Abstract
Intracellular protein complexes containing nucleic acids are common targets of autoantibodies in many autoimmune diseases. Central tolerance to these antigens is incomplete, yet nucleosomal DNA is expressed on the surface of cells dying by apoptosis. It is commonly believed that autoimmunity is prevented by the rapid uptake of apoptotic cells (ACs) by neighbors or professional phagocytes to which they deliver anti-inflammatory signals. Self-reactive, innate-like B cells contact and are selected by intracellular antigens expressed on ACs; however, how self-tolerance is maintained is not well understood. Here we report that IL-10 production by B cells, stimulated by contact with ACs, results from the engagement of Toll-like receptor 9 (TLR9) within the B cell after recognition of DNA-containing complexes on the surface of ACs. Until now, TLR9 ligation has been considered an inflammatory signal, but we have confirmed a hitherto unexpected immunoregulatory role by demonstrating the absence of the protective effect of ACs during experimental autoimmune encephalitis (EAE) in TLR9-deficient mice. Human circulating CD27+ B cells also respond to DNA-bearing ACs, but not to DNase-treated cells, by secreting IL-10. Chronic autoimmune disease may arise if this tolerance mechanism is not reimposed after episodes of inflammation, or if the regulatory B-cell response is subverted.
Apoptotic cells (ACs) are immunomodulatory, dampening the inflammation mediated by innate immune cells (1–5). ACs also protect mice from autoimmune-mediated inflammation (6, 7) and induce B cells to adopt an IL-10–secreting regulatory B-cell phenotype (Breg) (8). Innate-like B cells have many self-reactive B-cell receptors (BCRs) and are selected by intracellular antigens expressed on ACs (9), but this is generally compatible with health (10–13). However, ACs express many of the antigens associated with autoimmune disease on their cell surface (14–17) and thus are thought to be a target of autoimmune responses. The BCR can deliver chromatin complexes from the AC to the endosome, allowing Toll-like receptor 9 (TLR9)-mediated signaling (18, 19). Despite this, lupus-related renal disease is exacerbated in TLR9-deficient mice (20–23), suggesting a regulatory role for TLR9-induced activation of self-reactive B cells in health that breaks down when tolerance is lost, leading to autoimmunity (24, 25).
How B cells maintain tolerance to AC-expressed antigens is not known and is the focus of the present work. We show that marginal zone B (MZB) cells and B1a B cells recognize DNA-containing chromatin complexes and secrete IL-10 in response to signaling through TLR9. In vivo, mice given DNase-treated ACs are no longer protected from arthritis, and AC-mediated protection from experimental autoimmune encephalitis (EAE) is lost in TLR9-deficient mice. In agreement with previous studies (26, 27), we found that human circulating CD27+ B cells secrete IL-10 in response to DNA complexes expressed by ACs. Thus, in health, TLR9-mediated recognition of ACs by B cells allows maintenance of tolerance to self.
Results
IL-10 Production by B Cells Requires Direct Contact with Whole ACs but Is Absent in B Cells Specific for Hen Egg Lysozyme.
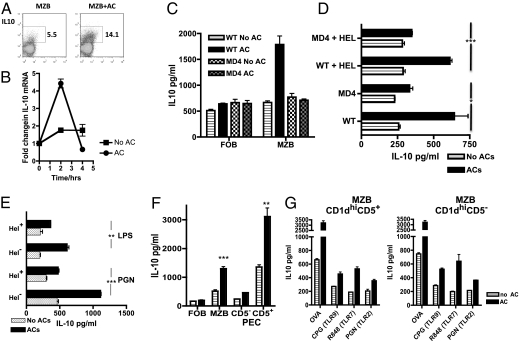
B cells secrete IL-10 in response to ACs, but only when they are able to make direct contact with whole ACs rather than with cellular debris (Fig. S1 A and B). Little IL-10 protein is detectable when resting B cells are cocultured with ACs, although the majority of B cells die rapidly in culture in the absence of concomitant stimulation (28) (Fig. S1D). To address this in another way, we injected ACs into IL-10 reporter mice and 1 wk later analyzed IL-10 secretion from splenic MZB cells by FACS analysis. We found IL-10 expression in 5.15% of the MZB cells from mice that had received vehicle alone (Fig. 1A), and in 14.1% of the MZB cells in mice that had received ACs, indicating that B cells can respond to ACs in vivo in the absence of additional stimulation. In addition, short-term cocultures of MZB cells and ACs alone induced a fourfold increase in mRNA for IL-10 (Fig. 1B), again showing that B cells can respond to ACs in the absence of additional stimuli. The IL-10 protein level increased further when the B cells were activated by T cells or TLR ligands.
Fig. 1.
