Abstract
TGF-β modulates immune response by suppressing non-regulatory T (Treg) function and promoting Treg function. The question of whether TGF-β achieves distinct effects on non-Treg and Treg cells through discrete signaling pathways remains outstanding. In this study, we investigated the requirements of Smad-dependent and -independent TGF-β signaling for T-cell function. Smad2 and Smad3 double deficiency in T cells led to lethal inflammatory disorder in mice. Non-Treg cells were spontaneously activated and produced effector cytokines in vivo on deletion of both Smad2 and Smad3. In addition, TGF-β failed to suppress T helper differentiation efficiently and to promote induced Treg generation of non-Treg cells lacking both Smad2 and Smad3, suggesting that Smad-dependent signaling is obligatory to mediate TGF-β function in non-Treg cells. Unexpectedly, however, the development, homeostasis, and function of Treg cells remained intact in the absence of Smad2 and Smad3, suggesting that the Smad-independent pathway is important for Treg function. Indeed, Treg-specific deletion of TGF-β–activated kinase 1 led to failed Treg homeostasis and lethal immune disorder in mice. Therefore, Smad-dependent and -independent TGF-β signaling discretely controls non-Treg and Treg function to modulate immune tolerance and immune homeostasis.
Keywords: inflammation, immune-suppression
TGF-β is a pleiotropic cytokine critical for immune regulation (1). The most prominent role of TGF-β in immune regulation is to suppress immune response, because deletion of TGF-β leads to lethal autoimmune syndrome in mice (2). TGF-β is central to suppressing T-cell function because the deletion of TGF-β receptor (TGF-βR) specifically in T cells results in lethal autoimmunity reminiscence of TGF-β1 deficiency (1, 3). TGF-β controls T-cell function through multiple mechanisms. It inhibits T-cell proliferation and T-cell differentiation (4). In addition, TGF-β is important for the generation of Foxp3-expressing immune-suppressive regulatory T cells. TGF-β signaling is required for the development of naturally occurring regulatory T (nTreg) cells in the thymus (5, 6), potentially by promoting their survival (7). In addition, TGF-β promotes the generation of induced regulatory T (iTreg) cells through promoting Foxp3 expression in activated non-Treg cells (8, 9). Therefore, TGF-β controls T-cell response by multiple means through Treg-dependent and -independent mechanisms.
Binding of TGF-β to TGF-βR activates structurally similar transcription factors Smad2 and Smad3 in the target cells (10, 11). Activated Smad2 or Smad3 heterodimerizes with Smad4 and translocates into the nucleus to regulate target gene expression. Smad-independent pathways also exist to mediate TGF-βR signaling (12). TGF-β induces rapid activation of TGF-β–activated kinase 1 (TAK1), Ras-Erk, and PI3K-Akt pathways.
Although the importance of TGF-β in controlling T-cell function is undeniable, it remains poorly understood as to what intracellular components are critical to mediate its function in target cells. More importantly, it is unclear as to whether Treg-dependent and -independent functions of TGF-β can be attributed to different signaling pathways downstream of TGF-βR. Efforts have been devoted to study the involvement of Smad proteins in T-cell function. T cells deficient in Smad3 or Smad2 are normal in development and homeostasis (13, 14), displaying distinct phenotypes from the mice whose T cells lack TGF-βR (3, 5, 6). These findings suggested that Smad-dependent pathways may be dispensable for TGF-β–mediated effects in T cells. Yet, a recent report showed that deficiency of both Smad2 and Smad3 in T cells led to development of autoimmunity in mice (15). When both Smad2 and Smad3 were deleted during early T-cell development, T-cell thymic development was impaired and peripheral T cells were aberrantly activated, associating with reduced Treg numbers. These findings suggest that Smad2 and Smad3 are redundantly required to control the functions of both non-Treg cells and Treg cells. Nevertheless, such observation could be confounded by the drastic defect in T-cell development, and thereby central tolerance. Thus, the question of whether Smad2 and Smad3 are essential for the normal functions of mature T cells remains outstanding.
Results
CD4Cre:Smad2fl/fl:Smad3−/− (CD4:DKO) Mice Developed Lethal Inflammation.
