Abstract
Cardiovascular manifestations of lysosomal storage disease (LSD) are a significant health problem for affected patients. Infantileonset cardiac disease, because of its rapid progression, is usually treated symptomatically. Therapy in older patients includes valve replacement and bone marrow (BM) transplantation, both of which are life threatening in the already debilitated patients. Enzyme replacement therapy has potential benefit but has not yet been demonstrated to provide long-term relief for cardiac disease. Here, we demonstrate prevention of severe cardiac manifestations in β-glucuronidase (GUSB) null mice BM-transplanted i.v. as neonates without myeloablative pretreatment. The mice, a model of mucopolysaccharidosis type VII (MPSVII, Sly syndrome), develop progressive LSD unless provided with GUSB early in life. The BM recipients retained GUSB+ donor cells in the peripheral blood and heart until necropsy at >11 months of age. The enzyme β-hexosamindase increased in tissues of GUSB null MPSVII mice was reduced significantly (P = 0.001) in treated MPSVII hearts. Electrocardiography demonstrated normalization of heart rate, PR, PQ, and QRS intervals in BM recipients. Storage was markedly reduced in the stroma of heart valves, adventitial cells of the aortic root, perivascular and interstitial cells of the myocardium, and interstitial cells of the conduction tissue. Heart/body weight ratio normalized. The aortic root was still grossly distended, and the conductive myocytes retained storage, suggesting neither plays a major role in ECG normalization. We conclude that transplantation of MPSVII neonates without toxic intervention can prevent many of the cardiovascular manifestations of LSD.
Cardiac failure is a major cause of death in patients with lysosomal storage diseases (LSDs). Lesions can be extensive and include cardiomegaly, coronary stenosis, marked thickening of the atrioventricular and aortic valves, and endocardial fibroelastosis. Heart disease may occur in infants with LSD, such as mucopolysaccharidosis (MPS) type I (MPSI, Hurler's syndrome), glycogenosis type II (Pompe's syndrome), MPS type VI (Maroteaux-Lamy syndrome), and MPS type VII (MPSVII) (1–5). The ECG of patients with LSD is often abnormal because of cardiac lesions and chamber hypertrophy. The early onset and rapid progression of cardiovascular disease require management of symptoms (6–10). Therapeutic interventions for children and adults with LSD and later-onset heart problems include bone marrow (BM) transplantation postmyeloablation (11–13) and surgical replacement of damaged arteries and valves (14–16), although this treatment is rarely used (17). Both create attendant risks to the patient and neither are currently feasible treatments for infants, many of whom succumb to heart and respiratory problems within the first year of life. Allogeneic BM transplantation in children with MPS resulted in cardiac improvement at 1 year posttransplantation by providing cross-correcting enzyme (18). Attempts to treat human storage diseases in utero with allogeneic transplantation have, thus far, been unsuccessful (19). Treatment objectives are to provide the mannosylated enzyme that is recognized by a mannose 6-phosphate receptor on the recipient cells, internalized, and targeted to the lysosomes (20). The heart has higher receptor levels, making it a good target for correction.
Newer technologies that replace the deficient enzyme offer promise for treatment of infantile-onset heart disease. One of these is enzyme replacement therapy (ERT). Studies in animal models have been seminal to the translation of ERT to patients. Canine MPSI was treated at ≥5.5 months of age with the recombinant human form of the missing enzyme α-l-iduronidase (21, 22). Whereas storage decreased in some visceral tissues, there was no improvement in the heart valves up to 13 months of age after long-term low doses or short-term higher doses. ERT was subsequently approved for clinical trials and has proven effective in delaying disease progression. The associated heart disease has shown some improvement in humans (23). In feline MPS type VI neonates, weekly injections of recombinant human N-acetylgalactosamine-4-sulfatase to replace the deficient enzyme (24) resulted in less lysosomal storage in heart valves and the tunica media of the aorta. Clinical trials of ERT in MPS type VI patients are in progress (23). ERT in quails with acid-α-glucosidase deficiency (glycogenosis type II) improved muscle function (25). In four infants with Pompe's disease, ERT resulted in improved cardiac structure and function and prolonged survival (26–28), but long-term results are not yet available. Major problems persist with ERT. The high cost of ERT, generation of antibodies against the enzyme by the recipient, impracticality of lifelong ERT, limitation of ERT to the more common storage diseases, and failure to resolve storage in the brain are all concerns.
Gene therapy also offers promise for the treatment of LSD although it is currently limited to experimental animals. Neonatal MPSVII mice injected i.v. with a recombinant adeno-associated virus encoding β-glucuronidase (GUSB) retained serum enzyme levels of ≥10% for 8 weeks before dropping to 1% at 16 weeks (29). Hepatic cells of neonatal MPSVII mice transduced in vivo with Moloney murine leukemia virus produced up to 127-fold normal levels of GUSB for 6 months (30). After injection of the same retroviral construct into MPSVII neonatal dogs, normal serum levels of GUSB were maintained for 14 months (31) and cross-corrected many tissue storage sites.