Regulatory B cells from the MZB cell and B1a cell subsets recognize whole ACs via the BCRs and secrete IL-10. (A) IL-10–GFP reporter mice were injected with 20 × 106 ACs, and MZB cells were harvested 1 wk later. IL-10 expression was determined by FACS analysis. (B) MZB cells were stimulated with ACs for up to 4 h in the absence of further stimulation and mRNA for IL-10 measured by quantitative PCR. (C) B cells from WT or transgenic B cells from mice specific for HEL (MD4) were separated into FOB and MZB cells. After stimulation for 72 h in the presence of OVA-specific transgenic T cells and OVA peptide with or without ACs, supernatants were collected and IL-10 was measured by ELISA. (D) MZB cells from MD4 and WT mice stimulated as in C, but with the cognate protein HEL added to some of the cultures. (E) Polyclonal MZB cells (HEL−) and HEL-specific MZB cells (HEL+) derived from the same SWHEL mice were stimulated with the TLR4 ligand LPS or the TLR2 ligand PGN for 72 h with and without ACs, and IL-10 was measured. *Indicates a significant difference between IL-10 in stimulated B cells cocultured with ACs and HEL− and HEL+ MZB cells. (F) Peritoneal-derived (PEC) CD5− and CD5+ CD19 B cells and splenic MZB and FOB cells were stimulated as in C. (G) MZB cells were sorted into CD1dhiCD5− or CD1dhiCD5+ve subsets and stimulated for 72 h as in C (OVA) or with various TLRs, including CpG (TLR9), R848 (TLR7), and PGN (TLR2), and IL-10 was measured after 72 h. Each figure is representative of at least three experiments performed with three mice per group. Error bars represent SEM. ***P ≤ 0.0004; **P ≤ 0.004; *P ≤ 0.04. The threshold for detection of IL-10 was 25 pg/mL
MZB and B1 B cells cocultured with ovalbumin (OVA)-specific T cells, OVA peptide, and ACs secreted significantly more IL-10 compared with those in cultures containing follicular B (FOB) cells (Fig. 1 C and D and Fig. S1 C and D), which lack self-reactive BCRs (29). However, IL-10 secretion was not enhanced in FOBs and MZB cell populations drawn from BCR transgenic (MD4) animals carrying a single hen egg lysozyme (HEL) specificity (30) not expressed by the ACs (even when HEL was included in in vitro cultures) (Fig. 1 C and D). To further clarify the role of the BCRs, we used SWHEL Ig knock-in mice that have a large proportion of B cells specific for HEL, but these can class switch, and these mice also contain populations of polyclonal B cells (31). MD4 and SWHEL mice have an increased number of MZB cells, but their B1 B cells maintain a FOB phenotype (Fig. S1 E–G). When highly purified MZB cells from SWHEL mice were separated into polyclonal HEL− and HEL+ MZB cells, only polyclonal MZB cells produced significantly higher amounts of IL-10 in response to ACs and stimulation with the TLR ligands LPS and peptoglycan (PGN) (Fig. 1E). This suggests that MZB cells with a polyclonal BCR repertoire are required for the induction of IL-10 after the recognition of ACs. In WT mice, B1a B cells (i.e., CD5+) secreted more IL-10 in response to ACs compared with B1b B cells (CD5−) (Fig. 1F). However, in cocultures with ACs, activated splenic CD1dhiCD5+ and CD1dhiCD5− MZB cells responded similarly (Fig. 1G and Fig. S1I), suggesting that IL-10 secretion in response to AC is not restricted to a small subset of splenic B cells with defined cell surface markers.
DNase Treatment Abolishes the Capacity of ACs to Enhance IL-10 Secretion and Inhibits Regulatory Responses in Vivo.
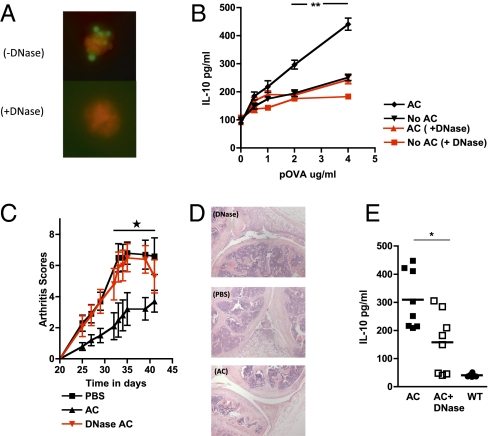
After apoptosis, chromatin complexes containing DNA are rapidly translocated to the AC surface (14). Given that recognition of CpG DNA motifs is a potent inducer of IL-10 in B cells (32), we reasoned that DNA-bearing molecular patterns on ACs are a potential candidate for recognition by Bregs. DNase treatment of ACs removed DNA from the surface of ACs (Fig. 2A and Fig. S2A) and abolished the AC-mediated enhancement of IL-10 production when these cells were used in cocultures with OVA-specific DO11.10 T cells, OVA peptide, and B cells (Fig. 2B). This effect was specific for DNA but not for RNA-containing complexes (Fig. S2B). DNase-treated ACs also offered no protection in the mouse model of collagen-induced arthritis (CIA) in contrast to mice given untreated ACs (Fig. 2C), which was confirmed histologically (Fig. 2D). The level of protection were correlated with the amount of IL-10 secreted by spleen cells after in vitro restimulation with collagen (Fig. 2E). This provides clear evidence that DNA-bearing molecular patterns expressed on the surface of intact ACs are responsible for signaling the production of IL-10 by B cells.
