μm.)
Using live cell imaging, we found that some of the Cul3-depleted cells contained numerous large, spherical vacuoles (Fig. 6B). The vacuoles stained positive with LysoTracker, a marker for acidic compartments, although the largest showed somewhat weaker staining. Consistent with the elevated LE/LY marker staining (Fig. 6A and Fig. S4A), we observed a general increase in LysoTracker-positive vacuoles (Fig. 6B).
The enlarged vacuoles probably represented modified LEs because they were Rab7 positive and largely devoid of Rab5 (Fig. 6C). In stably transfected HeLa cells expressing EGFP-Rab7, ≈30% acquired enlarged Rab7-positive vacuoles upon Cul3 depletion, compared with 6% in siControl cells (Fig. 6D). Moreover, the fluid-phase marker, Dextran-Alexa Fluor 488 accumulated in the enlarged endosomes, indicating that the vacuoles were connected to the endocytic system (Fig. 6E).
Two pharmacological inhibitors were found to induce a similar vacuolation effect (Fig. S4C). MLN4924 inhibits the Nedd8-activating enzyme, required to activate cullin-based ligases (32). The percentage of cells filled with enlarged EGFP-Rab7–positive endosomes increased over time, reaching 80% after 24 h of drug treatment. The other compound, YM201636 (33), inhibits PIKfyve, a kinase that converts PtdIns(3)P into PtdIns(3,5)P2 during endosome maturation. PIKfyve inhibition resulted in a similar vacuolated phenotype with highly enlarged Rab7–positive endosomes in all cells.
Electron microscopy (EM) of Cul3-depleted cells revealed the existence of enlarged vacuoles, in both HeLa and A549 cells (Fig. 6F and Fig. S5). These vacuoles appeared empty, largely devoid of ILVs and other visible luminal content, with a diameter up to 1–1.5 μm. They were present only in a fraction of Cul3-depleted cells and, thus, probably represented the aforementioned Rab7 positive/fluid-filled enlarged endosomes.
Together, the light and electron microscopy data revealed the accumulation of LEs with distorted morphology upon Cul3 depletion, consistent with defects in LE maturation.
Discussion
Here, we provide evidence for a previously undescribed role of Cul3 as a regulator of the endolysosomal pathway. Depletion of Cul3 resulted in the deformation of LEs and a defect in the transport of endocytic cargo to LYs. By tracking the fate of EGF/EGFR and IAV, we could characterize the block and determine some of its consequences.
The early steps in the processing and trafficking of EGF and its receptor were not affected by Cul3 depletion. Upon EGF binding, EGFR was phosphorylated and ubiquitinated. Endocytic internalization was normal, although somewhat more efficient than in control cells, possibly due to the increased amount of receptor. The EGF/EGFR complexes entered EEs from which they were sorted into LEs with a slight delay. Whether EGFR was sequestered into ILVs as in normal cells is not clear. Nevertheless, the EGF/EGFR complexes were transported to compartments containing the LE/LY marker LAMP1. Because degradation of EGFR and EGF was slower than normal, these probably represented modified LEs, which failed to efficiently fuse with LYs.
The fate of incoming IAV told a similar story. In Cul3-depleted cells, the virus particles were transported to LAMP1-positive vacuoles. However, uncoating of the viral capsid and transport of vRNPs to the nucleus did not occur and, as a result, the cells were not infected. Importantly, the Cul3 requirement was bypassed when virus infection was artificially induced without involvement of endocytosis. Moreover, VSV, a virus that is acid-activated in the EE compartment, had no problem infecting Cul3-depleted cells, supporting the conclusion that Cul3 depletion affected mainly late endosomal compartments.
Judging by the A1 antibody staining, the HA of IAV converted to the acid-induced conformation in Cul3-depleted cells, but the vRNPs and the M1 protein remained associated with endosomal vacuoles, indicating that capsid uncoating and vRNP release did not take place. It is unclear whether this phenotype was because the virus failed to fuse, whether fusion was incomplete (hemifusion), or whether the capsids failed to undergo disassembly after release into the cytosolic surface of endosomes. The block resembled the effect of amantadine, an inhibitor of the M2 ion channel in the viral membrane. M2 allows acidification of the viral interior before viral membrane fusion inducing a disassembly-competent state in the capsid (16). Thus, in the presence of amantadine, fusion takes place, but the capsids remain associated with endosomes and fail to release the vRNPs for nuclear import (14). Although acidic enough to convert the HA, the ionic environment in modified LEs may not fulfill all requirements for proper M2 action. It was recently proposed that M2 has antiporter-like activity for cations such as K+ and Na+, and that this activity is required for full acidification of the IAV core (34). During maturation, endosomes undergo a number of ionic changes within the lumen (35), and such changes may be used by the virus to initiate viral core acidification. A similar block in IAV infection has been demonstrated upon inhibition of protein kinase C βII, where viruses are trapped in LEs, although the acid-mediated HA conversion has occurred (10). A requirement of an endosomal membrane potential for uncoating or membrane fusion has been proposed for other viruses, including human rhinovirus type 2 (HRV2) and Semliki forest virus (SFV) (36, 37).
