Abstract
Global climate change is having profound impacts on the natural world. However, climate influence on faunal dynamics at macroevolutionary scales remains poorly understood. In this paper we investigate the influence of climate over deep time on the diversity patterns of Cenozoic North American mammals. We use factor analysis to identify temporally correlated assemblages of taxa, or major evolutionary faunas that we can then study in relation to climatic change over the past 65 million years. These taxa can be grouped into six consecutive faunal associations that show some correspondence with the qualitative mammalian chronofaunas of previous workers. We also show that the diversity pattern of most of these chronofaunas can be correlated with the stacked deep-sea benthic foraminiferal oxygen isotope (δ18O) curve, which strongly suggests climatic forcing of faunal dynamics over a large macroevolutionary timescale. This study demonstrates the profound influence of climate on the diversity patterns of North American terrestrial mammals over the Cenozoic.
Keywords: fossil record, Paleogene, Neogene
Understanding the influence of climate on the evolution and ecology of today’s organisms is crucial to avert future biodiversity collapse (1). However, establishing the relationship of climate to ecological and evolutionary patterns is a difficult task (2). To this respect, studies of biodiversity dynamics at macroevolutionary timescales provide power to understand past biotic responses to climate change and to predict biotic reactions in future climatic changes (3).
Here, we explore the relationship between long-term patterns of evolutionary diversity and climate using mammalian faunal dynamics over the Cenozoic era (the past 65 Ma) of North America. Large terrestrial mammals (i.e., greater than ∼5 kg) are an excellent group for this investigation because they are clearly influenced by climatic change today (1), and are well-preserved in the fossil record (4).
During the past few decades there has been debate about the influence of climate change on mammalian evolutionary dynamics, either arguing for a strong influence (e.g., 5–7), or against it (e.g., 8, 9). However, recent studies of Cenozoic mammals in restricted temporal intervals (e.g., 2, 10–16), or in restricted taxonomic groups (e.g., 15, 17, 18) have demonstrated that climatic change may indeed have profound effects on patterns of mammalian evolution. In this paper we analyze the macroevolutionary dynamics of all large terrestrial mammals from North America during the last 65 Ma.
To explore the relationship between mammal faunal dynamics and the Cenozoic climatic events, we performed a Q-mode factor analysis (FA) to identify objective associations of taxa of large terrestrial mammals that we classify as evolutionary faunas (19), whose dynamics can then be studied in relation to climatic change. Specifically, we address the following: (i) Can the fossil record of large North American mammals be summarized in a relatively simple pattern of successive faunas? (ii) Do the members of these faunas share the same patterns of origination, diversification, and extinction? (iii) What might be the role of climatic change in shaping the rise and fall of these faunal associations?
Results
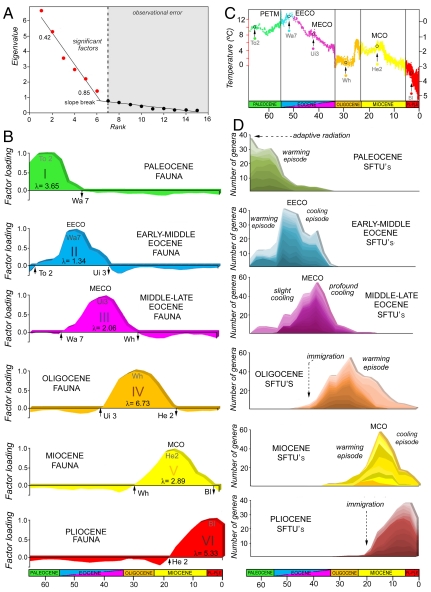
The FA performed from the abundance of genera within the subfamily taxonomic units (SFTUs) distributed over the 26 time intervals produced six significant eigenvectors, or factors, that explained > 80% of the original variance (Fig. 1A). The record of large mammals can be summarized by six successive faunas (Fig. 1B), each composed of a specific association of taxa (Table 1) that share times of origination, diversification, and extinction (Fig. 1D). We recognize these faunal associations as follows: (i) Paleocene fauna; (ii) early-middle Eocene fauna; (iii) middle-late Eocene fauna; (iv) Oligocene fauna; (v) Miocene fauna; and (vi) Pliocene fauna.
Fig. 1.