Fig. 2.
DNase treatment abolishes the capacity of AC to enhance IL-10 secretion and inhibits regulatory responses in vivo. (A) ACs expressed DNA-containing complexes, stained with Sytox (molecular probes, Invitrogen) on the cell surface, which was lost after treatment with DNase. (B) B cells were stimulated by OVA-specific T cells, OVA peptide, and ACs along with 50 μg/mL of DNase, and IL-10 was measured in supernatants after 72 h. (C) A single injection of vehicle (PBS) or ACs pretreated with DNase (DNase-AC) or untreated ACs (AC) was injected into mice at the time of immunization with type II collagen (CII) in complete Freund's adjuvant (CFA), and arthritis was assessed clinically. (D) Histology of H&E-stained sagittal sections through the knee joints of mice taken from Fig. 3C. Both the DNase-treated mice and the PBS-treated mice exhibited active synovitis associated with a fibrinopurulent exudate within the joint space, with less inflammation in the AC-treated mice. (E) Splenocytes from AC-treated mice (filled squares), DNase-treated mice (open squares), and WT mice (filled circles) obtained at the end of the experiment shown in Fig. 3C were restimulated in vitro with CII for 3 d, and IL-10 levels were measured. The CIA experiment is representative of two experiments with eight mice per group. The remaining figures are representative of at least three experiments performed with three mice per group. Error bars represent SEM. ***P ≤ 0.0004; **P ≤ 0.004; *P ≤ 0.04.
B-Cell TLR9 Signaling Is Required for Immunosuppressive Responses to ACs.
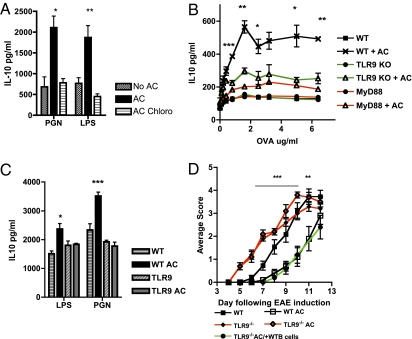
After endocytosis, DNA is sensed by several innate receptors (33). One of these, the MyD88-dependent receptor TLR9, recognizes mammalian DNA in endosomes and requires acidification of that compartment for signaling to occur (34). Chloroquine, which inhibits endosomal acidification, prevents signaling through TLR7 and TLR9 but not through TLR1, TLR2, or TLR4 (Fig. S3A). When B cells were stimulated with TLR2 (PGN) and TLR4 (LPS), chloroquine inhibited the AC-induced enhancement of IL-10 secretion (Fig. 3A). B cells deficient in TLR9 or its MyD88 signaling adapter (but not in TLR2 or TLR4) also were unable to induce a significant rise in IL-10 secretion in cocultures with T cells (TLR9/MyD88-sufficient DO11.10 plus pOVA) when exposed to ACs (Fig. 3B and Fig. S3B). To confirm that this result is a direct effect of TLR9 signaling on B cells, TLR9-deficient B1 cells were stimulated with TLR4 or TLR2 with and without ACs; the cells failed to respond to ACs by secreting IL-10 (Fig. 3C).
Fig. 3.
B-cell TLR9 signaling is required for the immunosuppressive response to ACs. (A) B cells were stimulated with the TLR ligands PGN (TLR2) and LPS (TLR4) alone or in the presence of ACs or ACs and chloroquine (AC Chloro) for 72 h, after which IL-10 in the supernatants was measured. (B) WT, MyD88-deficient (MyD88), and TLR9-deficient (TLR9) B cells were cocultured with ACs, OVA-specific T cells, and OVA peptide in vitro. After 72 h, IL-10 secretion was measured by ELISA. (C) PEC B1 cells were stimulated with the same TLR ligands as in Fig. 1A with and without AC for 72 h, and IL-10 was measured. (D) WT and TLR9-deficient mice were immunized with MOG/CFA, and EAE was scored. A single i.v. injection of ACs also was administered on day 0. Some TLR9-deficient mice also received an injection of 10 × 106 CD19 B cells on day 0. The EAE experiment is representative of three experiments with five mice per group. The remaining figures are representative of at least three experiments performed with three mice per group. Error bars represent SEM. ***P ≤ 0.0004; **P ≤ 0.004; *P ≤ 0.04. The threshold for detection of IL-10 was 25 pg/mL.
