Abstract
Positive and negative selection of thymocytes in the thymus are critical for the development of a mature and self-tolerant T-cell repertoire. The proapoptotic Bcl-2 family member Bim is important for negative selection by inducing apoptosis in thymocytes receiving a strong signal through their antigen receptor. However, in the case of ubiquitous self-antigens (UbA), Bim is not required for the clonal deletion of self-reactive thymocytes, suggesting the existence of nonapoptotic clonal deletion mechanisms. Unlike UbA, clonal deletion to tissue-restricted antigens (TRAs) requires positive selection and CCR7-mediated migration to the medulla. This led us to hypothesize that Bim is required for the latter. To study the role of Bim in clonal deletion to TRA, we constructed bone marrow (BM) chimeras using OT-I Bim-deficient or -sufficient donor bone marrow and recipients that express membrane bound chicken ovalbumin under control of the rat insulin promoter (Rip-mOVA). We found that clonal deletion to TRA was completely abrogated in the absence of Bim and large numbers of mature OT-I CD8 T cells survived in the periphery. Despite the large numbers of autoreactive T cells, the chimeras did not develop diabetes and OT-I Bim-deficient T cells from these chimeras were functionally impaired. Collectively, these data provide unique evidence of a differential, thymocyte-intrinsic, molecular requirement downstream of the T-cell receptor (TCR) for clonal deletion to UbA versus TRA and highlight the profound ability of other tolerance mechanisms to control T-cell autoreactivity in the absence of thymic clonal deletion.
Keywords: autoimmunity, thymocyte development
During T-cell development, the T-cell receptor (TCR) is generated by random rearrangements at the TCRα and TCRβ loci. This allows for a large variety of TCR specificities to be generated but also requires that T cells be educated. The education process ensures that the T cells can respond to antigen in the context of self-major histocompatibility complex (MHC) molecules but cannot respond to self-antigens in the context of self-MHC. These selection events begin at the CD4+CD8+ double-positive (DP) stage of thymocyte development within the cortex of the thymus. DP thymocytes may undergo one of three fates depending on the affinity of their TCR for self-peptide presented on self-MHC (pMHC) on cortical thymic epithelial cells (1). If the TCR cannot functionally interact with self-pMHC, the thymocytes die by neglect. Thymocytes expressing a TCR with high affinity for self-pMHC undergo negative selection, resulting in the elimination of that particular TCR specificity from the T-cell repertoire. Low or moderate affinity interactions between the TCR and self-pMHC result in positive selection, leading to thymocyte survival and subsequent differentiation into CD4+ or CD8+ single-positive (SP) thymocytes (1). These positively selected thymocytes then undergo CCR7-dependent migration to the thymic medulla (2). During the 3- to 4-d residence in the medulla (3), SP thymocytes are subjected to a further round of negative selection through interactions with medullary thymic epithelial cells (mTECs) and medullary dendritic cells (DCs) (4, 5). Interestingly, some T-cell subsets are positively selected by high-affinity antigen encounter in the thymus (6). One such example are T-regulatory (Treg) cells, which are believed to require high-affinity interactions to develop in the medulla (7). It remains unknown what drives this distinction between negative selection and Treg development, although secondary signals, such as TGF-β, may play a role in overcoming cell death (8).
Although it is unclear how the same TCR can transduce a signal for positive or negative selection, differential mitogen-activated protein kinase signaling appears to play a role, ultimately leading to the activation of different transcriptional programs (9). Negative selection induces expression of proapoptotic molecules such as the Bcl-2 homology domain 3 (BH3) only Bcl-2 family member Bim (10, 11), which is essential for the intrinsic pathway of apoptosis (12). It was generally held that Bim-mediated apoptosis was required for negative selection (13). This was supported by the fact that Bim-deficient mice develop late onset autoimmune disease and that nonobese diabetic mice, which spontaneously develop diabetes, have a defect in the induction of Bim (11, 14, 15). In contrast to this paradigm, recent studies demonstrated that, whereas Bim is required for thymocyte apoptosis, it is not required for clonal deletion to the ubiquitous s-mcy male peptide (16, 17) or superantigen-mediated clonal deletion (18).
It is clear that negative selection is induced by two different classes of self-antigens: ubiquitous self-antigens (UbA) and tissue-restricted antigens (TRAs). TRA expression is restricted to the medulla with expression of many TRAs controlled by the AIRE gene (19). After much debate, recent data suggest that negative selection of DP thymocytes to UbA occurs in the cortex without medullary involvement (20), whereas positive selection and CCR7-dependent migration to the medulla are required for negative selection to TRA (4). Once in the medulla, CD4+CD8lo or CD4loCD8lo thymocytes can interact with mTECs that directly present TRA or DCs that can cross-present TRAs derived from mTECs (5). Therefore, negative selection to UbA and TRA differ with respect to the developmental stage of the thymocyte and the anatomical location within the thymus. These factors may result in differential molecular requirements for negative selection to UbA versus TRA, although no such thymocyte intrinsic differences downstream of the TCR have been reported.
