Abstract
The strength of T-cell receptor (TCR) stimulation and subsequent T-cell response depend on a combination of peptide-major histocompatibility complex (pMHC) density and potency. By comparing two different pMHC at doses yielding similar proliferation in vivo, we have highlighted unexpected differences in the qualitative and quantitative effects of TCR ligand. Measurements of cytokine sensitivity and two-photon imaging of T cell–dendritic cell (T–DC) interactions reveal discrimination between comparably weak stimuli resulting from either decreased pMHC potency or pMHC density. In addition, TCR-induced genes in broad gene expression profiles segregate into two groups: one that responds to cumulative TCR signal and another that responds to pMHC quality, independent of quantity. These observations suggest that models of TCR ligand discrimination must account for disparate sensitivity of downstream responses to specific influences of pMHC potency.
Keywords: affinity, IL-2, STAT5
T cells recognize peptide antigen in the context of MHC. During this process, the quality and quantity of the ligands determine the cumulative level of downstream signaling. Evidence suggests that T-cell receptor (TCR) can detect pMHC with remarkable sensitivity, responding to fewer than 10 pMHC complexes (1, 2), and several models of TCR triggering have been proposed to explain the ability of TCR to recognize various pMHC ligands with such sensitivity, above the noise of abundant self-pMHC (3). Both TCR/pMHC affinity and off-rate can correlate with the potency of stimulation, depending on the on-rate of the ligand (4, 5). Thus, the diverse nature of TCR/pMHC binding properties may limit the applicability of characterizing ligand quality on the basis of any single biochemical or thermodynamic parameter. Here we use the term potency to more generally reflect qualitative differences in TCR/pMHC binding parameters.
It is known from in vitro studies that the strength of TCR/pMHC interactions sets dose requirements for initiation of a variety of T-cell responses (6). Higher peptide concentrations can often compensate for weaker TCR/pMHC interactions to yield equivalent maximal responses in vitro. Thus, in studying the effects of altering the individual components of cumulative TCR signal (i.e., the potency or density of antigen), it is difficult to discriminate between distinct influences of TCR/pMHC binding parameters and the impact of shifting the overall TCR signal. For example, whereas recent in vivo studies have demonstrated that altered TCR ligands can induce large differences in the degree of proliferation, cytokine production and memory formation (7, 8), it is unclear whether the observed functional consequences are specific to qualitative differences in these ligands or could be similarly influenced by the amount of antigen. Only by manipulating both variables within the same system is it possible to determine whether T cells can discern specific influences of pMHC quality and quantity. Thus, we compared weak stimuli in vivo, resulting from either decreased pMHC potency (large quantities of weak pMHC) or decreased pMHC density (small quantities of strong pMHC). We used two previously characterized 5C.C7 TCR ligands presented in the context of I-Ek: moth cytochrome c peptide 88–103 (MCC) (9) and 102S, a weak agonist peptide resulting from an amino acid substitution at a single TCR contact residue (10). The half-life of pMHC/TCR interactions is shorter for 102S than for MCC, as measured by either tetramer dissociation (t1/2 = 33 ± 0.4 and 188.1 s, respectively) (8) or as calculated from monomeric surface plasmon resonance measurements of off-rate (t1/2 = 0.82 and 5.77 s, respectively) (11).
Our results provide unique in vivo evidence for discrimination of pMHC potency, independent of the cumulative level of TCR signal, clearly demonstrating distinct influences of pMHC quality and quantity. Although pMHC density and potency appear interchangeable in the induction of proliferation in vivo, there are specific effects of pMHC potency on the regulation of the IL-2 pathway. Furthermore, two-photon imaging indicates that T cells respond to comparably weak low-potency and low-density stimuli in the context of distinct T cell–dendritic cell (T–DC) interactions. Finally, broad gene expression patterns reveal a segregation of TCR-induced responses into two groups: one that responds to the cumulative level of TCR signal and one that is sensitive to pMHC potency independent of ligand density. Together these findings inform improved models of TCR ligand discrimination and highlight the importance of distinguishing between pMHC density and potency during the design of experimental systems and immunotherapies.
Results
Peptide-MHC Density Can Compensate for Potency in the Induction of Proliferation in Vivo.
