Abstract
An important feature of many chronic parasitic infections is the ability of the invading pathogen and host to establish a compromise, which ensures successful parasitism without killing the infected host. For many helminth infections, down-modulating the immune response is critical because persistent inflammation can become more damaging to the host than the invading pathogen itself. Such is the case with schistosomiasis mansoni, where chronic granulomatous inflammation in the liver causes portal hypertension, porto-pulmonary shunting, bleeding from collateral bypass vessels, and eventual death if not suppressed effectively. CD4+ T helper type 2 cells (Th2) (secreting IL-4, IL-5, and IL-13) characterize the host response after Schistosoma mansoni infection, and recent studies have identified IL-13 as the principal mediator of hepatic fibrosis. Here, we show that the IL-13 receptor α 2 (IL-13Rα2) is a critical mediator of immune down-modulation, identifying the receptor as a life-sustaining off signal for chronic and pernicious inflammation in schistosomiasis.
Schistosomes infect >200 million people worldwide, most often populations in developing countries where exposure to infectious Schistosoma cercariae in snail-infested waters is high (1). Depending on the course of infection and patient symptoms, schistosomiasis mansoni can progress to a severe life-threatening form of disease (2). Indeed, the highest mortality in human schistosomiasis mansoni occurs in the minority of people who develop hepatosplenic schistosomiasis, characterized by periportal fibrosis, portosystemic shunts, and hematemesis (3, 4). In the murine model of Schistosoma mansoni infection, adult female worms begin laying eggs 5–6 wk postinfection. Many of the eggs become lodged in the portal venules of the liver, inducing a vigorous inflammatory response, resulting in eosinophil-rich granulomas that peak in size soon after egg deposition commences (6–9 wk). Thereafter, the granuloma volume around newly deposited eggs gradually diminishes as the infection progresses to the chronic stage (2). Nevertheless, in a subset of chronically infected patients, this process of “endogenous desensitization” or “immune down-modulation” is defective or inadequate, which may allow the disease to progress to the severe hepatosplenic form (5). Therefore, identifying mechanisms that facilitate immune down-modulation in schistosomiasis is critical to fully understanding pathogenesis and could ultimately provide new strategies for vaccine development (6, 7).
Suppressing the host T helper type 2 (Th2) response markedly reduces granuloma formation in schistosomiasis (8), implicating Th2 cytokines as important mediators in the disease. This hypothesis was verified in studies conducted with signal transducer and transactivator-6–/– (Stat6) (9), IL-4 receptor α–/– (IL-4Rα) (10), and IL-13–/– mice (11, 12). The type II IL-4 receptor is comprised of IL-4Rα and IL-13Rα1, with IL-4 and IL-13 both using the IL-4Rα chain for signal transduction (13). In addition to binding the signaling type II receptor, IL-13 binds the high-affinity receptor IL-13Rα2. The IL-13Rα2 is expressed in various tissues, exists as a soluble receptor in the urine and serum of mice (14, 15), exhibits inducible expression in vivo that is IL-13-, IL-10-, and Stat6-dependent (16), and regulates tissue and serum levels of IL-13 (16, 17). Moreover, functional studies conducted with IL-13Rα2–/– mice showed enhanced responsiveness to IL-13, including increased serum IgE and reduced macrophage IL-12 production after exposure to lipopolysaccharide, thus providing evidence of “decoy” activity for IL-13Rα2 in vivo (16, 17). Given, the central role of IL-13 in the pathogenesis of schistosomiasis, in the current paper we investigate whether the critical process of immune down-modulation observed during chronic infection is controlled by the IL-13Rα2.
Methods
Mice and Parasites. Female and male BALB/c and C57BL/6 mice were purchased from the National Cancer Institute, Frederick, MD. BALB/c IL-13Rα2–/– mice were generated as described (17). All mice were infected percutaneously via the tail with 25–30 cercariae of a Puerto Rican strain of S. mansoni (NMRI) obtained from infected Biomphalaria glabrata snails (Biomedical Research Institute, Rockville, MD).