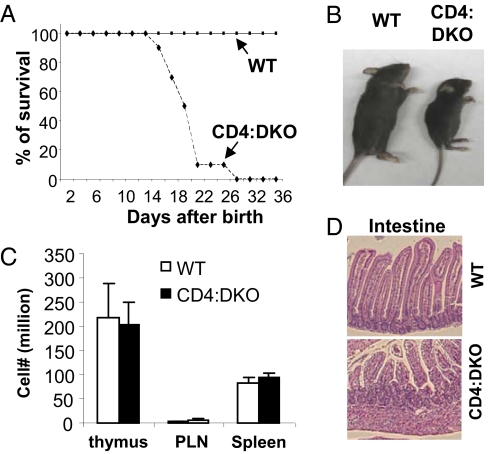
To address whether Smad2 and Smad3 are essential for the normal function of mature T cells, we crossed CD4Cre transgenic mice (16) with Smad2fl/fl and Smad3−/− mice to obtain CD4Cre:Smad2fl/fl:Smad3−/− [CD4:DKO (double KO)] mice. Although CD4Cre:Smad2fl/fl and Smad3−/− mice were viable and phenotypically grossly normal (13, 15, 17) (Fig. S1), CD4:DKO mice succumbed to an inflammatory disorder by 4 wk of age (Fig. 1A). Compared with WT littermates, CD4:DKO mice were substantially smaller in size (Fig. 1B). Nonetheless, comparable numbers of lymphocytes from CD4:DKO mice and the WT littermates were recovered from the peripheral lymph nodes (PLNs) and spleens (Fig. 1C). Immune pathological findings were detected in the nonlymphoid organs, such as the intestines, from CD4:DKO mice (Fig. 1D).
Fig. 1.
CD4:DKO mice developed lethal inflammation. (A) Comparison of the survival rates between CD4:DKO (dashed line) and WT (solid line) mice after birth. (B) Size comparison between a 3-wk-old CD4:DKO mouse and its sex- and age-matched WT littermate. The result is representative of at least three experiments. (C) Numbers of total lymphocytes recovered from the thymus, PLNs, and spleens of 3-wk-old WT (open bars) and CD4:DKO (solid bars) mice. The mean ± SD of the results from four pairs of mice are shown. (D) H&E staining and histological analysis of the intestines isolated from WT and CD4:DKO mice. Representative results from two experiments are shown.
Phenotypic Characterization of T Cells from CD4:DKO Mice.
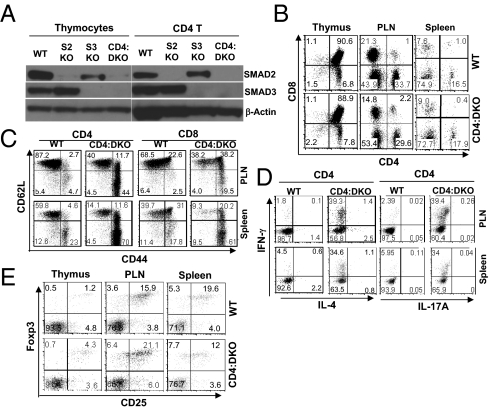
To understand the cellular mechanisms underlying the observed immune disorder in CD4:DKO mice, we characterized the phenotypes of thymic and peripheral T cells from these mice. We first confirmed that Smad2 and Smad3 were efficiently deleted in the mature T cells as well as in the majority of the thymocytes from CD4:DKO mice (Fig. 2A). In the thymus, the distribution of different thymocyte populations in CD4:DKO mice was comparable to that observed in the WT littermates (Fig. 2B). In addition, thymocyte development in CD4:DKO mice was largely normal, because the expression of thymocyte maturation and activation markers, such as CD5, CD24, and CD69, was similar between CD4:DKO mice and WT mice (Fig. S2). In addition, the distribution of mature CD4 and CD8 T-cell populations in the PLNs and spleens was comparable between CD4:DKO and WT mice (Fig. 2B). Nevertheless, CD4 non-Treg cells and CD8 T cells from CD4:DKO mice displayed activated phenotypes with down-regulation of CD62L expression and up-regulation of CD44 expression (Fig. 2C). CD4:DKO T cells predominantly acquired T helper (TH) 1 function because a great number of T cells from CD4:DKO mice produced TH1 signature cytokine IFN-γ (18, 19) but less TH2 signature cytokine IL-4 (18, 19) or TH17 signature cytokine IL-17A (20, 21) (Fig. 2D and Fig. S3). These findings are similar to the previous reports showing that disruption of TGF-βR in T cells led to a dominant TH1 response in mice (3, 5). We further investigated whether the Treg population was perturbed when Smad2 and Smad3 were deficient. Unexpectedly, the percentages of Foxp3+ Treg cells were not decreased in the thymus, PLNs, or spleens (Fig. 2E). These findings suggest that both Smad2 and Smad3 are required to suppress the activation and effector function of non-Treg cells yet are not essential for Treg homeostasis.