Clearly, to substantiate an immunosuppressive, regulatory role for B-cell TLR9, we needed to examine in vivo the effects of TLR9 deficiency on AC suppression of autoimmunity. We used myelin oligodendrocyte glycoprotein (MOG) peptide to induce EAE in TLR9−/− and TLR9+/+ mice, with ACs given at the time of disease induction. Here ACs were able to confer protection only in the WT mice, confirming a crucial role for TLR9 in mediating protection (Fig. 3D). Restimulation of spleen cells from the mice with EAE on day 12 demonstrated that TLR9-deficient mice made significantly less IL-10, but more proinflammatory IL-6 and IL-17 (Fig. S3C). Importantly, when TLR9-deficient mice were injected with WT B cells (but not TLR9−/− B cells) along with the ACs at the time of EAE induction, the protective phenotype of ACs was restored (Fig. 3D and Fig. S3D).
Human B Cells Generate an IL-10 Response After Interaction with ACs That Is Prevented by DNase Treatment.
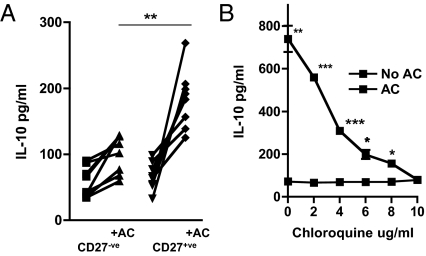
Finally, we asked whether human B cells also secreted IL-10 in response to DNA-bearing molecular patterns on ACs. We cocultured peripheral blood B cells (derived from healthy volunteers) with ACs and IL-4 to prevent B-cell apoptosis (28). CD27+ B cells secreted significantly more IL-10 (Fig. 4A). This increase did not occur when chloroquine (Fig. 4C) or DNase (Fig. S4A) was included in the cocultures, suggesting that both human and mouse Bregs are dependent on DNA-containing complexes expressed on ACs to secrete IL-10.
Fig. 4.
Human CD27+ B cells respond to ACs by secreting IL-10. (A) Human B cells isolated from healthy volunteers were separated into CD27+ and CD27− fractions and cultured in the presence of IL-4 for 3 d with AC (+AC) or without AC, after which IL-10 in the supernatants was measured. (B) B cells were stimulated with ACs and IL-4 in the presence of increasing concentrations of chloroquine for 3 d, and IL-10 secretion was measured by ELISA. A shows data collected from 14 healthy volunteer blood donors; B is representative of at least two separate experiments. Error bars represent SEM. ***P ≤ 0.0004; **P ≤ 0.004; *P ≤ 0.04. The threshold for detection of IL-10 was 25 pg/mL
Discussion
Healthy mice and humans exhibit significant autoreactivity to self-antigens expressed on ACs, especially within the MZB and B1 B-cell repertoires (12, 13, 35, 36). The mechanism by which tolerance is maintained in these peripheral B-cell populations has remained obscure, although these cells are able to contact and be selected by intracellular antigens expressed by ACs (37). Likewise, the mechanism by which regulatory function can be imparted to B cells by interaction with ACs is not known. In this paper, by way of explanation, we find that DNA-containing complexes on the surface of whole ACs are sensed by Bregs and delivered to TLR9-containing endosomes, where they induce IL-10 secretion. This provides a means for tolerizing self-reactive B and T cells, as well as modulating the severity of ongoing immune responses. For full-memory T-cell responses to occur, T cells must interact with both dendritic cells (DCs) and antigen-specific B cells (38, 39). Indeed, the role of B cells in shaping the T effector response through antigen presentation, costimulation, and cytokine production is being increasingly recognized (40). However, DCs that ingest infected ACs are able to up-regulate costimulatory molecules and to effectively present antigens derived from the ACs (41). Therefore, after an infectious insult, DCs are at risk of activating self-reactive T cells. Of note, our data clearly show that Bregs secrete IL-10 in response to ACs despite the presence of activating stimuli, and thus they are able to mediate a dominant tolerogenic signal despite an inflammatory milieu, which should ensure that self-reactive T cells that have been primed by DCs are induced to become regulatory IL-10–secreting cells when they contact Bregs.