Another newer technology is the use of “nonmyeloablative” marrow transplantation to create patient/donor chimeras and supply the missing enzyme. Even these measures are not without dangers to infants because pretransplantation treatment usually includes toxic preparative regimens. We have developed a completely nonablative technology for neonatal transplantation (32) and have used it successfully (33) in the MPSVII mouse model of Sly syndrome (34). The advantages include: early treatment; dissemination of donor GUSB+ macrophage progeny to bone, BM, visceral organs, and brain; provision of sufficient enzyme to alleviate lysosomal storage by cross-correcting host cells; 3-fold increase in the average lifespan; alleviation of functional defects; and lack of immune response to the GUSB that is provided by donor cells (35). As noted above, ERT in the null host can cause anaphylactic shock in most animal models and must be managed in human recipients.
The role of cardiac morphological changes on impairing heart function in the MPSVII mice has not been tested previously to our knowledge. Accordingly, we investigated electrocardiographic and phenotypic changes in conscious neonatally treated, untreated, and normal adult mice and relate these findings to changes in cardiac histology and lysosomal enzyme concentration posttransplantation.
Materials and Methods
Animals. C57BL/6J (B6)-gusmps/gusmps (MPSVII) neonates were injected i.v. (superficial temporal vein) with 1 × 1010 normal (B6-+/+) congenic donor marrow cells per kg on the first day after birth as described (36). The B6 donor cells were GUSB+ and homozygous for glucose phosphate isomerase 1A. MPSVII hosts were GUSB null and homozygous for glucose phosphate isomerase 1B. Controls were untreated B6-MPSVII and B6-+/+ mice.
Analytical Procedures Prenecropsy. A single 75-μl heparinized hematocrit tube of peripheral blood (PBl) was collected from the orbital sinus of treated MPSVII mice at various intervals before ECG and separated into RBCs and WBCs (buffy coat) after centrifugation (37). Donor (glucose phosphate isomerase 1A) cell repopulation was determined by quantitive densitometry of cellulose acetate strips after electrophoresis of lysed PBl samples as described (38). One week after the final bleed at 333 days, ECGs were performed. Because untreated male MPSVII mice do not breed, and a second pregnancy is usually lethal in untreated females, the treated mice were bred as a functional test of disease correction.
ECG Recording and Analyses. ECGs were recorded in conscious mice as described (39, 40). Briefly, mice were gently removed from their cages and positioned on a raised ECG recording platform (AnonyMOUSE, Mouse Specifics). An array of gel-coated ECG electrodes was embedded in the floor of the platform and spaced to provide contact between the electrodes and animals' paws. The electrodes were connected to an amplifier by three shielded conductive leads. The signals were digitized with 16-bit precision at a sampling rate of 2,000 samples per s. Only data from continuous recordings of 20–30 ECG signals were used in the final analyses. Data were transmitted to mousespecifics.com (Mouse Specifics) by using standard file-transfer protocols for ECG signal.
Each signal was analyzed by using e-mouse, an internet-based physiologic waveform analysis platform. The software uses a peak detection algorithm to find the peak of the R waves and calculate heart rate. Heart rate variability was calculated as the mean of the differences between sequential heart rates for the complete set of ECG signals. Subsequently, determination of first and second derivatives and algebraic “if-thens” search the ECG signals for probable P-wave peaks and for onset and termination of QRS complexes. The inverted and/or biphasic portions of the T wave (41) were included in calculations of the QT interval. The T wave was defined as the point where the signal returned to the isoelectric line (42, 43). The QT intervals were rate-corrected (QTc) by application of the equation for use in mice (39). The P, Q, R, S, and T intervals were interpreted by the software for each beat to eliminate spurious data from unfiltered noise or motion artifacts. The mean of the ECG time intervals for each set of waveforms were then calculated by e-mouse.
Analyses at Necropsy. All mice in the experiment reported here were necropsied immediately after ECG analyses. Body and heart weights were recorded. Each tissue from each mouse was cut into small pieces, blotted to remove blood cells, and distributed separately for each of the procedures. Tissue samples collected for biochemistry and histochemistry were nitrogen-frozen, the latter in Tissue-Tek (Sakura Finetek, Torrance, CA). Samples for histopathology were fixed in 4% paraformaldehyde, 2% glutaraldehyde. For histochemistry, 10-μm cryostat sections stained for GUSB and counterstained with methyl green were evaluated microscopically for donor cell dispersion (44). The specific activity of GUSB enzyme and β-hexosamindase (HEXB) was determined in tissue lysates by using the fluorescent substrates 4-methylumbelliferyl-β-d-glucuronide and 4-methylumbelliferyl-N-acetyl-β-d-glucosaminide, respectively (Sigma). HEXB is increased dramatically in the absence of GUSB and offers an additional test for posttreatment lysosomal storage reduction.
Hearts from comparably aged +/+, untreated MPSVII, and treated MPSVII adult mice were evaluated by using an Olympus SZH dissecting microscope. Atrioventricular and semilunar valves were examined and photographed in hearts cut coronally. Lysosomal storage was evaluated microscopically on tissues embedded in Spurr's resin, sectioned at 1–2 μ, and stained with Toluidine blue (45). To evaluate the morphology of the conduction system and the myocardium and coronary arteries, hearts from treated and control mice were sectioned freehand into three slices of 3–5 mm in thickness. The slices were embedded in glycol methacrylate resin, and multiple serial sections of 1-μm thickness were taken at 10-μm intervals through all three slices. The atrioventricular conduction tissue was identified at the level of the interior interatrial septum and included the superior portion of the ventricular septum as muscle fibers with sparse myofibrils without prominent striations in a subendocardial location.