Using OT-I TCR transgenic bone marrow (BM) and mice expressing membrane bound chicken ovalbumin (mOVA) under control of the chicken β-actin promoter (Act-mOVA) (21) or rat insulin promoter (RIP-mOVA) (22), we demonstrate that unlike clonal deletion to UbA, Bim is required for clonal deletion to TRA. Although OT-I Bim−/− CD8 T cells are abundant in RIP-mOVA chimeras, they do not cause diabetes, but rather are impaired in their response to antigen.
Results
Bim Is Not Required for Clonal Deletion to UbA.
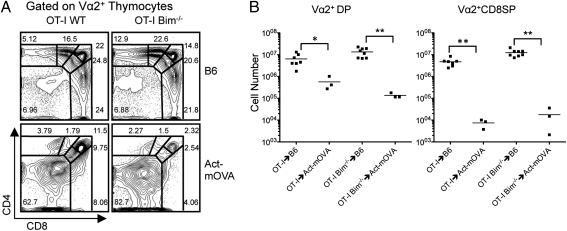
Recently, it has become clear that Bim is not required for clonal deletion in a number of physiological model systems (16, 17). However, in these models, the high-affinity antigen was expressed ubiquitously and therefore the role of Bim in clonal deletion to TRA is still unclear. As these models are not easily manipulated to examine clonal deletion to TRA, we sought to use the OT-I TCR transgenic system. OT-I thymocytes express a TCR specific for the SIINFEKL peptide of OVA in the context of H-2Kb and can be detected using an anti-Vα2 mAb. Furthermore, mice that express membrane OVA ubiquitously or in a tissue-restricted fashion are available. To ensure that clonal deletion of OT-I thymocytes to UbA was Bim independent, we generated BM chimeras using Act-mOVA recipients and either OT-I or OT-I Bim−/− BM donors. We found that Bim deficiency did not affect the CD4/CD8 thymic profiles of Vα2+ thymocytes in the OT-I Bim−/−→B6 chimeras compared with the OT-I→B6 chimeras (Fig. 1A). In both cases, DP, CD4+CD8int, and CD8SP thymocyte percentages were similar (Fig. 1A). In both Act-mOVA chimeras, the majority of thymocytes were CD4−CD8− double negative (DN) with reductions in DP, CD4+CD8int, and CD8SP populations, indicating deletion of OT-I thymocytes (Fig. 1A). The increased percentage of DN thymocytes was more pronounced in the OT-I Bim−/−→Act-mOVA chimeras. Cell numbers in the various compartments further supported Bim-independent clonal deletion to UbA. There was a similar reduction in Vα2+ DP and Vα2+ CD8SP cell numbers in OT-I→Act-mOVA compared with OT-I→B6 and in OT-I Bim−/−→Act-mOVA compared with OT-I Bim−/−→B6 chimeras (Fig. 1B). Furthermore, cleaved caspase 3 staining showed significant caspase 3 activity in the OT-I→Act-mOVA chimeras that was dramatically reduced in the OT-I Bim−/−→Act-mOVA chimeras (Fig. S1), indicating that Bim-independent mechanisms of clonal deletion are functioning. These data suggest that OT-I thymocytes are efficiently deleted in the thymus in response to ubiquitously expressed OVA regardless of Bim expression. These results recapitulate those observed in previous studies (16–18), providing further support that Bim is not required for clonal deletion to UbA and demonstrate that the OT-I model is comparable to other models.
Fig. 1.
Bim is not required for negative selection to UbA. (A) CD4 by CD8 profile of Vα2+ thymocytes from OT-I→B6 (n = 7), OT-I→Act-mOVA (n = 3), OT-I Bim−/−→B6 (n = 8), and OT-I Bim−/−→Act-mOVA (n = 3). (B) Number of Vα2+ DP and Vα2+ CD8+ thymocytes recovered from thymi of indicated strains. Data depict means and are representative of the indicated number of samples. *P < 0.05, **P ≤ 0.01.
Bim Is Required for Clonal Deletion to TRA.
Despite the lack of requirement for Bim in clonal deletion in the OT-I model and other models, the fact remains that Bim deficiency leads to autoimmune disorders (14). Whereas the previous models examined clonal deletion to UbA or superantigen they did not address clonal deletion to TRA, which differs with respect to the developmental stage of the thymocyte and anatomical location within the thymus. We hypothesized that these distinctions may result in a difference in the requirement for Bim in clonal deletion to TRA.