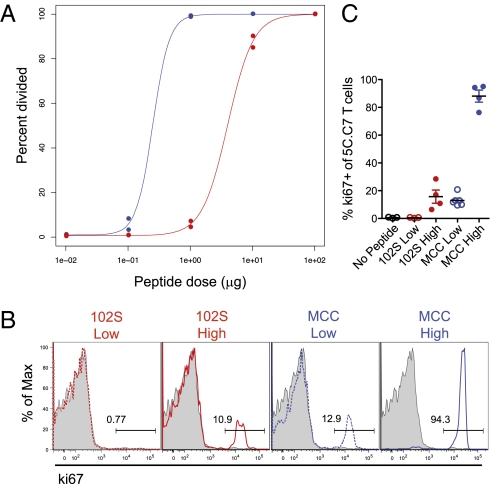
In vivo T-cell proliferation is influenced by both pMHC density and potency (12). We addressed the relationship between these two variables in the initiation of proliferation by titrating two previously characterized peptide ligands for the 5C.C7 TCR. The natural ligand, MCC peptide, behaves as a strong agonist both in vitro and in vivo (8, 9), whereas 102S peptide, with both a lower affinity and a shorter half-life of TCR/pMHC–tetramer interactions, has a higher in vitro EC50 and results in decreased proliferation, cytokine production, and memory formation in vivo (8, 10, 11). Both peptides bind comparably to MHC (13) and are cleared at a similar rate in vivo (14). Consistent with our previous work (14), in vivo titrations of stimulating peptide demonstrated that pMHC potency shifts the threshold for proliferation without altering the sharpness of the proliferative response; nearly all of the difference in proportion of dividing 5C.C7 occurs within a 10-fold range (0.1–1 μg for MCC and 1–10 μg for 102S) (Fig. 1A). This similarity in carboxyfluorescein succinimidyl ester (CFSE) dilution with respect to dose demonstrates that increased pMHC density can compensate for the low potency of 102S to initiate 5C.C7 T-cell division.
Fig. 1.
Peptide-MHC density can compensate for potency in the induction of proliferation in vivo. Adoptively transferred 5C.C7 T cells were isolated from LNs 48 h after injection of (A) a 10-fold titration or (B and C) high (2 μg) or low (0.2 μg) doses of MCC or 102S peptide. (A) Percentage of cells that had divided was assessed using dilution of CFSE. (B) Representative flow plots and (C) pooled frequencies of ki67 expression. Shaded histograms represent the no-peptide control. Data are representative of at least three independent experiments.
Considering that integration of pMHC quality and quantity determines the minimal TCR stimulation required for T-cell proliferation in vivo, we used cell division as a gauge of the cumulative TCR signal. Our previous work demonstrated that doses of 102S and MCC resulting in comparable proliferation also yielded similar Akt phosphorylation and induction of Foxp3 (14). After 48 h of in vivo stimulation, adoptively transferred 5C.C7 T cells were isolated and stained for the proliferation antigen ki67. We determined stimulation conditions, referred to here as “high-dose” (2 μg) 102S and “low-dose” (0.2 μg) MCC, which induced comparable proliferation, consistently resulting in expression of ki67 in fewer than half of 5C.C7 T cells (Fig. 1 B and C). Using these conditions, we compared weak stimuli resulting from either reduced pMHC potency or reduced pMHC density.
Peptide-MHC Potency Dissociates IL-2 Sensitivity, Receptor Expression, and Production from Cumulative TCR Signal.
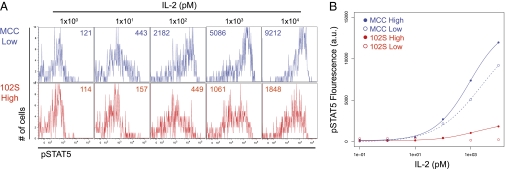
In seeking functional responses differentially regulated by TCR ligand quality and quantity, the IL-2 pathway was of particular interest in light of our previous work, which indicated differential survival induced by low-density and low-potency pMHC (14). In addition, the IL-2 pathway has known antiapoptotic properties (15, 16) and is regulated by a variety of factors downstream of the TCR (17). Thus, we assessed the effects of pMHC potency and density on TCR-induced IL-2 sensitivity. 5C.C7 T cells were isolated from lymph nodes (LNs) 48 h after injection of high- or low-dose MCC or 102S. The T cells were then stripped of bound cytokine and briefly treated with varying doses of IL-2, before fixing and staining for phosphorylated STAT5 as a measure of responsiveness to IL-2. Compared with 102S-stimulated 5C.C7, both doses of MCC resulted in increased IL-2 sensitivity (Fig. 2). High-dose 102S induced some sensitivity to IL-2; however, the amplitude of this response was reduced compared with cells responding to low-dose MCC (Fig. 2B). Thus, compared with the proliferative response (Fig. 1), increased pMHC density was unable to compensate for the low potency of the 102S ligand in terms of responsiveness to IL-2.