The defects in the late endocytic compartments in Cul3-depleted cells were reflected by a distorted morphology and aberrant behavior of LE/LYs. The size and number was increased in some of the cells showing a highly vacuolated phenotype with large, spherical, Rab7-positive, fluid-filled endosomes observed by light microscopy in live cells. EM studies further revealed a fraction of Cul3-depleted cells to contain enlarged empty vacuoles virtually devoid of ILVs. Vacuolation was comparable to cells treated with inhibitors of PIKfyve, a known regulator of LE maturation (20), as well as of neddylation, a crucial modification needed for Cul3-dependent E3 ligase activity (32). Together, the results indicated that Cul3 is involved in the regulation of endosome maturation, most probably through ubiquitination.
Interestingly, the phenotypes of cells treated with the proteasome inhibitor lactacystin have similarities to Cul3-depleted cells including a delay in lysosomal degradation of transmembrane proteins such as EGFR (38), a block of cargo deubiquitination (39), and inhibition of IAV infection at the level of endosomes (40). Lactacystin-treated cells also contain enlarged, empty endosomes with EGFR trapped in the limiting membrane instead of ILVs (38). At the same time, these cells still contain a population of ILV-filled endosomes. Similarly, temperature-sensitive Ts20 mutant cells that possess a thermolabile E1 ligase also accumulate acidic, hydrolase containing vacuoles, with similar characteristics as observed after Cul3 depletion (41). A Cul3-based E3 ligase could thus be responsible for the ubiquitination and proteasomal degradation of substrates involved in endosome maturation and, thus, ILV biogenesis. Moreover, subcellular fractionation of membranes revealed the presence of Cul3 on different early and late endosomal vesicles, suggesting that Cul3 might exert its function directly on these vesicles (Fig. S6).
Cul3-based E3 ligases recognize their substrate proteins through a family of adaptors bearing BTB motifs (42). The human genome encodes ≈200 BTB proteins. Recent siRNA screens suggest a role for some of them in the endolysosomal and recycling pathways (43). In addition, some voltage-gated potassium channels possess BTB-like T1 domains (42), raising the possibility that misregulation of ion channels could be a cause for endosome swelling upon Cul3 depletion. Clearly, the identification and characterization of relevant BTB-containing proteins required for maturation of LEs will be important. These findings, in turn, will aid in the identification of the possible substrates involved.
In conclusion, we identified a previously undescribed function for Cul3-mediated ubiquitination in endosome maturation. Considering the important role of the endolysosomal pathway in a number of physiological processes as well as pathological states such as infections, cancer, and neurodegeneration, deeper molecular understanding of these processes provides a rewarding research direction for the future.
Materials and Methods
Cells.
A549 and HeLa cells were cultured in DMEM (Invitrogen) supplemented with 10% FCS and glutamax.
RNAi.
All siRNAs (Microsynth/Qiagen) were reverse transfected with Lipofectamine RNAiMAX (Invitrogen) for 72 h at 20 nM. Transfection efficiency was controlled with Allstar death siRNA (Qiagen) and down-regulation efficiency assessed by immunoblotting or quantitative RT-PCR. The following siRNA duplexes (from Qiagen) were used to specifically down-regulate genes: Cul3-5 (oligo1), Cul3-10 (oligo2), Cul1-3, c-Cbl-8, ATP6V1B2-3, HRS-5, Rab7A-5, and as control siRNAs either “Allstar negative” (Qiagen) or “Scrambled” (Microsynth). For all Cul3 siRNA experiments, oligo1 was used unless otherwise indicated.
Further details on materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank 3-V Biosciences, Inc. for sharing their RNAi screening data; Chin Ha Chung, Jean Gruenberg, and Stephen Taylor for reagents; Alicia Smith and Jason Mercer for critical reading of the manuscript; and the A.H. and M.P. group members for helpful discussions. We acknowledge support by the Light- and Electron Microscopy Centers at ETH Zurich. N.M.-S. and H.M. were supported by grants from the Boehringer-Ingelheim-Fonds. The M.P. and A.H. laboratories were funded by the European Research Council, the Swiss National Science Foundation, and ETH Zurich; and the A.H. laboratory was supported by National Institutes of Health Grant 1UO1AI074523 and a Marie Curie Initial Training Networks grant.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1118744109/-/DCSupplemental.