Fig. 2.
Phenotypic characterization of T cells from CD4:DKO mice. (A) Efficient Smad2 and Smad3 deletion in T cells. Thymocytes and peripheral CD4 T cells were isolated from WT, CD4Cre:Smad2fl/fl (S2KO), Smad3−/− (S3KO), and CD4Cre:Smad2fl/fl:Smad3−/− (CD4:DKO) mice. The protein expression of Smad2 and Smad3 in isolated cells was detected by immunoblotting. β-Actin expression was also detected as loading controls. Results are representative of three experiments. (B) Distribution of T-cell populations. Lymphocytes were isolated from the thymus, PLNs, and spleens of WT and CD4:DKO mice. The distribution of CD4 and CD8 T cells in the recovered lymphocytes was assessed by flow cytometric analysis. Representative results from three experiments are shown. (C) Activation of T cells. Lymphocytes were isolated from the PLNs and spleens of WT and CD4:DKO mice. CD62L and CD44 expression on CD4 and CD8 T cells was assessed by flow cytometric analysis. Results are representative of at least three experiments. (D) Effector cytokine production of T cells. Lymphocytes were isolated from the PLNs and spleens of WT and CD4:DKO mice. IFN-γ, IL-4, and IL-17A production by CD4 T cells was assessed by flow cytometric analysis. Results are representative of at least three experiments. (E) Distribution of Treg cells. Foxp3-expressing Treg cells among CD4 single-positive T-cell populations in the thymus, PLNs, and spleens were detected by flow cytometric analysis. Representative results from at least three experiments are shown.
Cell-Intrinsic Defects of Non-Treg Cells Lacking Smad2 and Smad3.
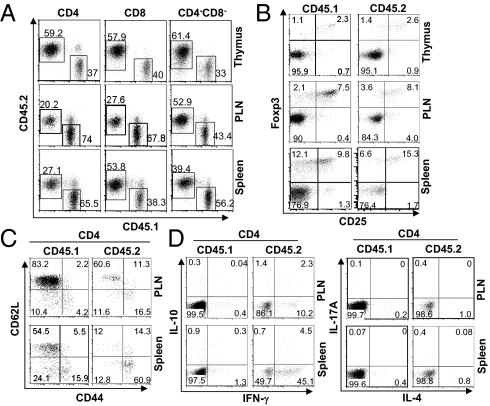
CD4:DKO mice developed lethal inflammation. Treg number may be aberrantly up-regulated under inflammatory conditions (6, 22–24). Thus, the inflammatory condition existing in CD4:DKO mice may mask potential Treg defects and confound the T-cell phenotypes observed. We therefore evaluated the cell-intrinsic effects of Smad2 and Smad3 deletion on T-cell function by generating mixed bone marrow chimeric mice, where WT and CD4:DKO T cells were compared directly in the same host. Equal numbers of bone marrow cells from CD45.2+ CD4:DKO mice and CD45.1+ WT mice were mixed and then transferred into irradiated Rag1−/− recipients. In the thymus of reconstituted recipients, the percentages of different thymocyte subsets were comparable between CD4:DKO and WT cells (Fig. 3A). The developmental markers were similarly expressed on CD4:DKO thymocytes and their WT counterparts (Fig. S4). In addition, mature CD4 and CD8 T cells of CD4:DKO origin were efficiently generated in the periphery (Fig. 3A). Moreover, similar percentages of Foxp3+ Treg cells of CD4:DKO and WT origins were found in the thymus, PLNs, and spleens of the recipients (Fig. 3B). Therefore, the development and peripheral maintenance of CD4:DKO T cells were largely normal. Nonetheless, although coexisting WT CD4 non-Treg and CD8 T cells displayed a naive phenotype, CD4:DKO CD4 non-Treg and CD8 T cells showed an activated phenotype (CD62LlowCD44high) (Fig. 3C and Fig. S5A). In addition, compared with coexisting WT CD4 non-Treg and CD8 T cells, wherein minimal effector cytokines were produced, CD4:DKO CD4 and CD8 T cells produced increased amounts of effector cytokines (Fig. 3D and Fig. S5B). These findings suggest that Smad2- and Smad3-deficient non-Treg cells are intrinsically prone to spontaneous activation and differentiation in vivo in the presence of functional WT Treg cells.
Fig. 3.