Fig. 2.
Peptide-MHC potency dissociates IL-2 sensitivity from cumulative TCR signal. Adoptively transferred 5C.C7 T cells were isolated from LNs 48 h after injection of high (2 μg) or low (0.2 μg) doses of MCC or 102S peptide. 5C.C7 were stripped of cytokine and treated with the indicated concentrations of IL-2 for 10 min before fixation and staining for pSTAT5. Representative (A) flow plots with GMFI Inset and (B) dose–response curves. Data are representative of at least three independent experiments.
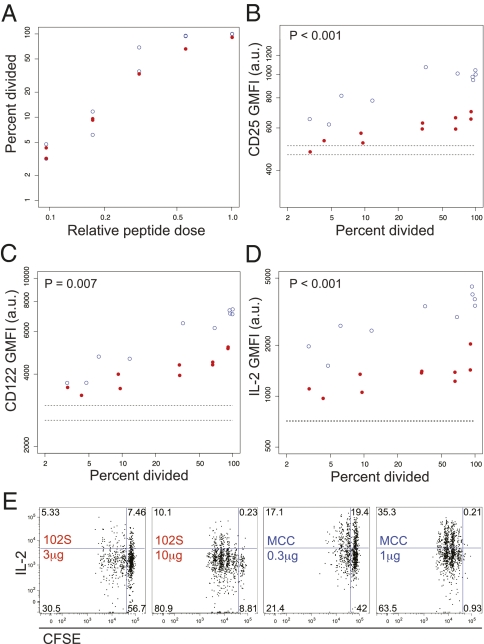
To better understand the specific influence of pMHC potency on these two responses, we assessed components of the IL-2 pathway across a 10-fold range of peptide, established as the proliferation threshold for each ligand, over which the frequency of divided cells increases from roughly 0 to 100% (Fig. 3A). Both peptides were normalized using the ratio of the maximum dose used (1 μg for MCC and 10 μg for 102S). Over this range of doses, we assessed expression of CD25 and CD122, the alpha and beta chains of the IL-2 receptor. For all amounts of division, there was a significant effect of pMHC potency, such that levels of CD25 and CD122 were higher in 5C.C7 stimulated with MCC in vivo (Fig. 3 B and C). This increased receptor expression is accordant with the increased IL-2 sensitivity of 5C.C7 stimulated with MCC (Fig. 2). Ex vivo restimulation revealed that IL-2 production is also dissociated from proliferation by the influence of TCR ligand potency (Fig. 3D). Even within equivalent CFSE peaks, 102S-stimulated cells expressed decreased amounts of IL-2, compared with MCC-stimulated cells (Fig. 3E). Differential TCR-induced IL-2 responsiveness is independent of 5C.C7 IL-2 production, as 5C.C7 T cells deficient in IL-2 also demonstrate increased IL-2 sensitivity in response to MCC stimulation (Fig. S1), suggesting direct regulation of both components downstream of TCR ligand quality. Together, these data demonstrate in vivo discrimination between weak stimuli resulting from either decreased pMHC potency or density and suggest increased importance of the qualitative effects of pMHC.
Fig. 3.
The affinity of TCR/pMHC interactions determines IL-2 and IL-2 receptor expression levels. (A) 5C.C7 division was assessed using dilution of CFSE 48 h after injection of doses ranging from 0.1 to 1 μg for MCC and 1 to 10 μg for 102S. The threshold ratio is the ratio of 1 μg for MCC and 10 μg for 102S. Samples from A were analyzed for (B and C) total CD25 and CD122 directly ex vivo or (D and E) IL-2 postrestimulation, compared with percentage of cells divided. Dashed lines (B–D) indicate the position of no-peptide controls. P values compare CD25, CD122, or IL-2 values between groups. Data are representative of at least three experiments.
Potency of pMHC Is a Greater Influence on T–DC Interactions than pMHC Density for a Given Rate of Proliferation.