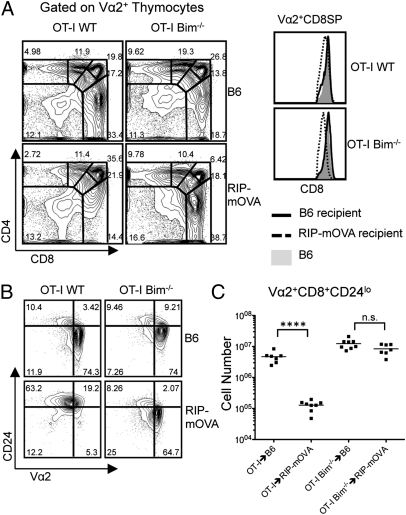
To generate a model of clonal deletion to TRA, we grafted OT-I or OT-I Bim−/− BM to RIP-mOVA recipients. The rat insulin promoter drives expression of mOVA in the pancreas, the kidney, and the medulla of the thymus. In this model, OT-I thymocytes will first undergo positive selection in the cortex before encountering their cognate antigen in the medulla. The CD4/CD8 thymic profiles of Vα2+ thymocytes from OT-I→B6 and OT-I Bim−/−→B6 chimeras were not drastically altered by Bim deficiency (Fig. 2A). In contrast and as previously demonstrated (23), the OT-I→RIP-mOVA chimeras displayed a significant reduction in the proportion of Vα2+ CD8SP thymocytes with an increase in the DN compartment (Fig. 2A). Contrary to the OT-I→RIP-mOVA chimeras, the OT-I Bim−/−→RIP-mOVA chimeras showed no evidence of clonal deletion compared with the OT-I Bim−/−→B6 chimeras. Interestingly, CD8 expression was slightly down-regulated in the OT-I Bim−/−→RIP-mOVA chimeras and may be indicative of high-affinity antigen encounter (Fig. 2A). With the exception of the Vα2+ CD8SP compartment, the number of Vα2+ thymocytes in the other compartments was similar (Table 1). We also examined CD24 expression on Vα2+ CD8SP thymocytes to assess the maturity of the thymocytes. Compared with either OT-I or OT-I Bim−/−→B6, OT-I→RIP-mOVA chimeras showed a considerable reduction in the proportion of mature, CD24lo, Vα2+ CD8SP thymocytes, whereas the proportion of mature OT-I thymocytes in OT-I Bim−/−→RIP-mOVA chimeras was equivalent (Fig. 2B). Cell numbers reinforced these observations with OT-I and OT-I Bim−/−→B6 and OT-I Bim−/−→RIP-mOVA displaying similar numbers of mature OT-I CD8SP thymocytes while the number of these cells was reduced approximately 50-fold in OT-I→RIP-mOVA chimeras compared with OT-I→B6 chimeras (Fig. 2C). Collectively, this rescue of the mature OT-I CD8SP population by Bim deficiency suggests that clonal deletion of TRA-specific thymocytes is completely abrograted in the absence of Bim.
Fig. 2.
Bim is required for negative selection to TRA. (A) CD4 by CD8 profile of Vα2+ thymocytes from OT-I→B6 (n = 7), OT-I→RIP-mOVA (n = 8), OT-I Bim−/−→B6 (n = 8), and OT-I Bim−/−→RIP-mOVA (n = 7) and CD8 expression of Vα2+ CD8+ thymocytes from indicated strains. (B) CD24 by Vα2 profile of CD8 thymocytes from indicated strains. (C) Cell numbers of indicated populations from indicated mice. Data are representative of the indicated number of samples over four independent experiments. ****P ≤ 0.0001. NS, no significance.
Table 1.
Bim is required for clonal deletion to TRA
| Chimeras | OT-I→B6 | OT-I→RIP-mOVA | OT-I Bim−/−→B6 | OT-I Bim−/−→RIP-mOVA |
| Vα2+DPBright | 6.38 ± 4.00 | 4.44 ± 2.52 | 13.4 ± 6.26 | 4.90 ± 3.15 |
| Vα2+CD4+CD8int | 4.81 ± 2.15 | 2.32 ± 0.672 | 13.1 ± 3.04 | 5.71 ± 3.41 |
| Vα2+DPDull | 5.81 ± 3.20 | 4.61 ± 1.52 | 9.26 ± 4.12 | 7.81 ± 5.97 |
| Vα2+CD8SP | 4.69 ± 1.94 | 0.129 ± 0.045 | 12.4 ± 4.44 | 8.43 ± 3.17 |
Absolute number of Vα2+ thymocytes from the indicated strains. Data are compiled from seven to eight mice (mean ± SD × 106).
TRA-Specific T Cells Persist in the Periphery in the Absence of Bim.
