Abstract
Misregulation of a pluripotency-associated transcription factor network in adult tissues is associated with the expansion of rare, highly malignant tumor-initiating stem cells (TISCs) through poorly understood mechanisms. We demonstrate that robust and selective expression of the receptor for the adipocyte-derived peptide hormone leptin (OB-R) is a characteristic feature of TISCs and of a broad array of embryonic and induced pluripotent stem cells and is mediated directly by the core pluripotency-associated transcription factors OCT4 and SOX2. TISCs exhibit sensitized responses to leptin, including the phosphorylation and activation of the pluripotency-associated oncogene STAT3 and induction of Oct4 and Sox2, thereby establishing a self-reinforcing signaling module. Exposure of cultured mouse embryonic stem cells to leptin sustains pluripotency in the absence of leukemia inhibitory factor. By implanting TISCs into leptin-deficient ob/ob mice or into comparably overweight Leprdb/db mice that produce leptin, we provide evidence of a central role for the leptin-TISC–signaling axis in promoting obesity-induced tumor growth. Differential responses to extrinsic, adipocyte-derived cues may promote the expansion of tumor cell subpopulations and contribute to oncogenesis.
Solid tumors and blood cell malignancies are composed of heterogeneous cell types that exhibit striking differences in their capacity to initiate and sustain tumor growth (1, 2). Tumor-initiating stem cells (TISCs) are rare, highly malignant cells identified within diverse tumor types that share important similarities with undifferentiated embryonic stem cells (ESCs) in the inner cell mass of blastocyst-stage embryos (3–5). TISCs, like ESCs, are capable of unlimited, self-renewing cell divisions and express a pluripotency-associated transcription factor (TF) network that includes the core constituent regulators OCT4, SOX2, and NANOG (6–8).
During normal embryonic development, this transcriptional regulatory network ensures that the generation of undifferentiated stem cells is matched appropriately to the demand for new cells as the organism engages in de novo tissue formation. In the context of TISCs, however, this same genetic network is thought to participate in the related processes of tumor initiation, malignant progression, and the acquisition of resistance to radiation and chemotherapy (9–11). Misregulation of Nanog and other constituents of the pluripotency-associated TF network can antagonize key tumor suppressor pathways and promote self-renewing divisions, thereby expanding the pool of malignant progenitor cells (12, 13).
Genetic and biochemical approaches to characterize TISCs have begun to yield important mechanistic insights into these events. TISCs display aberrant patterns of chromatin modification, including hypermethylation of promoter sequences and an associated silencing of tumor-suppressor genes, in a process mediated by the action of Polycomb group genes (14, 15). TISCs also fail to properly regulate the activity of major signal transduction pathways. Notably, TISCs isolated from human hepatocellular carcinoma (HCC) exhibit both loss of function of the type II TGF-β receptor and excessive activation of the IL-6 cytokine-signaling pathway, including the downstream effector STAT3 (16, 17). In a mouse model of HCC, a subpopulation of highly tumorigenic CD133+/CD49f+/Nanog+ liver TISCs was identified on the basis of significantly elevated expression of Toll-like receptor 4, a transmembrane receptor that activates NF-κB and induces a proinflammatory and tumorigenic gene expression program upon direct interaction with bacteria-derived lipopolysaccharide (LPS) ligands (18).
These observations raise the possibility that identification of cell-surface receptors and associated signal transduction pathways that are selectively expressed or activated in TISCs can provide vital insights into the molecular basis for the potent tumorigenic capacity that is characteristic of TISCs. Such insights in turn may lead to the identification of TISC-specific molecular vulnerabilities that can be exploited therapeutically. In this report, we show that the leptin receptor (OB-R), the transmembrane receptor for the adipocyte-derived peptide hormone leptin, is expressed strongly and selectively in TISCs and in a broad array of pluripotent stem cell types, including ESCs and induced pluripotent stem cells (iPSCs). By using gain- and loss-of-function strategies, we show that leptin serves as a key intermediary linking the accumulation of excess adipose tissue to activation of a pluripotency-associated TF network and obesity-induced oncogenesis.
Results
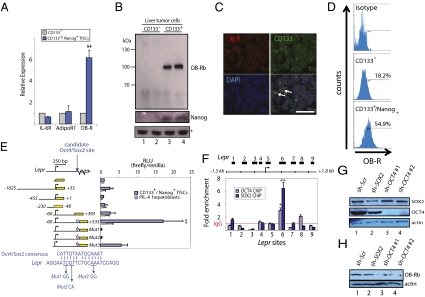
We set out to examine whether CD133+/Nanog+ liver TISCs isolated from a transgenic mouse model of HCC (18) exhibit differential expression of cytokine or adipokine receptors relative to nonprogenitor tumor cells. Analysis by quantitative reverse-transcription PCR (qRT-PCR) of a panel of transcripts, including those encoding receptors for IL-6 (IL-6R) and adiponectin (AdipoR1), showed comparable expression levels between TISCs and their nonprogenitor counterparts (Fig. 1A). By contrast, analysis of the leptin receptor (OB-R, encoded by the Lepr gene) revealed a striking, 6.3-fold increase in TISCs relative to CD133− control cells isolated in parallel from the same tumors (Fig. 1A). Immunoblotting of lysates prepared from two independent isolates of liver TISCs or CD133− controls confirmed that expression of the signaling-competent, long-form of OB-R (OB-Rb) occurs selectively in CD133+/Nanog+ TISCs (Fig. 1B).