Summary of the Q-mode factor analysis performed on the large Cenozoic terrestrial mammals of North America. (A) Bivariate plot of the eigenvalues against their rank for the first 15 factors. (B) Factor loadings of each time interval within the first six rotated factors plotted against geologic time showing their distribution through the Cenozoic. Factors are reordered into temporal sequence. The importance of each rotated factor is denoted by its relative eigenvalue (λ). (C) Stacked deep-sea benthic foraminiferal oxygen isotope values (y axis) on geologic time in Ma (x axis) used as a proxy for global paleotemperature (4). (D) Diversity curves performed as summed areas of those taxa with scores above more than 1.0 in each fauna (Table 1). Note that the diversity peaks coincide approximately with the peaks of factor loadings of each fauna (see Fig. 1C for time intervals). (PETM, Paleocene-Eocene thermal maximum; EECO, early Eocene climate optimum; MECO, middle Eocene climatic optimum; MCO, Miocene climate optimum; To, Torrejonian; Wa, Wasatchian; Ui, Uintan; Wh, Whitneyan; He, Hemingfordian; Bl, Blancan.)
Table 1.
Summary of the scores of each SFTUs on the first six rotated factors
| Scores |
Evolutionary faunas |
|||||
| Paleocene | Early-middle Eocene | Middle-late Eocene | Oligocene | Miocene | Pliocene | |
| > 2.0 | Viverravidaea | Proviverrinaeb | Miacidaea | Hesperocyoninaec | Borophaginaec | Caninaec |
| Hyopsodontidaed | Miacidaea | Homacodontinaee | Nimravinaef | Oligobuninaeg | Mustelinaeg | |
| Mioclaeninaed | Basal Ceratomorphah | Protoreodontinaei | Merycoidodontinaei | Merycodontinaej | Camelinaek | |
| Arctocyoninael | Hyracotheriinaem | Oromerycidaek | Hypertragulidaen | Moschidaej | Antilocaprinaej | |
| Pantolambdidaeo | Diacodexeinaee | Leptotragulinaek | Tapiridaeh | Anchitheriinaem | Equinaem | |
| — | Viverravidaea | Brontotheriinaep | — | Equinaem | Gomphotheriidaeq | |
| — | Oxyaeninaeb | Helohyinaee | — | Amphicyoninaer | Felinaes | |
| — | Mesonychinael | Helaletidaeh | — | — | — | |
| — | — | Amynodontidaet | — | — | — | |
| > 1.0 | Anisonchinaed | Limnocyonidaeb | Hyaenodontinaeb | Daphoeninaer | Synthetoceratinaek | Machairodontinaes |
| Triisodontinael | Meniscotherinaed | Viverravidaea | Bothriodontinaeu | Miolabinaek | Procyoninaeg | |
| Barylambdidaeo | Isectolophidaeh | Antiacodontinaee | Entelodontidaeu | Tapiridaeh | Tayassuinaeu | |
| Periptychinaed | Stylinodontidaeo | Tapiroidsh | Poebrotherinaek | Protolabinaek | Cervidaej | |
| — | Homacodontinaee | Mesonychinael | Leptomerycidaen | Cranioceratinaej | Bovidaej | |
| — | Hyopsodintidaee | Hyracodontidaet | Tapiroidsh | Hesperhyinaee | Mephitidaeg | |
| — | — | Teleaceratinaet | Anchitheriinaem | Procyoninaeg | Melinaeg | |
| — | — | Hyracotheriinaem | Borophaginaec | — | — | |
| — | — | Dolichorhininaep | Basal rhinocerotidst | — | — | |
| — | — | — | Diceratheriinaet | — | — | |
The values of the SFTU scores indicate their relative contribution of each SFTU to the total diversity of each evolutionary fauna. Key to the higher taxa containing the SFTUs (quotes indicate paraphyletic taxa, daggers indicate extinct taxa)
a“Miacoids”†
bCreodonta†
cCanidae
d“Condylarthra”†: Herbivorous or omnivorous taxa
eDichobunidae†
fNimravidae†
g“Musteloids”
h“Tapiroids”
iOreodontoidea†
jPecora
k“Tylopods” (inc. camels, protoceratids,† and oromerycids†)
l“Condylarthra”†: Carnivorous taxa.