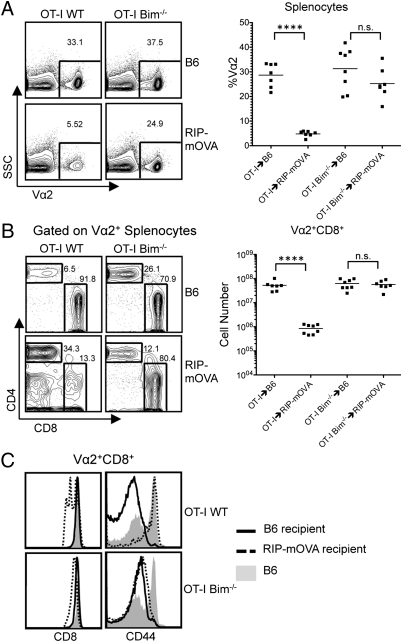
To determine the fate of the OT-I Bim−/− CD8SP thymocytes that escaped clonal deletion in the thymus, we examined the periphery of the chimeras. The proportion of Vα2+ splenocytes was similar in the OT-I→B6 chimeras and the OT-I Bim−/−→B6 chimeras. In the OT-I→RIP-mOVA chimeras, the proportion of Vα2+ splenocytes was significantly reduced compared with the OT-I→B6 control chimera, whereas in the OT-I Bim−/−→RIP-mOVA chimeras, the proportion of Vα2+ cells was similar to what was seen in the OT-I Bim−/−→B6 chimeras (Fig. 3A). Further examination of the Vα2+ splenocytes for CD4 and CD8 expression revealed a similar proportion of CD8+ T cells in the OT-I→B6 and OT-I Bim−/−→B6 chimeras, although the CD4+ T-cell population was larger in the OT-I Bim−/−→B6 mice (Fig. 3B). OT-I→RIP-mOVA chimeras had a dramatically reduced proportion of CD8+ T cells within the Vα2+ population compared with the OT-I→B6 and OT-I Bim−/−→B6 chimeras (Fig. 3B). Similar to the OT-I→B6 and OT-I Bim−/−→B6 chimeras, the Vα2+ splenocytes from OT-I Bim−/−→RIP-mOVA chimeras were largely CD8+. As in the thymus, we observed down-regulation of the CD8 coreceptor, suggesting antigen encounter in the periphery (Fig. 3 B and C). OT-I→B6 and OT-I Bim−/−→B6 chimeras had similar numbers of Vα2+ CD8+ T cells, demonstrating that Bim does not significantly alter mature peripheral T-cell numbers (Fig. 3B). As expected, Vα2+ CD8+ T-cell numbers were significantly reduced in the OT-I→RIP-mOVA chimeras, demonstrating effective deletion of these autoreactive T cells through central and peripheral tolerance mechanisms (Fig. 3B). However, in the OT-I Bim−/−→RIP-mOVA chimeras, there was an abundance of OT-I Bim−/− T cells in the periphery (Fig. 3B), further suggesting that not only was thymic clonal deletion abrogated but peripheral clonal deletion was impaired as well. The few OT-I T cells that did enter the periphery in OT-I→RIP-mOVA chimeras expressed high levels of CD44 (Fig. 3C). It is unclear whether CD44 induction occurred in response to antigen or lymphopenia. Interestingly, Vα2+ CD8+ T cells from the OT-I Bim−/−→RIP-mOVA chimeras had a CD44lo phenotype, suggesting that they were not overtly activated in the periphery, even though cognate antigen was present and CD8 dulling was apparent (Fig. 3C). It has been previously shown that adoptive transfer of 5 × 106 OT-I T cells into a RIP-mOVA mouse will result in rapid onset of diabetes (24). However, blood glucose readings obtained for up to 14 wk post-BM transplant demonstrated that these OT-I Bim−/−→RIP-mOVA chimeras did not develop diabetes despite that, on average, there were 6.5 × 107 OT-I T cells present in the spleen. These data suggest that although OT-I Bim−/− thymocytes escaped deletion in the thymus of RIP-mOVA mice and survived in the periphery, they were unable to cause diabetes.
Fig. 3.
TRA-specific T cells persist in the periphery in the absence of Bim. (A) SSC by Vα2 profile of splenocytes from indicated strains. OT-I→B6 (n = 7), OT-I→RIP-mOVA (n = 8), OT-I Bim−/−→B6 (n = 8), and OT-I Bim−/−→RIP-mOVA (n = 7) and percentage of Vα2+ splenocytes from indicated strains. (B) CD4 by CD8 profile of Vα2+ splenocytes from indicated strains and Vα2+ CD8+ cell numbers from indicated strains. (C) CD8 and CD44 expression of Vα2+ CD8+ T cells from indicated strains. Data are representative of at least five mice from each strain over four independent experiments. ****P ≤ 0.0001. NS, no significance.
TRA-Specific T Cells Are Functionally Impaired in the Periphery of OT-I Bim−/−→RIPmOVA Chimeras.