Fig. 1.
Selective expression of leptin receptor OB-Rb in CD133+ TISCs is mediated by the pluripotency factors OCT4 and SOX2. (A) Quantitative RT-PCR analysis of mRNA transcript levels for the IL-6 receptor (IL-6R), adiponectin receptor (AdipoR1), and leptin receptor (OB-R) in CD133+/Nanog+ TISCs and CD133− cells. (B) Selective expression of the long-form leptin receptor OB-Rb in CD133+/Nanog+ TISCs. Two independent sets of CD133+ and matched CD133− cells were isolated in parallel by dissociation of Ns5a-Tg mouse liver tumors. Tumor lysates were probed using antisera specific for OB-R (Top) or NANOG (Middle). Asterisk (*) indicates a nonspecific cross-reacting band and is included as a loading control. (C) Immunostaining of mouse liver tumors showing distribution of CD133 and OB-R. CD133 localizes prominently to plasma membranes. Colocalization of OB-R and CD133 occurs in a subpopulation of tumor cells. (Scale bar, 50 μm.) (D) FACS analysis and quantification of OB-R expression in CD133− controls and CD133+/Nanog+ TISCs isolated from dissociated tumors. (E) Lepr promoter-reporter assay. The indicated Lepr-luciferase fusions or a promoter-less vector were examined in CD133+/Nanog+ TISCs and PIL-4 hepatoblasts. A candidate OCT4/SOX2-binding site identified by sequence homology search is indicated by the blue triangle. Promoter activity is expressed as relative light units (RLU) normalized to the activity of cotransfected Renilla luciferase. Error bars represent the SD from at least three independent biological replicates (**P < 0.01). (F) ChIP-qPCR assay showing direct association of OCT4 and SOX2 on Lepr. Error bars represent SD from at least three independent biological replicates. (**P < 0.01; *P < 0.05). (G) Lentiviral-mediated shRNA knockdown of OCT4 and SOX2. Lysates prepared from CD133+ TISCs infected with lentivirus encoding nontargeting, scrambled control shRNA (sh-Scr), sh-SOX2 (lane 2), or sh-OCT4 (lanes 3 and 4) were analyzed by immunoblotting to determine the levels of SOX2 and OCT4. An actin immunoblot of the same lysates is included as a loading control. (H) Immunoblot analysis of OB-Rb in lysates prepared from CD133+ TISCs stably expressing a nontargeting shRNA control (sh-Scr, lane 1) or shRNAs targeting either SOX2 (lane 2) or OCT4 (lanes 3 and 4).
Examination of OB-R expression by immunohistochemical analysis of mouse tumor specimens showed significant overlap in immunoreactivity between OB-R and CD133 (Fig. 1C), with 85.6 ± 6.2% (n = 647, P < 0.01) of OB-R+ cells from multiple tumor samples (n = 8) costaining with antisera specific for CD133. To obtain an additional quantitative assessment of the relationship between OB-R and CD133 expression in these tumors, we performed FACS analysis of cultured, early passage mouse liver tumor cells (Fig. 1D). Results from this approach corroborated the immunohistochemical studies, indicating that 54.9 ± 4.1% of CD133+ TISCs strongly coexpress OB-R, whereas only 18.2 ± 1.7% of CD133− cells exhibit staining for OB-R. Thus, the expression of OB-R occurs preferentially in CD133+ cells, and OB-R+ cells compose a distinct subset of the overall CD133+ progenitor population.
To determine the molecular basis for the markedly enriched expression of OB-Rb in TISCs, we generated a series of promoter reporter constructs in which defined regions of the mouse Lepr promoter and flanking region were placed upstream of a reporter gene encoding firefly luciferase. Cultured liver TISCs and PIL-4 hepatoblast controls (19) were transfected with each reporter vector, and the normalized firefly luciferase activity was subsequently determined. This approach delineated a discrete sequence encompassing the proximal 0.5 kb downstream of the transcription start site (TSS) in exon 1 of Lepr as a critical mediator of TISC-specific expression of OB-R (Fig. 1E).
In parallel with the reporter assays, we used the multiple sequence alignment algorithm ClustalW to search the Lepr gene and associated promoter sequences for matches to consensus target motifs recognized by the pluripotency-associated transcription factors OCT4, SOX2, NANOG, STAT3, and KLF4 (6, 7). Using this in silico approach, we identified a candidate site located 0.5 kb downstream of the TSS, which closely matches (12/15 bases) the consensus target sequence for the OCT4/SOX2 heterodimer (Fig. 1E). To evaluate the function of this sequence in TISC-selective expression of OB-R, we generated a series of three mutants (Mut1–Mut3), each of which harbors mutations in two consecutive nucleotides within the candidate site. Introduction of these mutations sharply diminished the expression of the (−60/+535) Lepr-luciferase reporter in TISCs, implicating the OCT4/SOX2 heterodimer in TISC-specific induction of Lepr (Fig. 1E).