Histopathology and Fibrosis Measurements. Hepatic granulomas, liver fibrosis, and egg burdens were measured as described (18).
Detection of Soluble IL-13Rα2 in Serum. Immulon-2 plates were coated with recombinant murine (rm) IL-13 (0.5 μg/ml) (provided by Wyeth) in PBS overnight. Plates were washed with 0.05% Tween 20 in PBS (PBST) and blocked with 5% milk in PBST. Mouse serum (at 1:10 or 1:40) and assay standard diluted in PBST plus 1% BSA were added for 2 h at 37°C. The detection Ab biotinylated goat anti-mouse IL-13Rα2 (R & D Systems) was added for 2 h at 37°C. Peroxidase-labeled streptavidin (1:1,000 for 1 h at 37°C) and ABTS (2,2′-azino-di(3-ethylbenzthiazoline-6-sulfonate) peroxidase substrate (both from Kirkegaard & Perry Laboratories) were used to detect biotinylated Ab. The concentration of IL-13Rα2 in the sample was determined from a serial-fold diluted standard of rmIL-13Rα2 Fc/chimera (R & D Systems). The sensitivity of the assay was ≈98 pg/ml.
For detection of human soluble IL-13Rα2, murine anti-human IL-13Rα2 monoclonal Ab (5 μg/ml) was used to coat Immulon-2 plates overnight. The plate was washed and blocked as described above. Plasma prepared from North American normal control blood donors (National Institutes of Health blood bank) or Ugandan schistosomiasis patients (19) was added at 1:10 (diluted as above) for 3 h at 37°C. The detection Ab, biotinylated goat anti-human IL-13Rα2, was added for 2 h at 37°C. Peroxidase-labeled streptavidin and ABTS substrate were used as described above. The concentration of IL-13Rα2 in the sample was determined from a serial-fold diluted standard of recombinant human IL-13Rα2Fc/chimera. The antibodies and standard used in this assay were all purchased from R & D Systems. The minimum level of detection for the assay was 3.12 ng/ml. Some of the Ugandan patients analyzed in this study had infections apart from S. mansoni. Of the study cohort, 30.2% had trichuris infections, 1.6% had ascaris infections, 0.8% had trichuris and ascaris infections, 11.5% had malaria, and 5% had both malaria and either trichuris or ascaris infections. Nevertheless, neither malaria nor other infections had any influence on the level of IL-13Rα2 [Student's t test on log(.+1) transformed data and Mann–Whitney test].
Results
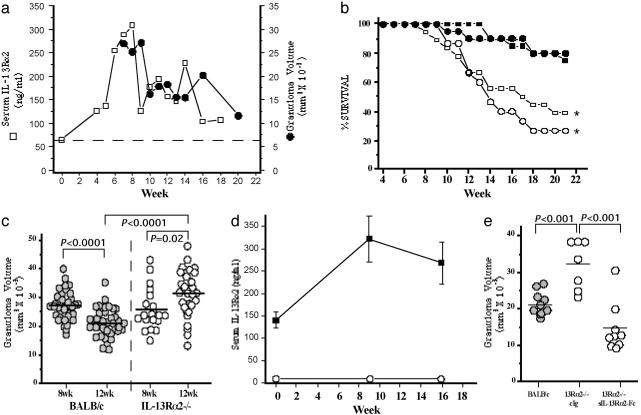
IL-13Rα2 Expression Increases After S. mansoni Infection and Is Required for Down-Modulation of Granuloma Formation During Chronic Infection. To examine the kinetics of serum IL-13Rα2 expression during schistosome infection, we exposed large numbers of mice to 25–30 S. mansoni cercariae and followed the course of infection for 5 months. The average serum levels of IL-13Rα2 protein were evaluated along with liver granuloma size at weekly intervals. Granuloma volumes peaked between 7 and 9 wk postinfection, with significant down-modulation by the tenth week of infection (Fig. 1a). Thereafter, only modest decreases in granulomatous inflammation were observed. To quantify soluble IL-13Rα2, a serum-based ELISA was generated. Before exposure to S. mansoni, IL-13Rα2 was detected in the serum of all naive mice but was significantly up-regulated after infection, with peak levels occurring during the acute phase of the granulomatous response (Fig. 1a) when the Th2 response is vigorous (4). Furthermore, although expression decreases as the disease progresses, the levels of IL-13Rα2 remain significantly above background. Consequently, there was remarkable congruence between the serum levels of IL-13Rα2 and the size of the egg-induced inflammatory reaction at acute and chronic time points.