Although both pMHC potency and density have been described as independent influences of T cell–dendritic cell interactions (18–20), it was unclear how naïve T cells might differentially sense weakly stimulating antigens resulting from manipulation of either variable. We reasoned that visualizing 5C.C7 recognition of low-potency and low-density pMHC could provide insight into differences in subsequent responses. Thus, 15 h after peptide injection, during the time when T–DC interactions form and persist (21, 22), we monitored cellular dynamics using two-photon microscopy. Surprisingly, cell velocities were more similar between 5C.C7 T cells responding to either dose of MCC than between low-dose MCC and high-dose 102S, the conditions resulting in comparable suboptimal proliferation (Fig. 4A). Analysis of interactions between 5C.C7 and CD11c-YFP+ DCs confirmed that long-lasting interactions with DCs (>300 s) occurred more frequently when 5C.C7 T cells sensed low-density pMHC, compared with low-potency pMHC; less stable contacts were observed in response to 102S than in response to MCC, regardless of peptide dose (Fig. 4 B and C and Movie S1). There was also a significant difference between the two weak stimuli when comparing the overall percentage of time spent in contact with a DC throughout the duration of imaging (Fig. 4D). Of note, although only a small percentage of cells sensing high-dose 102S form long-lasting contacts (Fig. 4C), there is notable 5C.C7 down-regulation of CD62L and up-regulation of CD69 (Fig. 4E), over levels of CD69 present after adoptive transfer alone; pMHC-independent expression of CD69 is consistent with previous reports (23–25). The population shift in the expression of CD69 and CD62L suggests that the majority of cells have encountered pMHC by the time of imaging. Thus, we do not believe the small fraction of 5C.C7 T cells observed stably interacting with DCs to represent the frequency of the cells that have been stimulated.
Fig. 4.
The potency of pMHC is a greater influence on T-DC interactions than pMHC density after controlling for proliferation. 5C.C7 Ub-GFP T cells were imaged using two-photon microscopy on intact LN 15 hours after injection of the indicated peptide (High: 2μg, Low: 0.2μg). (A) Cumulative frequency plot for velocity is shown from one representative experiment of three. (B) Representative images are shown with circles indicating 5C.C7 interacting with CD11c-YFP DC (solid line indicates ≥300 sec). (C) Tabulated duration of interactions and (D) percent of time in contact with DC are shown. Contact duration was analyzed for two of three representative two-photon experiments. 5C.C7 surface expression of CD69 and CD62L (E) was assessed in LN cells isolated at the time of imaging. Shaded histograms represent the no-peptide control.
Together these data demonstrate distinct T–DC interactions in response to comparably weak low- and high-potency stimuli, the latter resulting in more stable contact formation. This visual evidence is consistent with subsequent responses being independent of the cumulative level of TCR signal. In addition, it suggests that there are inherent differences in how T cells engage DCs according to the strength of TCR/pMHC interactions, making pMHC quality a greater influence than quantity in determining duration of T–DC contacts.
TCR-Stimulated Genes Can Be Apportioned on the Basis of Sensitivity to Distinct Influences of pMHC Potency.