mEquidae
n“Traguloids”
o“Ungulate-like mammals”†
pOther perissodactyls (inc. brontotheres† and chalicotheres†)
qProboscidea
rAmphicyonidae†
sFelidae
tRhinocerotoidea
u“Suoids”
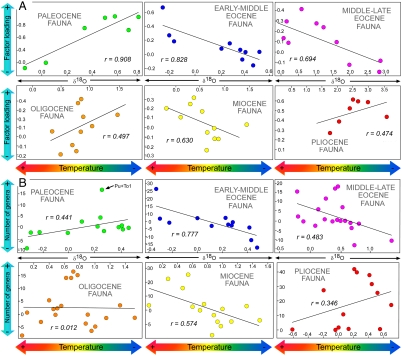
The results of the bivariate regression analysis between both the positive factor loadings (PFLs) and the number of genera (NG) of those SFTUs scoring above more than 1.0 in each fauna (Fig. 1D; Table 1) against the δ18O isotopic values in their corresponding time intervals using the method of generalized differences (GDRA) are shown in Fig. 2 and Table 2. These results clearly indicate that the rise and fall of the majority of these faunas is correlated with the major climatic events of the Cenozoic.
Fig. 2.
Bivariate plots of δ18O isotopic values on the diversity of each mammalian evolutionary fauna. (A) The PFLs of each fauna are used as proxy for faunal diversity. (B) The NG of those SFTUs scoring above 1.0 within each fauna is used as proxy for faunal diversity (see also Fig. 1D and Table 1). Note that both variables have been corrected for serial correlation using GDRA method (SI Text).
Table 2.
Bivariate regression analysis performed between the PFLs and the NG of each fauna versus the δ18O isotopic values for their corresponding time intervals
| Evolutionary faunas | N | Using PFLs | N | Using NG |
| Paleocene | 7 | r = 0.908; F = 23.494; p = 0.005a | 13 | r = 0.441; F = 2.649; p = 0.132; p = 0.036b |
| Early-middle Eocene | 10 | r = 0.828; F = 17.439; p = 0.003a | 13 | r = 0.777; F = 16.721; p = 0.002a |
| Middle-late Eocene | 10 | r = 0.694; F = 7.417; p = 0.026ac | 19 | r = 0.483; F = 5.161; p = 0.036a |
| Oligocene | 10 | r = 0.497; F = 2.634; p = 0.143 | 19 | r = 0.012; F = 0.002; p = 0.962 |
| Miocene | 11 | r = 0.630; F = 5.932; p = 0.038a | 15 | r = 0.574; F = 6.393; p = 0.025a |
| Pliocene | 7 | r = 0.474; F = 1.451; p = 0.282c | 13 | r = 0.346; F = 1.497; p = 0.247 |
The time series variables were corrected for serial correlation using the generalized differences method of Wonnacott and Wonnacott (42) and McKinney and Oyen (41). The bivariate regressions between the PFLs of the late Eocene and Pliocene faunas on their corresponding isotopic values of δ18O were not possible to perform using GDRA I. In both cases only the GDRA II was used instead (SI Text).
aSignificant at 95% level.
bResults performed excluding Puercan plus Torrejonian-1 (see Results).
cRegression performed using GDRA II.
Discussion
The temporal distribution of the Paleocene fauna (Table 1) begin at the Cretaceous-Paleogene (K/Pg) boundary (Fig. 1B). None of these taxa are known from the Cretaceous (20) and the rise of this fauna may be due to extremely rapid evolution following the K/Pg extinctions, possibly involving immigration from Asia (21). The Paleocene fauna reached its maximum diversity in the late early Paleocene (∼61.5 Ma; Fig. 1B) and there is a significant correlation between the PFLs and the oxygen isotopic values (δ18O) (Fig. 2A; Table 2). The regression analysis of the NG against the oxygen isotopic values (δ18O) results in a similarly positive, but not significant, trend (Fig. 2A; Table 2), possibly reflecting a partial decoupling between climate and mammalian biodiversity at this time (22). However, repeating the analysis excluding the first sampled interval (Puercan plus Torrejonian 1) did result in a significant correlation (Table 2). The Paleocene fauna’s demise is related with a warming trend in the late Paleocene that peaked at the Paleocene–Eocene thermal maximum (PETM; Fig. 1C). This same warming heralded the rise of the early Eocene fauna (Fig. 1B; Table 1), which reached its maximum diversity at 52-50 Ma during the early Eocene climatic optimum (EECO) (2, 23–25; Fig. 1 B and C). Therefore, the late early Paleocene temperature rise may be the cause of the transition between the Paleocene fauna and the early-middle Eocene one. Both the early-middle Eocene fauna PFLs and the NG show a significant correlation with the δ18O isotopic values (Fig. 2 A and B; Table 2). After the EECO, there was a rapid climatic change leading to a 17 Ma period of cooling (23, 26; Fig. 1C), which correlates with the progressive demise of the early-middle Eocene fauna (Fig. 1B). Thus, whereas both the rise and fall of the early-middle Eocene fauna can be related to long-term climatic trends (the warming of the early Eocene and the later cooling, respectively) the transition between the early-middle and the middle-late Eocene faunas should be related with the EECO.