Results
Treated Mice Are Partially Repopulated and Functionally Improved. Using the genetic differences in donor/host glucose phosphate isomerase 1 isoforms, we demonstrated that a significant proportion of donor isoform was present in the PBl of all six nonablated MPSVII hosts (Table 1) used subsequently for ECG. Although there was variability between time points, the donor population was retained in both RBCs (averaging 9% overall) and WBCs (averaging 11% overall) for 11 months. The final PBl analysis on all six mice was performed 1 week before ECG.
Table 1. Percent repopulation of the host PBI.
| Evaluation at various intervals posttreatment
|
|||||
|---|---|---|---|---|---|
| Cells analyzed | 42 days, 6 mice | 168 days, 6 mice | 228 days, 3 mice | 315 days, 3 mice | 333 days, 6 mice |
| RBCs, % donor | 10.08 ± 2.03 | 17.05 ± 2.08 | 8.44 ± 0.52 | 4.35 ± 0.81 | 7.41 ± 1.88 |
| WBCs, % donor | 17.42 ± 1.63 | 9.62 ± 0.99 | 10.90 ± 3.24 | 7.25 ± 1.95 | 9.69 ± 1.09 |
Values are mean ± SEM.
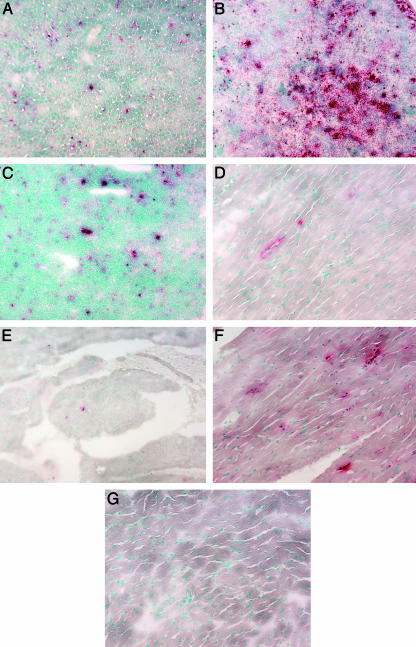
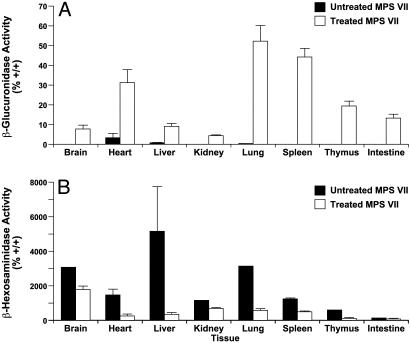
Donor cells identified by histochemical staining after necropsy were present in liver, spleen, and thymus (Fig. 1 A–C), bone, marrow, kidney, intestine, and brain (data not shown), and heart of the treated mice (Fig. 1 D and E) but not the untreated mice (Fig. 1G). Donor cells were dispersed in the interstitial cell population of the cardiac muscle and in the stroma of the heart valves. Biochemical analyses of GUSB enzyme confirmed the wide dispersion (Fig. 2A). The enzyme HEXB increased in the absence of GUSB and decreased in tissues with GUSB enzyme activity (Fig. 2B). The decrease in HEXB in the heart was statistically significant (P = 0.001).
Fig. 1.
Histochemical evaluation of GUSB+ (red staining) cells. Liver (A), spleen (B), thymus (C), heart myocardium (D), and heart valve (E) from an 11.5-month-old treated MPSVII mouse. The myocardium from an adult +/+ mouse (F) and an untreated MPSVII mouse (G) are presented for comparison. (Magnification: ×120.)
Fig. 2.
Evaluation of enzyme levels in untreated and treated MPSVII mice. (A) GUSB enzyme levels are expressed as a percent of +/+ values in treated (open bars) and untreated (filled bars) MPSVII tissues. Tissue fragments were blotted during collection to eliminate PBl cells. The increased level of GUSB in the untreated MPSVII heart is from a single mouse. Multiple repeats of the assay gave the same result. This finding may represent the activity of another enzyme acting on the substrate or authentic GUSB enzyme activity. Parallel bars represent the standard deviations between samples within a tissue. (B) HEXB enzyme levels are expressed as a percent of +/+ values in treated (open bars) and untreated (filled bars) MPSVII tissues.
To confirm that the treated mice enjoyed better health, a functional test of disease correction was used (46). The limitations on reproductive potential, detected in the untreated MPS-VII animals, were corrected in the treated MPSVII mice. As adults, the neonatally treated males bred on average 16.5 times with 8.5 successful pregnancies per male as defined by birth of live pups. Three treated females bred 4.7 times on average and produced an average of 5.8 pups per litter during the three successful pregnancies of each female. Lifespan was prolonged from an average of 6 months to at least ≥11 months, at which time the treated mice were necropsied.