Cell-intrinsic defects of T cells lacking Smad2 and Smad3. Bone marrow cells isolated from WT (CD45.1+) and CD4:DKO (CD45.2+) mice were mixed at a 1:1 ratio. Cell mixtures were transferred into sublethally irradiated Rag1−/− mice. Eight weeks after transfer, T cells from reconstituted, mixed bone marrow chimeric mice were analyzed. (A) Distributions of CD4 T, CD8 T, and CD4−CD8− cells generated from the bone marrow cells of WT (CD45.1+) and CD4:DKO (CD45.2+) mice were determined by flow cytometric analysis. (B) Foxp3-expressing Treg cells among CD4 single-positive T-cell populations in the thymus, PLNs, and spleens were detected by flow cytometric analysis. The cells originating from WT (CD45.1+) and CD4:DKO (CD45.2+) mice were distinguished by congenic markers CD45.1 and CD45.2, respectively. (C) Lymphocytes were isolated from the PLNs and spleens of mixed bone marrow chimeric mice. CD62L and CD44 expression on CD4 T cells originating from WT (CD45.1+) and CD4:DKO (CD45.2+) mice was assessed by flow cytometric analysis. (D) Lymphocytes were isolated from the PLNs and spleens of mixed bone marrow chimeric mice. IFN-γ, IL-10, IL-4, and IL-17A production by CD4 T cells originating from WT (CD45.1+) and CD4:DKO (CD45.2+) mice was assessed by flow cytometric analysis. Results shown are representative of at least three experiments.
Evaluation of the Function of CD4:DKO Non-Treg and Treg Cells in Vitro.
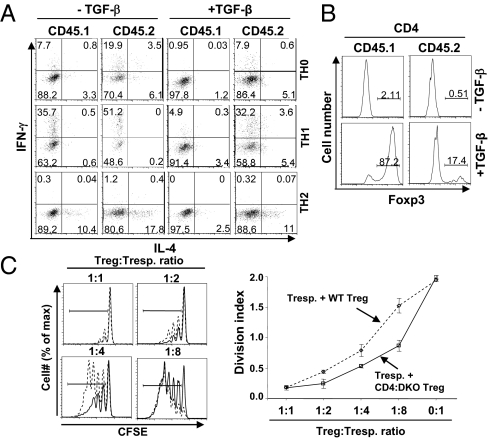
The spontaneous T-cell activation and effector cytokine production of CD4:DKO CD4 T cells are likely attributable to the enhanced ability of these cells to differentiate into effector T cells. We thus compared the ability of WT and CD4:DKO CD4 T cells to differentiate into TH1 and TH2 cells under polarizing conditions. Because naive CD4 T cells could hardly be obtained from CD4:DKO mice, we sorted CD25−CD62LhighCD44low naive CD4:DKO and WT CD4 T cells from bone marrow chimeric mice as described in Fig. 3. Purified cells were activated under nonpolarizing (TH0) as well as TH1 and TH2 polarizing conditions. TH1 and TH2 differentiation of CD4 T cells was assessed 4 d after activation. Higher percentages of IFN-γ–expressing cells were detected in CD4:DKO cells than in WT cells under TH0 and TH1 conditions. Higher percentages of IL-4–expressing cells were detected in CD4:DKO cells than in WT cells under TH0 and TH2 conditions (Fig. 4A). Therefore, in the absence of Smad2 and Smad3, T cells displayed enhanced ability to differentiate into effector TH cells.
Fig. 4.
Evaluation of the function of CD4:DKO T cells in vitro. (A) WT (CD45.1+) and CD4:DKO (CD45.2+) CD25−CD62LhighCD44low naive CD4 T cells were purified from the mixed bone marrow chimeric mice described in Fig. 3. Purified cells were activated with plate-bound anti-CD3 and anti-CD28 mAb under nonpolarizing (TH0) and TH1 or TH2 polarizing conditions in the presence (+) or absence (−) of exogenous recombinant TGF-β. IFN-γ and IL-4 production was assessed by intracellular cytokine staining and flow cytometric analysis. Results are representative of three experiments. (B) WT (CD45.1+) and CD4:DKO (CD45.2+) CD25−CD62LhighCD44low naive CD4 T cells were purified from the mixed bone marrow chimeric mice described in Fig. 3. Purified cells were activated with plate-bound anti-CD3 and anti-CD28 mAb in the presence (+) or absence (−) of TGF-β. The generation of iTreg cells was determined by Foxp3 intracellular staining and flow cytometric analysis. Results are representative of three experiments. (C) CD4+CD25+ Treg cells were sorted from WT (dashed line, open diamonds) and CD4:DKO (solid line, open squares) mice. An in vitro T-cell suppression assay was performed. (Left) Proliferation of responder T cells was assessed by carboxyfluorescein succinimidylester (CFSE) dilution. Results are representative of three experiments. (Right) Mean division index ± SD of three samples in one experiment representative of three are plotted.