The rise of the middle-late Eocene fauna (Fig. 1B; Table 1) also correlates with this 17 Ma cooling following the EECO, as shown by the significant correlation of both the PFLs and the NG with the δ18O isotopic values for this time interval (Fig. 2 A and B; Table 2). Faunal diversity peaked following a slight respite in this cooling, the middle Eocene climatic optimum (MECO) (∼41.5 Ma) (23) at Ui-3 (∼44 Ma), but progressively diminished in correlation with the continued cooling trend in the late Eocene (Fig. 1 B and C). Thus, the rise of the late Eocene fauna can be related to initially cooler conditions following the EECO, but diversity fell in correlation with the lower absolute values following the MECO (Fig. 1 B and C). Members of this fauna apparently favored somewhat cooler conditions from the temperature peak of the Cenozoic, but not the increasingly cooler conditions of the later Eocene. Note that the later Eocene also marks the decline and eventual extinction of the North American primates, animals that are clearly sensitive to temperature and seasonality (27, 28).
The transition between the middle-late Eocene fauna and the succeeding Oligocene one might be expected to be related to the MECO temperature peak. However, although the rise of the Oligocene fauna is coincident with the late Eocene cooling (Fig. 1 B and C), the absence of a significant correlation between both the PFLs and the NG of this fauna with the late Eocene δ18O isotopic values suggests that climatic change alone was not a factor (Fig. 2 A and B; Table 2). Rather, the rise of the Oligocene fauna may relate to the major immigration event from Asia in the middle-late Eocene following a fall in sea level at this time (29). In fact, many members of this fauna represent immigrant groups (e.g., nimravids, amphicyonids, diceratheriine rhinos, camelids, and traguloids; see Table 1), which may also have been adapted to cooler climatic conditions (15). This immigration event could also have influenced the demise of the middle-late Eocene fauna. The Oligocene fauna reached its maximum diversity in the mid-Oligocene at approximately 30–32 Ma (Fig. 1B) and clearly captures the classic White River Chronofauna (30–32).
The initial late Oligocene rapid temperature rise also correlates with the rise of the Miocene fauna (Fig. 1 B and C; Table 1) and this rapid climatic change was probably the cause for the transition between the Oligocene and the Miocene faunas. After this initial temperature peak, a warming trend continued (23) peaking at 17-15 Ma in the Miocene climatic optimum (MCO) (Fig. 1C). This warming coincides both with the demise of the Oligocene fauna and the rise of the Miocene fauna. Both the PFLs and the NG of the Miocene fauna are significantly correlated with the δ18O isotopic values of the early Miocene (Fig. 2; Table 2). The Miocene fauna appears to be a warm-adapted fauna: Its maximum diversity coincides with the MCO at approximately 16.5 Ma (Fig. 1 B and C), and its demise correlates with the subsequent long-term cooling trend. However, this fauna begins too early and peaks too soon to capture the classic early-late Miocene Clarendonian Chronofauna of Webb et al. (30), characterized by a high diversity of savanna-adapted hoofed mammals. The Clarendonian diversity may be due to a mixture of the later members of the Miocene fauna (which follows a slow decline) and the earlier members of the rapidly rising succeeding Pliocene fauna (see Fig. 1D).