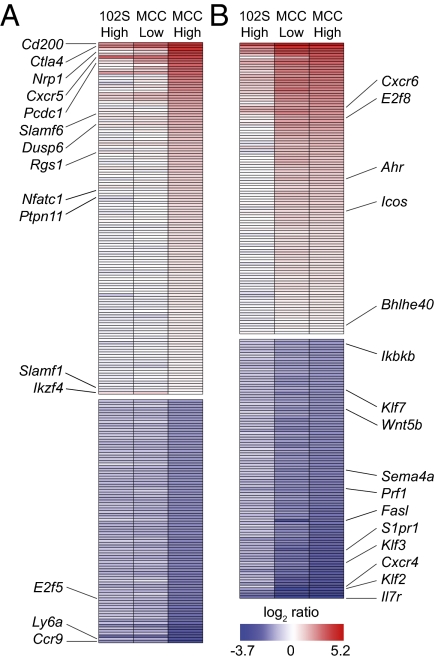
We found it of considerable interest that low-potency and low-density weak stimuli resulted in comparable proliferation, but disparate induction of the IL-2 pathway, in the context of distinct T–DC interactions. To better understand how T cells distinguish between engagement of few strong ligands and many weaker ones, we used microarray analysis to compare transcript profiles of adoptively transferred 5C.C7 T cells, 48 h after stimulation in vivo with high- or low-dose 102S or MCC peptide. The levels of expression for large subsets of TCR-regulated genes correlated either with cumulative TCR signal (Fig. 5A), defined by comparable proliferation induced by either weak stimulus, or with pMHC potency (Fig. 5B); few genes grouped according to pMHC density (Fig. S2). Most genes were less strongly up-regulated by weak stimuli. Ikzf4 was a notable exception (Fig. 5A), in addition to Foxp3, which was below the twofold cutoff (1.4-, 1.5-, and −1.1-fold change for high-dose 102S, low-dose MCC, and high-dose MCC, respectively) but is consistent with our previous work (14). Additionally, the degree of expression for a variety of negative regulators was determined by the cumulative TCR signal, including Ctla4, Pcdc1, Dusp6, Rgs1, and Ptpn11. These data suggest that, in addition to proliferation, a variety of genes (Fig. 5A) are similarly influenced by a decrease in TCR ligand quality or quantity. However, another set of genes demonstrates discrimination between the weak stimuli (Fig. 5B), including Il7r, Klf2, and several G protein coupled receptors, suggesting further examples of responses where discrimination of pMHC potency occurs independently of pMHC density. Consistent with our IL-2 and IL-2 receptor expression data (Figs. 2 and 3), gene-set enrichment analysis (26) highlighted an IL-2 signaling gene set (27), which was enriched in low-dose MCC-stimulated 5C.C7 compared with high-dose 102S (Fig. S3). Together, our microarray analysis demonstrates that a large variety of TCR-induced genes can be separated into two groups on the basis of whether their expression levels reflect distinct influences of pMHC quality and quantity. This segregation of transcriptional programs not only highlights the noninterchangeable nature of these two variables in vivo, but also suggests disparate ability of increased pMHC density to compensate for potency for given sets of responses.
Fig. 5.
TCR-stimulated genes can be apportioned on the basis of sensitivity to distinct influences of pMHC potency. Adoptively transferred 5C.C7 T cells were isolated from LNs 48 h after injection of high (2 μg) or low (0.2 μg) doses of MCC or 102S peptide. Affymetrix microarray analysis of mRNA from sorted 5C.C7 T cells, with three independent samples per condition. TCR-regulated genes, with a difference in expression of twofold or more compared with low-dose 102S, were batched on the basis of similar expression due to (A) cumulative weak TCR stimulation or (B) pMHC potency.
Discussion
Both pMHC potency and density can influence signaling downstream of the TCR and determine the robustness of effector T-cell responses (12), and their effects are not independent of each other. In vitro, increasing the affinity of stimulating peptide ligand results in T-cell proliferation at lower concentrations of antigen (6). In polyclonal responses in vivo, higher pMHC density results in a lower overall affinity of the responding T-cell population for pMHC tetramer (28, 29). We aimed to address whether T cells discriminate TCR ligand quality independent of quantity, by seeking distinct responses to weak stimuli resulting from low-potency or low-density pMHC in vivo. Increased doses of weak agonist 102S peptide resulted in 5C.C7 T-cell proliferation comparable to low doses of the strong agonist MCC, with the shape of the dose–response curve not notably influenced by TCR ligand potency. This may suggest an equally important contribution of pMHC density and potency in the initiation of the proliferative responses, as one variable can compensate for the other. We had previously noted similar integration of ligand quality and quantity in vivo in the initiation of Akt phosphorylation and initial Foxp3 induction, in that weak stimulation resulting from either low-affinity or low-density pMHC yielded comparable results (14). Thus, the degree of proliferation was used to normalize the cumulative TCR signal, although we cannot exclude the contribution of 5C.C7 T-cell extrinsic effects of MCC or 102S administration, because cell division (and all responses measured) can be influenced by additional stimuli such as costimulation and cytokine production.