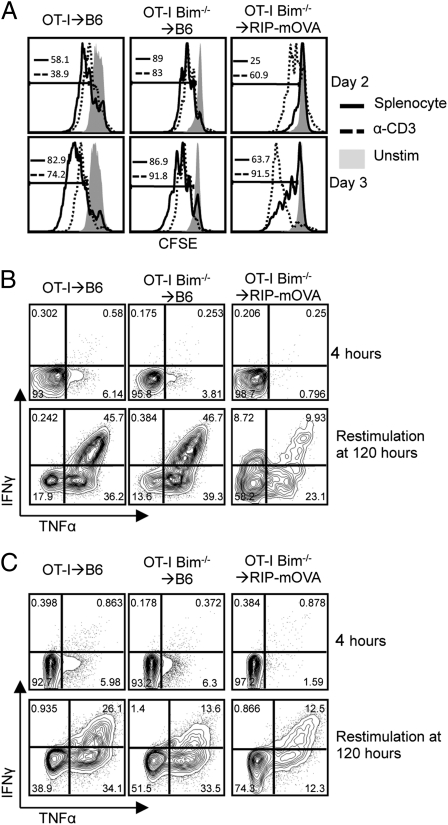
Recently, it was demonstrated that Bim−/− CD4+ T cells were unable to produce cytokines following activation (25). Therefore, if OT-I Bim−/− T cells were functionally impaired, it would explain why the OT-I Bim−/−→RIP-mOVA chimeras did not develop diabetes. However, pathogen challenge experiments with OT-I Bim−/− T cells showed no such impairment in effector function (26). To further study the effector capacity of peripheral Vα2+ CD8+ T cells from the OT-I Bim−/−→RIP-mOVA chimeras, we stimulated carboxy fluorescein succinimidyl ester (CFSE)-labeled bulk splenocytes from the chimeras with either SIINFEKL-pulsed congenic splenocytes or plate-bound anti-CD3/28 antibodies and examined their ability to proliferate and produce cytokines. While OT-I and OT-I Bim−/− splenocytes from the B6 chimeras proliferated equally on days 2 and 3 poststimulation, only a minority of splenocytes from the OT-I Bim−/−→RIP-mOVA chimeras proliferated in response to SIINFEKL-pulsed splenocytes (Fig. 4A). Interestingly, proliferation of CD8 T cells from OT-I Bim−/−→RIPmOVA chimeras was comparable to B6 controls upon stimulation with plate-bound anti-CD3/28 (Fig. 4A). At 4 h poststimulation with SIINFEKL-pulsed splenocytes, a minor fraction of Vα2+ CD8+ splenocytes from the B6 control chimeras were producing low levels of TNFα, whereas the splenocytes from the OT-I Bim−/−→RIP-mOVA chimera were not producing any cytokines (Fig. 4B). To further examine the effector function of the OT-I and OT-I Bim−/− T cells, following 3 d of stimulation with SIINFEKL-pulsed splenocytes, we rested splenocytes for 2 d in the presence of IL-2 before restimulating them with SIINFEKL-pulsed splenocytes for another 4 h. After restimulation, the majority of Vα2+ CD8+ splenocytes from both OT-I→B6 and OT-I Bim−/−→B6 chimeras produced cytokines. In contrast, fewer of the OT-I Bim−/− splenocytes from the OT-I Bim−/−→RIP-mOVA chimeras produced cytokines (Fig. 4B). Interestingly, in contrast to the proliferation data, anti-CD3/28 stimulation did not rescue cytokine production (Fig. 4C). Furthermore, it was apparent that the subset of cells undergoing division was responsible for the cytokine production we observed (Fig. S2B). To ensure that OT-I T cells from OT-I Bim−/−→RIPmOVA chimeras were able to interact with antigen as well as those from OT-I→B6 and OT-I Bim−/−→B6 chimeras, we examined CD69 surface expression 1 d poststimulation. The percentage of OT-I T cells that up-regulated CD69 in the OT-I Bim−/−→RIPmOVA chimeras was similar to those from the OT-I→B6 and OT-I Bim−/−→B6 chimeras. However, the median fluorescence intensity of CD69 was lower on the OT-I T cells from the OT-I Bim−/−→RIPmOVA chimeras compared with the OT-I→B6 and OT-I Bim−/−→B6 chimeras. OT-I T cells from OT-I→RIPmOVA chimeras did not up-regulate CD69 following antigen challenge (Fig. S2A). Collectively, these data indicate that the OT-I Bim−/− T cells from RIP-mOVA chimeras are functionally impaired. As the OT-I Bim−/− T cells from B6 chimeras are unaffected, this impaired function is not due to the absence of Bim but rather suggests that recognition of high-affinity self-antigen in the thymus or periphery induces nonresponsiveness. It is currently unclear whether the lack of responsiveness is due to dominant suppression by Treg or recessive anergy, but these data are consistent with recent data showing that defects in the Bcl-2 regulated apoptotic pathway leads to an increase in CD25loFoxp3+ anergic CD4+ T cells (27).
Fig. 4.
CD8 T cells from OT-I Bim−/−→RIP-mOVA chimeras are functionally impaired. (A) CFSE stain dilution of Vα2+ CD8+ splenocytes from indicated strains 2 and 3 d after stimulation. (B and C) IFNγ by TNFα staining of indicated strains at indicated time points after splenocyte or plate-bound α-CD3/28 stimulation, respectively. Data are representative of at least three mice per strain over three independent experiments.
Discussion
It was previously thought that Bim-mediated apoptosis was required for clonal deletion of thymocytes; however, recent data demonstrate that in the case of clonal deletion to UbA, Bim is not required (16–18). Our data from the Act-mOVA chimeras supports the findings of these previous reports, where Bim is essential for thymocyte apoptosis but not clonal deletion to UbA, suggesting Bim-independent clonal deletion mechanisms exist. One such candidate protein that may mediate Bim-independent clonal deletion is the orphan nuclear receptor Nur77 or its family member Nor1; however, the mechanism underlying this form of clonal deletion has not yet been determined.