The transition between the Miocene fauna and the Pliocene fauna seems to be marked by the MCO. In fact, the Pliocene fauna (Fig. 1B; Table 1) peaks in diversity in the early Pliocene (∼3.5 Ma), coinciding with the early Pliocene warming, and its decline coincides with the later cooling (Fig. 1 B and C). However, the correlation between both the PFLs and the NG with the δ18O isotopic values is not statistically significant (Fig. 2 A and B; Table 2). Again, the rise of the Pliocene fauna may be related with a major immigration that occurred near the end of the early Miocene (7). The Pliocene fauna began to diversify at approximately 16.5 Ma, shortly after an extensive interchange between Eurasian and North American faunas, and its members include groups immigrating at this time (Felinae, Procyonidae, Mustelinae, Antilocapridae, and Gomphotheriidae). Thus, the rise of the Pliocene fauna likely relates to this immigration event, which may also have played a role in the decline of the Miocene fauna. However, some of the early Miocene immigrants became extinct before the end of the epoch (e.g., Hemicyoninae, Moschidae, Dromomerycinae, Cranioceratinae, Merycodontinae, and Aceratheriniinae), being present during the decline of the Miocene fauna, and thus included within this fauna (contributing to the high diversity observed in the Clarendonian, as previously noted).
Conclusions
Our analysis demonstrates that, despite a great deal of faunal diversity and turnover throughout the North American Cenozoic, there is a relatively simple underlying pattern of faunal dynamics. FA shows that most of the temporal variation can be accounted for by six successive taxonomic associations or evolutionary faunas (Fig. 1 A and B). In addition, the individual taxonomic groupings associated with each fauna (Table 1) share times of origination, diversification, and extinction (Fig. 1D). Consequently, the diversity dynamics of terrestrial mammals through the North American Cenozoic can be described in terms of these six faunal associations. Therefore, our results differ from those (27) who claimed that the Cenozoic history of mammalian biodiversity could be summarized by only three major evolutionary faunas.
The significant correlation between both the PFLs and the NG for the majority of these faunas with the stacked deep-sea benthic foraminiferal oxygen isotope (δ18O) curve strongly implies that climatic change has had a profound impact on North American mammalian biodiversity over the last 65 Ma. The waxing and waning of faunas is mostly correlated to long-term sustained climatic trends, either toward warming or cooling. The only exceptions are the Oligocene and the Pliocene faunas, whose origination coincides with major immigration events from the Old World. Furthermore, the transitions between faunas are mostly correlated with short-term perturbation events such as rapid climatic changes or temperature peaks (Fig. 3). However, the exact nature of the transitions between the faunas—whether the primary process is the decline of old species or rather the dispersal of new ones tracking newly available habitats and resources—is difficult to determine at this time, but will be the focus of future investigation.
Fig. 3.
Long-term sustained trends and short-term perturbation events proposed as the main causes for the waning/waxing of the six mammalian evolutionary faunas and their transitions.
In summary, we show here that, over the last 65 Ma in North America, mammalian diversity can be partitioned into successive faunal associations that expand and decline over time. These faunas tend to increase in diversity in association with sustained periods of climatic trends (warming or cooling) until some kind of perturbation occurs (temperature peaks or rapid climatic changes), which correlates with transitions between successive faunas. Such perturbations, related to anthropogenic climatic change, are currently challenging the fauna of the world today (33), emphasizing the importance of the fossil record for our understanding of how past events affected the history of faunal diversification and extinction, and hence how future climatic changes may continue to influence life on earth.
Materials and Methods
We collected a number of genera within subfamilies (SFTUs)—or within families for the smaller taxonomic units—of Cenozoic North American, large terrestrial mammals. (SI Text). Data for the Tertiary mammals were derived from Janis et al. (20) with updates from Janis et al. (28) (sources that collate locality information for every species), and some further literature updates [registered at the Neogene Old World Database (NOW) database (34)]. Data for Pleistocene mammals were derived from the Quaternary distribution of the mammalian species in the United States database (FAUNMAP) (35).
We used the timescale and divisions of the North American Land Mammal Ages (NALMAs) into biochrons (i.e., NALMA subdivisions) following Woodburne and Woodburne (36). As these biochrons are of different durations, which could result in bias in the analyses (longer intervals could show more abundance of genera), we grouped them so as to create time bins that were as equal in length as possible (SI Text), following Sepkoski (19) for the Phanerozoic intervals. In so doing we reduced the number of time intervals from 62 (X = 1.05 Ma; σ = 0.94 Ma) to 26 (X = 2.5; σ = 0.68 Ma). We also performed the same multivariate analysis using the original 62 biochrons and obtained similar results (Fig. S1).