Anomalous MPSVII ECGs Are Corrected in the Treated MPSVII Mice. To determine the effect of treatment on heart anomalies, ECG was performed on the conscious mice. Analyses of the digitized ECG signals demonstrated a significantly depressed mean heart rate in untreated MPSVII mice that returned to normal rate after treatment (Table 2). Heart rate variability indices in MPSVII mice, regardless of whether or not mice were treated as neonates, were reduced with respect to +/+ mice although these differences did not reach significance. The PR interval duration was significantly (P < 0.05) prolonged in untreated MPSVII mice compared to treated MPSVII mice and +/+ mice. The QRS interval in untreated MPSVII mice was significantly longer (P < 0.05) than that from +/+ and treated MPSVII mice. Overall, there was significant correction of ECG indices in the treated MPSVII mice (39, 40).
Table 2. ECG values obtained from the test mice.
| ECG measurements | Untreated MPSVII | Treated MPSVII | +/+ |
|---|---|---|---|
| No. of animals tested | 11 | 6 | 10 |
| Heart rate, bpm | 672 ± 26.97 | 746 ± 5.43 | 746 ± 10.42 |
| Heart rate variability, bpm* | 3.9 ± 0.8 | 7.3 ± 2.1 | 11.4 ± 2.4 |
| PR, ms | 33.8 ± 1.9 | 27.4 ± 1.6 | 25.7 ± 0.9 |
| PQ, ms | 28.2 ± 1.7 | 22.2 ± 1.6 | 20.6 ± 1.0 |
| QRS, ms | 9.4 ± 0.6 | 8.2 ± 0.1 | 8.2 ± 0.1 |
| QT, ms | 53.6 ± 5.0 | 46.3 ± 1.5 | 47.8 ± 2.0 |
Values are mean ± SEM. Bold numbers in the untreated MPSVII column are significantly different (P < 0.05) from both +/+ and treated MPSVII mice. There was no significant difference between +/+ and treated MPSVII mice.
An index of autonomic nervous control of heart rhythm was calculated as the mean of the square of differences between successive heart beats.
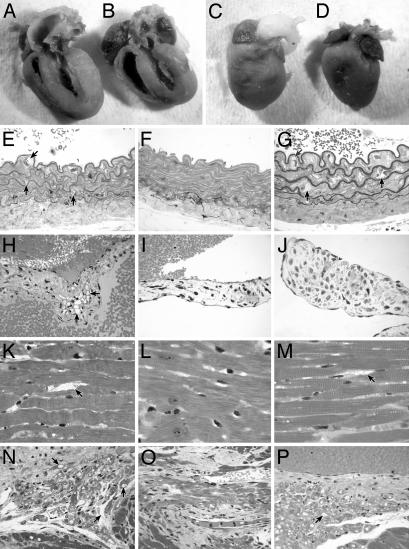
Heart Morphology Is Improved and Lysosomal Storage Is Decreased in the Treated MPSVII Mice. A series of evaluations was performed to assess differences in the treated and untreated MPSVII cardiac tissues. Hearts were enlarged with respect to the body weight in the untreated MPSVII mice. The heart weight/body weight ratio in mg/g was 8.6 compared to 6.4 mg/gin +/+ mice and 6.5 mg/g in treated MPSVII mice. The differences were significant between untreated and both +/+ (P = 0.047) and treated MPSVII mice (P = 0.036) but not between +/+ and treated mice (P = 0.87). Gross morphology also reflected the enlarged heart size between the smaller untreated MPSVII (average body weight, 21.3 g) (Fig. 3A) and the +/+ mice (average body weight, 25.5 g) (Fig. 3B).
Fig. 3.
Gross and microscopic comparisons of heart pathology. (A–D) For gross pathology, tissues were fixed in formalin. The untreated MPSVII heart and left ventricle (A) are enlarged with respect to the +/+ heart (B). The untreated aortic root (C) is grossly distended when compared to the normal aortic root (D). The heart and ventricle in treated MPSVII mice are similar in size to the +/+ mice, but the aortic root distension is not corrected (data not shown). (E–P) Lysosomal storage is noted with an arrow. These sections compare lysosomal storage in various cardiac structures. (E) The wall of the untreated MPSVII aorta has irregular elastic lamina. There is extensive storage in the intimal, medial, and adventitial cells by comparison with the +/+ aorta (F). (G) The treated MPSVII aorta shows similar storage as in the untreated mouse aorta. The only reduction in storage occurred in adventitial cells. (H)An untreated MPSVII heart valve has abundant storage in stromal cells when compared to a normal heart valve (I) and a treated MPSVII valve (J). The heart valve stromal cells had less storage in the treated MPSVII mice. In the myocardium, untreated mice (K) have abundant storage in the perivascular and interstitial cells when compared to those in +/+ (L) and treated (M) mice. The interstitial cells and myocytes of the conduction system in untreated mice (N) have storage when compared to those in normal mice (O). The interstitial cells but not the conduction tissue myocytes are cleared of storage in the treated MPSVII mice (P). (Magnification: ×100.)
The dorsal aortic root was distended in both the untreated (Fig. 3C) and treated (data not shown) MPSVII mice when compared to +/+ mice (Fig. 3D). Microscopically, the elastic lamina of the untreated MPSVII aorta (Fig. 3E) were more irregular than +/+ aortas (Fig. 3F) with zones of lamina thinning and abundant lysosomal storage in the intimal, medial, and adventitial cells. Storage persisted in the media and intima of the treated MPSVII mice, but was reduced in the adventitial cells (Fig. 3G).