Although in the absence of Bim clonal deletion to UbA appears to be intact, the fact remains that Bim-deficient mice develop autoimmunity (14). In the present study, we demonstrated that unlike clonal deletion to UbA, Bim deficiency completely abrogates clonal deletion to TRA. Whereas defective antigen encounter may explain these data, we find this unlikely as CD8 dulling in the thymic Vα2+ CD8SP compartment in OT-I Bim−/−→RIP-mOVA chimeras suggests antigen encounter. Furthermore, recent work by the Hogquist group determined that Nur77, a gene induced by TCR stimulation, is up-regulated in OT-I CD8SP thymocytes from OT-I Bim−/−→RIP-mOVA chimeras, which indicated there is TCR stimulation and activation of a transcriptional program (28). Finally, it is unlikely that the abrogated clonal deletion resulted from the killing of mOVA expressing APC in the thymus (29) because we did not find CD44hi OT-I Bim−/− cells in the thymus of Rip-mOVA recipients and the OT-I Bim−/− cells from Rip-mOVA recipients had impaired effector function and did not induce diabetes. Collectively, these data demonstrate that, unlike clonal deletion to UbA, thymic clonal deletion to TRA is impaired in the absence of Bim. Importantly, this is a unique report of differential thymocyte intrinsic molecular requirements for clonal deletion to UbA versus TRA downstream of the TCR. These data also demonstrate that other mediators of clonal deletion, such as Nur77 or Nor1, cannot compensate for the absence of Bim in clonal deletion to TRA.
Although we have demonstrated that the absence of Bim allows escape of TRA-specific T cells from clonal deletion, we were unable to replicate autoimmune disorders associated with Bim deficiency. Again, CD8 coreceptor dulling on peripheral OT-I T cells from OT-I Bim−/−→RIP-mOVA chimeras suggested antigen encounter in the periphery and this finding is supported by recent data from Moran et al. that demonstrated elevated Nur77 expression in Vα2+ CD8+ T cells from OT-I Bim−/−→RIP-mOVA chimeras (28). This lack of autoimmunity suggests that in the absence of thymic clonal deletion, either a dominant or recessive mechanism of tolerance was established to control these TRA reactive T cells in the OT-I Bim−/−→RIP-mOVA chimeras. There are several mechanisms by which OT-I Bim−/− T cells may be controlled in the periphery of RIP-mOVA mice. First, the OT-I Bim−/− T cells could be rendered anergic upon self-antigen encounter either in the thymus or periphery. It is currently unclear whether the OT-I Bim−/− thymocytes that encountered OVA as a TRA in the thymus, but were not deleted, are functional. Because thymic anergy is considered a form of negative selection, we cannot discount the thymus as the location of anergy induction. Previous work has demonstrated that transfer of Bim-deficient DO11.10 T cells into mice that express a soluble form of OVA are anergized (30). However, in this situation, the anergic DO11.10 Bim−/− T cells proliferated and up-regulated CD44 following adoptive transfer. Because the peripheral OT-I Bim−/− T cells were CD44lo, we find it unlikely that they underwent antigen-driven proliferation, and therefore the mechanism of tolerance induction may be different. We cannot discount lymphopenia-induced proliferation because it was recently demonstrated that self-specific CD8 T cells that underwent lymphopenia-induced proliferation remained CD44lo (31). It is also possible that anergy was induced in the thymus but maintained by continuous cognate antigen encounter in the periphery. Because we did not observe PD-1 to be expressed on thymocytes or peripheral OT-I Bim−/− T cells from RIP-mOVA chimeras, this state of anergy is unlikely to result from T-cell exhaustion (Fig. S3B). However, we cannot rule out the expression of other inhibitory cell surface receptors implicated in T-cell exhaustion.
Second, OT-I Bim−/− T cells from RIP-mOVA chimeras could become subject to regulation by Treg. We did not detect any appreciable increase in CD25+CD4+ T cells or thymocytes in the OT-I Bim−/−→RIP-mOVA chimeras (Fig. S3A). However, it has been shown that high-affinity antigen encounter can result in differentiation into CD4+Foxp3+CD25+ Treg cells. Therefore, it is possible that survival of high-affinity antigen encounter in the thymus could lead to the generation of an unknown CD8 regulatory population from the OT-I thymocytes. The Santamaria group has recently identified low-affinity autoregulatory CD8+ T cells that can potently suppress diabetogenic T cells (32). The possibility exists that either a traditional CD4+ Treg or a CD8+ regulatory cell population was induced in the OT-I Bim−/−→RIP-mOVA chimeras. Experiments designed to test for the presence of a dominant regulatory population are required to address this possibility.
Finally, the OT-I Bim−/− T cells may be signaled to further differentiate into another inactive subset (27). This possibility has recently been demonstrated for CD4+ T cells (27). However, further experiments are necessary to examine this possibility.
As plate-bound anti-CD3/28 stimulation rescued proliferation but not cytokine production compared with splenocyte stimulations, our data suggest that proliferation may be inhibited by regulatory populations or coinhibitory receptors, whereas intrinsic mechanisms may inhibit cytokine production. This finding again reinforces the fact that the immune system has developed “fail-safe” mechanisms to control autoreactive T cells and limit the potential for autoimmunity.