To evaluate a potential role for OCT4 and SOX2 in the stem cell-specific induction of Lepr, we performed chromatin immunoprecipitation (ChIP) using antibodies specific for OCT4 and SOX2, followed by quantitative PCR (qPCR) with primer sets designed to probe for association of these transcription factors across a 3.3-kb region centered at the Lepr TSS (Fig. 1F). In agreement with findings from the reporter assay, the ChIP–qPCR experiments demonstrated significant enrichment relative to IgG control immunoprecipitates for OCT4- and SOX2-bound DNA at or near the candidate site in exon 1 of Lepr (Fig. 1F).
We also conducted electrophoretic mobility-shift assays in which TISC nuclear lysates were incubated with a biotinylated, double-stranded oligonucleotide probe corresponding to the candidate OCT4/SOX2 site. The wild-type probe, but not a mutant probe harboring both Mut1 and Mut2 mutations, yielded a band that was diminished upon addition of excess, unlabeled competitor probe (Fig. S1, lanes 1–4). Preincubation of nuclear lysates with SOX2 antibody decreased the intensity of this band, and an OCT4 antibody produced an apparent shift in mobility (Fig. S1, lanes 6 and 7), supporting a direct association of OCT4 and SOX2 with the candidate OCT4/SOX2 site in Lepr.
We next examined whether OCT4 and SOX2 are required for expression of OB-R in TISCs. Lentiviral shRNA-mediated knockdown of OCT4 or SOX2 was confirmed by immunoblotting (Fig. 1G) and resulted in an apparent reduction in the levels of OB-Rb in a manner that corresponded closely to the efficiency of OCT4 or SOX2 gene silencing (Fig. 1H). TISCs harboring shRNAs targeting either OCT4 or SOX2 exhibited reduced activity of the Lepr (−60/+535) luciferase reporter (Fig. S2A) and decreased levels of endogenous OB-R as determined by qRT-PCR analysis (Fig. S2B).
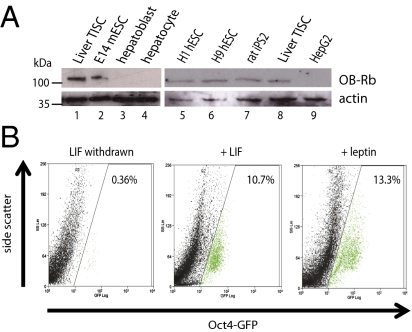
The involvement of OCT4 and SOX2 in expression of OB-Rb prompted us to explore whether elevated expression of OB-Rb might likewise occur in other stem cell lines. Comparison by immunoblotting of lysates prepared from human hepatoblasts, mature human hepatocytes, or HepG2 hepatocellular carcinoma cells with a panel of stem cell lines, including the mouse ESC line E14, the human ESC lines H1 and H9, and iPSCs derived from rat fibroblasts (iPS2), demonstrated a direct correspondence between pluripotency and the robust expression of OB-Rb (Fig. 2A). These findings suggest that elevated expression of OB-Rb represents a general feature of pluripotent cells.
Fig. 2.
Expression of OB-Rb in diverse classes of stem cells and role of leptin in sustaining pluripotency. (A) Immunoblot of lysates prepared from mouse CD133+ liver TISCs (lanes 1 and 8), E14 mESCs (lane 2), and human liver hepatoblasts and hepatocytes (lanes 3 and 4). OB-Rb expression is also shown for the H1 and H9 human ESCs (lanes 5 and 6), rat iPS2 cells (lane 7), and human HepG2 hepatocellular carcinoma cells (lane 9). (B) Leptin sustains pluripotency in cultured E14 mouse embryonic stem cells. E14 Oct4-GFP cells were cultured for 11 d in media with or without LIF (1,000 U/mL) or in the presence of leptin (200 ng/mL), harvested, and analyzed by FACS for quantification of GFP-expressing cells. The graphs shown are representative of results from three independent biological replicates.
Cultured mouse ESCs (mESCs) can be maintained in the pluripotent state through sustained activation of STAT3 by the cytokine leukemia inhibitory factor (LIF) (20), which engages a high-affinity, heterodimeric coreceptor complex composed of LIFR and gp-130 (21). Mouse embryos genetically deleted for LIFR develop normally, however, suggesting that in vivo multiple, redundant signaling pathways collaborate to maintain pluripotency in blastocyst-stage embryos (22). To examine whether leptin can substitute for LIF to maintain pluripotency in cultured mESCs, we used ESCs that carry a chromosomally integrated green fluorescent protein (GFP) reporter expressed under the control of the Oct4 promoter (Oct4-GFP), which is therefore active specifically in pluripotent stem cells (23). E14 Oct4-GFP cells were cultured for 11 d in the presence or absence of LIF or in media supplemented with 200 ng/mL recombinant mouse leptin. As expected, propagation of E14 Oct4-GiP cells in the absence of LIF diminished expression of the Oct4-GFP reporter (Fig. 2B). Strikingly, addition of recombinant leptin to the culture media maintained the expression of the Oct4-GFP reporter at a level comparable to that measured in the presence of LIF (Fig. 2B). Leptin therefore can serve as a substitute for LIF to sustain the pluripotent state in cultured mESCs.