Our finding that HEL-specific MZB cells from MD4 and SWHEL mice are refractory to the regulatory effects of ACs leads us to speculate that self-reactive BCRs are responsible for the recognition and uptake of these chromatin complexes. Both of these subsets are replete with self-reactive BCRs (42, 43) and AC DNA, which is more hypomethylated than DNA from viable cells (44), can activate TLR9 (45), even though TLR9's endosomal location was previously thought to prevent its “accidental” stimulation by self-DNA (46). Despite this, the regulatory responses of B cells to ACs were abolished after treatment with chloroquine, which is known to prevent TLR7 or TLR9 signaling in the endosomal compartment, but not signaling through other TLRs (Fig. S3A). Although CpG is able to gain access to the B cells independent of the BCRs and can induce B cells to secrete cytokines and proliferate (32), recent studies have demonstrated that B cells cannot take up longer lengths of DNA found physiologically, unless they are initially internalized through the BCRs (47). This will prevent large-scale activation of TLR9+ memory B cells in vivo and restrict this activation to self-reactive B cells. In fact, ligation of BCRs have been shown to control the subcellular distribution of TLR9 in B cells, allowing relocation of TLR9 from the endoplasmic reticulum to the endosomal compartment, where interaction with antigen internalized through the BCRs can occur (48, 49). This again adds substance to the hypothesis that regulatory responses to AC-expressed chromatin complexes occurs via the BCRs.
Human CD27+ B cells respond to ACs by secreting IL-10, but only in the presence of DNA-containing chromatin complexes on the ACs, which also may help maintain peripheral tolerance in humans. The conclusion that TLR9 is involved in preventing the development of potentially damaging autoreactive responses fits with the observations from mouse disease models. Treatment of mice with CpG to stimulate TLR9 has alleviated disease severity in colitis, arthritis, and diabetes (50–53). In addition, lupus-related renal disease is exacerbated in TLR9-deficient autoimmune prone mice (20–23), and recent reports suggest that TLR9 is required to prevent pathological responses that result from TLR7-mediated signaling (24, 25). Other studies, however, show that TLR9 stimulation has an adjuvant effect driving Th1 responses allied with IgG2a antibodies, thereby potentially exacerbating autoimmune disease (19, 54–57). In addition, hydroxychloroquine is used to good effect in patients with systemic lupus erythematosus and rheumatoid arthritis, which suggests that in the rheumatic diseases, autoreactive B cells, which do not have regulatory activity, dominate the immune response.
Thus, it seems likely that TLR9 can mediate both proinflammatory and immunoregulatory signals, depending on the context in which the DNA is sensed. What factors might lead to changes in the balance between regulatory and inflammatory responses? The experiments that ascribe an inflammatory response of AC-derived DNA to TLR9 (19, 54, 55), in which DNA-antibody (IgG2a) complexes are taken up by rheumatoid factor-expressing B cells, may represent one of the situations that leads to autoimmune disease, for instance, when one or more of these low-affinity B cells receives signals that allow affinity maturation and drive antibody production. Alternatively, the difference might be quantitative (e.g., amount of TLR9 ligand delivered as a result of antibody affinity) or qualitative (e.g., availability of T-cell help or the form of the TLR9 ligand). Interestingly, the delivery of IgG2a–chromatin complexes to B cells causes proliferation, but not cytokine production (49), in contrast to the B cell response to ACs, which results in both effects (Fig. S4B). Another factor influencing the inflammatory/regulatory balance is the underlying genetic control of TLR9 expression and function. A study of Japanese patients reported lower expression of TLR9 in patients with systemic lupus erythematosus (58). In addition, components of the innate immune system, such as C1q (59–62) and natural IgM (6), may decorate DNA-associated molecular patterns on ACs, which can alter the way DNA complexes are sensed within the endosome.
In summary, our data suggest that naturally occurring MZB and B1a B cells bind and internalize chromatin complexes from the surface of ACs, which invokes both B cell proliferation and IL-10 secretion. We believe that this effect provides a regulatory mechanism to prevent the differentiation of low-affinity autoreactive B cells and also obviates any autoimmune consequence of antigen presentation by these cells. Thus, in health these self-reactive B cells are kept in check and perform a TLR9-dependent broad-based tolerance function, but circumstances can cause them to switch from a regulatory mode to a pathogenic mode. A failure along the pathway from AC recognition to TLR9 signaling may be the crucial switch between health and disease secondary to autoimmunity. Clearly when this layer of immune regulation breaks down, self-reactive B cells may start to secrete high-titer, high-affinity antibody to apoptotic self, amplifying an autoinflammatory loop and leading to the multitudinous symptoms that characterize the rheumatic diseases.
Materials and Methods
Cell Stimulation and Treatments.