By normalizing the degree of proliferation, we demonstrated distinct influences of pMHC potency on the in vivo IL-2 response. Across doses spanning the threshold of proliferation for either peptide, we found that pMHC potency had a significant influence on the expression of the IL-2 receptors CD25 and CD122 and on IL-2 production upon restimulation. These results were of particular interest considering our published findings demonstrating increased persistence of Foxp3+ 5C.C7 resulting from stimulation with low-dose MCC, compared with high-dose 102S (14). Increased production and/or sensitivity to IL-2 by MCC-stimulated cells could result in enhanced survival (15, 16), thus offering a potential mechanism behind our previous observations. This would also be consistent with a more recent report showing that strong agonist TCR stimulation is required for CD25 up-regulation and subsequent regulatory T-cell formation in the thymus, whereas both strong and weak ligands can activate pathways associated with thymocytes deletion (30). The dissociation of proliferation from the IL-2 pathway provides clear in vivo evidence for specific effects of pMHC potency, distinct from simply shifting the cumulative TCR signal, and suggests that potency outweighs the contribution of pMHC density in initiating signaling events triggering expression of IL-2 and its receptors.
Imaging of 5C.C7–DC interactions during recognition of low-density or low-potency pMHC revealed that the latter condition resulted in a decreased frequency of long-lasting contacts with DCs. Up-regulation of CD69 in the majority of these weak agonist-stimulated cells suggests that they had sensed pMHC by the time of imaging, likely in the context of shorter contacts compared with MCC-stimulated 5C.C7. It is possible that accumulation of TCR stimulation in the context of multiple shorter T–DC interactions may induce 5C.C7 T cells responding to high-dose 102S to reach levels of proliferation comparable to low-dose MCC. In this scenario, multiple short contacts may not be equivalent to more stable interactions in the induction of functional responses, such as IL-2 production and sensitivity. Considering that addition of more peptide may result in an increased concentration of antigen-bearing DCs, in addition to a higher density of pMHC per DC, it is interesting to speculate that these variables could have distinct influences on the ability of pMHC quantity to compensate for pMHC quality.
In addition to proliferation and IL-2 receptor expression, our microarray data suggest that the segregation of responses downstream of the TCR applies to a variety of pathways. Whereas a large subset of genes was similarly expressed in cells stimulated by either low-density or low-potency pMHC, another group of genes demonstrated discrimination between these weak stimuli. The presence of distinct gene expression patterns suggests that the influences of pMHC potency on in vivo T-cell responses vary on the basis of the genetic program's dependence on ligand density. Ligand discrimination is likely dependent on TCR-proximal events, as differential phosphorylation and recruitment have been demonstrated for several important early signaling intermediates downstream of stimulation with altered peptide ligands (31–35). However, it seems likely that, whereas more TCR-proximal mechanisms facilitate ligand discrimination, more distal signaling intermediates segregate responses on the basis of the ability of pMHC density to compensate for potency. This would be consistent with a model whereby accumulation of stable downstream signaling intermediates allows pMHC potency and pMHC density to contribute to a cumulative TCR signal (36–38). In contrast, pathways that rely on less stable intermediates may exhibit greater dependence on pMHC potency for the induction of downstream responses, because their rapid decay would not allow for signal accumulation with increased pMHC density over time. A role for temporal components in distinguishing pathways more or less sensitive to pMHC potency is consistent with our two-photon observations, discussed above; more stable signaling intermediates may accumulate during multiple shorter T–DC interactions, whereas prolonged contacts may be required for efficient induction of signaling pathways reliant on more transient intermediates. Differential half-life of transcription factor activity has previously been suggested to account for distinct calcium oscillation frequency requirements of such factors (39). In addition, it has been shown in vitro that 102S peptide induces less efficient conjugate formation (40) and reduced calcium signal (38) compared with MCC. Thus, these data are consistent with our hypothesis that in our in vivo system, the low-potency 102S results in bursts of signal, in the context of shorter contacts, and that only signaling intermediates that persist between these stimuli are able to induce a downstream response.
It has long been appreciated from in vitro studies that pMHC affinity sets dose thresholds for initiation of a variety of T-cell responses (6). By comparing ligands of varying TCR/pMHC affinity in vivo at equivalent points in the proliferation dose–response curve, we have provided rare in vivo evidence for ligand discrimination beyond sensing of cumulative TCR signal. The data for distinct readouts of pMHC quality and quantity suggest that the noninterchangeable nature of these variables should be taken into consideration during the design of experimental systems and immunotherapies. Finally, we have demonstrated that in vivo T-cell responses vary in their distinction between low-density and low-potency weak stimuli, supporting a model of ligand discrimination in which temporal aspects of TCR signaling may account for the disparate sensitivity to specific influences of pMHC potency.
Experimental Procedures
Mice.