Grossly, the untreated MPSVII aortic valves, unlike the +/+ and treated MPSVII valves, were thickened with focal areas of hemosiderin deposition in the valve stroma (data not shown). Microscopically, the untreated MPSVII valves had abundant lysosomal storage within the connective tissue stromal cells (Fig. 3H). By comparison, the treated MPSVII valves were grossly thin and translucent with little storage in the stromal cells (Fig. 3J) and much less sclerosis. A +/+ valve is shown for comparison (Fig. 3I). In the myocardium, untreated mice had a small amount of storage in the coronary artery medial cells and abundant storage in the perivascular and interstitial cells (Fig. 3K). Storage was alleviated by neonatal transplantation (Fig. 3M). The myocardium of a +/+ mouse is shown for comparison (Fig. 3L). Neither the treated nor untreated MPSVII mice had histologically apparent myocardial fibrosis, myocardial necrosis, endocardial fibroelastosis, or coronary artery narrowing caused by intimal sclerosis.
The myocytes in the conduction tissue from an untreated MPSVII mouse had a few large vacuoles, whereas the interstitial cells had abundant small vacuoles (Fig. 3N). After neonatal hematopoietic cell transplantation, the amount of vacuolization of the interstitial cells was markedly reduced (Fig. 3P) and resembled more that of a +/+ mouse (Fig. 3O) but vacuoles in the myocytes were retained.
Discussion
It has been estimated that the overall incidence of LSDs is 1/5,000–7,000 live births (47). Prevalence is higher for some, such as MPSI and Gaucher's disease, but others such as MPSVII, occur at a low incidence in the population. Cardiovascular and respiratory problems are major causes of death. In fact, early onset of cardiac manifestations can be a presenting feature of MPSI (2) and MPS type IH (1). Heart disease can become severe in MPSVI infants before other clinical manifestations (3, 6, 10). Therapy for the infantile-onset forms has been mainly supportive. There is one promising report of a significant decrease in the left ventricular mass at 36 weeks of age after ERT in four infants with Pompe's disease (28). Older children have shown modest clinical improvement after BM transplantation (12, 18). Interpretation of the transplantation results is difficult given the necessity of treating after the disease is established because of a delay in diagnosis, the high death rate from complications of either the disease or the treatment, and the phenotypic heterogeneity. BM transplantation for infantile-onset MPSI cardiac disease resolved left ventricular restriction in two patients within 1 year (18).
Here, we demonstrate some curative effects of neonatal nonablative BM transplantation for heart manifestations in a model for the LSD, MPSVII. Significant long-term correction of the heart rate, PR, and QRS intervals are associated with greatly extended lifespan (average 19 months, unpublished data) in the treated mice and alleviation of lysosomal storage in multiple organs. The average lifespan of untreated mice is 6 months (34). None have lived beyond 381 days. The relationship between lifespan and onset/severity of the heart lesions has not, as yet, been examined in detail to our knowledge. We have evidence that abnormal ECG values are not limited to the untreated older mice but can occur earlier. A survey of mice 100 days of age and older detected aberrant ECGs in at least one MPSVII mouse at the first time point. The early onset of heart manifestations suggests heart failure may be a cause of death. Sudden death occurs in 11% of patients with MPS, at least one of whom had an abnormality of the cardiac conduction system with first-degree atrioventricular block (48).
The relationship between ECG improvement and correction of lesions had not been studied in an animal LSD model previously to our knowledge. Here, the major improvements noted after necropsy of the neonatally treated MPSVII mice include changes in both the gross and microscopic pathology. The heart size normalizes, valve architecture improves, and storage in perivascular and interstitial cells of the conduction system and myocardium is reduced. The cardiac response to ERT is not as well documented. Valve improvements and disappearance of storage in both the aorta and heart valves are noted after retroviral gene transfer therapy in the murine MPSVII neonate (30). These changes are consistent with gene therapy results in MPSVII newborn dogs where mitral valve regurgitation assessed by echocardiography was minimal or absent by 8–9 months posttreatment (49). Here, the distension of the dorsal aortic root and storage in the intimal and medial layers of the aorta and in the conduction system myocytes are detected in both treated and untreated mice and likely have little effect on the ECG pattern. This finding does not mean the remaining defects are without consequence.
The voltage interval alterations in the untreated MPSVII mouse can be assessed with respect to the morphologic changes. The P wave is associated with atrial depolarization; the PR interval is associated with atrioventricular conduction; the QRS wave occurs during ventricular depolarization; and the T wave is consonant with ventricular repolarization. In the untreated MPSVII mice, the PR and QRS intervals are prolonged compared to those in normal and treated MPSVII mice. Lysosomal storage in the interstitial cells of the nodes in untreated MPSVII mice and its alleviation after neonatal BM transplantation suggest that interstitial cell storage may contribute to the prolonged PR and QRS intervals. Alternatively, a change in the character of the interstitial extracellular connective tissue without morphologic correlate but related to more normal glycosaminoglycan metabolism by interstitial cells could also impact conduction velocity. Sudden death in a patient with Hunter syndrome occurred during Holter ECG examination and was traced to atrioventricular block (48). Loss of 70% of the cells in the penetrating portion of the bundle of His was noted at autopsy. In the current evaluation, the number of cells did not appear to differ, but the accumulation of lysosomal storage material in support cells was significantly higher in conduction tissue of untreated animals. Notably, heart rate variability, an index of autonomic nervous control, was lowest in untreated MPSVII mice. This finding may reflect damage to neurons, thereby disrupting a healthier balance of sympathetic and parasympathetic signaling to the heart.