References
- 1.Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. [DOI] [PubMed] [Google Scholar]
- 2.Sumara I, et al. A Cul3-based E3 ligase removes Aurora B from mitotic chromosomes, regulating mitotic progression and completion of cytokinesis in human cells. Dev Cell. 2007;12:887–900. doi: 10.1016/j.devcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Jin Z, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–735. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa M, Xiong Y. BTB protein Keap1 targets antioxidant transcription factor Nrf2 for ubiquitination by the Cullin 3-Roc1 ligase. Mol Cell Biol. 2005;25:162–171. doi: 10.1128/MCB.25.1.162-171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell. 2009;35:841–855. doi: 10.1016/j.molcel.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Meyer-Schaller N, et al. The human Dcn1-like protein DCNL3 promotes Cul3 neddylation at membranes. Proc Natl Acad Sci USA. 2009;106:12365–12370. doi: 10.1073/pnas.0812528106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnatwinkel C, et al. The Rab5 effector Rabankyrin-5 regulates and coordinates different endocytic mechanisms. PLoS Biol. 2004;2:E261. doi: 10.1371/journal.pbio.0020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: A Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137:938–948. doi: 10.1016/j.cell.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2009;315:683–696. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 10.Sieczkarski SB, Brown HA, Whittaker GR. Role of protein kinase C betaII in influenza virus entry via late endosomes. J Virol. 2003;77:460–469. doi: 10.1128/JVI.77.1.460-469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sieczkarski SB, Whittaker GR. Differential requirements of Rab5 and Rab7 for endocytosis of influenza and other enveloped viruses. Traffic. 2003;4:333–343. doi: 10.1034/j.1600-0854.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 12.White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1:217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skehel JJ, Wiley DC. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu Rev Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 14.Martin K, Helenius A. Nuclear transport of influenza virus ribonucleoproteins: The viral matrix protein (M1) promotes export and inhibits import. Cell. 1991;67:117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- 15.Martin K, Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991;65:232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palese P, Shaw ML. In: Orthomyxoviridae: The Viruses and Their Replication. Fields Virology. Knipe DM, Howley PM, editors. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1647–1690. [Google Scholar]
- 17.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD. Fab1p is essential for PtdIns(3)P 5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol. 1998;143:65–79. doi: 10.1083/jcb.143.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acconcia F, Sigismund S, Polo S. Ubiquitin in trafficking: The network at work. Exp Cell Res. 2009;315:1610–1618. doi: 10.1016/j.yexcr.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Raiborg C, Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 23.Johannsdottir HK, Mancini R, Kartenbeck J, Amato L, Helenius A. Host cell factors and functions involved in vesicular stomatitis virus entry. J Virol. 2009;83:440–453. doi: 10.1128/JVI.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White J, Matlin K, Helenius A. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J Cell Biol. 1981;89:674–679. doi: 10.1083/jcb.89.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster RG, Brown LE, Jackson DC. Changes in the antigenicity of the hemagglutinin molecule of H3 influenza virus at acidic pH. Virology. 1983;126:587–599. doi: 10.1016/s0042-6822(83)80015-4. [DOI] [PubMed] [Google Scholar]
- 26.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 27.Helenius A, Kartenbeck J, Simons K, Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980;84:404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matlin KS, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright MH, Berlin I, Nash PD. Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination. Cell Biochem Biophys. 2011;60:39–46. doi: 10.1007/s12013-011-9181-9. [DOI] [PubMed] [Google Scholar]
- 31.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Rabut G, Peter M. Function and regulation of protein neddylation. ‘Protein modifications: Beyond the usual suspects’ review series. EMBO Rep. 2008;9:969–976. doi: 10.1038/embor.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jefferies HB, et al. A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep. 2008;9:164–170. doi: 10.1038/sj.embor.7401155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leiding T, Wang J, Martinsson J, DeGrado WF, Arsköld SP. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc Natl Acad Sci USA. 2010;107:15409–15414. doi: 10.1073/pnas.1009997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott CC, Gruenberg J. Ion flux and the function of endosomes and lysosomes: PH is just the star. BioEssays. 2011;33:103–110. doi: 10.1002/bies.201000108. [DOI] [PubMed] [Google Scholar]
- 36.Berka U, Khan A, Blaas D, Fuchs R. Human rhinovirus type 2 uncoating at the plasma membrane is not affected by a pH gradient but is affected by the membrane potential. J Virol. 2009;83:3778–3787. doi: 10.1128/JVI.01739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helenius A, Kielian M, Wellsteed J, Mellman I, Rudnick G. Effects of monovalent cations on Semliki Forest virus entry into BHK-21 cells. J Biol Chem. 1985;260:5691–5697. [PubMed] [Google Scholar]
- 38.Longva KE, et al. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geetha T, Wooten MW. TrkA receptor endolysosomal degradation is both ubiquitin and proteasome dependent. Traffic. 2008;9:1146–1156. doi: 10.1111/j.1600-0854.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 40.Khor R, McElroy LJ, Whittaker GR. The ubiquitin-vacuolar protein sorting system is selectively required during entry of influenza virus into host cells. Traffic. 2003;4:857–868. doi: 10.1046/j.1398-9219.2003.0140.x. [DOI] [PubMed] [Google Scholar]
- 41.Lenk SE, Dunn WA, Jr, Trausch JS, Ciechanover A, Schwartz AL. Ubiquitin-activating enzyme, E1, is associated with maturation of autophagic vacuoles. J Cell Biol. 1992;118:301–308. doi: 10.1083/jcb.118.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stogios PJ, Downs GS, Jauhal JJ, Nandra SK, Privé GG. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005;6:R82. doi: 10.1186/gb-2005-6-10-r82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collinet C, et al. Systems survey of endocytosis by multiparametric image analysis. Nature. 2010;464:243–249. doi: 10.1038/nature08779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.