Cells were treated with the following: DNase, 50 μg/mL (Roche); Rnase, 10 μg/mL (Sigma-Aldrich); chloroquine, 2 μg/mL (Sigma-Aldrich); TLR1/2 (PAM(3)CSK), 0.2 μg/mL (InVivoGen); TLR4 ligand LPS, 2 μg/mL (Sigma-Aldrich); TLR3 ligand (poly IC), 25 μg/mL (InVivoGen); TLR2 ligand peptidoglycan, 10 μg/mL (InVivoGen); TLR7 ligand R848, 0.1 μg/mL (InVivoGen); and TLR9 ligand CpG (ODN 1826) (InVivoGen), 1 μg/mL except in one experiment in which a dose of 25 μg/mL was used (Fig. S1D). Biotinylated HEL protein was used at 5.8 μg/mL.
Mice.
DO.11.10 TcR transgenic mice (H2Ad-restricted, OVA peptide 323–339-specific) (63), MD4 HEL-specific BCR transgenic mice (30), IL-10–GFP mice (64), TLR9−/− mice, (56) and MyD88−/− mice (65) were bred and maintained under specific pathogen-free conditions in the animal facilities at the University of Edinburgh. The IL-10–GFP mice were kindly provided by Dr Richard Flavell (Yale University, New Haven, CT), and the TLR2−/−, TLR4−/−, TLR9−/−, and MyD88−/− mice were all generously provided by Prof. S. Akira (Hyogo College of Medicine, Nishinomiya, Japan). The SWHEL mice were a kind gift from Dr. Robert Brink (Garvan Institute, Darlinghurst, NSW, Australia) (31). Male DBA1 mice were purchased from Harlan, and C57BL/6 and BALB/c mice were bred in house. Mice were used at 8–12 wk of age and were age- and sex-matched in experiments. All experiments were covered by a project license granted by the Home Office under the Animal (Scientific Procedures) Act of 1986. Locally, this license was approved by the University of Edinburgh's Ethical Review Committee.
Cell Isolation and Culture.
Human mononuclear cells were extracted from the peripheral blood of healthy volunteers or from blood donor buffy coats using dextran sedimentation and a Percoll gradient as described previously (66) with Lothian Research Ethics Committee approval (LREC/2001/4/56). CD19+ B cells were isolated in accordance with the manufacturer's instructions using a negative selection kit (Miltenyi Biotech). Viable CD19+ B cells were further sorted into CD27+, CD27−, IgM+, and IgM− subsets using a FACSAria cell sorter (BD Biosciences) to generate highly purified populations. Human B cells (3 × 105) were cocultured with ACs (1 × 106) in the presence of 10 ng/mL of IL-4 for 72 h in RPMI medium supplemented with FCS. CD4+ T cells and CD19+ B cells from single-cell suspensions of spleen or lymph nodes were separated using CD4 and CD19 microbeads, respectively (Miltenyi Biotech), in accordance with the manufacturer's instructions. In some experiments, viable CD19+ B cells were further separated into FOB and MZB B cells after staining with anti-CD21 and anti-CD23, and PEC cells were separated into CD5+/− cell populations by cell sorting with a FACSAria. For experiments with SWHEL mice, viable CD19+CD23−CD21+ B cells (MZB cells) were further stained with biotinylated HEL protein and sorted to high purity to yield HEL+- and HEL−-specific B cells.
Cytokine Quantification.
Single-cell spleen suspensions were cultured at 5 × 106 cells/mL with serial dilutions of CII, OVA peptide (OVA323–339; Albachem), or MOG peptide for 72 h in a volume of 200 μL in 96-well round-bottomed plates (Costar; Corning Life Sciences). For T- and B-cell coculture, T and B cells were sorted to >98% purity using anti-CD4 and anti-CD19 magnetic beads (Miltenyi), and then 2 × 105 B cells were pulsed with peptide and incubated with 1 × 105 DO11.10 CD4+ T cells. Cytokines were quantitated using standard ELISA (R&D Systems). Transwell plates were purchased from Corning Life Sciences. All experiments were performed in triplicate. Using the optimized kits (R&D Systems), the threshold for detection was 25 pg/mL for IL-10, 7.8 pg/mL for IL-6, and 3.9 pg/mL for IL-17.
Generation of ACs.
Apoptotic mouse thymocytes were generated by incubating cells for 4 h with 0.5 μM dexamethasone, followed by extensive washing. For in vivo experiments, ACs were injected i.v. For in vitro cultures, ACs were added to B cells at a 5:1 ratio and then left in the wells for the duration of the assay.
Induction of CIA.