Because Smad2 and Smad3 are involved in the TGF-β signaling that is important to suppress TH differentiation and to promote Foxp3 expression in activated T cells (4, 8, 9), we hypothesized that CD4:DKO T cells were refractory to TGF-β–mediated inhibition of TH differentiation and TGF-β–promoted iTreg generation. Indeed, although addition of exogenous TGF-β potently inhibited TH1 and TH2 differentiation of WT T cells, such effects of TGF-β were greatly reduced in CD4:DKO T cells (Fig. 4A). In addition, TGF-β–promoted iTreg generation was impaired in CD4:DKO T cells (Fig. 4B). These findings suggest that Smad2 and Smad3 are required for TGF-β–mediated suppression of non-Treg cells as well as TGF-β–promoted conversion of non-Treg cells to Treg cells.
Although we have found that Smad2 and Smad3 were not required for the generation and maintenance of Treg cells (Figs. 2 and 3), whether the suppressive function of CD4:DKO is intact remains to be addressed. To answer this question, we performed in vitro T-cell suppression assays as previously described (25). CD4+CD25+ Treg cells were sorted from CD4:DKO mice and WT littermates as “suppressor T cells.” Carboxylfluorescein succinimidylester-labeled WT CD25−CD45RBhi CD4 non-Treg cells were used as “responder T cells.” CD4:DKO Treg cells suppressed proliferation of responder T cells as efficiently as, if not more efficiently than, WT Treg cells, suggesting that CD4:DKO Treg cells possess intact immune suppressive function (Fig. 4C).
Treg Homeostasis and Function in Mice with Treg-Specific Double Deletion of Both Smad2 and Smad3.
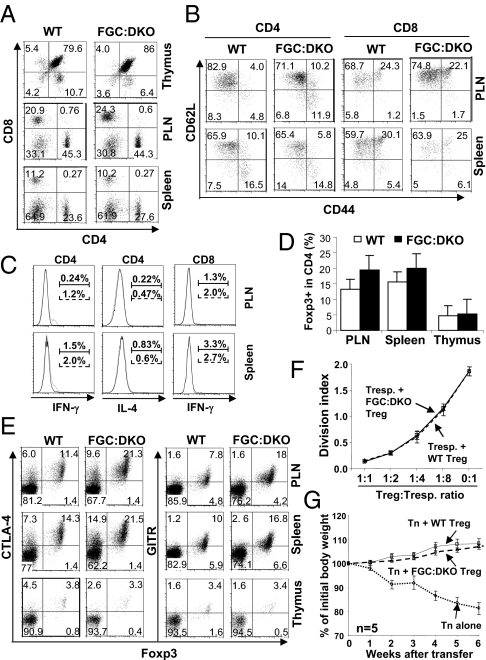
TGF-β is required for the peripheral maintenance of Treg cells (5, 26). Whether the Smad-dependent or -independent pathway mediates TGF-β–promoted Treg maintenance remains unclear. Our aforementioned findings suggest that Smads may not be essential for Treg generation and function. To investigate further the specific requirements of Smad2 and Smad3 for Treg homeostasis and function under physiological conditions, we generated mice with Treg-specific Smad2 and Smad3 double deficiency. Smad2fl/fl and Smad3−/− mice were crossed with Foxp3-EGFP-Cre (FGC) transgenic mice, where a BAC transgenic construct encoding both EGFP and Cre under the control of Foxp3 promoter was inserted into mouse genome (27). Thus, in FGC:Smad2fl/fl:Smad3−/− mice (hereafter referred to as FGC:DKO mice), only Foxp3-expressing Treg cells are deficient in both Smad2 and Smad3. FGC:DKO mice were grossly normal. The development and homeostasis of T cells of FGC-DKO mice were comparable to those of WT littermates (Fig. 5A). In addition, minimal activation (Fig. 5B) and cytokine production (Fig. 5C) were detected in non-Treg cells from FGC:DKO mice. These findings suggest that Smad2 and Smad3 deletion in Treg cells did not compromise immune homeostasis. Further analysis revealed that Treg cells were efficiently generated in the thymus and maintained in the periphery of FGC:DKO mice (Fig. 5D). A modestly higher percentage of Treg cells was detected in FGC:DKO mice compared with WT littermates. In addition, compared with WT Treg cells, Treg cells in FGC:DKO mice expressed normal levels of Treg signature genes, including cytotoxic T-lymphocyte antigen 4 (CTLA-4) and glucocorticoid-induced TNFR-related protein (GITR) (Fig. 5E). Moreover, compared with WT Treg cells, FGC:DKO Treg cells suppressed naive T-cell proliferation in vitro (Fig. 5F) and inhibited naive T cell-elicited inflammatory bowel disease (IBD) in vivo (Fig. 5G) to a similar extent. These findings suggest that Smad2 and Smad3 are dispensable for Treg function.