Fig. 1.
IL-13Rα2 down-modulates granulomatous inflammation and prolongs survival in chronic S. mansoni infection. (a) C57BL/6 mice were infected percutaneously with 25–30 cercariae of S. mansoni. Uninfected (“wk 0,” n = 5) and infected mice (n = 5 weekly) were bled from wk 4 to wk 18 postinfection (open squares) to measure serum IL-13Rα2 levels; mean granuloma volumes were also determined (n = 5) from wk 7 to wk 20 (filled circles). The dashed line represents the mean level of serum IL-13Rα2 in naive mice. (b) BALB/c male (filled squares, n = 20), BALB/c female (filled circles, n = 20), IL-13Rα2–/– male (open squares, n = 18), and IL-13Rα2–/– female (open circles, n = 15) mice were infected percutaneously with 25–30 cercariae of S. mansoni. The median survival time for all IL-13Rα2–/– mice after infection was 15 wk (*, P = 0.0002). Survival was analyzed and compared with WT mice by log-rank test using prism 3.0 (GraphPad, San Diego). (c) BALB/c (wk 8, n = 36; wk 12, n = 36) and IL-13Rα2–/– (wk 8, n = 20; wk 12, n = 28) mice were infected and killed on wk 8 and wk 12 postinfection. The circles represent average granuloma measurements for individual mice from three independent experiments. Approximately 30 granulomas were measured in each animal. Relevant statistical comparisons are shown. (d) Uninfected and infected BALB/c(n = 5 per time point, filled squares) and IL-13Rα2–/– (open circles) mice were bled to quantify serum IL-13Rα2 levels at the times indicated. (e) Mice were infected, and liver granuloma size was measured at wk 12 postinfection in the surviving animals: BALB/c mice (n = 9), IL-13Rα2–/– mice treated with control IgG (n = 7), and IL-13Rα2–/– mice treated from wk 6 through wk 12 with 200 μg of soluble IL-13Rα2-Fc (sIL-13Rα2-Fc) (n = 9) injected three times weekly. BALB/c vs. sIL-13Rα2-Fc treated IL-13Rα2–/–, P = 0.011. Other relevant statistical comparisons are shown.
To determine whether the increase in IL-13Rα2 influenced disease progression, the next studies investigated the functional role of the receptor during chronic S. mansoni infection. In initial experiments, we monitored the survival of large groups of WT and IL-13Rα2–/– mice (Fig. 1b). Strikingly, the IL-13Rα2–/– mice displayed marked mortality as the infection progressed. By wk 15, only 50% of the IL-13Rα2–/– mice survived whereas little mortality was observed among the WT animals up to wk 21. Severe hemorrhage was suspected as the primary cause of death because blood was frequently found in the lumen of the small intestine on autopsy. Because the IL-13 receptors are located on the X chromosome (15), the data were also plotted according to sex, but no significant differences were noted for either strain. Next, to determine whether the increased morbidity of the IL-13Rα2–/– mice was related to a dysregulated inflammatory response, granuloma size was compared at the 8-wk (acute) and 12-wk time points. Wk 12 was selected as the optimal chronic time point for these studies because it falls well within the period where granuloma down-modulation is expected (Fig. 1a) yet precedes the time at which significant mortality is observed among the IL-13Rα2–/– mice (Fig. 1b). On wk 8, hepatic granuloma volumes did not differ between WT and IL-13Rα2–/– mice (Fig. 1c), which agreed with earlier observations (16). Furthermore, by wk 12, WT mice had significantly down-regulated their granulomatous response. In marked contrast, however, granuloma volumes not only failed to decrease in the IL-13Rα2–/– mice during this period, they increased significantly. As reported previously, at the acute stage of schistosomiasis (16), IL-13 production actually decreased in the IL-13Rα2–/– mice compared with WT mice at the 12 wk time point, providing evidence