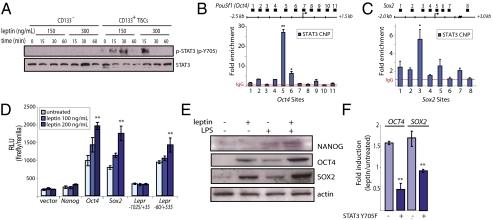
We next examined the biochemical response of TISCs to leptin. Consistent with the elevated expression of OB-Rb in TISCs, exposure to 150 or 300 ng/mL leptin triggered the rapid tyrosine phosphorylation and activation of STAT3, a downstream effector of OB-Rb, in TISCs but not in CD133− tumor cells, as determined by immunoblotting using antisera specific for phospho-STAT3 (p-Y705) (Fig. 3A). The levels of phospho-STAT3 increased within 15 min of exposure to leptin, followed by a rapid decrease, which has been described and is due in part to induction of the feedback inhibitor SOCS3 (24, 25).
Fig. 3.
Leptin stimulates the activity of a pluripotency-associated TF network in TISCs. (A) CD133− nonprogenitor tumor cells or CD133+ TISCs were exposed to leptin (150 or 300 ng/mL) for the indicated times, followed by lysis and immunoblotting using antisera specific for STAT3 or phospho-STAT3 (p-Y705). (B and C) STAT3 ChIP-qPCR analysis. Fragmented, cross-linked chromatin isolated from leptin-treated (150 ng/mL, 24 h) TISCs was immunoprecipitated using anti-STAT3 antibody, followed by qPCR using primers designed to amplify the indicated sites at the Oct4 (B) or Sox2 (C) genomic loci. (D) Promoter-reporter assays. Cultured TISCs transfected with the indicated reporter constructs were either untreated or exposed to recombinant mouse leptin (100 or 200 ng/mL, 24 h) before harvest and determination of luciferase activity. (E) Immunoblot analysis of lysates from TISCs exposed to leptin (150 ng/mL), LPS (5 ng/mL), or both ligands for 24 h. (F) qRT-PCR analysis of OCT4 and SOX2 in TISCs stably transfected with either empty vector or dominant-negative STAT3 (STAT3 Y705F). Fold induction is calculated as the ratio of transcript levels present in cells exposed to leptin (200 ng/mL) for 24 h relative to untreated controls.
Components of the pluripotency-associated TF network including STAT3, OCT4, and SOX2 coordinately occupy target promoters of downstream effector genes and modulate the expression of other network constituents through extensive reciprocal and cross-regulation (6, 7, 26). We found that in TISCs exposed for 24 h to leptin (150 ng/mL), STAT3 binds directly to the proximal promoter regions of Oct4 and Sox2, as determined by ChIP-qPCR using an antibody specific for STAT3, with the greatest enrichment of STAT3 occurring in the region between 0.5 and 1 kb upstream of the TSS for both Oct4 and Sox2 (Fig. 3 B and C). These findings prompted us to examine the effect of leptin on the induction of Oct4, Sox2, and Nanog. Exposure to leptin produced dose-dependent increases in the transcriptional induction of a luciferase reporter from the Oct4 (−5068) and Sox2 (−3.3 kb) promoters, although no effect was detected on the Nanog (−4828/+190) promoter (Fig. 3D). Consistent with the induction of Oct4 and Sox2 and in keeping with our earlier findings that OCT4 and SOX2 mediate expression of OB-R in TISCs (Fig. 1 F and H), leptin also stimulated the Lepr (−60/+535) luciferase reporter but not the Lepr (−1025/+35) reporter that lacks the OCT4/SOX2 response element (Fig. 3D).
Immunoblot analysis of lysates prepared from TISCs exposed for 24 h to either leptin (150 ng/mL) or LPS (5 ng/mL), a known inducer of Nanog, or to both stimuli showed that the levels of OCT4 and SOX2, but not NANOG, are increased to similar degrees in response to leptin (Fig. 3E). Interestingly, the strongest induction of OCT4, SOX2, and NANOG occurred following concurrent exposure to both leptin and LPS ligands. Quantitative RT-PCR analysis of RNA isolated from TISCs exposed to leptin (150 ng/mL) for 48 h substantiated these findings, demonstrating comparable increases in the levels of OCT4 and SOX2 as well as the induction of multiple constituents of the pluripotency-associated TF network (Fig. S3). Stable expression of the nonphosphorylatable, dominant-negative STAT3 mutant STAT3-Y705F in TISCs abolished the induction of endogenous Oct4 and Sox2 by leptin, as judged by qRT-PCR analysis (Fig. 3F), indicating that activation of STAT3 is the basis for induction by leptin of downstream components of the pluripotency-associated TF network.