Collectively, our data demonstrate that whereas the proapoptotic molecule Bim is not required for clonal deletion to UbA, it is required for clonal deletion to TRA. This is the first demonstration of differences in the molecular requirements of clonal deletion to TRA versus UbA downstream of the TCR. Although clonal deletion to TRA is abrograted in the absence of Bim, the OT-I T cells remained tolerant. Although the exact nature of this tolerance is unknown, understanding the regulatory mechanisms at play in this situation should provide insight into understanding how autoimmunity progresses beyond impaired clonal deletion.
Materials and Methods
Mice.
C57BL/6 mice were purchased from the National Cancer Institute and The Jackson Laboratory. C57BL/6-Tg(Ins2-TFRC/OVA)296Wehi/WehiJ (RIP-mOVA) mice (22), C57BL/6-Tg(TcraTcrb)1100Mjb/J (OT-I) mice (33), and C57BL/6-Tg(TcraTcrb)1100Mjb/J (Act-mOVA) mice (21) were purchased from The Jackson Laboratory. OT-I Bim−/− mice were kindly provided by Dr. Maureen McGargill (St. Jude Children's Research Hospital, Memphis, TN). All mice, except Act-mOVA, were bred and maintained in our colony at the University of Alberta, treated in accordance with protocols approved by the University of Alberta Animal Care and Use Committee.
Bone Marrow Chimeras.
Donor mice were injected i.p. with 100 μg of purified anti-CD8 Ab (clone 2.43) on days −2 and −1 before BM harvest to deplete CD8 T cells. BM from the femur, tibia, and humerus was harvested in EasySep media (PBS, 2% FCS, 2 mM EDTA) and was passed through 70-μm nylon cell strainers (Fisherbrand). BM was depleted of T cells using the EasySep Mouse FITC Selection kit (Stemcell Technologies) and FITC conjugated anti-Thy1.2 Ab (eBiosciences). Between 5 and 10 × 106 BM cells were injected into the tail vein of lethally irradiated (1,000 Gy) recipient mice. Mice were provided with antibiotic water (40 mg neomycin, 15 mg polymyxin per 1 L) for 4 wk postinjection. Mice were allowed to reconstitute for at least 8 wk before analysis.
Antibodies and Flow Cytometry.
All fluorochrome-conjugated and biotinylated antibodies (Ab) were purchased from eBiosciences except for anti-active caspase 3 (Asp-175), which was purchased from Cell Signaling Technology. Cells were stained with Ab mixtures in FACS buffer (PBS, 1% FCS, 0.02% sodium azide) for 30 min on ice. Cells were washed twice with FACS buffer between primary and secondary staining mixtures. Cells were treated with the BD Fix/Perm kit (BD Biosciences) for intracellular staining for active caspase 3 or intracellular cytokines. Cell events were collected on a FACSCanto II (BD Biosciences) and analyzed by FlowJo software (Tree Star).
Proliferation Assay.
Stimulator splenocytes were harvested from B6 Ly5.1/5.2 mice, washed, and resuspended at 20 × 106 cells/mL in pure FCS and pulsed with 100 nM SIINFEKL peptide at 37 °C for 1 h with gentle shaking every 15–20 min. Stimulators were washed three times with 2% FCS–PBS and resuspened in RP10 (RPMI, 10% FCS, 5 mM Hepes, 50 units (mg)/mL penicillin/streptocycin, 2 mM l-glutamine, 50 mM 2-mercaptoethanol, 50 mg/mL gentamicin sulfate) at 20 × 106 cells/mL. Plates were prepared with 10 μg/mL of α-CD3 and 5 μg/mL of α-CD28 for 1 h at 37 °C. Plates were blocked with 2% BSA in PBS. Effectors were harvested from indicated mice, washed, and resuspended in sterile PBS at 10 × 106 cells/mL. A total of 1 μL of a 1.25-mM solution of CFSE in DMSO was added per 10 × 106 cells, which were then incubated for 10 min at 37 °C with regular mixing. Staining was quenched with RP10 and cells were washed once in RP10 and the cells were resuspended in RP10 to 10 × 106 cells/mL. Effectors were mixed with stimulators at a ratio of 3:1 and allowed to incubate at 37 °C for the indicated time points.
Cytokine Assay.
Effectors and stimulators were treated as above, but without CFSE labeling, and mixed at a ratio of 3:1 in RP10. On day 3 poststimulation, cells were washed and resuspended in RP10 plus 1 ng/mL IL-2. On day 5, effectors and freshly pulsed stimulators were mixed at a 3:1 ratio. A total of 3 μg of Brefeldin A per 1 mL of culture volume was added to each culture 4 h before harvest and anaylsis. Cytokines where detected by internal staining and flow cytometry.
Blood Glucose Levels.
Blood glucose levels were determined using a OneTouch UltraMini system with OneTouch Ultra Test Strips. Blood samples (∼5 μL) were obtained by tail vein bleeding. Readings were taken once per week and mice were considered diabetic when blood glucose levels exceeded 300 mg/dL for 2 consecutive weeks. Blood glucose levels were measured for 8–14 wk after BM graft.
Statistical Analysis.
Mean, SD, and P values were calculated using Prism software (GraphPad).