Because differences in sampling efforts among different time intervals could result in a potential bias in the diversity patterns analyzed here, we used bivariate regression analysis to test whether the relationship between the abundance of genera (i.e., dependent variable) was dependent on the number of paleontological localities available for the used time intervals—as a proxy of sampling efficiency (i.e., independent variable). We found no systematic effects of sampling efficiency or stratigraphic resolution on large mammal diversity (Fig. S2 A–D). This fact indicates that there is no temporal trend of increase in the observational completeness of the Cenozoic mammalian record of North America that could be masking the major patterns of diversification obtained for the faunas. Furthermore, as the use of stratigraphic intervals of different durations could be a potential bias in FA, longer intervals tend to have more abundance of genera and vice versa, we performed a bivariate regression analysis to test whether the abundance of genera present in each time interval (i.e., dependent variable) was dependent on their durations (i.e., independent variable). The results clearly indicated that the taxonomic richness is not biased by the duration of the time intervals used here (Fig. S2E). However, it should be noted that the linear regression approach used here to test for sampling biases is not fully appropriate, because the diversity recorded at a given stage depends in part on diversity at the preceding one. Such limitation could be avoided with a nonparametric test of correlation, but this would preclude the obtaining of regression residuals.
We performed a Q-mode FA following Imbrie (37). FA was then computed from the matrix of NG within SFTUs present in each of the 26 temporal intervals. We used as variables the NG within SFTUs and as cases the temporal bins through the Cenozoic of North America. Subsequently, this matrix was transposed so that the temporal intervals were the columns (variables) and the SFTUs were the row data (cases). As in Sepkoski (19), we standardized the data within temporal bins and we performed an ordinary FA using correlation matrix with program SPSSv.13. Also, we performed FA with the Paleontological Statistics Software (PAST) (38) using the original algorithm of the Q-mode factor analysis developed by Klovan and Imbrie (39).
The number of factors was selected using the following criteria: (i) factors with eigenvalues greater than 1 because these eigenvectors at least explain the same proportion of the variance than one variable by itself; and (ii) breaks in the slope from the bivariate graph of the eigenvalues against their rank, following the procedure used by Sepkoski (19) for discarding those factors that account for random variation in the dataset (i.e., those which eigenvalues show a slope against rank order that does not differ significantly from zero). The selected eigenvectors were extracted and rotated using a method that maximizes the sum of the within-factor variances (VARIMAX) of squared factor loadings (40).
We performed the method of generalized differences (41, 42) to assess if climatic change played a role in the rise and fall of mammalian chronofaunas (SI Text). We used the δ18O isotope values from deep-sea record (23) as a proxy for paleotemperature (Fig. 1C). Average values of δ18O were computed for each of the 26 time intervals. For a pancontinental analysis such as the one performed here, the use of the oceanic temperature curve as a proxy for terrestrial climate should not be problematic (8, 17). We used two different proxies of faunal diversity: (i) the PFLs of each fauna (Fig. 1B), and (ii) the diversity curve of each fauna (Fig. 1D), counted as the total NG of those SFTUs scoring above 1.0 (Table 1). Accordingly, the two serially corrected time series variables (i.e., the PFLs or NG of each reported chronofauna and the δ18O values for their corresponding time intervals) were regressed using the ordinary least square regression analysis.
Supplementary Material
Acknowledgments.
We are grateful to several colleagues (David Polly, Jussi Eronen, and Thompson Webb III) whose comments on an earlier version of this manuscript are greatly appreciated. We also thank two anonymous reviewers whose suggestions and comments have helped us to improve the rigor of the manuscript contents. This study was supported by Fulbright Postdoctoral Grant FU2009-0184 (to B.F.), a Bushnell Foundation grant to Brown University (C.J.), and Spanish Ministry of Science and Innovation research projects CGL2008-04896 and CGL2010-15326 (to J.A.P.-C., M.R., and P.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110246108/-/DCSupplemental.