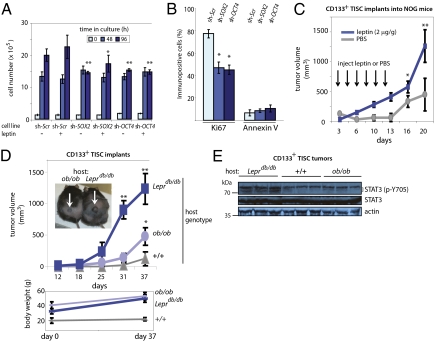
Leptin can act as a mitogen (27, 28), and STAT3 is rapidly activated during liver regeneration and has been implicated in cell proliferation following partial hepatectomy (29) and in the proliferation of transformed cells (30). We therefore asked whether OCT4 or SOX2, acting downstream of STAT3, can drive TISC proliferation. TISCs stably expressing lentiviral shRNAs targeting either OCT4 or SOX2 displayed an attenuated rate of cell growth after 96 h in culture and were largely refractory to the stimulative effect of leptin (200 ng/mL) on cell proliferation relative to control TISCs expressing a nontargeting, scrambled shRNA (Fig. 4A). Whereas 78.7 ± 6.1% of control TISCs were immunopositive for Ki67, a marker of cell proliferation, knockdown of SOX2 or OCT4 reduced the fraction of Ki67-positive cells to 44.7 ± 4.5% and 42.2 ± 3.8%, respectively (P < 0.01), without significantly affecting the proportion of cells undergoing apoptosis as determined by Annexin V immunoreactivity (Fig. 4B). We further found that in vivo knockdown of OCT4 impaired tumor growth by TISCs implanted s.c. into immune-compromised NOG (NOD/Shi-scid/IL-2Rγnull) mice. Two weeks after cells were implanted, tumors that grew from control TISCs expressing a scrambled shRNA (sh-Scr) were significantly larger than those obtained from sh-OCT4–expressing TISCs (415 ± 120 mm3 vs. 151 ± 59 mm3; P < 0.01) (Fig. S4).
Fig. 4.
Functional analysis of leptin and downstream effectors OCT4 and SOX2 in proliferation and TISC-mediated oncogenesis. (A) In vitro cell proliferation assay. Equal numbers (1.5 × 105) of TISCs expressing either nontargeting, scrambled shRNA (sh-Scr) or shRNAs targeting either SOX2 or OCT4 were plated and cultured in low-serum media or in the same media supplemented with recombinant mouse leptin (200 ng/mL). At 48-h intervals, cells were harvested and viable cells were counted. Error bars represent SD from at least three independent biological replicates (**P < 0.01 and *P < 0.05 relative to identically treated sh-Scr controls harvested at the same time). (B) Ki67 and Annexin V immunostaining shows decreased proliferation in TISCs stably expressing shRNAs targeting SOX2 or OCT4. Cells were cultured for 96 h in media supplemented with leptin (200 ng/mL) before harvest, fixation, and immunostaining. Asterisks indicate statistical significance (*P < 0.05) relative to identically cultured sh-Scr controls. (C) Leptin stimulates tumor development by implanted TISCs. CD133+ TISCs (4 × 104) were implanted s.c. into the dorsal hind flanks of NOG mice. Mice were randomly assigned to cohorts (n = 8 mice) receiving i.p. injections of either PBS (vehicle) or recombinant mouse leptin (2 μg/g body weight) every other day for 2 wk. (D) TISCs (4 × 104) were implanted s.c. into the dorsal hind flanks of ob/ob or Leprdb/db mice or into lean littermate controls. (Lower) Mouse body weight. (E) Immunoblot analysis of phospho-STAT3 (p-Y705) and STAT3 levels in lysates prepared from TISC tumors recovered from ob/ob, Leprdb/db, or lean control hosts. Tumors from all hosts were allowed to attain similar volumes (400–500 mm3) before surgical recovery and preparation of lysates.
To evaluate the function of leptin in TISC-mediated oncogenesis, NOG mice implanted with CD133+ TISCs (4 × 104) were randomly assigned to cohorts (n = 8 mice/cohort) receiving i.p. injections of either PBS vehicle or leptin (2 μg/g body weight) every other day for a total of six injections. TISCs implanted into the leptin-treated mice grew more rapidly and after 3 wk attained significantly greater tumor volumes compared with tumors implanted into the vehicle-treated cohort (1,260 ± 275 mm3 vs. 448 ± 246 mm3; P < 0.01) (Fig. 4C).