5C.C7 TCR transgenic RAG2−/− mice purchased from Taconic Farms were bred to B10.A CD45.1 (provided by W. Paul via the National Institute of Allergy and Infectious Diseases (NIAID) contract facility at Taconic Farms) to generate 5C.C7 RAG2−/− CD45.1 mice, which were used as donor mice in adoptive transfer experiments. Male B10.A mice from Taconic were used as adoptive transfer recipients between 6 and 9 wk of age. For two-photon imaging experiments, 5C.C7 RAG2−/− mice were bred to B6 Ub-GFP mice purchased from The Jackson Laboratory and backcrossed three times to generate 5C.C7 RAG2−/− Ub-GFP mice. Donor cells from these mice were transferred into CD11c-YFP mice (provided by M. Nussenzweig, Rockefeller University, New York, NY.), backcrossed six times to B10.A. Mice were treated in accordance with the regulations of the National Institutes of Health and the American Association of Laboratory Animal Care. All experiments were approved by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Animal Care and Use Committee.
Peptides.
Peptides were synthesized and HPLC purified (≥95%) by Biosynthesis, Lewisville, TX.
In Vivo TCR Stimulation.
Between 5 × 105 and 1 × 106 5C.C7 RAG2−/− CD45.1 T cells were transferred into B10.A recipients by tail vein. Mice were subsequently injected i.v. with 0.01–100 μg of MCC or 102S peptide diluted in PBS. In some experiments cells were CFSE labeled before transfer. Upon harvest, LNs were pooled.
IL-2 Production and Sensitivity.
The ability of 5C.C7 to produce IL-2 was assessed ex vivo by restimulating LN cells for 5 h with MCC-pulsed B10.A DCs, in the presence of Brefeldin A. In experiments assessing 5C.C7 sensitivity to IL-2, LN cells were stripped of cytokine using a pH 4 glycine solution (0.1 M glycine in PBS, with HCl added to pH 4), rested in complete RPMI, and treated with varying doses of IL-2 for 10 min at 37 °C before immediate centrifugation and fixation.
Flow Cytometry.
Flow cytometry was done on a BD LSRII. For detection of phosph-STAT5 (Tyr694), we used a rabbit monoclonal antibody from Cell Signaling Techology (C11C5) followed by APC-conjugated polyclonal donkey antirabbit (Jackson ImmunoResearch Laboratories), after fixation with 1.6% paraformaldehyde and subsequent permeabilization with cold methanol. To detect total expression of ki67 (BD Pharmingen; B56), IL-2 (eBioscience; JES6-5H4), CD25 (BioLegend; PC61) and CD122 (eBioscience; 5H4), staining was performed after fixation and permeabilization, as above, or using BD cytofix/cytoperm in the case of IL-2.
Two-Photon Imaging and Analysis.
Two-photon imaging was performed using a Zeiss LSM-710 inverted microscope with a 40× objective on intact lymph nodes, maintained at 37 °C, and perfused with media bubbled with O2. Time-lapse Z stacks, 10–15 z planes spaced 3 μm apart, were taken at 30-s intervals. Collected imaging series were tracked automatically with Imaris 6. Only continuous tracks that could be followed for at least 2 min were used for analysis. Contact duration was calculated by examining overlap of 5C.C7-GFP and CD11c-YFP fluorescent signals in individual z planes.
Microarray Sample Preparation and Analysis.
Adoptively transferred CD45.1+ 5C.C7 T cells were sorted from LNs, 48 h after injection of the indicated peptide. A total of 100 ng of total RNA, isolated using TRIzol reagent, was labeled using the GeneChip 3′ IVT express kit (Affymetrix) with the in vitro transcription step being carried out for 16 h. Ten micrograms of labeled and fragmented cRNA were then hybridized to the mouse genome MOE430A2.0 array (Affymetrix) by the MSKCC genomics core facility. Raw expression data were analyzed using GCOS 1.4 (Affymetrix) and normalized to a target intensity of 500 to account for differences in global chip intensity. Heat maps of TCR-induced genes were generated in R using median ratio values, relative to 102S Low, derived from Partek after Robust Multichip Analysis background correction and quantile normalization; genes were considered differentially expressed if fold change over 102S low was greater or equal to 2, with false discovery rate correction set to 0.05. Division of heat maps was accomplished by normalizing logarithmically scaled fold-change values by the difference between the largest and smallest values (the range). A gene's expression was considered similar between two conditions if the intermediate value was less than 33% of the range from the highest or lowest ratio value.