Supplementary Material
Acknowledgments
The authors thank Qian Hu and Dr. Kristin Hogquist for helpful suggestions and critical review of the manuscript, Dr. Maureen McGargill for the OT-I Bim−/− mice, and Mr. Bing Zhang for excellent technical assistance. This work was supported by Canadian Institutes of Health Research Grant MOP-86595 (to T.A.B). T.A.B. is supported by Alberta Heritage Foundation for Medical Research Scholar and Canadian Institutes for Health Research New Investigator Awards; and A.S. was supported by a Canadian Institutes of Health Post Graduate Scholarship Masters Award.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114834109/-/DCSupplemental.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: Learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–782. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Ueno T, et al. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCaughtry TM, Wilken MS, Hogquist KA. Thymic emigration revisited. J Exp Med. 2007;204:2513–2520. doi: 10.1084/jem.20070601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitta T, Nitta S, Lei Y, Lipp M, Takahama Y. CCR7-mediated migration of developing thymocytes to the medulla is essential for negative selection to tissue-restricted antigens. Proc Natl Acad Sci USA. 2009;106:17129–17133. doi: 10.1073/pnas.0906956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin TA, Hogquist KA, Jameson SC. The fourth way? Harnessing aggressive tendencies in the thymus. J Immunol. 2004;173:6515–6520. doi: 10.4049/jimmunol.173.11.6515. [DOI] [PubMed] [Google Scholar]
- 7.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang W, Beckett O, Ma Q, Li MO. Transforming growth factor-beta signaling curbs thymic negative selection promoting regulatory T cell development. Immunity. 2010;32:642–653. doi: 10.1016/j.immuni.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labrecque N, Baldwin T, Lesage S. Molecular and genetic parameters defining T-cell clonal selection. Immunol Cell Biol. 2011;89:16–26. doi: 10.1038/icb.2010.119. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin TA, Hogquist KA. Transcriptional analysis of clonal deletion in vivo. J Immunol. 2007;179:837–844. doi: 10.4049/jimmunol.179.2.837. [DOI] [PubMed] [Google Scholar]
- 11.Liston A, et al. Gene dosage—limiting role of Aire in thymic expression, clonal deletion, and organ-specific autoimmunity. J Exp Med. 2004;200:1015–1026. doi: 10.1084/jem.20040581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasser A, Puthalakath H, O'Reilly LA, Bouillet P. What do we know about the mechanisms of elimination of autoreactive T and B cells and what challenges remain. Immunol Cell Biol. 2008;86:57–66. doi: 10.1038/sj.icb.7100141. [DOI] [PubMed] [Google Scholar]
- 13.Bouillet P, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 14.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 15.Zucchelli S, et al. Defective central tolerance induction in NOD mice: Genomics and genetics. Immunity. 2005;22:385–396. doi: 10.1016/j.immuni.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-mediated apoptosis is not necessary for thymic negative selection to ubiquitous self-antigens. J Immunol. 2009;183:7761–7767. doi: 10.4049/jimmunol.0902181. [DOI] [PubMed] [Google Scholar]
- 17.Kovalovsky D, Pezzano M, Ortiz BD, Sant'Angelo DB. A novel TCR transgenic model reveals that negative selection involves an immediate, Bim-dependent pathway and a delayed, Bim-independent pathway. PLoS ONE. 2010;5:e8675. doi: 10.1371/journal.pone.0008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen TN, et al. Bim and Bcl-2 mutually affect the expression of the other in T cells. J Immunol. 2007;179:3417–3424. doi: 10.4049/jimmunol.179.6.3417. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi RT, Anderson MS. The role of Aire in clonal selection. Immunol Cell Biol. 2011;89:40–44. doi: 10.1038/icb.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 22.Kurts C, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J Exp Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MS, et al. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Kurts C, et al. CD4+ T cell help impairs CD8+ T cell deletion induced by cross-presentation of self-antigens and favors autoimmunity. J Exp Med. 1997;186:2057–2062. doi: 10.1084/jem.186.12.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ludwinski MW, et al. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. J Clin Invest. 2009;119:1706–1713. doi: 10.1172/JCI37619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prlic M, Bevan MJ. Exploring regulatory mechanisms of CD8+ T cell contraction. Proc Natl Acad Sci USA. 2008;105:16689–16694. doi: 10.1073/pnas.0808997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhan Y, et al. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187:1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelmann SL, Marconi P, Brocker T. Peripheral T cells re-enter the thymus and interfere with central tolerance induction. J Immunol. 2011;186:5612–5619. doi: 10.4049/jimmunol.1004010. [DOI] [PubMed] [Google Scholar]
- 30.Barron L, Knoechel B, Lohr J, Abbas AK. Cutting edge: Contributions of apoptosis and anergy to systemic T cell tolerance. J Immunol. 2008;180:2762–2766. doi: 10.4049/jimmunol.180.5.2762. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LD, Jameson SC. Self-specific CD8+ T cells maintain a semi-naive state following lymphopenia-induced proliferation. J Immunol. 2010;184:5604–5611. doi: 10.4049/jimmunol.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai S, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity. 2010;32:568–580. doi: 10.1016/j.immuni.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.