We next implanted TISCs (4 × 104) s.c. into obese ob/ob or Leprdb/db mice or into lean littermate controls. As anticipated, TISCs implanted into obese hosts formed tumors more rapidly than those implanted into the lean controls (31) (Fig. 4D). Notably, however, TISCs implanted into Leprdb/db mice, which lack a functional leptin receptor but produce leptin, grew more rapidly than TISCs implanted into comparably overweight ob/ob mice, which are unable to produce the leptin hormone (1,230 ± 244 mm3 vs. 477 ± 149 mm3; P < 0.01) (Fig. 4D). Biochemical analysis of tumors allowed to reach comparable volumes (400–500 mm3) before surgical removal showed that levels of tyrosine phosphorylated STAT3 (p-Y705) were reduced in tumors obtained from ob/ob mice compared with those from Leprdb/db hosts (Fig. 4E), indicating that leptin serves as a central activator of STAT3 in these TISC populations.
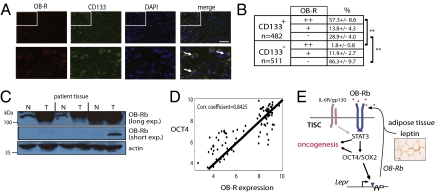
To determine the significance of these findings in human cancers, we analyzed the expression of OB-R in human hepatocellular carcinoma specimens and matched normal liver tissue obtained from the same patients. Immunostaining analysis revealed that a majority of CD133+ liver tumor cells strongly coexpressed OB-R (57.3 ± 8.6%; n = 482) whereas very few CD133− cells did so (1.8 ± 0.8%; n = 511) (P < 0.01) (Fig. 5 A and B). In support of these findings, immunoblotting of lysates prepared from flash-frozen human HCC clinical specimens and matched, noncancerous tissues demonstrated significantly greater abundance of OB-Rb specifically within tumor tissues (Fig. 5C).
Fig. 5.
Elevated OB-R expression in human HCC and correspondence of OB-R and OCT4 in multiple tumor types. (A) Immunohistochemical analysis of HCC tumors. Surgically resected HCC patient tumor specimens from age-matched specimens were fixed and stained with antisera to CD133 and OB-R and counterstained with DAPI to indicate nuclei. White arrows indicate examples of cells strongly coexpressing CD133 and OB-R. (Scale bar, 100 μm.) (B) Quantification of CD133 and OB-R reactivity. CD133+ and CD133− cells were scored for the degree of OB-R immunopositivity. Brackets indicate statistically significant (**P < 0.01) comparisons between groups. (C) Immunoblot analysis of lysates prepared from surgically resected HCC patient specimens (T) and matched normal tissue (N). Immunoblot of actin serves as a control for protein loading. (D) Scatter plots showing results from meta-analysis comparing the expression levels of OB-R and OCT4 across multiple tumor samples. Expression data were collected from pancreatic and breast cancers, multiple myeloma, and CaCo-2 colon adenocarcinoma cells. (E) Model of leptin action on TISCs in promoting tumor growth. Leptin signaling triggers phosphorylation and activation of STAT3 to induce OCT4 and SOX2, thereby completing a self-reinforcing oncogenic signaling circuit in TISCs. Cytokines such as IL-6 and TNF may also contribute to this signaling network.
We also conducted an extensive meta-analysis comparing expression levels of OCT4 and OB-R using micorarray data generated from human pancreatic cancer, breast cancer, and multiple myeloma tissue specimens and from cultured human epithelial colorectal adenocarcinoma (CaCo-2) cells. Through this approach, we elucidated a robust, statistically significant (P < 0.05) positive correlation between expression of OCT4 and OB-R across these diverse human cancers (Fig. 5D), extending the clinical significance of the causal relationship between OCT4 and OB-R first identified in mouse TISCs.
Discussion
We demonstrate that profound and selective expression of the leptin receptor OB-R occurs across a broad array of pluripotent cell types, including in nontransformed human and mouse-derived ESC and iPSC lines, implicating robust expression of OB-R as an underlying feature of the pluripotent state. These findings reveal a signaling route from white adipose tissue to tumor stem cell subpopulations and indicate that TISCs are intrinsically primed to modulate the expression and activity of a pluripotency-associated TF network in response to fluctuations in body-fat levels. The identification of this physiological signaling circuit has implications for our understanding of how systemic hormonal cues can influence tumor growth and malignant progression.
Epidemiological studies have demonstrated that excess body fat increases the incidence of cancers from multiple tissue types and is associated with an elevated risk of cancer mortality (32, 33). Elevated serum insulin and IGF-1 (34–36) and colonization of tumors by adipose-derived stromal and vascular endothelial cells (37, 38) have been put forward as mechanisms to explain the tumor-promoting effect of obesity. Genetic studies in mice have further demonstrated an essential role for the proinflammatory cytokines IL-6 and tumor necrosis factor (TNF) in the onset and progression of experimental obesity-induced cancer (31, 39). However, the question of whether distinct subpopulations of tumor cells exhibit differential responses to these and other extrinsic cues has been largely overlooked. The demonstration that solid tumors and hematological malignancies are composed of distinct subpopulations of cells (1, 2) raises the possibility that a given signaling molecule can elicit varying effects on conversion to a malignant phenotype in a manner that depends critically on characteristics intrinsic to the responding cells.