of a partially attenuated Th2 response in the absence of IL-13Rα2 (data not shown). However, consistent with the kinetic studies performed in C57BL/6 mice (Fig. 1a), the infected WT BALB/c mice showed significant elevations in serum IL-13Rα2 during this period (Fig. 1d). The ELISA was also highly specific because no receptor was detectable in the IL-13Rα2–/– mice at any time point. Therefore, to determine whether the failure to down-modulate the granulomatous response was due to the absence of the decoy IL-13 receptor, additional IL-13Rα2–/– mice were treated with soluble receptor (sIL-13Rα2-Fc) for 6 wk starting at wk 6 postinfection (15). Granuloma down-modulation was completely restored in the receptor-treated IL-13Rα2–/– mice, with granuloma size falling well below that observed in the control IgG-treated IL-13Rα2–/– mice (Fig. 1e). Granulomas in the treated mice were also significantly smaller than the control WT BALB/c group (Fig. 1e).
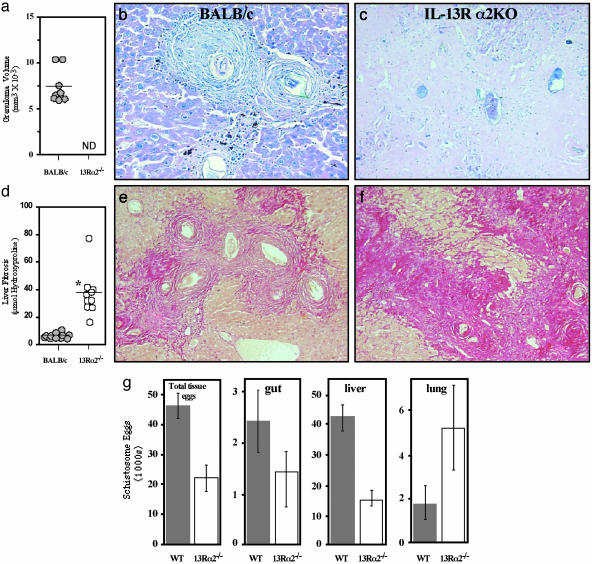
Chronically Infected IL-13Rα2-Deficient Mice Display Reduced Egg Burdens While Developing Markedly Increased Disease. To better understand the cause of the increased morbidity and mortality in the absence of IL-13Rα2, subsequent studies examined the pathology of the IL-13Rα2–/– mice in advanced disease. Here again, a large group of IL-13Rα2–/– mice was infected, and, when a sufficient number remained alive for analysis (wk 38), the surviving animals were killed and compared with WT mice. Distinct granulomas surrounding live miracidium-containing eggs were observed in the livers of all chronically infected WT mice (Fig. 2 a and b). Unexpectedly, live eggs were virtually absent in the livers of the IL-13Rα2–/– mice at this late stage (Fig. 2c), making granuloma measurements impossible (Fig. 2a). Histological examination of deeper cuts from the paraffin blocks also revealed no live eggs, confirming a remarkable absence of new egg deposition in the liver, thus mirroring observations made in patients with advanced disease (20). Strikingly, instead of finding active granulomas, the livers from infected IL-13Rα2–/– mice were a nearly solid mass of collagen (Fig. 2 c and f), exhibiting hydroxyproline levels 5- to 10-fold higher than the WT group (Fig. 2 d–f). The absence of viable eggs in the IL-13Rα2–/– liver tissue was not due to a significant difference in the number of worm pairs [WT 2.0 ± 0.22 (n = 11); IL-13Rα2–/– 1.4 ± 0.15 (n = 10)]. Although the gross appearance of the male and female worms also seemed normal, it is possible the parasites in IL-13Rα2–/– mice were less fertile and laid fewer eggs or, alternatively, the mice were destroying liver eggs more rapidly. Quantitative egg counts confirmed that there were significantly fewer eggs in the IL-13Rα2–/– mice (Fig. 2g). Nevertheless, the lower number of liver eggs could also result in part from the shunting of eggs to the lungs (21). Indeed, when egg burdens were quantified in individual organs, there was evidence of increased numbers of eggs per worm pair in the lungs of the IL-13Rα2–/– mice (Fig. 2g). This result contrasted with the gut and liver where the total tissue egg burden was less than in WT mice. Importantly, egg deposition in the lung provides the best evidence for the formation of collateral blood vessels (21), thus providing evidence for marked portal obstruction in the surviving chronically infected IL-13Rα2–/– mice. Such features were likely exacerbated in the mice that hemorrhaged and succumbed to the infection at earlier time points. Thus, although the influence of the IL-13Rα2 on granuloma formation seems minimal at the early acute stage (wk 8) (16), the relative importance of the receptor expands dramatically in the chronic phase of the disease.