Fig. 5.
Normal Treg homeostasis and function in mice with Treg-specific double deficiency of Smad2 and Smad3. (A) Distribution of CD4 and CD8 T cells in the thymus, PLNs, and spleens of WT and FGC:DKO mice, wherein both Smad2 and Smad3 were deleted specifically in Treg cells. Representative results from three experiments are shown. (B) Activation of CD4 and CD8 T cells in the PLNs and spleens of WT and FGC:DKO mice was assessed by CD62L and CD44 staining and flow cytometric analysis. Results are representative of three experiments. (C) Effector cytokine production by CD4 and CD8 T cells in the PLNs and spleens of WT (solid lines) and FGC:DKO (dashed lines) mice was assessed by intracellular cytokine staining and flow cytometric analysis. The percentages of cytokine-producing cells are indicated above the brackets. Representative results of three experiments are shown. (D) Percentages of Foxp3+ Treg cells among CD4 single-positive T cells in the PLNs, spleens, and thymus of WT and FGC:DKO mice were determined by intracellular staining and flow cytometric analysis. The mean ± SD of three pairs of mice are shown. (E) Flow cytometric analysis of coexpression of cytotoxic T-lymphocyte antigen 4 (CTLA-4), glucocorticoid-induced TNFR-related protein (GITR) with Foxp3 in Treg cells from the PLNs, spleens, and thymus of WT and FGC:DKO mice. Results are representative of three experiments. (F) GFP+ Treg cells were sorted from WT (dashed line) or FGC:DKO (solid line) mice. An in vitro T-cell suppression assay was performed. The mean division index ± SD of three samples in one experiment representative of three are plotted. (G) GFP+ Treg cells were sorted from WT (solid line) or FGC:DKO (dashed line) mice. Treg-mediated protection of naive CD4+ T cell-elicited inflammatory bowel disease (IBD) was assessed. The body weight changes of recipient mice were recorded. The mean ± SD of five mice in one experiment representative of two are shown.
TAK1 Is Essential for Treg Homeostasis.
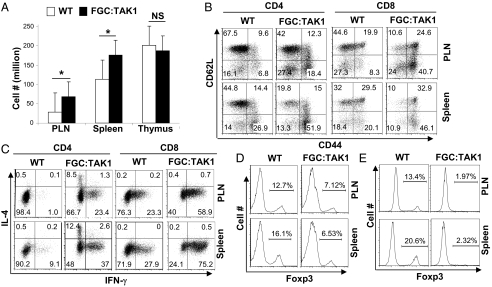
Smad-independent pathways are also involved in TGF-β signaling (12, 28). TAK1 can be activated by TGF-β independent of Smads (29) and is important for T-cell development, function, and survival (30). Whether and how TAK1 is involved in Treg function have not been addressed. We hypothesized that TAK1-mediated signaling is critical for the maintenance of Treg cells. To test this hypothesis, we crossed TAK1fl/fl mice (31) with FGC mice to delete TAK1 specifically in Treg cells. FGC:TAK1fl/fl mice succumbed to an inflammation disorder by 6 wk of age. FGC:TAK1fl/fl mice developed lymphadenopathy and splenomegaly. In agreement with this observation, the total numbers of lymphocytes recovered from the PLNs and spleens of FGC:TAK1fl/fl mice were higher compared with those from WT littermates (Fig. 6A). In addition, non-Treg CD4 T cells and CD8 T cells in FGC:TAK1fl/fl mice displayed an activated phenotype (Fig. 6B) with increased production of effector cytokines (Fig. 6C). These findings suggested a defect in Treg population. Indeed, the numbers of Foxp3-expressing Treg cells were significantly reduced in FGC:TAK1fl/fl mice (Fig. 6D). A closer examination revealed that the remaining Treg cells in FGC:TAK1fl/fl mice are “escaping” Treg cells failing to express functional Cre, because when FGC:TAK1fl/fl mice were crossed with Cre-reporter mice, where YFP is expressed only when functional Cre is present in the cells, few YFP cells could be detected in these mice (Fig. S6). This observation suggests that TAK1 is essential for Treg homeostasis. To validate this finding further and to study the cell-intrinsic defects of TAK-deficient Treg cells, we generated mixed bone marrow chimeric mice to compare TAK1-deficient and -sufficient Treg cells in the same hosts. Equal numbers of bone marrow cells from FGC:TAK1fl/fl mice (45.1.2) and WT mice (45.2.2) were mixed and transferred into irradiated Rag1−/− recipients. The contribution of the Treg population from different donor origins was determined 8 wk after transfer. Although 10–20% of WT CD4 T cells expressed Foxp3, ∼2% of FGC:TAK1fl/fl CD4 T cells expressed Foxp3 in the chimeric mice. Therefore, TAK1 is essential for homeostasis of Treg cells.