CIA was induced as described previously (8). The first signs of arthritis appeared between day 21 and day 35, with a prevalence of 60–90% of immunized mice. Each arthritic limb was scored by an investigator blinded to the treatment regimen as 0, normal; 1, erythema or swelling in a single digit; 2, erythema and swelling in two or more joints; or 3, swelling of whole paw, including the hock joint. The scores of all four joints were added together, and the sum was taken as a measure of the degree of arthritis, with a maximum possible score of 12 (i.e., 4 × 3). For groups, the mean of this score was calculated.
Induction and Assessment of EAE.
EAE was induced by s.c. injection of 100 μg of MOG peptide (35–55: (MEVGWYRSPFSRVVHLYRNGK) emulsified in CFA containing 500 μg of heat-killed Mycobacterium tuberculosis H37RA (Sigma-Aldrich). Mice were given 200 ng of pertussis toxin (Speywood Pharmaceuticals) on days 0 and 2, and 20 × 106 ACs i.v. on day 0. Clinical signs of EAE were assessed daily on a scale of 0 to 6: 0, no signs; 1, flaccid tail; 2, impaired righting reflex and/or gait; 3, partial hindlimb paralysis; 4, total hindlimb paralysis; 5, hindlimb paralysis with partial forelimb paralysis; 6, moribund or dead.
Histology.
Hindlimbs were prepared as described previously (16). Sections were analyzed blind by a histopathologist (D.S.).
Statistics.
Data are expressed, when appropriate, as mean ± SEM. Significance was assessed using unpaired t tests, and P values <0.04 are considered significant.
Supplementary Material
Acknowledgments
This work was supported by grants from the Arthritis Research UK and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109173109/-/DCSupplemental.
References
- 1.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 3.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol. 2002;168:1627–1635. doi: 10.4049/jimmunol.168.4.1627. [DOI] [PubMed] [Google Scholar]
- 5.Voll RE, et al. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 6.Notley CA, Brown MA, Wright GP, Ehrenstein MR. Natural IgM is required for suppression of inflammatory arthritis by apoptotic cells. J Immunol. 2011;186:4967–4972. doi: 10.4049/jimmunol.1003021. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, et al. Regulation of dendritic cells and macrophages by an anti-apoptotic cell natural antibody that suppresses TLR responses and inhibits inflammatory arthritis. J Immunol. 2009;183:1346–1359. doi: 10.4049/jimmunol.0900948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc Natl Acad Sci USA. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferry H, Jones M, Vaux DJ, Roberts IS, Cornall RJ. The cellular location of self-antigen determines the positive and negative selection of autoreactive B cells. J Exp Med. 2003;198:1415–1425. doi: 10.1084/jem.20030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steele EJ, Cunningham AJ. High proportion of Ig-producing cells making autoantibody in normal mice. Nature. 1978;274:483–484. doi: 10.1038/274483a0. [DOI] [PubMed] [Google Scholar]
- 11.Souroujon M, White-Scharf ME, Andreschwartz J, Gefter ML, Schwartz RS. Preferential autoantibody reactivity of the preimmune B cell repertoire in normal mice. J Immunol. 1988;140:4173–4179. [PubMed] [Google Scholar]
- 12.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plotz PH. The autoantibody repertoire: Searching for order. Nat Rev Immunol. 2003;3:73–78. doi: 10.1038/nri976. [DOI] [PubMed] [Google Scholar]
- 16.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol. 2002;169:159–166. doi: 10.4049/jimmunol.169.1.159. [DOI] [PubMed] [Google Scholar]
- 17.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 18.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 20.Christensen SR, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lartigue A, et al. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 23.Yu P, et al. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- 24.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Nickerson KM, et al. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bouaziz JD, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 27.Iwata Y, et al. Characterization of a rare IL-10–competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Illera VA, Perandones CE, Stunz LL, Mower DA, Jr, Ashman RF. Apoptosis in splenic B lymphocytes: Regulation by protein kinase C and IL-4. J Immunol. 1993;151:2965–2973. [PubMed] [Google Scholar]
- 29.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 30.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 31.Phan TG, et al. B cell receptor-independent stimuli trigger immunoglobulin (Ig) class switch recombination and production of IgG autoantibodies by anergic self-reactive B cells. J Exp Med. 2003;197:845–860. doi: 10.1084/jem.20022144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: Distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilaysane A, Muruve DA. The innate immune response to DNA. Semin Immunol. 2009;21:208–214. doi: 10.1016/j.smim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Yi AK, et al. CpG motifs in bacterial DNA activate leukocytes through the pH-dependent generation of reactive oxygen species. J Immunol. 1998;160:4755–4761. [PubMed] [Google Scholar]