Fig. 2.
Chronically infected IL-13Rα2–/– mice lack viable parasite eggs and newly formed granulomas in the liver and develop significant portal obstruction. BALB/c and IL-13Rα2–/– mice were infected with S. mansoni, and survivors were killed at wk 38 (n ≥ 10 analyzed per group). (a) Liver granuloma size was measured in infected BALB/c mice but could not be determined (ND) in IL-13Rα2–/– mice due to the absence of newly deposited mature eggs. (b) Representative hepatic granuloma from chronically infected BALB/c mice (Giemsa stain). (c) Chronically infected IL-13Rα2–/– mice lack viable parasite eggs in the liver and are devoid of active granulomatous lesions (Giemsa stain). (d) Fibrosis (μmol of hydroxyproline per 1.0 × 104 eggs) is significantly exacerbated in IL-13Rα2–/– mice after 38 wk of infection (*, P < 0.001). (e) Liver sections were prepared from chronically infected (38 wk) BALB/c mice and stained with the collagen-specific stain picrosirius red. Collagen deposition was localized within the granulomas. (f) Liver sections were prepared from chronically infected (38 wk) IL-13Rα2–/– mice and stained with picrosirius red. Large densely staining bands of collagen were seen throughout the liver. (g) Tissues were digested to enumerate egg burdens. The total tissue egg burden per worm pair, total eggs per gut (small and large intestine), and total eggs per liver and lung are shown as averages ± SE.
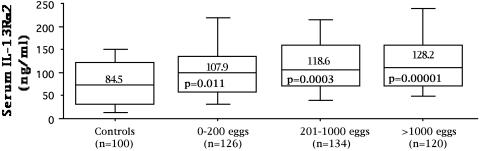
Serum IL-13Rα2 Levels Increase in Human Subjects in Response to S. mansoni Infection. Finally, although convincing evidence is provided showing increased levels of IL-13Rα2 in the sera of infected mice, no previous studies have quantified receptor levels in normal human serum or examined whether expression is modulated during an ongoing infection. To address this problem, we developed an assay to quantify the levels of IL-13Rα2 in human serum or plasma and then examined a large number of schistosomiasis mansoni subjects living in a fishing community on Lake Albert in Uganda. Stool samples were taken at the time of enrollment to determine whether S. mansoni eggs were present, which served as evidence of ongoing chronic infection and as a direct measure of infection intensity (19). All individuals were bled and then treated with praziquantel. Patient data were stratified by their egg counts to generate three equal size groups: those with 0–200, 201–1,000, and >1,000 eggs per g of feces. One hundred uninfected controls were also analyzed to determine the normal range for the receptor. Consistent with levels detected in naive mice, the uninfected controls displayed abundant serum IL-13Rα2 (average 84.5 ng/ml) (Fig. 3). More importantly, however, there was evidence of elevated expression in the schistosomiasis patients, with greater increases in individuals exhibiting higher intensity infections (Fig. 3). The overall level of receptor in the infected subjects was also remarkably similar to the chronically infected mice (Fig. 1a). In contrast to IL-13Rα2, however, serum levels of IL-13 were much more variable in the patients and no correlation was observed with their IL-13Rα2 response (data not shown).