References
- 1.Blois JL, Hadly EA. Mammalian response to Cenozoic climatic change. Annu Rev Earth Planet Sci. 2009;37:181–208. [Google Scholar]
- 2.Woodburne MO, Gunnell GF, Stucky RK. Climate directly influences Eocene mammal faunal dynamics in North America. Proc Natl Acad Sci USA. 2009;106:13399–13403. doi: 10.1073/pnas.0906802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erwin DH. Climate as a driver of evolutionary change. Curr Biol. 2009;19:R575–R583. doi: 10.1016/j.cub.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 4.Behrensmeyer AK, Kidwell SM, Gastaldo RA. Taphonomy and paleobiology. Paleobiology. 2000;26:103–147. [Google Scholar]
- 5.Barry JC, Johnson NM, Raza SM, Jacobs LL. Neogene mammalian faunal change in southern Asia: Correlations with climatic, tectonic, and eustatic events. Geology. 1985;13:637–640. [Google Scholar]
- 6.Janis CM. A climatic explanation for patterns of evolutionary diversity in ungulate mammals. Palaeontology. 1989;32:463–481. [Google Scholar]
- 7.Janis CM. Tertiary mammal evolution in the context of changing climates, vegetation, and tectonic events. Annu Rev Ecol Syst. 1993;24:467–500. [Google Scholar]
- 8.Alroy J, Koch PL, Zachos JC. Global climate change and North American mammalian evolution. Paleobiology. 2000;26:259–288. [Google Scholar]
- 9.Prothero DR. Did impacts, volcanic eruptions, or climate change affect mammalian evolution? Palaeogeogr Palaeoclimatol Palaeoecol. 2004;214:283–294. [Google Scholar]
- 10.Badgley C, et al. Ecological changes in Miocene mammalian record show impact of prolonged climatic forcing. Proc Natl Acad Sci USA. 2008;105:12145–12149. doi: 10.1073/pnas.0805592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnosky AD, Koch PL, Feranec RS, Wing SL, Shabel AB. Assessing the causes of late Pleistocene extinctions on the continents. Science. 2004;306:70–75. doi: 10.1126/science.1101476. [DOI] [PubMed] [Google Scholar]
- 12.Blois JL, McGuire JL, Hadly EA. Small mammal diversity loss in response to late-Pleistocene climatic change. Nature. 2010;465:771–775. doi: 10.1038/nature09077. [DOI] [PubMed] [Google Scholar]
- 13.Casanovas-Vilar I, Garcia-Paredes I, Alba DM, Ostende LWV, Moya-Solá S. The European far Wwest: Miocene mammal isolation, diversity and turnover in the Iberian Peninsula. J Biogeogr. 2010;37:1079–1093. [Google Scholar]
- 14.Eronen JT, et al. Distribution history and climatic controls of the late Miocene Pikermian chronofauna. Proc Natl Acad Sci USA. 2009;106:11867–11871. doi: 10.1073/pnas.0902598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janis CM, Damuth J, Theodor JM. Miocene ungulates and terrestrial primary productivity: Where have all the browsers gone? Proc Natl Acad Sci USA. 2000;97:7899–7904. doi: 10.1073/pnas.97.14.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dam JA, et al. Long-period astronomical forcing of mammal turnover. Nature. 2006;443:687–691. doi: 10.1038/nature05163. [DOI] [PubMed] [Google Scholar]
- 17.Mihlbachler MC, Rivals F, Solounias N, Semprebon GM. Dietary change and evolution of horses in North America. Science. 2011;331:1178–1181. doi: 10.1126/science.1196166. [DOI] [PubMed] [Google Scholar]
- 18.Smith T, Rose KD, Gingerich PD. Rapid Asia-Europe-North America geographic dispersal of earliest Eocene primate Teilhardina during the Paleocene-Eocene Thermal Maximum. Proc Natl Acad Sci USA. 2006;103:11223–11227. doi: 10.1073/pnas.0511296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepkoski JJ. A factor analytic description of the phanerozoic marine fossil record. Paleobiology. 1981;7:36–53. [Google Scholar]
- 20.Janis CM, Scott KM, Jacobs LL. Evolution of Tertiary Mammals of North America Vol. 1: Terrestrial Carnivores, Ungulates, and Ungulate-like Mammals. Cambridge, UK: Cambridge University Press; 1998. p. 691. [Google Scholar]