- 35.Ehrenstein MR, Notley CA. The importance of natural IgM: Scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 36.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 37.Ferry H, Crockford TL, Leung JC, Cornall RJ. Signals from a self-antigen induce positive selection in early B cell ontogeny but are tolerogenic in adults. J Immunol. 2006;176:7402–7411. doi: 10.4049/jimmunol.176.12.7402. [DOI] [PubMed] [Google Scholar]
- 38.Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185:2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 40.Yanaba K, et al. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 41.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 42.Baumgarth N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 43.Qian Y, Wang H, Clarke SH. Impaired clearance of apoptotic cells induces the activation of autoreactive anti-Sm marginal zone and B-1 B cells. J Immunol. 2004;172:625–635. doi: 10.4049/jimmunol.172.1.625. [DOI] [PubMed] [Google Scholar]
- 44.Wen ZK, et al. DNA hypomethylation is crucial for apoptotic DNA to induce systemic lupus erythematosus-like autoimmune disease in SLE-non-susceptible mice. Rheumatology (Oxford) 2007;46:1796–1803. doi: 10.1093/rheumatology/kem275. [DOI] [PubMed] [Google Scholar]
- 45.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 46.Barton GM, Kagan JC, Medzhitov R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat Immunol. 2006;7:49–56. doi: 10.1038/ni1280. [DOI] [PubMed] [Google Scholar]
- 47.Roberts TL, et al. B cells do not take up bacterial DNA: An essential role for antigen in exposure of DNA to toll-like receptor-9. Immunol Cell Biol. 2010;89:517–525. doi: 10.1038/icb.2010.112. [DOI] [PubMed] [Google Scholar]
- 48.Chaturvedi A, Dorward D, Pierce SK. The B cell receptor governs the subcellular location of Toll-like receptor 9 leading to hyperresponses to DNA-containing antigens. Immunity. 2008;28:799–809. doi: 10.1016/j.immuni.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busconi L, et al. Functional outcome of B cell activation by chromatin immune complex engagement of the B cell receptor and TLR9. J Immunol. 2007;179:7397–7405. doi: 10.4049/jimmunol.179.11.7397. [DOI] [PubMed] [Google Scholar]
- 50.Wu HJ, et al. Inflammatory arthritis can be reined in by CpG-induced DC-NK cell cross-talk. J Exp Med. 2007;204:1911–1922. doi: 10.1084/jem.20070285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quintana FJ, Rotem A, Carmi P, Cohen IR. Vaccination with empty plasmid DNA or CpG oligonucleotide inhibits diabetes in nonobese diabetic mice: Modulation of spontaneous 60-kDa heat shock protein autoimmunity. J Immunol. 2000;165:6148–6155. doi: 10.4049/jimmunol.165.11.6148. [DOI] [PubMed] [Google Scholar]
- 52.Rachmilewitz D, et al. Immunostimulatory DNA ameliorates experimental and spontaneous murine colitis. Gastroenterology. 2002;122:1428–1441. doi: 10.1053/gast.2002.32994. [DOI] [PubMed] [Google Scholar]
- 53.Gilkeson GS, et al. Modulation of renal disease in autoimmune NZB/NZW mice by immunization with bacterial DNA. J Exp Med. 1996;183:1389–1397. doi: 10.1084/jem.183.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yasuda K, et al. Requirement for DNA CpG content in TLR9-dependent dendritic cell activation induced by DNA-containing immune complexes. J Immunol. 2009;183:3109–3117. doi: 10.4049/jimmunol.0900399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hemmi H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 57.Leadbetter EA, Rifkin IR, Marshak-Rothstein A. Toll-like receptors and activation of autoreactive B cells. Curr Dir Autoimmun. 2003;6:105–122. doi: 10.1159/000066858. [DOI] [PubMed] [Google Scholar]
- 58.Tao K, et al. Genetic variations of Toll-like receptor 9 predispose to systemic lupus erythematosus in Japanese population. Ann Rheum Dis. 2007;66:905–909. doi: 10.1136/ard.2006.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: Complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–4528. [PubMed] [Google Scholar]
- 60.Nauta AJ, et al. Direct binding of C1q to apoptotic cells and cell blebs induces complement activation. Eur J Immunol. 2002;32:1726–1736. doi: 10.1002/1521-4141(200206)32:6<1726::AID-IMMU1726>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 61.Rosenberg AM, Prokopchuk PA, Lee JS. The binding of native DNA to the collagen-like segment of Clq. J Rheumatol. 1988;15:1091–1096. [PubMed] [Google Scholar]
- 62.Uwatoko S, Mannik M. The location of binding sites on C1q for DNA. J Immunol. 1990;144:3484–3488. [PubMed] [Google Scholar]
- 63.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 64.Kamanaka M, et al. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knock-in tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 65.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1– and IL-18–mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 66.Haslett C, Guthrie LA, Kopaniak MM, Johnston RB, Jr, Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am J Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.