Fig. 3.
Serum IL-13Rα2 increases in subjects chronically infected with S. mansoni. Schistosomiasis mansoni subjects (380) living in an endemic region in Uganda with evidence of chronic infection (eggs detectable in stool) were bled and then treated with praziquantel. One hundred uninfected controls from North America were also bled to establish a normal range for the soluble IL-13Rα2. Receptor levels in serum are shown in box and whisker plots. The median response is represented by the horizontal black line within a box that represents the 25th to 75th percentile range. Whiskers represent the range of data excluding outliers. The means and P values for each group are also shown (each patient group compared with uninfected controls). Patients in the low 0–200 eggs per g group were also significantly different from the heavily infected (>1,000 eggs per g of feces) group (P = 0.03).
Discussion
Induction of a functional decoy receptor system for the Th2 response was only recently described, and the data presented here represent a detailed examination of the role of IL-13Rα2 during a persistent infection. The findings described above document that the IL-13 decoy receptor critically controls the progression of murine schistosomiasis by reducing many key pathological changes that characterize the severe hepatosplenic form of the disease in humans (5, 21). Most notably, these data illustrate a vital role for the IL-13Rα2 in the down-regulation of the granulomatous inflammatory response, findings that translated into the long-term survival of the parasitized murine host. Elucidating the mechanisms by which the host suppresses chronic inflammation has been an important and growing area of research (22) because exploiting these natural regulatory systems in a therapeutic setting may help ameliorate a wide variety of inflammatory diseases. Based on the findings described here, it is now clear that the IL-13Rα2 system should be added to the list of important “stop signals” regulating the immune response (22). The fact that serum IL-13Rα2 levels were elevated in infected patients emphasizes the need for detailed studies on its role during human schistosomiasis, as well as in other diseases (23, 24) in which sustained Th2 cytokine production is thought to be pathogenic.
Acknowledgments
We thank Fred Lewis and his colleagues at the Biomedical Research Institute for providing the parasite materials, the animal staff at the National Institutes of Health for excellent technical assistance, Ms. Klaudia Walter for statistical assistance, and the schistosomiasis patients in Uganda for participating in the study. We also appreciate the helpful comments provided by Alan Sher, Stephanie James, David Sacks, and Fred Lewis during the preparation of the manuscript. M.J.G. is a Scholar of the Leukemia and Lymphoma Society of America and is supported by National Institutes of Health Grant AI4040171, a grant from the Sandler Program for Asthma Research, and by the Mathers Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Th2, T helper type 2; IL-13Rα2, IL-13 receptor α 2.