The increase in the QRS interval in untreated MPSVII mice may reflect the significant cardiac hypertrophy in these animals. Humans with Pompe's syndrome also have a prolonged QRS (8). The QRS interval is increased as well in a knockout model of Pompe's disease. We witnessed an increase in QRS interval in the untreated MPSVII mouse that is indicative in glycogenosis type II humans of biventricular or left ventricular hypertrophy. A transgenic mouse line with a mutation in the γ2 subunit (PRKAG2) of AMP-activated protein kinase accumulates large amounts of cardiac glycogen and develops significant cardiac hypertrophy (50). These mutant mice exhibited multiple ECG anomalies, including prolonged QRS interval duration. α-Glucosidase knockout mice demonstrate progressive glycogen accumulation and cardiac hypertrophy (51). Several knockout mice had abnormal ECGs with a high-voltage QRS complex as seen in human glycogenosis type II. It may not have been possible for those authors to detect prolonged QRS interval durations in those anesthetized mice. There was a recent clinical report of a patient with Fabry's disease, a glycosphingolipid storage disorder, who presented with a prolonged PR interval duration and a QRS complex suggestive of left ventricular hypertrophy (52). We found that GUSB null mice display aberrant glycosaminoglycan storage in the heart, increased QRS interval duration, and cardiac hypertrophy. Taken together, these findings indicate that the storage accumulation may account for the cardiac hypertrophy that is indexed by a prolonged QRS interval duration.
The aberrant ECG and many of the progressive heart lesions are corrected long term in the MPSVII mice by neonatal nonablative BM transplantation. With the exception of the brain, lysosomal storage is relieved to some extent in all tissues in the donor/host chimera, and the lifespan is dramatically lengthened. Allogeneic neonatal transplantation in MPSVII mice is now feasible, and donor cells can be boosted to 100% thereafter (36). This level of chimerism may overcome concerns about the decreased effectiveness of partial engraftments in the human. Our rationale for performing nonablative congenic transplantation in neonates was to establish an earlier, less toxic approach for autologous gene transfer therapy. The major challenge remaining in gene therapy, such as carcinogenicity (53), make this route less likely in the immediate future. The major challenge in neonatal BM transplantation is to prevent the accumulation of lysosomal storage in brain. GUSB ERT in MPSVII neonates protects the brain for at least 6 weeks in mice (54), although protection in the human may be limited by earlier closure of the blood brain barrier. With simultaneous BM transplantation, ERT may provide even more therapeutic benefit than BM transplantation alone. The effect of such combined treatments, for example, may provide better therapy for cardiovascular manifestations of disease as well. An example is the persistence of aortic lesions even after neonatal BM transplantation. In this case, the aortic distension and wall abnormalities may be developmental defects that occurred before the establishment of sufficient donor cells to provide corrective enzyme.
Acknowledgments
We thank Babette Gwynn, Dr. Brian W. Soper, and Dr. Greg Cox for presubmission reviews and Dr. Robert Gourdie at the Medical University of South Carolina (Charleston) for describing techniques to section the cardiac conduction system in the mouse. This research was supported by National Institutes of Health Grants DK49525 (to J.E.B. and C.A.V.) and DK27726 (to J.E.B., Brian W. Soper, and C.A.V.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LSD, lysosomal storage disease; BM, bone marrow; ERT, enzyme replacement therapy; GUSB, β-glucuronidase; MPS, mucopolysaccharidosis; MPSI, MPS type I; MPSVII, MPS type VII; PBl, peripheral blood; HEXB, β-hexosamindase.
References
- 1.Donaldson, M. D., Pennock, C. A., Berry, P. J., Duncan, A. W., Cawdery, J. E. & Leonard, J. V. (1989) J. Pediatr. 114, 430–432. [DOI] [PubMed] [Google Scholar]
- 2.Stephan, M. J., Stevens, E. L., Wenstrup, R. J., Greenberg, C. R., Gritter, H., Hodges, G. F. & Guller, B. (1989) Am. J. Dis. Child. 143, 782–784. [DOI] [PubMed] [Google Scholar]