Our findings here indicate that a rare subpopulation of CD133+ liver tumor progenitor cells is primed to respond to leptin and that leptin can promote malignancy through activation of TISCs in tumors of overweight animals. Whereas obesity stimulates tumor growth in overweight, leptin-producing Leprdb/db hosts, this effect is blunted in ob/ob mice, which cannot produce leptin (Fig. 4D). We envision a scenario whereby IL-6 and TNF signaling to the bulk of nonprogenitor tumor cells, and a self-reinforcing leptin-signaling module active within CD133+ TISCs (Fig. 5E), define parallel, nonredundant pathways that cooperatively drive obesity-induced oncogenesis. Notably, this proposal does not rule out a critical role for IL-6 or TNF signaling directly to TISCs in driving tumor growth. Elevated expression and plasma concentrations of these cytokines have been noted in different rodent models of obesity (40), including in ob/ob mice (41), and we detected increased levels of phospho-STAT3 in tumors harvested from ob/ob mice relative to those obtained from lean controls (Fig. 4E). Analysis of experimental obesity-induced tumor growth in mice harboring targeted deletions of Lepr, IL-6R, or TNF-R, or a combination of these loci, will be required to evaluate the respective contributions of inflammatory cytokine and adipokine pathways to obesity-linked oncogenesis.
The striking correspondence between elevated OB-Rb levels and the pluripotent state, and the finding that leptin can maintain pluripotency of cultured mESCs (Fig. 2), together raise the possibility that OB-Rb performs this function in blastocyst-stage embryos. Although LIF sustains pluripotency in cultured mouse ESCs, self-renewal is not abolished in the complete absence of either LIF or its receptor LIFR (22, 42), pointing to a role for other factors in sustaining pluripotency in vivo. Leptin is secreted by uterine endothelial cells and is present in the uterine fluid during implantation (43, 44), and female ob/ob mice are sterile but can be restored to fertility by treatment with recombinant leptin during the first 6.5 d post coitus (45), a time interval that corresponds to the expansion of naive, pluripotent ESCs in the blastocyst (8). These findings raise the possibility that leptin signaling to OB-Rb functions as an independent signaling axis to maintain the pluripotent state in mouse blastocysts. Such a function may extend to human embryonic development, a proposal that is consistent with a recent report implicating leptin in supporting both the proliferation and the pluripotency of cultured human embryonic stem cells (46).
Materials and Methods
Vectors.
Lepr-luciferase promoter reporter constructs were generated by PCR using Pfu polymerase and C57BL/6 mouse genomic DNA as a template. Point mutants were introduced into the −60/+535 reporter by the Pfu/DpnI method. All clones were confirmed by sequencing on both strands.
Cells and Reagents.
Liver tumors were removed surgically before euthanasia from NS5a-Tg mice (18) maintained for 12 mo on a Lieber–Decarli alcohol diet (3.5% alcohol). TISCs were isolated using the anti-prominin1 (CD133) MACS affinity column (Miltenyi Biotech) following mechanical dissociation of liver tumors.
Statistical Analysis.
Statistical significance was estimated by unpaired, two-tailed Student's t test. Bars represent the mean and error bars the SD. For all figures, statistical significance is represented by asterisks above each column: *P < 0.05; **P < 0.01.
Supplementary Material
Acknowledgments
This study was supported by funding from National Cancer Institute Grant P01CA123328, National Institute on Alcohol Abuse and Alcoholism Grants R01 AA018857 and RC2 AA019392-01, National Institute of Allergy and Infectious Diseases Grants U19AI083025, and National Institutes of Health T32 Training Grant AA07578 to the Southern California Research Center for ALPD and Cirrhosis. D.E.F. was also supported by a California Institute for Regenerative Medicine Postdoctoral Scholarship. Microscopy was performed by the Cell and Tissue Imaging Core of the University of Southern California.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114438109/-/DCSupplemental.