Fig. 6.
TAK1 is essential for the homeostasis of Treg cells. (A) Numbers of lymphocytes in the PLNs, spleens, and thymus of WT and FGC:TAK1 mice, wherein TAK1 was specifically deleted in Treg cells. The mean ± SD of three pairs of mice are shown. *P < 0.05; NS, nonsignificant. (B) Activation of CD4 and CD8 T cells in the PLNs and spleens of WT and FGC:TAK1 mice was assessed by CD62L and CD44 staining and flow cytometric analysis. Results are representative of at least three experiments. (C) Effector cytokine production by CD4 and CD8 T cells in the PLNs and spleens of WT and FGC:TAK1 mice was assessed by intracellular cytokine staining and flow cytometric analysis. Results are representative of at least three experiments. (D) Percentages of Foxp3+ Treg cells among CD4 single-positive T cells in the PLNs, spleens, of WT and FGC:TAK1 mice were determined by intracellular staining and flow cytometric analysis. The values are indicated above the brackets. Representative results from at least three experiments are shown. (E) Equal numbers of bone marrow cells isolated from WT (CD45.2.2) and FGC:TAK1 (CD45.1.2) mice were transferred into sublethally irradiated Rag1−/− recipient mice. Eight weeks after transfer, CD4 T cells were isolated from the recipient mice. The percentages of Foxp3+ Treg cells of different origins were determined by flow cytometric analysis. The values are indicated above the brackets. Results representative of at least three experiments are shown.
Discussion
TGF-βR signaling cascades are complex, involving Smad-dependent and -independent pathways. The question remains as to whether the diverse function of TGF-β is differentially mediated through discrete signaling pathways. Using genetic approaches, we investigated this issue. Our results suggest that Smad-dependent pathways are obligatory to suppress the activation and TH differentiation of non-Treg cells and to maintain immune homeostasis. In addition, Smads are required for TGF-β–mediated immune suppression in T cells and for TGF-β–promoted iTreg generation. Therefore, Smads are likely the dominant factors mediating the TGF-β effect on non-Treg cells. In contrast, Smad2 and Smad3 deficiency did not noticeably affect the generation, homeostasis, or function of thymic-derived Treg cells, whereas deletion of TAK1, a critical component of the Smad-independent pathway, abrogated Treg homeostasis, suggesting that TGF-β controls Treg homeostasis through Smad-independent pathways. These findings support the notion that Smad-dependent and -independent pathways mediate discrete T-cell functions (Fig. S7). By carrying out distinct functions, Smad-dependent and -independent pathways cooperate to mediate TGF-β–controlled self-tolerance and immune homeostasis through Treg-dependent and -independent mechanisms.
Although nTreg and iTreg cells are both immune-suppressive, they are distinct in certain aspects. For instance, Foxp3 expression is more stable in nTreg cells than in iTreg cells (32–34), and the genetic program and epigenetic modification of gene loci of nTreg and iTreg cells are different (35–37), suggesting that the generation and maintenance of nTreg and iTreg cells may be mediated through distinct mechanisms. We have found that Smads are required for the generation of iTreg cells but not for the generation of nTreg cells, indicating that Smad signaling may be critically involved in the genetic and/or epigenetic distinction between nTreg and iTreg cells.
