NRG1 is a schizophrenia candidate gene which regulates brain development and neural function. The minor allele of rs7014762 in the NRG1 5′ core promoter was associated with schizophrenia (p=0.031) and significant predicted reduced NRG1 Type III isoform expression in postmortem human brain of schizophrenia cases (p=0.001). Our results provide additional evidence for transcriptional dysregulation as a biological mechanism implicating NRG1 in schizophrenia risk.
Association between NRG1 and schizophrenia was originally discovered via haplotype analysis in an Icelandic sample (HAPICE) at the 5′ end of the gene1, further replicated in a Scottish population2. In the present report, we examined association between schizophrenia and the NRG1 SNP rs7014762 because it is situated within a core promoter region3 and is physically proximal (87bp) to a functional SNP which has been shown to influence NRG1 type IV isoform expression4. In addition to clinical association analyses, we validated the functional effects of rs7014762 by testing for effects on mRNA expression in postmortem human brain.
Cases (N=296) and controls (N=365) were ascertained as part of the Clinical Brain Disorders Branch Sibling Study. Probands met DSM-IV criteria for a broad diagnosis category including schizophrenia, schizoaffective disorder, psychosis NOS, delusional disorder, schizotypal, schizoid, or paranoid personality disorder. Control individuals were screened to exclude individuals with psychiatric diagnoses. All participants gave informed consent and self-identified as Caucasian. Blood was collected and DNA was extracted using standard methods. Genotypes were obtained using the Taqman 5′-exonuclease allelic discrimination assay.
Postmortem brain tissue was collected with informed consent from the legal next-of-kin. The sample was previously described in detail along with the NRG1 primer and probe sets3,4. Briefly, hippocampi from 84 normal controls (22 females/62 males, 53 African American/25 American Caucasian/5 Hispanic and 1 Asian individual, mean age 40.5 ±(SD) 15.4 years, post mortem interval (PMI) 30.7 ± 13.9 hrs, pH 6.59 ± 0.32); and 44 schizophrenic patients (15 females/29 males, 24 African Americans/20 Caucasians, mean age 49.7 ± 17.2 years, PMI, 36.3 ± 17.7 hrs, pH 6.48 ± 0.28) were available for study. Diagnoses were determined by independent reviews of clinical records and family interviews by two psychiatrists using DSM-IV criteria. Macro- and microscopic neuropathological examinations and toxicology screening were performed prior to inclusion. No differences were observed on variables that potentially affect gene expression in human postmortem brain (i.e. age, PMI, pH and RIN) by rs7014762 genotype group. NRG1 (types I-IV) mRNA splice isoform expression was measured by real-time quantitative RT-PCR using an ABI Prism 7900 sequence detection system with 384-well format (Applied Biosystems, Foster City, CA, USA).
Case-control analyses used unconditional logistic regression. Effects of rs7014762 on NRG1 isoform mRNA expression were examined using ANOVA with genotype and diagnosis as independent factors, controlling for race. Where there was a significant genotype bydiagnosis interaction, individual group post hoc tests were examined. P-values were not adjusted for multiple testing.