References
- 1.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 2.Marusyk A, Polyak K. Tumor heterogeneity: Causes and consequences. Biochim Biophys Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke MF, Fuller M. Stem cells and cancer: Two faces of eve. Cell. 2006;124:1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 5.Clevers H. The cancer stem cell: Premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 6.Kim J, Chu J, Shen X, Wang J, Orkin SH. An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132:1049–1061. doi: 10.1016/j.cell.2008.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Buecker C, Geijsen N. Different flavors of pluripotency, molecular mechanisms, and practical implications. Cell Stem Cell. 2010;7:559–564. doi: 10.1016/j.stem.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Diehn M, Clarke MF. Cancer stem cells and radiotherapy: New insights into tumor radioresistance. J Natl Cancer Inst. 2006;98:1755–1757. doi: 10.1093/jnci/djj505. [DOI] [PubMed] [Google Scholar]
- 10.Gupta PB, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 12.Jeter CR, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011; 30:3833–3845. doi: 10.1038/onc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell. 2010;143:313–324. doi: 10.1016/j.cell.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukacs RU, Memarzadeh S, Wu H, Witte ON. Bmi-1 is a crucial regulator of prostate stem cell self-renewal and malignant transformation. Cell Stem Cell. 2010;7:682–693. doi: 10.1016/j.stem.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Valk-Lingbeek ME, Bruggeman SW, van Lohuizen M. Stem cells and cancer: The polycomb connection. Cell. 2004;118:409–418. doi: 10.1016/j.cell.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Tang Y, et al. Progenitor/stem cells give rise to liver cancer due to aberrant TGF-beta and IL-6 signaling. Proc Natl Acad Sci USA. 2008;105:2445–2450. doi: 10.1073/pnas.0705395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baek HJ, et al. Hepatocellular cancer arises from loss of transforming growth factor beta signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128–1137. doi: 10.1002/hep.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machida K, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jellicoe MM, et al. Bioenergetic differences selectively sensitize tumorigenic liver progenitor cells to a new gold(I) compound. Carcinogenesis. 2008;29:1124–1133. doi: 10.1093/carcin/bgn093. [DOI] [PubMed] [Google Scholar]
- 20.Nichols J, Evans EP, Smith AG. Establishment of germ-line-competent embryonic stem (ES) cells using differentiation inhibiting activity. Development. 1990;110:1341–1348. doi: 10.1242/dev.110.4.1341. [DOI] [PubMed] [Google Scholar]
- 21.Gearing DP, et al. The IL-6 signal transducer, gp130: An oncostatin M receptor and affinity converter for the LIF receptor. Science. 1992;255:1434–1437. doi: 10.1126/science.1542794. [DOI] [PubMed] [Google Scholar]
- 22.Dani C, et al. Paracrine induction of stem cell renewal by LIF-deficient cells: A new ES cell regulatory pathway. Dev Biol. 1998;203:149–162. doi: 10.1006/dbio.1998.9026. [DOI] [PubMed] [Google Scholar]
- 23.Szabó PE, Hübner K, Schöler H, Mann JR. Allele-specific expression of imprinted genes in mouse migratory primordial germ cells. Mech Dev. 2002;115:157–160. doi: 10.1016/s0925-4773(02)00087-4. [DOI] [PubMed] [Google Scholar]
- 24.Dunn SL, et al. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin receptor and suppressor of cytokine signaling 3. Mol Endocrinol. 2005;19:925–938. doi: 10.1210/me.2004-0353. [DOI] [PubMed] [Google Scholar]
- 25.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280:31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 27.Ramani K, et al. Leptin's mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2beta. Hepatology. 2008;47:521–531. doi: 10.1002/hep.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoda MR, et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- 29.Leu JI, Crissey MA, Leu JP, Ciliberto G, Taub R. Interleukin-6-induced STAT3 and AP-1 amplify hepatocyte nuclear factor 1-mediated transactivation of hepatic genes, an adaptive response to liver injury. Mol Cell Biol. 2001;21:414–424. doi: 10.1128/MCB.21.2.414-424.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park EJ, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 33.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 34.Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem. 2008;114:63–70. doi: 10.1080/13813450801954451. [DOI] [PubMed] [Google Scholar]
- 35.Key TJ. Diet, insulin-like growth factor-1 and cancer risk. Proc Nutr Soc. 2011:1–4. doi: 10.1017/S0029665111000127. [DOI] [PubMed] [Google Scholar]
- 36.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 37.Dirat B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, et al. White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69:5259–5266. doi: 10.1158/0008-5472.CAN-08-3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khasawneh J, et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc Natl Acad Sci USA. 2009;106:3354–3359. doi: 10.1073/pnas.0802864106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 41.Harkins JM, et al. Expression of interleukin-6 is greater in preadipocytes than in adipocytes of 3T3-L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673–2677. doi: 10.1093/jn/134.10.2673. [DOI] [PubMed] [Google Scholar]
- 42.Li M, Sendtner M, Smith A. Essential function of LIF receptor in motor neurons. Nature. 1995;378:724–727. doi: 10.1038/378724a0. [DOI] [PubMed] [Google Scholar]
- 43.Kawamura K, et al. Leptin promotes the development of mouse preimplantation embryos in vitro. Endocrinology. 2002;143:1922–1931. doi: 10.1210/endo.143.5.8818. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura K, et al. The role of leptin during the development of mouse preimplantation embryos. Mol Cell Endocrinol. 2003;202(1-2):185–189. doi: 10.1016/s0303-7207(03)00081-9. [DOI] [PubMed] [Google Scholar]
- 45.Malik NM, et al. Leptin requirement for conception, implantation, and gestation in the mouse. Endocrinology. 2001;142:5198–5202. doi: 10.1210/endo.142.12.8535. [DOI] [PubMed] [Google Scholar]
- 46.Anisimov SV, et al. Identification of molecules derived from human fibroblast feeder cells that support the proliferation of human embryonic stem cells. Cell Mol Biol Lett. 2011;16:79–88. doi: 10.2478/s11658-010-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.