Being able to integrate signals from multiple stimuli is a common and useful feature of intracellular signaling transducers. Such ability allows signal transducers to sense different environment cues and to integrate and translate such cues into appropriate responses. As intracellular signal transducers, Smad2 and Smad3 predominantly mediate TGF-β signaling in T cells. It is nevertheless recognized that Smad2 and Smad3 can relay signaling of other TGF-β superfamily members, such as activins (38). Similarly, TAK1 can mediate signals not only from TGF-β but from T-cell receptor and other cytokines in T cells (30). Thus, the defects observed in T cells lacking Smad2, Smad3, and TAK1 could be the combined effects of abrogation of TGF-β and other signaling pathways. Although it remains to be revealed how much the effects of Smad deletion and/or TAK1 deletion on T cells can be attributed to the defects in TGF-β–dependent or –independent signals, this study provides genetic evidence to suggest that Smad-dependent and -independent pathways control distinct functions in different T-cell types, and thus offer unique insights into how pleiotropic effects of TGF-β on T cells could be mediated.
Deletion of Smad2 and Smad3 during the early [double-negative (DN)] stage of thymic development using LckCre resulted in drastic alternation of T-cell development (15). However, we found that thymocyte development was largely unperturbed when Smad2 and Smad3 were deleted at the later [double-positive (DP)] stage of T-cell development using CD4Cre. The reason for defective thymocyte development in Lck:DKO mice is unlikely to be attributable to inflammation because lethal inflammation developed in both models. Therefore, these findings suggest a critical role for Smads during the DN-to-DP transition in T-cell development. In addition, in Lck:DKO mice but not CD4:DKO mice, the generation of thymic Treg cells was defective, suggesting that the expression of Smad2 and Smad3 during the DN stage but not the DP stage of thymocyte development is essential for the induction of Treg cells. Therefore, Smad-dependent pathways may be required for the development of T cells at specific stage(s) in the thymus. Considering the fact that T-cell thymic selection establishes central tolerance, whether and how Smad-dependent pathways may regulate self-tolerance by modulating thymic selection of non-Treg and Treg cells are remaining questions to be addressed.
Materials and Methods
Mice.
SMAD3−/−, SMAD2fl/fl, TAK1fl/fl, CD4Cre, FGC, Rag1−/−, YFP Cre reporter, and CD45.1 mice were kept under specific pathogen-free conditions in the animal care facility at the University of North Carolina. All mouse experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina.
Cell Function Analysis in Vitro and in Vivo.
T-cell isolation, activation, differentiation, flow cytometric analysis, cell sorting, in vitro and in vivo suppression assays, and bone marrow chimera generation were performed as described previously (39). Details are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank E. Robertson and E. Bikoff (University of Oxford, Oxford, United Kingdom) for Smad2fl/fl mice, M. Schneider (Imperial College London, London, United Kingdom) for TAK1fl/fl mice, N. Fisher and J. Kalnitsky (University of North Carolina flow cytometry facility) for cell sorting, and J. Ting and M. Su (University of North Carolina) for helpful discussions. This study was supported by National Natural Science Foundation of China Grant 30801334 (to A.-D.G.), National Institutes of Health Grant R00AI072956, the Lupus Research Institute, and the University Cancer Research Fund (Y.Y.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108352109/-/DCSupplemental.
References
- 1.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 2.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2(1):46–53. doi: 10.1038/nri704. [DOI] [PubMed] [Google Scholar]
- 5.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, et al. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 11.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 12.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184:4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takimoto T, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185:842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 16.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 17.Martinez GJ, et al. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010;285:29039–29043. doi: 10.1074/jbc.C110.155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 19.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 20.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 21.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouyang W, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11:618–627. doi: 10.1038/ni.1884. [DOI] [PubMed] [Google Scholar]
- 23.Rudra D, et al. Runx-CBFbeta complexes control expression of the transcription factor Foxp3 in regulatory T cells. Nat Immunol. 2009;10:1170–1177. doi: 10.1038/ni.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitoh A, et al. Indispensable role of the Runx1-Cbfbeta transcription complex for in vivo-suppressive function of FoxP3+ regulatory T cells. Immunity. 2009;31:609–620. doi: 10.1016/j.immuni.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massagué J. How cells read TGF-beta signals. Nat Rev Mol Cell Biol. 2000;1(3):169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 29.Sorrentino A, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 30.Wan YY, Chi H, Xie M, Schneider MD, Flavell RA. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol. 2006;7:851–858. doi: 10.1038/ni1355. [DOI] [PubMed] [Google Scholar]
- 31.Xie M, et al. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA. 2006;103:17378–17383. doi: 10.1073/pnas.0604708103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 33.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 34.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.