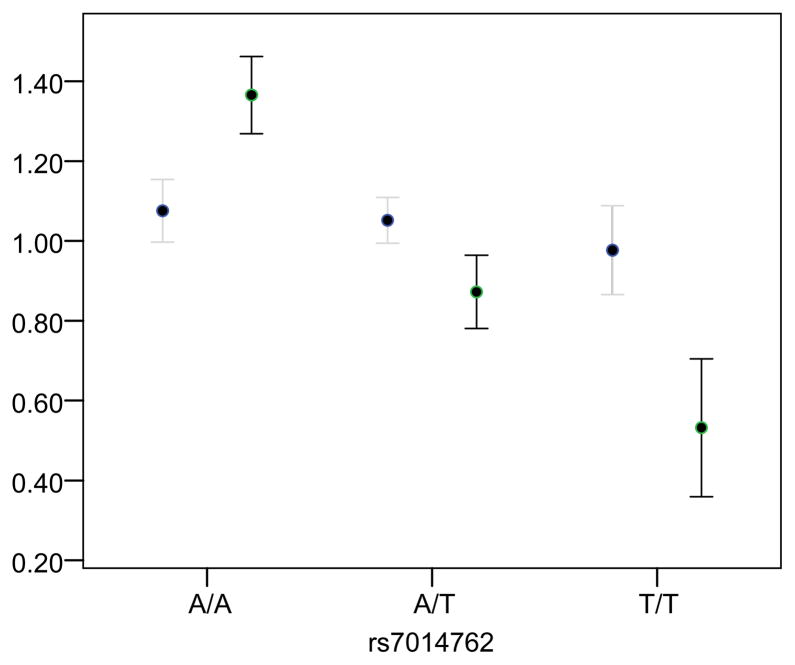
rs7014762 was in Hardy Weinberg equilibrium in cases and controls (p > 0.05). The minor allele of rs7014762 showed significant association with schizophrenia case status (minor allele (T) carrier OR = 1.49 (1.04, 2.15), p-value = 0.031). mRNA expression analysis revealed a significant diagnosis by genotype interaction on type III isoform expression in the hippocampus (F (5, 106) = 5.98; p-value = 0.003). Post hoc analysis showed the effect of rs7014762 was significant only in patients, whereby individuals heterozygous (LSD; p-value = 0.001) or homozygous (LSD p-value = 0.002) for the minor allele exhibited significantly lower levels of NRG1 type III expression compared to major allele homozygotes (Figure 1). No effects of race or race by genotype interactions were observed. No effects of genotype were observed for any other NRG1 isoform.
Figure 1. Normalized NRG1 Type III Expression by rs7014762 Genotype in Schizophrenia Cases and Healthy Controls.
Association between rs7014762 and NRG1 type III isoform mRNA expression in the hippocampus in schizophrenia cases and healthy controls. A diagnosis by genotype interaction was observed (p-value=0.003). Post hoc comparisons reveled that patients carrying the minor allele (heterozygous or homozygous) exhibited significantly lower levels of expression compared to major allele homozygotes. Bars represent mean ± SEM; case expression = black bars; control expression = gray bars.
We report association between schizophrenia and rs7014762 in a case-control sample and show that the same allele of rs7014762 in the NRG1 5′ promoter region significantly predicts lower type III isoform expression levels in patients. NRG1 is expressed throughout the human brain, including the hippocampus and prefrontal cortex5, two areas implicated in schizophrenia, and individuals with schizophrenia show abnormalities in ErbB4–NRG1 signaling in the brain versus healthy controls6. In animal models, NRG1 type III isoform has been associated with axonal myelination7, lateral ventricle enlargement, and reduced function in the prefrontal cortex and hippocampus8. Disturbances in myelination/oligodendroglial density in individuals with schizophrenia have been observed9 and suggest reduced structural connectivity may be part of the neurobiology of schizophrenia. The same allele at this SNP has been shown to be associated with increased risk for bipolar disorder10. In addition, rs7014752 is 87 bp from rs6994992 (HAPICE SNP8NRG1243177) and is in moderate LD with this HAPICE SNP (D′ = 0.96, r2 = 0.21). SNP rs6994992 has previously been reported to be a functional promoter variant associated with schizophrenia and the regulated expression of a novel brain-specific isoform of NRG1, type IV, in humans3,4 and was associated with alterations in activation in frontal/temporal lobes, higher risk of psychotic symptomology and reduction in premorbid IQ in schizophrenia patients11. Together these observations suggest that variation in the HAPICE region may impact risk for schizophrenia via transcriptional regulation of multiple NRG1 isoforms.
References
- 1.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefansson H, Sarginson J, Kong A, Yates P, Steinthorsdottir V, Gudfinnsson E, et al. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, et al. Proc Natl Acad Sci USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ, et al. J Biol Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- 5.Law AJ, Shannon Weickert C, Hyde TM, Kleinman JE, Harrison PJ. Neuroscience. 2004;127:125–136. doi: 10.1016/j.neuroscience.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Hahn C-G, Wang H-Y, Cho D-S, Talbot K, Gur RE, Berrittini WH, et al. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 7.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Proc Natl Acad Sci USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, et al. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, et al. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 10.Georgieva L, Dimitrova A, Ivanov D, Nikolov I, Williams NM, Grozeva D, et al. Biol Psychiatry. 2008;64:419–427. doi: 10.1016/j.biopsych.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, et al. Nat Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]