- 3.Hayflick, S., Rowe, S., Kavanaugh-McHugh, A., Olson, J. L. & Valle, D. (1992) J. Pediatr. 120, 269–270. [DOI] [PubMed] [Google Scholar]
- 4.Sly, W. S., Brot, F. E., Glaser, J. G., Stahl, P. D., Quinton, B. A., Rimoin, D. L. & McAllister, W. H. (1974) Birth Defects 12, 239–245. [PubMed] [Google Scholar]
- 5.Shipley, J. M., Klindenberg, M., Wu, B. M., Bachinsky, D. R., Grubb, J. H. & Sly, W. S. (1993) Am. J. Hum. Genet. 52, 517–526. [PMC free article] [PubMed] [Google Scholar]
- 6.Fong, L. B., Menahem, S., Wraith, J. E. & Chow, C. W. (1987) Clin. Cardiol. 10, 362–364. [DOI] [PubMed] [Google Scholar]
- 7.Gillette, P. C., Nihill, M. R. & Singer, D. B. (1974) Am. J. Dis. Child. 128, 622–626. [DOI] [PubMed] [Google Scholar]
- 8.Lenard, H. G., Schaub, J., Keutel, J. & Osand, M. (1974) Neuropadiatrie 5, 410–424. [DOI] [PubMed] [Google Scholar]
- 9.Seifert, B. L., Snyder, M. S., Klein, A. A., O'Loughlin, J., Magid, M. S. & Engle, M. A. (1992) Am. Heart J. 123, 239–242. [DOI] [PubMed] [Google Scholar]
- 10.Miller, G. & Partridge, A. (1983) Pediatr. Cardiol. 4, 61–62. [DOI] [PubMed] [Google Scholar]
- 11.Butman, S. M., Karl, L. & Copeland, J. G. (1989) Chest 96, 209–210. [DOI] [PubMed] [Google Scholar]
- 12.Tan, C. T., Schaff, H. V., Miller, F. A., Edwards, W. D. & Karnes, P. S. (1992) Circulation 85, 188–195. [DOI] [PubMed] [Google Scholar]
- 13.Fischer, T. A., Lehr, H.-A., Nixdorff, U. & Meyer, J. (1999) Heart 81, 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vinallonga, X., Sanz, N., Balaguer, A., Miro, L., Ortega, J. J. & Casaldaliga, J. (1992) Pediatr. Cardiol. 13, 107–109. [DOI] [PubMed] [Google Scholar]
- 15.Krivit, W., Peirpont, M. E., Ayaz, K., Tsai, M., Ramsay, N. K., Kersey, J. H., Weisdorf, S., Sibley, R., Snover, D., McGovern, M. K., et al. (1984) N. Eng. J. Med. 311, 1606–1611. [DOI] [PubMed] [Google Scholar]
- 16.Herskhovitz, E., Young, E., Rainer, J., Hall, C. M., Lidchi, V., Chong, K. & Vellodi, A. (1999) J. Inherited Metab. Dis. 22, 50–62. [DOI] [PubMed] [Google Scholar]
- 17.Wilson, C. S., Mankin, H. T. & Pluth, J. R. (1980) Ann. Intern. Med. 92, 496–498. [DOI] [PubMed] [Google Scholar]
- 18.Gatzoulis, M. A., Vellodi, A. & Redington, A. N. (1995) Arch. Dis. Child. 73, 259–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamback, B. J., Mosser, K., Blakemore, K., Corson, B. L., Griffin, C. A., Noga, S. J., Perlman, R., Zuckerman, R., Wenger, D. A. & Jones, R. J. (1997) Bone Marrow Transplant 19, 399–402. [DOI] [PubMed] [Google Scholar]
- 20.Natowicz, M. R., Chi, M., Lowry, O. H. & Sly, W. S. (1979) Proc. Natl. Acad. Sci. USA 76, 4322–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shull, R. M., Kakkis, E. D., McEntee, M. F., Kania, S. A., Jonas, A. J. & Neufeld, E. F. (1994) Proc. Natl. Acad. Sci. USA 91, 12937–12941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakkis, E. D., McEntee, M. F., Schmidtchen, A., Neufeld, E. F., Ward, D. A., Gompf, R. A., Kania, S., Bedolla, C., Chien, S.-L. & Shull, R. M. (1996) Biochem. Mol. Med. 58, 156–167. [DOI] [PubMed] [Google Scholar]
- 23.Kakkis, E. D., Muenzer, J., Tiller, G. E., Waber, L., Belmont, J., Passage, M., Izykowski, B., Phillips, J., Doroshow, R., Walot, I., et al. (2001) N. Engl. J. Med. 344, 182–188. [DOI] [PubMed] [Google Scholar]
- 24.Crawley, A. C., Niedzielski, K. H., Isaac, E. L., Davey, R. C. A., Byers, S. & Hopwood, J. J. (1997) J. Clin. Invest. 99, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi, T., Yang, H. W., Pennybacker, M., Ichihara, N., Mizutani, M., Van Hove, J. L. & Chen, Y. T. (1998) J. Clin. Invest. 101, 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van den Hout, H., Reuser, A. J., Vulto, A. G., Loonen, M. C., Cromme-Dijkhuis, A. & Van der Ploeg, A. T. (2000) Lancet 356, 397–398. [DOI] [PubMed] [Google Scholar]
- 27.Van den Hout, J. M., Reuser, A. J., de Klerk, J. B., Arts, W. F., Smeitink, J. A. & Van der Ploeg, A. T. (2001) J. Inherited Metab. Dis. 24, 266–274. [DOI] [PubMed] [Google Scholar]
- 28.Schiffmann, R. & Brady, R. (2002) Drugs 62, 733–742. [DOI] [PubMed] [Google Scholar]