References
- 1.Colley, D. G., LoVerde, P. T. & Savioli, L. (2001) Science 293, 1437–1438. [DOI] [PubMed] [Google Scholar]
- 2.Domingo, E. O. & Warren, K. S. (1968) Am. J. Pathol. 52, 369–379. [PMC free article] [PubMed] [Google Scholar]
- 3.Cheever, A. W. & Andrade, Z. A. (1967) Trans. R. Soc. Trop. Med. Hyg. 61, 626–639. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann, K. F., Wynn, T. A. & Dunne, D. W. (2002) Adv. Parasitol. 52, 265–307. [DOI] [PubMed] [Google Scholar]
- 5.Warren, K. S. (1975) Bull. N.Y. Acad. Med. 51, 545–550. [PMC free article] [PubMed] [Google Scholar]
- 6.Pearce, E. J. (2003) Acta Trop. 86, 309–313. [DOI] [PubMed] [Google Scholar]
- 7.Todd, C. W. & Colley, D. G. (2002) Am. J. Trop. Med. Hyg. 66, 348–358. [DOI] [PubMed] [Google Scholar]
- 8.Wynn, T. A., Cheever, A. W., Jankovic, D., Poindexter, R. W., Caspar, P., Lewis, F. A. & Sher, A. (1995) Nature 376, 594–596. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan, M. H., Whitfield, J. R., Boros, D. L. & Grusby, M. J. (1998) J. Immunol. 160, 1850–1856. [PubMed] [Google Scholar]
- 10.Jankovic, D., Kullberg, M. C., Noben-Trauth, N., Caspar, P., Ward, J. M., Cheever, A. W., Paul, W. E. & Sher, A. (1999) J. Immunol. 163, 337–342. [PubMed] [Google Scholar]
- 11.Chiaramonte, M. G., Donaldson, D. D., Cheever, A. W. & Wynn, T. A. (1999) J. Clin. Invest. 104, 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fallon, P. G., Richardson, E. J., McKenzie, G. J. & McKenzie, A. N. (2000) J. Immunol. 164, 2585–2591. [DOI] [PubMed] [Google Scholar]
- 13.Obiri, N. I., Husain, S. R., Debinski, W. & Puri, R. K. (1996) Clin. Cancer Res. 2, 1743–1749. [PubMed] [Google Scholar]
- 14.Zhang, J. G., Hilton, D. J., Willson, T. A., McFarlane, C., Roberts, B. A., Moritz, R. L., Simpson, R. J., Alexander, W. S., Metcalf, D. & Nicola, N. A. (1997) J. Biol. Chem. 272, 9474–9480. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson, D. D., Whitters, M. J., Fitz, L. J., Neben, T. Y., Finnerty, H., Henderson, S. L., O'Hara, R. M., Jr., Beier, D. R., Turner, K. J., Wood, C. R. & Collins, M. (1998) J. Immunol. 161, 2317–2324. [PubMed] [Google Scholar]
- 16.Chiaramonte, M. G., Mentink-Kane, M., Jacobson, B. A., Cheever, A. W., Whitters, M. J., Goad, M. E., Wong, A., Collins, M., Donaldson, D. D., Grusby, M. J. & Wynn, T. A. (2003) J. Exp. Med. 197, 687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood, N., Whitters, M. J., Jacobson, B. A., Witek, J., Sypek, J. P., Kasaian, M., Eppihimer, M. J., Unger, M., Tanaka, T., Goldman, S. J., et al. (2003) J. Exp. Med. 197, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheever, A. W., Finkelman, F. D., Caspar, P., Heiny, S., Macedonia, J. G. & Sher, A. (1992) J. Immunol. 148, 3244–3248. [PubMed] [Google Scholar]
- 19.Kabatereine, N. B., Vennervald, B. J., Ouma, J. H., Kemijumbi, J., Butterworth, A. E., Dunne, D. W. & Fulford, A. J. (1999) Parasitology 118, 101–105. [DOI] [PubMed] [Google Scholar]
- 20.von Lichtenberg, F. (1975) J. Toxicol. Environ. Health 1, 175–184. [DOI] [PubMed] [Google Scholar]
- 21.Cheever, A. W. (1972) Trans. R. Soc. Trop. Med. Hyg. 66, 947–948. [DOI] [PubMed] [Google Scholar]
- 22.Nathan, C. (2002) Nature 420, 846–852. [DOI] [PubMed] [Google Scholar]
- 23.Medeiros, M., Jr., Figueiredo, J. P., Almeida, M. C., Matos, M. A., Araujo, M. I., Cruz, A. A., Atta, A. M., Rego, M. A., de Jesus, A. R., Taketomi, E. A. & Carvalho, E. M. (2003) J. Allergy Clin. Immunol. 111, 947–951. [DOI] [PubMed] [Google Scholar]
- 24.Wills-Karp, M., Luyimbazi, J., Xu, X., Schofield, B., Neben, T. Y., Karp, C. L. & Donaldson, D. D. (1998) Science 282, 2258–2261. [DOI] [PubMed] [Google Scholar]