- 21.Alroy J. The fossil record of North American mammals: Evidence for a Paleocene evolutionary radiation. Syst Biol. 1999;48:107–118. doi: 10.1080/106351599260472. [DOI] [PubMed] [Google Scholar]
- 22.Rose PJ, Fox DL, Marcot J, Badgley C. Flat latitudinal gradient in Paleocene mammal richness suggests decoupling of climate and biodiversity. Geology. 2011;39:786–786. [Google Scholar]
- 23.Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451:279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
- 24.Prothero DR. In: Evolution of Tertiary Mammals of North America Vol. I: Terrestrial Carnivores, Ungulates, and Ungulate-like Mammals. Janis CM, Scott KM, Jacobs LL, editors. Cambridge, UK: Cambridge University Press; 1998. pp. 9–37. [Google Scholar]
- 25.Prothero DR. In: Evolution of Tertiary Mammals of North America Vol I: Terrestrial Carnivores, Ungulates, and Ungulate-like Mammals. Janis CM, Scott KM, Jacobs LL, editors. Cambridge, UK: Cambridge University Press; 1998. pp. 337–358. [Google Scholar]
- 26.Katz ME, et al. Stepwise transition from the Eocene greenhouse to the Oligocene icehouse. Nat Geosci. 2008;1:329–334. [Google Scholar]
- 27.Alroy J. Are Sepkoski’s evolutionary faunas dynamically coherent? Evol Ecol Res. 2004;6:1–32. [Google Scholar]
- 28.Janis CM, Gunell G, Uhen M. Evolution of Tertiary Mammals of North America Vol. 2: Small Mammals, Edentates, and Marine Mammals. Cambridge, UK: Cambridge University Press; 2008. p. 802. [Google Scholar]
- 29.Prothero DR. Mid-Oligocene extinction event in North-American land mammals. Science. 1985;229:550–551. doi: 10.1126/science.229.4713.550. [DOI] [PubMed] [Google Scholar]
- 30.Webb SD, Opdyke ND. In: Effects of Past Global Change on Life. Stanley SM, editor. Washington, DC: Natl Academy Press; 1995. pp. 184–208. [Google Scholar]
- 31.Emry R. Additions to the mammalian fauna of the type Duchesnean, with comments on the status of the Duchesnean. J Paleontol. 1981;5:563–570. [Google Scholar]
- 32.Prothero DR, Emry R. In: The Terrestrial Eocene-Oligocene Transition in North America. Prothero DR, Emry R, editors. Cambridge, UK: Cambridge University Press; 1996. pp. 646–664. [Google Scholar]
- 33.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471:51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 34.Fortelius M. Neogene of the Old World Database of Fossil Mammals (NOW) Finland: University of Helsinki; 2011. Available at: http://www.helsinki.fi/science/now/ [Google Scholar]
- 35.Faunmap Working group. FAUNMAP: A database documenting late Quaternary distributions of mammal species in the United States. 1994. Available at: ttp://gcmd.nasa.gov/records/GCMD_Faunmap.html.
- 36.Woodburne M. In: Late Cretaceous and Cenozoic Mammals of North America: Biostratigraphy and Geochronology. Woodburne MO, editor. New York: Columbia University Press; 2004. p. 391. [Google Scholar]
- 37.Imbrie J. Factor and vector analysis programs for analyzing geological data. US Office Naval Res Geogr Branch Tech Rep. 1963;6:83. [Google Scholar]
- 38.Hammer ØHD, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- 39.Klovan JE, Imbrie J. An algorithm and FORTRAN-IV program for large-scale Q-mode factor analysis and calculation of factor scores. Math Geol. 1971;3:61–77. [Google Scholar]
- 40.Jöreskog KG, Klovan JE, Reyment RA. Geological Factor Analysis. Amsterdam: Elsevier; 1976. p. 178. [Google Scholar]
- 41.McKinney ML, Oyen CW. Causation and nonrandomness in biological and geological time series temperature as a proximal control of extinction and diversity. Palaios. 1989;4:3–15. [Google Scholar]
- 42.Wonnacott TH, Wonnacott R. Introductory Statistics for Business and Economics. New York: Wiley; 1984. p. 745. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.