- 29.Daly, T. M., Vogler, C., Levy, B., Haskins, M. E. & Sands, M. S. (1999) Proc. Natl. Acad. Sci. USA 96, 2296–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu, L., Mango, R. L., Sands, M. S., Haskins, M. E., Ellinwood, N. M. & Ponder, K. P. (2002) Mol. Ther. 6, 745–758. [DOI] [PubMed] [Google Scholar]
- 31.Ponder, K. P., Melniczek, J. R., Xu, L., Weil, M. A., O'Malley, T. M., O'Donnell, P. A., Knox, V. W., Aguirre, G. D., Mazrier, H., Ellinwood, N. M., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 13102–13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barker, J. E., Kaysser-Kranich, T. M., Hamblen, N. & Deveau, S. (1999) Exp. Hematol. 27, 966–971. [DOI] [PubMed] [Google Scholar]
- 33.Soper, B. W., Lessard, M. D., Vogler, C. A., Levy, B., Beamer, W. G., Sly, W. S. & Barker, J. (2001) Blood 97, 1498–1504. [DOI] [PubMed] [Google Scholar]
- 34.Birkenmeier, E. H., Davisson, M. T., Beamer, W. G., Ganschow, R. E., Vogler, C. A., Gwynn, B., Lyford, K. A., Maltais, L. M. & Wawrzniak, C. J. (1989) J. Clin. Invest. 83, 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barker, J. E., Deveau, S., Lessard, M., Hamblen, N., Vogler, C. & Levy, B. (2001) Blood Cells Mol. Dis. 27, 861–873. [DOI] [PubMed] [Google Scholar]
- 36.Soper, B. W., Lessard, M. D., Jude, C. D., Schuldt, A. J. T., Bunte, R. & Barker, J. E. (2003) J. Immunol. 171, 3270–3277. [DOI] [PubMed] [Google Scholar]
- 37.Barker, J. E., Braun, J. & McFarland-Starr, E. C. (1988) Proc. Natl. Acad. Sci. USA 85, 7332–7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eppig, J. J., Kozak, L. P., Eicher, E. M. & Stevens, L. C. (1977) Nature 269, 517–518. [DOI] [PubMed] [Google Scholar]
- 39.DeAngelis, J. D., Lopez, O., Yepes, D., Otero, J. M. & Hampton, T. G. (1999) Circulation 100, 1–276. [Google Scholar]
- 40.Chu, V., Otero, J. M., Lopez, O., Morgan, J. P., Amende, I. & Hampton, T. G. (2001) BMC Physiol. 1, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell, G. F., Jeron, A. & Koren, G. (1998) Am. J. Physiol. 274, H747–H751. [DOI] [PubMed] [Google Scholar]
- 42.Kirchhoff, S., Nelles, E., Hagendorff, A., Kruger, O., Traub, K. & Willecke, K. (1998) Curr. Biol. 8, 299–302. [DOI] [PubMed] [Google Scholar]
- 43.Wang, L., Swirp, S. & Duff, H. (1998) Am. J. Physiol. 278, C73–C80. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe, J. H. & Sands, M. S. (1996) in Protocols for Gene Transfer in Neurological Disorders, eds. Lowenstein, P. R. & Enquist, L. W. (Wiley, Chichester, U.K.), pp. 263–274.
- 45.Vogler, C., Birkenmeier, E. H., Sly, W. S., Levy, B., Pegors, C., Kyle, J. W. & Beamer, W. G. (1990) Am. J. Pathol. 136, 207–217. [PMC free article] [PubMed] [Google Scholar]
- 46.Soper, B. W., Pung, A. W., Vogler, C. A., Grubb, J. H., Sly, W. S. & Barker, J. E. (1999) Pediatr. Res. 45, 180–186. [DOI] [PubMed] [Google Scholar]
- 47.Meikle, P. J., Ranieri, E., Ravenscroft, E. M., Hua, C. T., Brooks, D. A. & Hopwood, J. J. (1999) SE Asian J. Trop. Med. Public Health 30, 104–110. [PubMed] [Google Scholar]
- 48.Hisbitani, T. Wakita, S., Isoda, T., Katori, T. & Ishizawa, A. (2000) J. Pediatr. 136, 268–269. [DOI] [PubMed] [Google Scholar]
- 49.Sammarco, C., Weil, M., Just, C., Weimelt, S., Hasson, C., O'Malley, T., Evans, S. M., Wang, P., Casal, M. L., Wolfe, J., et al. (2000) Bone Marrow Transplant 25, 1289–1297. [DOI] [PubMed] [Google Scholar]
- 50.Arad, M., Moskowitz, I. P., Patel, V. V., Perez-Atayde, A. R., Sawyer, D. B., Walter, M., Li, G. H., Burgon, P. G., Maguire, C. T., Stapleton, D., et al. (2003) Circulation 107, 2850–2856. [DOI] [PubMed] [Google Scholar]
- 51.Bijvoet, A. G., van de Kamp, E. H. M., Kroos, M. A., Ding, J.-H., Yang, B. Z., Visser, P., Bakker, C. E., Verbeet, M. P., Oostra, B. A., Reuser, A. J. J., et al. (1998) Hum. Mol. Genet. 7, 53–62. [DOI] [PubMed] [Google Scholar]
- 52.Blum, A., Ashkenazi, H., Haromankov, I., Khazim, K. & Sheiman, J. (2003) South Med. J. 96, 212–223. [DOI] [PubMed] [Google Scholar]
- 53.Donsante, A., Vogler, C., Muzyczka, N., Crawford, J., Barker, J., Flotte, T. & Sands, M. (2001) Gene Ther. 8, 1343–1346. [DOI] [PubMed] [Google Scholar]
- 54.Vogler, C., Levy, B., Galvin, N. J., Thorpe, C., Sands, M. S., Barker, J. E., Baty, J., Birkenmeier, E. H. & Sly, W. S. (1999) Pediatr. Res. 45, 838–844. [DOI] [PubMed] [Google Scholar]