Statistical Analysis.
Flow data were analyzed using FlowJo version 8.8.6 and further processed using either Prism 5.0 (GraphPad) or the statistical software R (http://www.r-project.org). Sensitivity curves were fit to a Hill function with floating Hill coefficient using the nls() function in R. Statistical significance was determined for two-photon data using a two-tailed Student t test, whereas for flow cytometry data compared across multiple doses, an independent-samples t test was used.
Supplementary Material
Acknowledgments
We thank A. Trumble-Koncelik and W. Montalvo for technical assistance; G. Loeb, M. Hassimi, and R. Khanin for assistance with microarray analysis; and M. Huse and T. Pentcheva-Hoang for helpful discussion and critical reading of the manuscript. M.M.H. and G.A.-B. are supported by National Institute of Allergy and Infectious Diseases Grant R01-AI083408. R.A.G. is a predoctoral fellow of the Cancer Research Institute and J.P.A. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1119763109/-/DCSupplemental.
References
- 1.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–849. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 2.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 3.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 4.Aleksic M, et al. Dependence of T cell antigen recognition on T cell receptor-peptide MHC confinement time. Immunity. 2010;32:163–174. doi: 10.1016/j.immuni.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govern CC, Paczosa MK, Chakraborty AK, Huseby ES. Fast on-rates allow short dwell time ligands to activate T cells. Proc Natl Acad Sci USA. 2010;107:8724–8729. doi: 10.1073/pnas.1000966107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signaling events in human T cells. J Immunol. 1998;160:5807–5814. [PubMed] [Google Scholar]
- 7.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corse E, Gottschalk RA, Krogsgaard M, Allison JP. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 2010;8:e1000481 doi: 10.1371/journal.pbio.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MM, et al. Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 10.Reay PA, Kantor RM, Davis MM. Use of global amino acid replacements to define the requirements for MHC binding and T cell recognition of moth cytochrome c (93-103) J Immunol. 1994;152:3946–3957. [PubMed] [Google Scholar]
- 11.Huppa JB, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–967. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 13.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol Cell. 2003;12:1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 14.Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. J Exp Med. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng G, Podack ER. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci USA. 1993;90:2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Parijs L, et al. Functional responses and apoptosis of CD25 (IL-2R alpha)-deficient T cells expressing a transgenic antigen receptor. J Immunol. 1997;158:3738–3745. [PubMed] [Google Scholar]
- 17.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 19.Henrickson SE, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skokos D, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007;8:835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 21.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugues S, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 25.Moran AE, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marzec M, et al. Differential effects of interleukin-2 and interleukin-15 versus interleukin-21 on CD4+ cutaneous T-cell lymphoma cells. Cancer Res. 2008;68:1083–1091. doi: 10.1158/0008-5472.CAN-07-2403. [DOI] [PubMed] [Google Scholar]
- 28.Rees W, et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J Exp Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cozzo Picca C, et al. CD4(+)CD25(+)Foxp3(+) regulatory T cell formation requires more specific recognition of a self-peptide than thymocyte deletion. Proc Natl Acad Sci USA. 2011;108:14890–14895. doi: 10.1073/pnas.1103810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: Altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 32.Madrenas J, et al. Zeta phosphorylation without ZAP-70 activation induced by TCR antagonists or partial agonists. Science. 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 33.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: The importance of kinetics in TCR signaling. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 34.Stefanová I, et al. TCR ligand discrimination is enforced by competing ERK positive and SHP-1 negative feedback pathways. Nat Immunol. 2003;4:248–254. doi: 10.1038/ni895. [DOI] [PubMed] [Google Scholar]
- 35.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 36.Rosette C, et al. The impact of duration versus extent of TCR occupancy on T cell activation: A revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 37.Rachmilewitz J, Lanzavecchia A. A temporal and spatial summation model for T-cell activation: Signal integration and antigen decoding. Trends Immunol. 2002;23:592–595. doi: 10.1016/s1471-4906(02)02342-6. [DOI] [PubMed] [Google Scholar]
- 38.Wülfing C, et al. Kinetics and extent of T cell activation as measured with the calcium signal. J Exp Med. 1997;185:1815–1825. doi: 10.1084/jem.185.10.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 40.Egen JG, Allison JP. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.